Photodynamic and Sonodynamic Antibacterial Activity of Grape Leaf Extracts
Abstract
1. Introduction
2. Results and Discussion
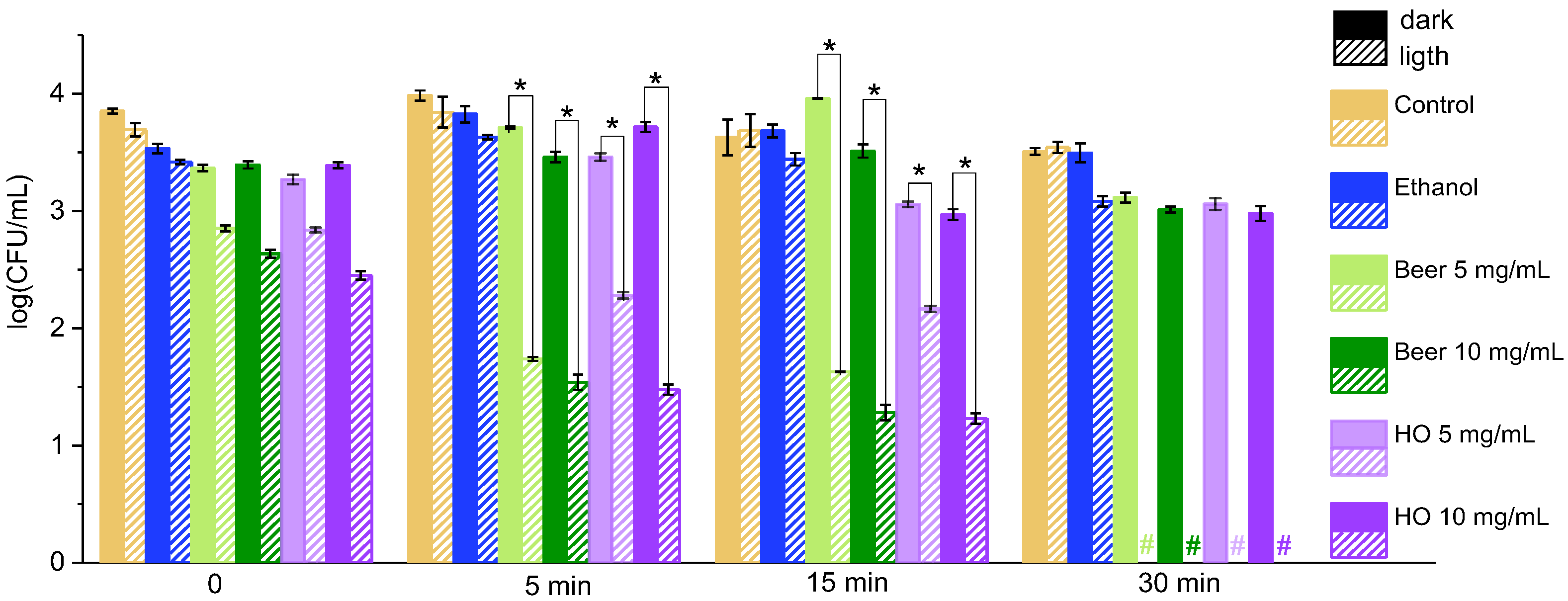
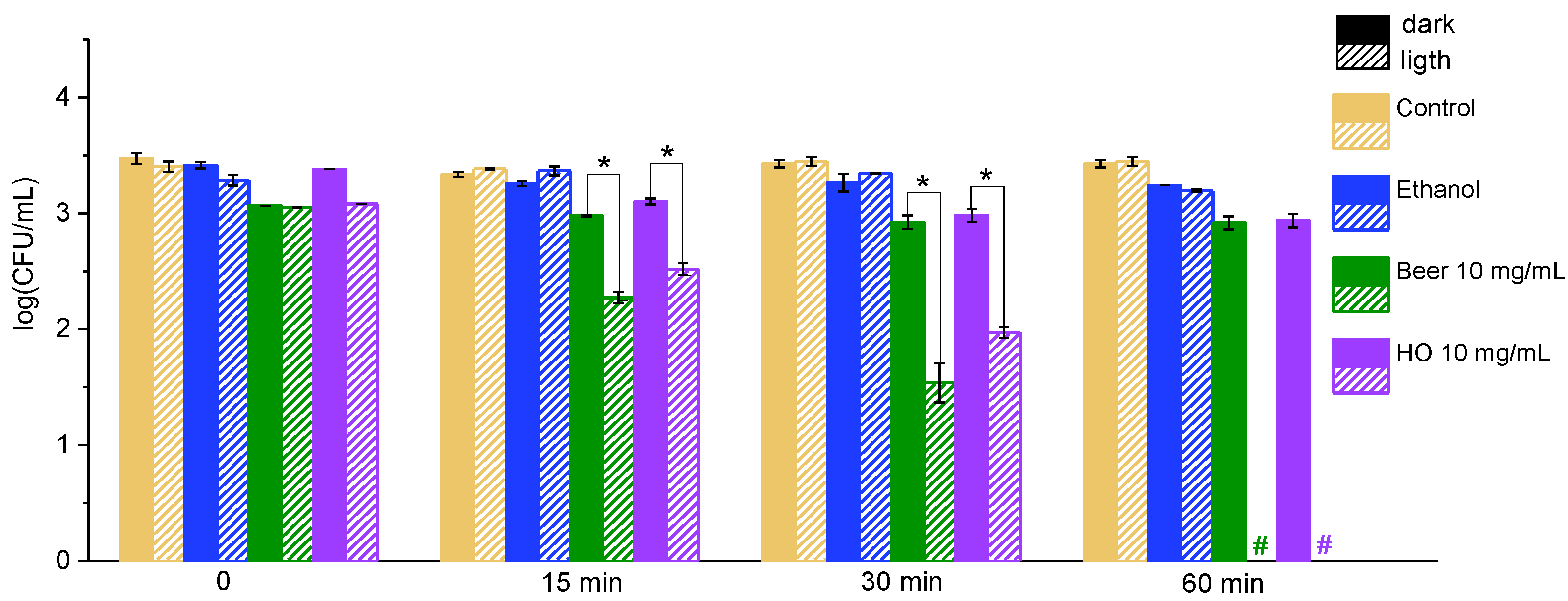
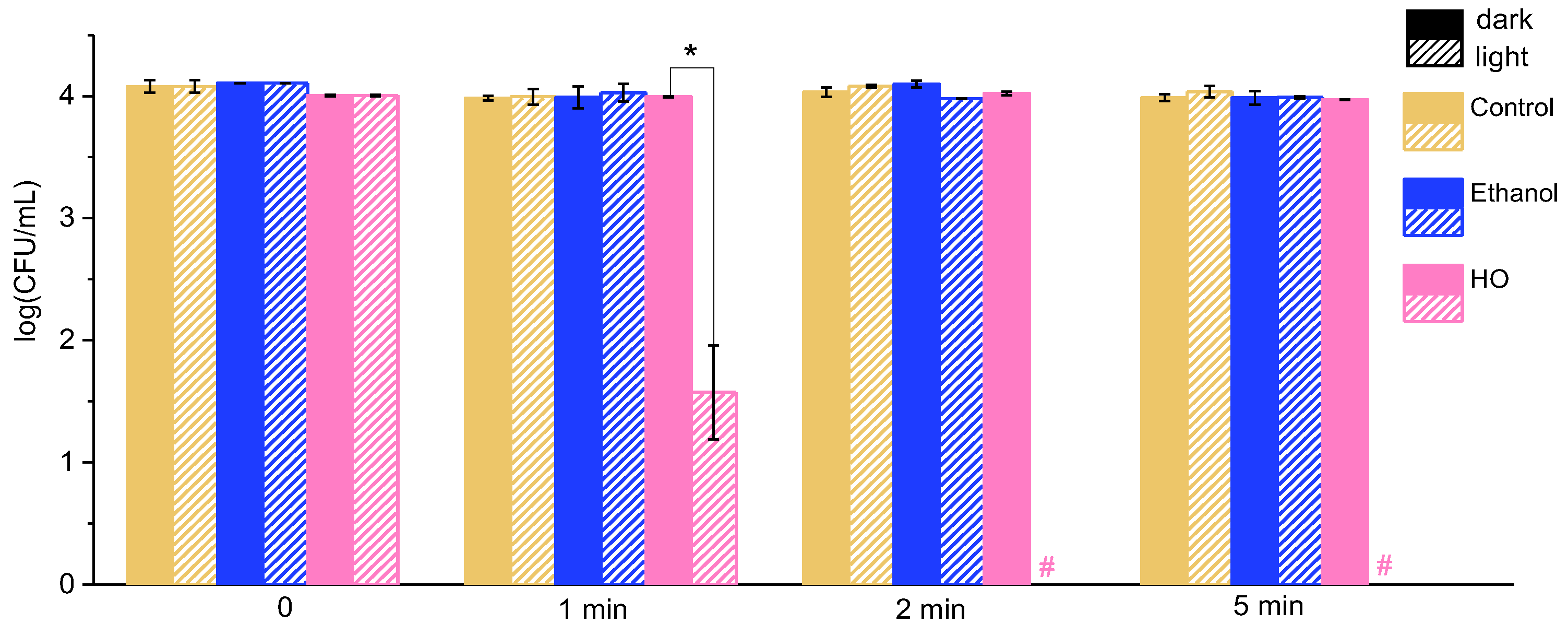
2.1. Effect of Grape Leaf Extracts on Bacteria Under Illumination
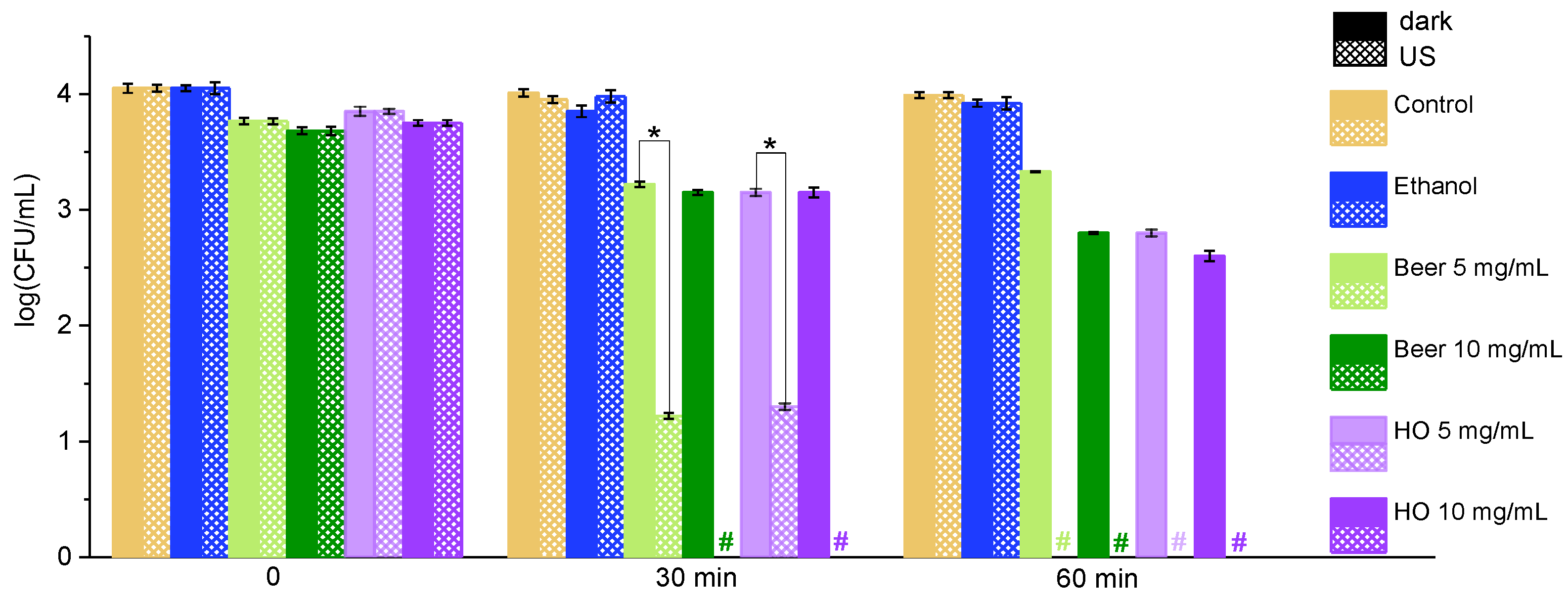
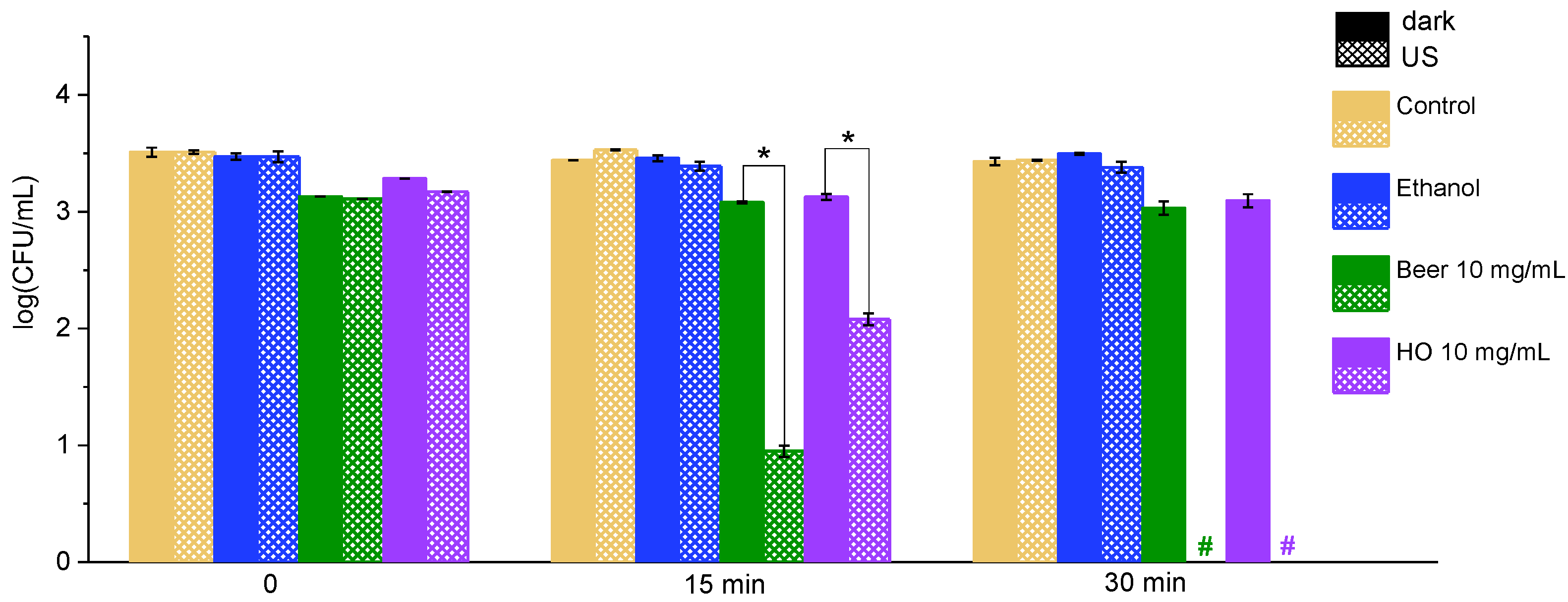
2.2. Effect of the Extracts on Bacteria Under Exposure to Ultrasound
2.3. Fractionation of Hanut Orcha Extract and Testing Antibacterial Activity of Various Fractions
2.4. Characterization of the Active Fraction from the Extract
2.4.1. Spectroscopic Analysis of the Active Fraction
2.4.2. Measurements of Singlet Oxygen Generation
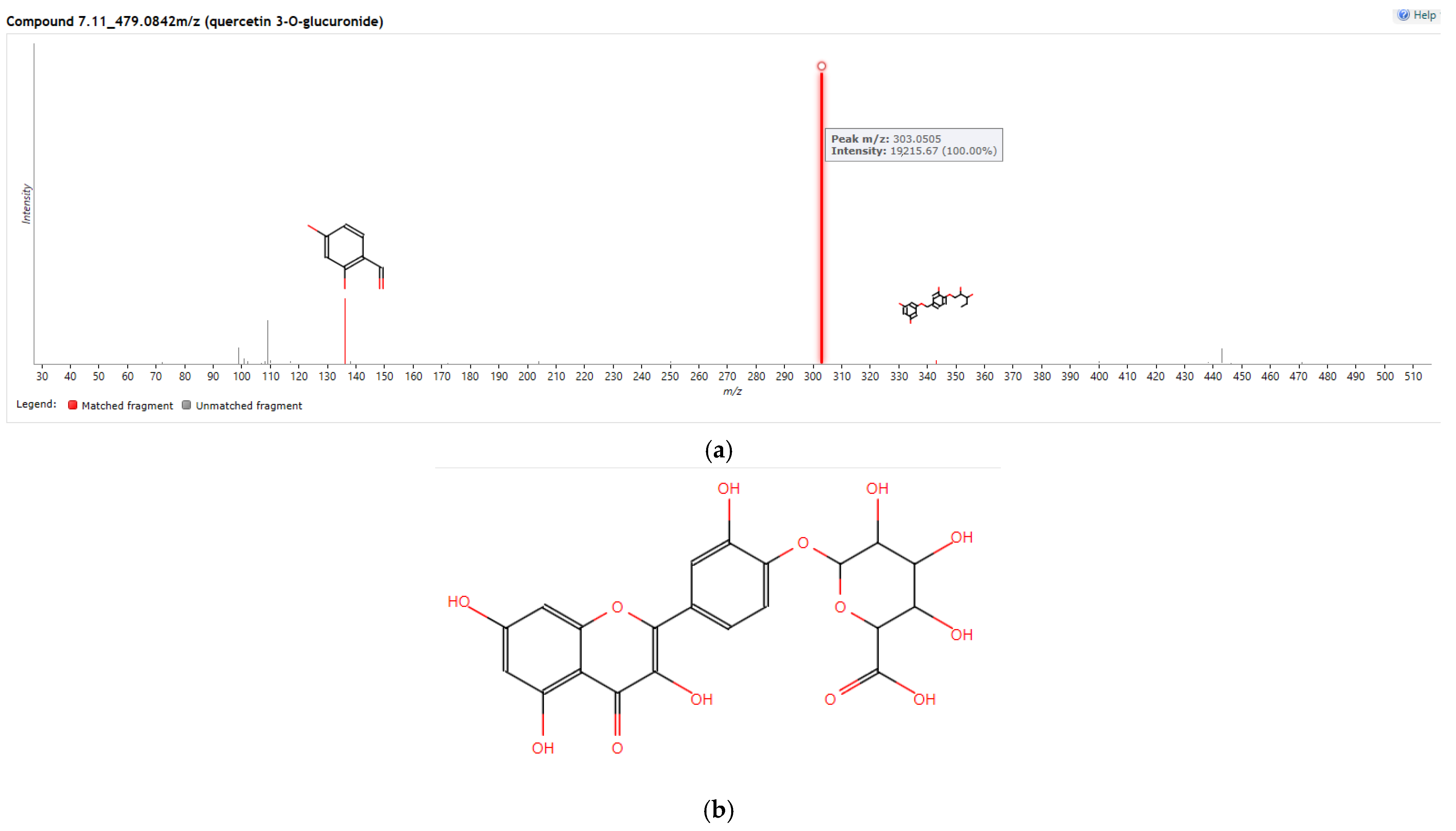
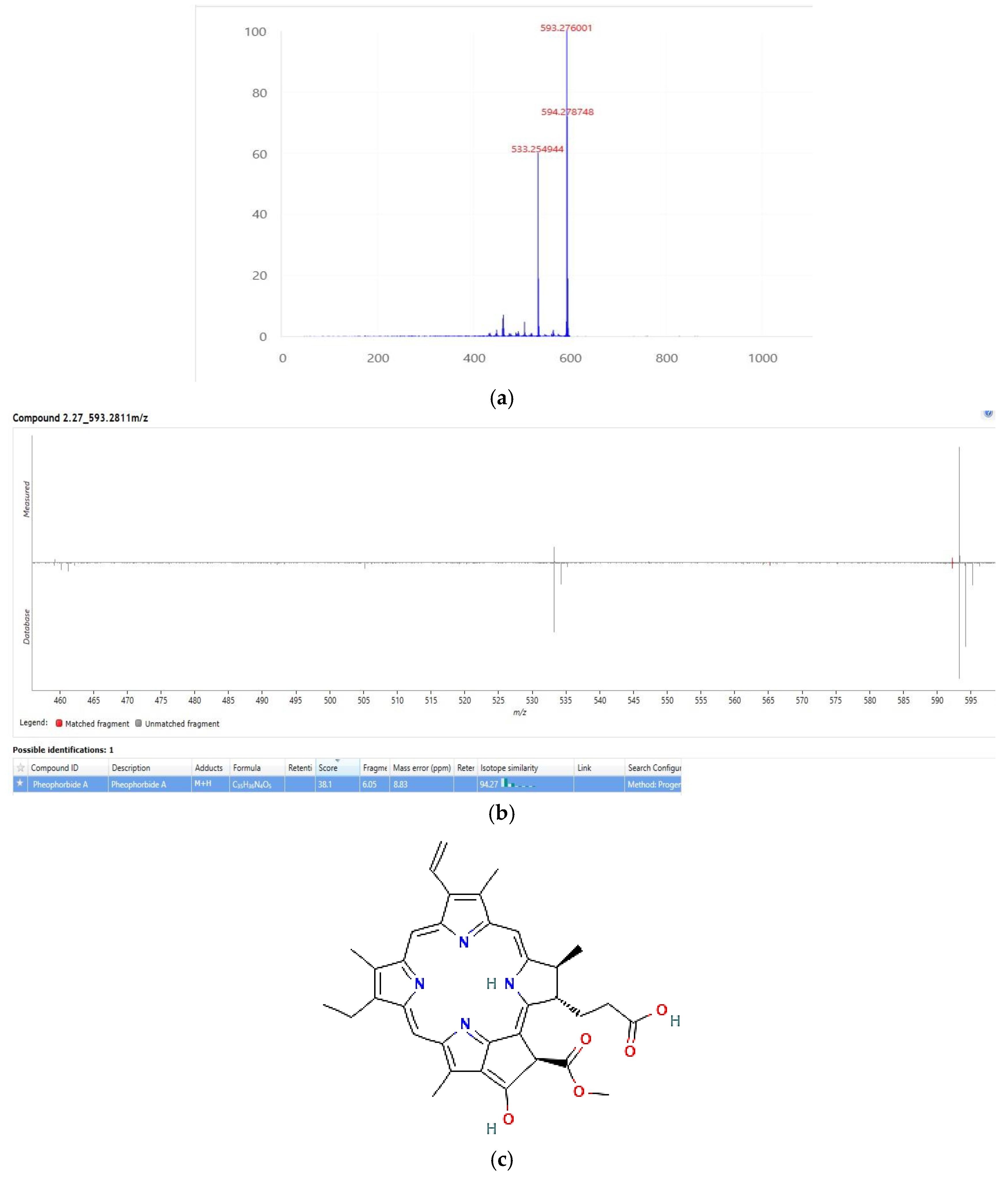
2.4.3. HPLC/MS Analysis of the Active Fraction
3. Materials and Methods
3.1. Extraction of Bioactive Compounds from Grape Leaves
3.2. Fractionation and Separation of Bioactive Compounds
3.3. Bacterial Strains and Culture Preparation
3.4. Testing Photodynamic and Sonodynamic Activity of the Crude and Fractionated Grape Leaf Extracts
3.5. Characterization of the Extracts and the Active Fraction from the Hanut Orcha Extract
3.5.1. Spectroscopic Assay
3.5.2. Generation of ROS
3.5.3. HPLC/MS Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| HPLC | High-performance liquid chromatography |
| MS | Mass spectrum |
| ACN | Acetonitrile |
