Catalyst Development for Dry Reforming of Methane and Ethanol into Syngas: Recent Advances and Perspectives
Abstract
1. Introduction
2. Dry Reforming of Methane
2.1. Nickel-Based Catalysts: Functionalization of Catalyst Supports
2.2. Bimetallic Ni-Based Catalysts
2.3. High-Entropy Alloy-Based Catalysts
| Catalyst | Method of Synthesis | Reaction Conditions (T, P) | Conversion (CH4/CO2) | Rate of Coke Formation | Stability (Running Time) | Key Features | Ref |
|---|---|---|---|---|---|---|---|
| 20 wt.% Ni-Ru/CeO2 (NR) | Wet impregnation | 450 °C, 1 atm | 92/70 | Low (TGA: 1.25 mg/g·h) | 1 h | Ni dispersion (2.19 nm), oxygen vacancies CeO2, Ru weakens the Ni-C bond | [65] |
| 7 wt.%Ni-3 wt.%Co/SiO2 | Sol–gel | 750 °C, 1 atm | 87/85 | Moderate (TGA: 3.5 mg/g·h) | 50 h | Ni-Co synergy, mesoporous SiO2 structure, sintering suppression | [66] |
| 15 wt.% Ni-5 wt.%Fe-Al (SCS) | Solution combustion | 900 °C, 1 atm | 93/94 | High (TGA: >10%) | 20 h | Ni3Fe alloy formation, NiAl2O4 spinel, deactivation resistance | [67] |
| 2.5 wt.% Ni-2.5 wt.% Co/MgO-Al2O3 | Impregnation | 700 °C, 1 atm | 73/76 | - | 30 h | Ni–Co synergy, basic MgO–Al2O3 (CO2 activation), NiO–MgO solid solution, MgAl2O4 matrix (anti-sintering) | [68] |
| 15 wt.% Ni-0.5 wt.%Re/MgAl2O4 | Wet impregnation | 750 °C, 1 atm | 79/67 | Moderate (TGA: 4.8 mg/g·h) | 50 h | Re increases Ni dispersion, reduces carrier acidity | [69] |
| 3.75 wt.% Ni-1.25 wt.%Co/ScCeZr | Solvothermal | 700 °C, 1 atm | 46.8/60 | Low (TGA: 2.5 mg/g·h) | 5.5 h | Mixed oxides Sc-Ce-Zr, strong metal-support interaction, isolated O2− | [70] |
| 1.2 wt.% Ni-0.3 wt.%Co/SiO2 | Sol–gel | 550 °C (light), 1 atm | 75/80 | Minimum (TGA: 0.8 mg/g·h) | 30 h | Photoactivation by hot carriers, selective oxidation *CHO→ CO | [71] |
2.4. Perovskite-Based Catalysts
2.5. MOF-Derived Catalysts
3. Ethanol Dry Reforming
3.1. General Aspects of the EDR Process
3.2. Catalytic Systems for the EDR Reaction
3.2.1. Influence of the Support on Catalyst Activity and Stability
3.2.2. Modified and Multifunctional Catalytic Systems
3.2.3. Advanced Catalytic Materials
4. Comparative Analysis of DRM and EDR
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gujar, J.P.; Verma, A.; Modhera, B. Optimizing Glycerol Conversion to Hydrogen: A Critical Review of Catalytic Reforming Processes and Catalyst Design Strategies. Int. J. Hydrogen Energy 2025, 109, 823–850. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Rajendran, S.; Al-Samydai, A.; Palani, G.; Trilaksana, H.; Sathish, T.; Giri, J.; Saravanan, R.; Lalvani, J.I.J.; Nasri, F. Replacement of Petroleum Based Products with Plant-Based Materials, Green and Sustainable Energy—A Review. Eng. Rep. 2025, 7, e70108. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Ng, K.H. An Overview on the Carbon Deposited during Dry Reforming of Methane (DRM): Its Formation, Deposition, Identification, and Quantification. Results Eng. 2025, 25, 104328. [Google Scholar] [CrossRef]