| SOSG | Singlet Oxygen Sensor Green |
| MB | Methylene blue |
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow®: The First European Food Safety Authority-Approved Natural Cardio-Protective Functional Ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Das, M.; Tripathi, A. Sodium Benzoate, a Food Preservative, Affects the Functional and Activation Status of Splenocytes at Non-Cytotoxic Dose. Food Chem. Toxicol. 2016, 88, 40–47. [Google Scholar] [CrossRef]
- Ren, L.; Meng, M.; Wang, P.; Xu, Z.; Eremin, S.A.; Zhao, J.; Yin, Y.; Xi, R. Determination of Sodium Benzoate in Food Products by Fluorescence Polarization Immunoassay. Talanta 2014, 121, 136–143. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; De Sun, C. Citrus Flavonoids and Their Antioxidant Evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Aiyegoro, O.A.; Okoh, A.I. Preliminary Phytochemical Screening and In Vitro Antioxidant Activities of the Aqueous Extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010, 10, 21. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.A.; Kahouadji, A.; Khedid, K.; Mousaddak, M.; Ouaffak, L.; Charof, R.; Mennane, Z. Evaluation of Antimicrobial Activity of Aqueous and Ethanolic Extracts of Leaves of Vitis vinifera Collected from Different Regions in Morocco. Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 85–90. [Google Scholar]
- Nermin, S.; Abdelhamid, N.; Hassan, H.; Mattar, Z.; Abou-Taleb, K.; Ramadan, E. Screening of Some Egyptian Plant Extracts for Biological Activity against Some Pathogenic Bacteria. Arab. Univ. J. Agric. Sci. 2017, 25, 377–386. [Google Scholar] [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, X.; Zhen, X. Development of Organic Photosensitizers for Antimicrobial Photodynamic Therapy. Biomater. Sci. 2023, 11, 5108–5128. [Google Scholar] [CrossRef] [PubMed]
- Mikulich, A.V.; Plavskii, V.Y.; Tretyakova, A.I.; Nahorny, R.K.; Sobchuk, A.N.; Dudchik, N.V.; Emeliyanova, O.A.; Zhabrouskaya, A.I.; Plavskaya, L.G.; Ananich, T.S.; et al. Potential of Using Medicinal Plant Extracts as Photosensitizers for Antimicrobial Photodynamic Therapy. Photochem. Photobiol. 2024, 100, 1833–1847. [Google Scholar] [CrossRef]
- Bonifácio, D.; Martins, C.; David, B.; Lemos, C.; Neves, M.G.P.M.S.; Almeida, A.; Pinto, D.C.G.A.; Faustino, M.A.F.; Cunha, Â. Photodynamic Inactivation of Listeria innocua Biofilms with Food-Grade Photosensitizers: A Curcumin-Rich Extract of Curcuma longa vs. Commercial Curcumin. J. Appl. Microbiol. 2018, 125, 282–294. [Google Scholar] [CrossRef]
- Marqués-Calvo, M.S.; Codony, F.; Agustí, G.; Lahera, C. Visible Light Enhances the Antimicrobial Effect of Some Essential Oils. Photodiagn. Photodyn. Ther. 2017, 17, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Hwang, Y.J. Natural Phytochemical and Visible Light at Different Wavelengths Show Synergistic Antibacterial Activity against Staphylococcus aureus. Pharmaceutics 2024, 16, 612. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Edel, F.; Manandhar, N.P.; Towers, G.H. Antimicrobial Activities of Southern Nepalese Medicinal Plants. J. Ethnopharmacol. 1996, 50, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Q.; Gao, A.; Tang, N.; Zhang, Q.; Zhang, A.; Cui, D. Recent Developments of Sonodynamic Therapy in Antibacterial Application. Nanoscale 2022, 14, 12999–13017. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, S.; Xu, T.; Huang, L.; Zhang, Y.; Lan, M.; Lin, C.; Zheng, X.; Wang, P. Advances and Perspectives in Organic Sonosensitizers for Sonodynamic Therapy. Coord. Chem. Rev. 2021, 445, 214087. [Google Scholar] [CrossRef]
- Wang, X.; Ip, M.; Leung, A.W.; Yang, Z.; Wang, P.; Zhang, B.; Ip, S.; Xu, C. Sonodynamic Action of Curcumin on Foodborne Bacteria Bacillus cereus and Escherichia coli. Ultrasonics 2015, 62, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Rahimi Esboei, B.; Hodjat, M.; Bahador, A. Sonodynamic Excitation of Nanomicelle Curcumin for Eradication of Streptococcus mutans under Sonodynamic Antimicrobial Chemotherapy: Enhanced Anti-Caries Activity of Nanomicelle Curcumin. Photodiagn. Photodyn. Ther. 2020, 30, 101780. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahrami, R.; Bazarjani, F.; Bahador, A. Anti-Multispecies Microbial Biofilms and Anti-Inflammatory Effects of Antimicrobial Photo-Sonodynamic Therapy Based on Acrylic Resin Containing Nano-Resveratrol. Photodiagn. Photodyn. Ther. 2023, 43, 103669. [Google Scholar] [CrossRef]