- Schleussner, C.-F.; Rogelj, J.; Schaeffer, M.; Lissner, T.; Licker, R.; Fischer, E.M.; Knutti, R.; Levermann, A.; Frieler, K.; Hare, W. Science and Policy Characteristics of the Paris Agreement Temperature Goal. Nat. Clim. Change 2016, 6, 827–835. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; De Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Vo, C.-M.; Cao, A.N.T.; Saleh Qazaq, A.; Pham, C.Q.; Nguyen, D.L.T.; Alsaiari, M.; Vu, T.V.; Sharma, A.; Phuong, P.T.T.; Tran Van, T.; et al. Toward Syngas Production from Simulated Biogas Dry Reforming: Promotional Effect of Calcium on Cobalt-Based Catalysts Performance. Fuel 2022, 326, 125106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Zeng, C.; Sun, W.; Fan, H.; Ma, Q.; Zhao, T.-S. Recent Research Progress of Methane Dry Reforming to Syngas. Fuel 2025, 398, 135535. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.; Caballero, C.G.T.; Cardoso, J.S.; Tarelho, L.A.C.; Eusébio, D. Future Prospects and Industrial Outlook of Syngas Applications. In Advances in Synthesis Gas: Methods, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 427–463. ISBN 978-0-323-91878-7. [Google Scholar]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Chernysheva, E.A.; Savelenko, V.D.; Makhmudova, A.E.; Potanin, D.A.; Salameh, T.; Abdelkareem, M.A.; Olabi, A.G. Innovative Conceptional Approach to Quantify the Potential Benefits of Gasoline-Methanol Blends and Their Conceptualization on Fuzzy Modeling. Int. J. Hydrogen Energy 2022, 47, 35096–35111. [Google Scholar] [CrossRef]
- Okonye, L.U.; Yao, Y.; Ren, J.; Liu, X.; Hildebrandt, D. A Perspective on the Activation Energy Dependence of the Fischer-Tropsch Synthesis Reaction Mechanism. Chem. Eng. Sci. 2023, 265, 118259. [Google Scholar] [CrossRef]
- Jang, W.-J.; Jeong, D.-W.; Shim, J.-O.; Kim, H.-M.; Roh, H.-S.; Son, I.H.; Lee, S.J. Combined Steam and Carbon Dioxide Reforming of Methane and Side Reactions: Thermodynamic Equilibrium Analysis and Experimental Application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Djinović, P.; Osojnik Črnivec, I.G.; Erjavec, B.; Pintar, A. Influence of Active Metal Loading and Oxygen Mobility on Coke-Free Dry Reforming of Ni–Co Bimetallic Catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Kumar Yadav, P.; Sharma, S. Ni, Co & Cu Substituted CeO2: A Catalyst That Improves H2/CO Ratio in the Dry Reforming of Methane. Fuel 2024, 358, 130163. [Google Scholar] [CrossRef]
- Agún, B.; Abánades, A. Comprehensive Review on Dry Reforming of Methane: Challenges and Potential for Greenhouse Gas Mitigation. Int. J. Hydrogen Energy 2025, 103, 395–414. [Google Scholar] [CrossRef]
- Rosha, P.; Singh, R.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Optimization of Hydrogen-Enriched Biogas by Dry Oxidative Reforming with Nickel Nanopowder Using Response Surface Methodology. Energy Fuels 2018, 32, 6995–7001. [Google Scholar] [CrossRef]
- Zafarnak, S.; Rahimpour, M.R. Dry Reforming of Methane for CO2 Conversion Using Sintering-Resistant Halloysite-Supported Ni Catalysts Enhanced by Alkaline-Earth Metal Oxide Promoters. Fuel 2025, 395, 135168. [Google Scholar] [CrossRef]
- Benajes, J.; García, A.; Monsalve-Serrano, J.; Guzmán-Mendoza, M. A Review on Low Carbon Fuels for Road Vehicles: The Good, the Bad and the Energy Potential for the Transport Sector. Fuel 2024, 361, 130647. [Google Scholar] [CrossRef]