- Orhan, D.D.; Orhan, N.; Özçelik, B.; Ergun, F. Biological Activities of Vitis vinifera L. Leaves. Turk. J. Biol. 2009, 33, 341–348. [Google Scholar] [CrossRef]
- Güvensen, N.C.; Keskin, D.; Zorlu, Z.; Uğur, A. In-Vitro Antimicrobial Activities of Different Extracts of Grapevine Leaves (Vitis vinifera L.) from West Anatolia against Some Pathogenic Microorganisms. J. Pure Appl. Microbiol. 2012, 6, 1303–1308. [Google Scholar]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic Natural Products as Photosensitizers for Antimicrobial Photodynamic Therapy: Recent Advances and Future Prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, D.; Ashokkumar, M.; Ye, X.; Jin, T.Z.; Guo, M. Antibacterial Mechanism of Ultrasound against Escherichia coli: Alterations in Membrane Microstructures and Properties. Ultrason. Sonochem. 2021, 73, 105509. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Goon, K.; Gilbert, A.; Nguyen Huu, C.; Walsh, M.; Nitin, N.; Wrenn, S.; Tikekar, R.V. Inactivation of Foodborne Pathogens Based on Synergistic Effects of Ultrasound and Natural Compounds during Fresh Produce Washing. Ultrason. Sonochem. 2020, 64, 104983. [Google Scholar] [CrossRef]
- Fan, L.; Muhammad, A.I.; Ismail, B.B.; Liu, D. Sonodynamic Antimicrobial Chemotherapy: An Emerging Alternative Strategy for Microbial Inactivation. Ultrason. Sonochem. 2021, 75, 105591. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial Activity of the Crude Extract, Fractions and Compounds from Stem Bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef]