- Shafiqah, M.-N.N.; Siang, T.J.; Kumar, P.S.; Ahmad, Z.; Jalil, A.A.; Bahari, M.B.; Van Le, Q.; Xiao, L.; Mofijur, M.; Xia, C.; et al. Advanced Catalysts and Effect of Operating Parameters in Ethanol Dry Reforming for Hydrogen Generation. A Review. Environ. Chem. Lett. 2022, 20, 1695–1718. [Google Scholar] [CrossRef]
- Abbasi, S.; Abbasi, M.; Tabkhi, F.; Akhlaghi, B. Syngas Production plus Reducing Carbon Dioxide Emission Using Dry Reforming of Methane: Utilizing Low-Cost Ni-Based Catalysts. Oil Gas Sci. Technol. Rev. D’IFP Energ. Nouv. 2020, 75, 22. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Hussien, A.G.S.; Polychronopoulou, K. A Review on the Different Aspects and Challenges of the Dry Reforming of Methane (DRM) Reaction. Nanomaterials 2022, 12, 3400. [Google Scholar] [CrossRef]
- Yousefi, S.; Tavakolian, M.; Rahimpour, M.R. Bio-Templated Ni/MgO-Al2O3 as an Efficient Catalyst toward Methane Dry Reforming. Inorg. Chem. Commun. 2025, 171, 113557. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric Studies and Impediments to Valorization of Dry Reforming of Methane for Hydrogen Production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Ro, K.S.; Chen, L.; Mahajan, D.; Siang, T.J.; Ashik, U.P.M.; Hayashi, J.; Pham Minh, D.; Vo, D.-V.N. Microwave-Assisted Dry Reforming of Methane for Syngas Production: A Review. Environ. Chem. Lett. 2020, 18, 1987–2019. [Google Scholar] [CrossRef]
- Ordoñez Ramos, F.; Sapag, K.; Múnera, J.F.; Tarditi, A.M. One-Pot Ni-ZSM-5 Catalysts for Hydrogen Production from the Dry Reforming of Methane. Mol. Catal. 2025, 577, 114963. [Google Scholar] [CrossRef]
- Park, H.-R.; Kim, B.-J.; Ryu, S.-J.; Jeon, Y.; Lee, S.S.; Bae, J.-W.; Roh, H.-S. Super-Dry Reforming of Methane over Surface Oxygen Mobility Enhanced Ni/MgO-Ce/SBA-15 Catalysts. Catal. Today 2025, 453, 115257. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Hussain, I.; Mohammed Aitani, A.; Alotaibi, S.; Abdul Jalil, A. The Critical Role of Intrinsic Catalytic Properties for Enhanced Dry Reforming of Methane (DRM): Recent Advances, Challenges and Techno-Feasibility Assessments. J. Ind. Eng. Chem. 2024, 133, 1–37. [Google Scholar] [CrossRef]
- Ahn, S.; Littlewood, P.; Liu, Y.; Marks, T.J.; Stair, P.C. Stabilizing Supported Ni Catalysts for Dry Reforming of Methane by Combined La Doping and Al Overcoating Using Atomic Layer Deposition. ACS Catal. 2022, 12, 10522–10530. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, H.; Zhang, M.; Liang, C.; Duan, L. Recent Advances in Promoting Dry Reforming of Methane Using Nickel-Based Catalysts. Catal. Sci. Technol. 2024, 14, 1712–1729. [Google Scholar] [CrossRef]
- Lee, S.; Thuan Khiet Nguyen, T.; Kweon, S.; Park, M.B. Nickel Silicate CHA-Type Zeolite Prepared by Interzeolite Transformation and Its Catalytic Activity in Dry Reforming of Methane. Chem. Eng. J. 2024, 498, 155602. [Google Scholar] [CrossRef]
- Han, D.; Yang, Y.; Wu, T.; Wang, Z.; Xiong, J.; Zou, J.; Wei, Y. Synergistic Effect of Ni and CeOx in NiCeOx/SSZ-13 Catalyst for Boosting Activity and Stability during Dry Reforming of Methane. Catal. Today 2024, 435, 114720. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Fakeeha, A.H.; Kasim, S.O.; Khatri, J.; Ibrahim, A.A.; Arasheed, R.; Alabdulsalam, M.; Lanre, M.S.; Osman, A.I.; et al. Promotional Effect of Magnesium Oxide for a Stable Nickel-Based Catalyst in Dry Reforming of Methane. Sci. Rep. 2020, 10, 13861. [Google Scholar] [CrossRef]