- Etame, R.E.; Mouokeu, R.S.; Pouaha, C.L.C.; Kenfack, I.V.; Tchientcheu, R.; Assam, J.P.A.; Poundeu, F.S.M.; Tiabou, A.T.; Etoa, F.X.; Kuiate, J.R.; et al. Effect of Fractioning on Antibacterial Activity of Enantia chlorantha Oliver (Annonaceae) Methanol Extract and Mode of Action. Evid. Based Complement. Altern. Med. 2018, 2018, 4831593. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Obiiyeke, G.E.; Chigor, V.N.; Okoh, A.I. Assessment of Tamarindus indica Extracts for Antibacterial Activity. Int. J. Mol. Sci. 2011, 12, 6385–6396. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Ngene, A.A.; Iroegbu, C.U.; Gc, O. Effects of Fractionation on Antibacterial Activity of Crude Extracts of Tamarindus indica. Afr. J. Biotechnol. 2010, 9, 7108–7113. [Google Scholar] [CrossRef]
- Sidjui, L.S.; Toghueo, R.M.K.; Menkem Zeu, E.; Mbouna, C.D.J.; Leddet, V.M.; Herbette, G.; Fekam, F.B.; Ollivier, E.; Folefoc, G.N. Antibacterial Activity of the Crude Extracts, Fractions and Compounds from the Stem Barks of Jacaranda mimosifolia and Kigelia africana (Bignoniaceae). Pharmacologia 2016, 7, 22–31. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Orhan, D.D.; Ergun, F.; Yeşilada, E. In-Vivo Assessment of Antidiabetic and Antioxidant Activities of Grapevine Leaves (Vitis vinifera) in Diabetic Rats. J. Ethnopharmacol. 2006, 108, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Salie, M.; Garcia, E.; Reyes, A.; Ebersole, S.C.; Naegele, R.P.; Van Zyl, S. A New Method for Fractionation and Characterization of Polyphenols and Tannins from Grapevine Leaf Tissue. Plants 2023, 12, 1706. [Google Scholar] [CrossRef]
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility Study on Quantitative Measurements of Singlet Oxygen Generation Using Singlet Oxygen Sensor Green. J. Fluoresc. 2013, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ragàs, X.; Jiménez-Banzo, A.; Sánchez-García, D.; Batllori, X.; Nonell, S. Singlet Oxygen Photosensitisation by the Fluorescent Probe Singlet Oxygen Sensor Green®. Chem. Commun. 2009, 2009, 2920–2922. [Google Scholar] [CrossRef]
- Shecori, S.; Kher, M.M.; Tyagi, K.; Lerno, L.; Netzer, Y.; Lichter, A.; Ebeler, S.E.; Drori, E. A Field Collection of Indigenous Grapevines as a Valuable Repository for Applied Research. Plants 2022, 11, 2563. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Kaur, R.; Kaur, H.; Garg, R.; Kaur, S.; Ercisli, S.; Choudhary, R.; Skrovankova, S.; Mlcek, J. Valorization of Grape (Vitis vinifera) Leaves for Bioactive Compounds: Novel Green Extraction Technologies and Food Pharma Applications. Front. Chem. 2023, 11, 1290619. [Google Scholar] [CrossRef] [PubMed]
- Chemical Book. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB51484947.htm (accessed on 10 August 2025).