- Liu, K.; Ye, L.; Cao, Z.; Li, M.; Zhang, L.; Liu, W.; Ma, Q.; Peng, H. Synergistic Enhancement of Coke Resistance in Methane Dry Reforming via Oxygen Vacancies and Spatial Confinement on Ni-ZrO2/DMS Catalysts. Fuel 2025, 388, 134588. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ayodele, B.V.; Cheng, C.K.; Khan, M.R. Syngas Production from Catalytic CO2 Reforming of CH4 over CaFe2O4 Supported Ni and Co Catalysts: Full Factorial Design Screening. Bull. Chem. React. Eng. Catal. 2018, 13, 57–73. [Google Scholar] [CrossRef]
- Yergaziyeva, G.Y.; Kutelia, E.; Dossumov, K.; Gventsadze, D.; Jalabadze, N.; Dzigrashvili, T.; Mambetova, M.M.; Anissova, M.M.; Nadaraia, L.; Tsurtsumia, O.; et al. Comparative Study the Activity in Dry Reforming of Methane of Bioxide NiO-Co3O4 and NiO-Fe2O3 Systems Supported on the Granulated Natural Diatomite. Combust. Plasma Chem. 2023, 21, 89–97. [Google Scholar] [CrossRef]
- Azeem, S.; Ashraf, M.; Hussain, S. Role of Stable Ni Nano Catalysts for Dry Reforming of Methane. Adsorpt. Sci. Technol. 2025, 43, 02636174251330376. [Google Scholar] [CrossRef]
- Guo, S.; Sun, Y.; Zhang, Y.; Zhang, C.; Li, Y.; Bai, J. Bimetallic Nickel-Cobalt Catalysts and Their Application in Dry Reforming Reaction of Methane. Fuel 2024, 358, 130290. [Google Scholar] [CrossRef]
- Mousavinejad, S.A.; Farsi, M.; Rahimpour, M.R. Role of Promoter on the Catalytic Activity of Novel Hollow Bimetallic Ni–Co/Al2O3 Catalyst in the Dry Reforming of Methane Process: Optimization Using Response Surface Methodology. J. Energy Inst. 2024, 113, 101524. [Google Scholar] [CrossRef]
- Zafarnak, S.; Rahimpour, M.R. Co-Ni Bimetallic Supported on Mullite as a Promising Catalyst for Biogas Dry Reforming toward Hydrogen Production. Mol. Catal. 2023, 534, 112803. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, T.; Lv, L.; Tang, W.; Zhang, G.; Kumar Gupta, R.; Wang, Y.; Tang, S. Preparation Adjacent Ni-Co Bimetallic Nano Catalyst for Dry Reforming of Methane. Fuel 2023, 343, 128013. [Google Scholar] [CrossRef]
- Mohamad Ali, M.; Chaghouri, M.; Tidahy, H.L.; Marinova, M.; Guy, H.; Durécu, S.; Royer, S.; Abi-Aad, E.; Ciotonea, C.; Gennequin, C. Catalytic Performance of Shaped Ni Co Extrudates for Dry Reforming of Methane. Chem. Eng. J. 2025, 522, 167269. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Che, Y.; Shi, W.; Wang, D.; Cui, R.; Feng, X.; Zhang, J.; Liu, H.; Tian, Y. In Situ Synthesizable High-Performance Ni-Fe5C2 Composite Catalysts for Dry Reforming of Methane. Chem. Eng. J. 2025, 517, 164441. [Google Scholar] [CrossRef]
- Yan, X.; Liang, H.; Wang, R.; Li, D.; Li, J.; Peng, W.; Zhang, J. Preparation of High Stability Hierarchical Bimetallic Ni-Mo Catalysts for CO2 Reforming with Methane. Mol. Catal. 2025, 573, 114835. [Google Scholar] [CrossRef]
- Zhao, W.; Fu, Q.; Xie, B.; Ni, Z.; Xia, S. Mechanistic Study of Transition Metal Loaded/Doped Ni − MgO Catalyzed Dry Reforming of Methane: DFT Calculations. Chem. Phys. Lett. 2024, 853, 141538. [Google Scholar] [CrossRef]