- Cioffi, E.; Comune, L.; Piccolella, S.; Buono, M.; Pacifico, S. Quercetin 3-O-glucuronide from Aglianico Vine Leaves: A Selective Sustainable Recovery and Accumulation Monitoring. Foods 2023, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; Kim, S.-H.; Kang, D.-H. Quercetin-Mediated Antimicrobial Photodynamic Treatment Using Blue Light on Escherichia coli O157:H7 and Listeria monocytogenes. Curr. Res. Food Sci. 2023, 6, 100428. [Google Scholar] [CrossRef]
- Condat, M.; Babinot, J.; Tomane, S.; Malval, J.P.; Kang, I.K.; Spillebout, F.; Mazeran, P.-E.; Lalevée, J.; Andalloussi, S.A.; Versace, D.-L. Development of Photoactivable Glycerol-Based Coatings Containing Quercetin for Antibacterial Applications. RSC Adv. 2016, 6, 18235–18245. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Alaeddini, M.; Etemad-Moghadam, S.; Rahimi Esboei, B.; Bahrami, R.; Miri Mousavi, R.S.; Bahador, A. Quorum Quenching of Streptococcus mutans via the Nano-Quercetin-Based Antimicrobial Photodynamic Therapy as a Potential Target for Cariogenic Biofilm. BMC Microbiol. 2022, 22, 125. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bazarjani, F.; Bahador, A. In Silico and In Vitro Insights into the Prediction and Analysis of Natural Photosensitive Compounds Targeting Acinetobacter baumannii Biofilm-Associated Protein. Photodiagn. Photodyn. Ther. 2022, 40, 103134. [Google Scholar] [CrossRef]
- Paul, S.; Pallavi, A.; Gandasi, N.R. Exploring the Potential of Pheophorbide A, a Chlorophyll-Derived Compound, in Modulating GLUT for Maintaining Glucose Homeostasis. Front. Endocrinol. 2024, 15, 1330058. [Google Scholar] [CrossRef]
- Anić, M.; Kontić, J.K.; Rendulić, N.; Čarija, M.; Osrečak, M.; Karoglan, M.; Andabaka, Ž. Evolution of Leaf Chlorophylls, Carotenoids and Phenolic Compounds during Vegetation of Some Croatian Indigenous Red and White Grape Cultivars. Plants 2024, 13, 971. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Dharmaratne, P.; Wang, B.; Lau, K.M.; Lee, C.C.; Cheung, D.W.S.; Chan, J.Y.W.; Yue, G.G.L.; Lau, C.B.S.; Wong, C.K.; et al. Hypericin- and Pheophorbide A-Mediated Photodynamic Therapy Fighting MRSA Wound Infections: A Translational Study from In Vitro to In Vivo. Pharmaceutics 2021, 13, 1399. [Google Scholar] [CrossRef]
- Pheophorbide α. Available online: https://epic.awi.de/id/eprint/28854/1/Jef1997aj.pdf (accessed on 10 August 2025).
- Wang, K.-K.; Li, J.; Kim, B.-J.; Lee, J.-H.; Shin, H.-W.; Ko, S.-H.; Lee, W.-Y.; Lee, C.-H.; Jung, S.H.; Kim, Y.-R. Photophysical Properties of Pheophorbide-A Derivatives and Their Photodynamic Therapeutic Effects on a Tumor Cell Line In Vitro. Int. J. Photoenergy 2014, 793723. [Google Scholar] [CrossRef]
- Yoon, H.-E.; Oh, S.-H.; Kim, S.-A.; Yoon, J.-H.; Ahn, S.-G. Pheophorbide A-Mediated Photodynamic Therapy Induces Autophagy and Apoptosis via the Activation of MAPKs in Human Skin Cancer Cells. Oncol. Rep. 2014, 31, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Atrash, M.; Hovor, I.; Gurianov, Y.; Barel, M.; Semenova, O.; Brider, T.; Nisnevitch, M.; Nakonechny, F. Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3330. [Google Scholar] [CrossRef]
- Syed, H.K.; Liew, K.B.; Loh, G.O.K.; Peh, K.K. Stability Indicating HPLC-UV Method for Detection of Curcumin in Curcuma longa Extract and Emulsion Formulation. Food Chem. 2015, 170, 321–326. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Comprehensive Evaluation of the Photo-Transformation Routes of Trans-Resveratrol. J. Chromatogr. A 2015, 1410, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Singlet Oxygen Sensor Green. Available online: https://www.lumiprobe.com/k/singlet-oxygen-sensor-green (accessed on 13 February 2025).
- Usui, Y. Determination of the Quantum Yield of Singlet Oxygen Formation by Photosensitization. Chem. Lett. 1973, 2, 743–744. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, B.M.; Bennett, T.F.; Monsour, C.G.; Selke, M.; Liu, Y. Determination of Singlet Oxygen Quantum Yield of a Porphyrinic Metal-Organic Framework. J. Phys. Chem. C 2021, 125, 7392–7400. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A–Rose Bengal Bioconjugate for Targeted Gram-Negative Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B 2020, 206, 111852. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ale, T.H.; Hovor, I.; Atrash, M.; Semenova, O.; Zemliana, N.; Kogan, N.M.; Nisnevitch, M.; Nakonechny, F. Photodynamic and Sonodynamic Antibacterial Activity of Grape Leaf Extracts. Appl. Sci. 2025, 15, 10738. https://doi.org/10.3390/app151910738
Ale TH, Hovor I, Atrash M, Semenova O, Zemliana N, Kogan NM, Nisnevitch M, Nakonechny F. Photodynamic and Sonodynamic Antibacterial Activity of Grape Leaf Extracts. Applied Sciences. 2025; 15(19):10738. https://doi.org/10.3390/app151910738
Chicago/Turabian StyleAle, Tigabu Haddis, Iryna Hovor, Melad Atrash, Olga Semenova, Natalia Zemliana, Natalya M. Kogan, Marina Nisnevitch, and Faina Nakonechny. 2025. "Photodynamic and Sonodynamic Antibacterial Activity of Grape Leaf Extracts" Applied Sciences 15, no. 19: 10738. https://doi.org/10.3390/app151910738
APA StyleAle, T. H., Hovor, I., Atrash, M., Semenova, O., Zemliana, N., Kogan, N. M., Nisnevitch, M., & Nakonechny, F. (2025). Photodynamic and Sonodynamic Antibacterial Activity of Grape Leaf Extracts. Applied Sciences, 15(19), 10738. https://doi.org/10.3390/app151910738