- Saconsint, S.; Srifa, A.; Koo-Amornpattana, W.; Assabumrungrat, S.; Sano, N.; Fukuhara, C.; Ratchahat, S. Development of Ni–Mo Carbide Catalyst for Production of Syngas and CNTs by Dry Reforming of Biogas. Sci. Rep. 2023, 13, 12928. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Pupucevski, M.; Impeng, S.; Yang, B.; Chen, G.; Kuboon, S.; Zhong, Q.; Faungnawakij, K.; Zheng, L.; et al. High-Performance Binary Mo–Ni Catalysts for Efficient Carbon Removal during Carbon Dioxide Reforming of Methane. ACS Catal. 2021, 11, 12087–12095. [Google Scholar] [CrossRef]
- Majewski, A.J.; Singh, S.K.; Labhasetwar, N.K.; Steinberger-Wilckens, R. Nickel–Molybdenum Catalysts for Combined Solid Oxide Fuel Cell Internal Steam and Dry Reforming. Chem. Eng. Sci. 2021, 232, 116341. [Google Scholar] [CrossRef]
- Duan, S.; Wang, X.; Ren, R.; Lv, Y. Effects of Preparation Methods on the Size and Interface of Ni–W Bimetallic Catalysts for Dry Reforming of Methane. Int. J. Hydrogen Energy 2024, 54, 1469–1477. [Google Scholar] [CrossRef]
- Zhao, R.; Cao, K.; Ye, R.; Tang, Y.; Du, C.; Liu, F.; Zhao, Y.; Chen, R.; Shan, B. Deciphering the Stability Mechanism of Pt-Ni/Al2O3 Catalysts in Syngas Production via DRM. Chem. Eng. J. 2024, 491, 151966. [Google Scholar] [CrossRef]
- Ji, R.; Su, W.; Men, X.; Yang, J.; Song, X.; Bai, Y.; Wang, J.; Lv, P.; Yu, G. Ultra-Low Amount of Ag Substantially Improves the Stability for Dry Reforming of Methane on Ni/Ag/MgAlO Bimetallic Catalyst. Appl. Surf. Sci. 2025, 688, 162320. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Alavi, S.M.; Akbari, E. Effect of Promoters and Calcination Temperature on the Performance of Nickel Silica Core-Shell Catalyst in Biogas Dry Reforming. J. Energy Inst. 2025, 120, 102062. [Google Scholar] [CrossRef]
- Li, B.; He, J.; Yuan, X. Effect of Lanthanum Modification on Core–Shell Structured Nitrogen-Doped Carbon Coated Nickel Catalysts for Dry Reforming of Methane. Fuel 2025, 388, 134495. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, S.; Liu, H.; Zhang, X.; Cui, Y.; Zhang, G.-R.; Nie, L.; Mei, D. Synergistic Ni-Ce-SSZ-13 Catalysts for Enhanced Dry Reforming of Methane. Int. J. Hydrogen Energy 2025, 104, 202–211. [Google Scholar] [CrossRef]
- Jin, S.; Ye, D.; Zhang, T.; Lv, L.; Tang, W.; Wang, Y.; Zou, Z.; Tang, S. Selective Sublimation of Zn to Prepare NiZn/SiO2 Catalysts for Dry Reforming of Methane: Insight of Synergistic Catalysis and Anti-Coking. Chem. Eng. J. 2025, 509, 161198. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Moser, T.; Klötzer, B.; Penner, S. Regeneration of Ni–Zr Methane Dry Reforming Catalysts in CO2: Reduction of Coking and Ni Redispersion. ACS Catal. 2025, 15, 3314–3327. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically Dispersed Nickel as Coke-Resistant Active Sites for Methane Dry Reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Menegazzo, F.; Pizzolitto, C.; Ghedini, E.; Di Michele, A.; Cruciani, G.; Signoretto, M. Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. C 2018, 4, 60. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the Size and Support Effects of Ni Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Chen, C.; Wang, W.; Ren, Q.; Ye, R.; Nie, N.; Liu, Z.; Zhang, L.; Xiao, J. Impact of Preparation Method on Nickel Speciation and Methane Dry Reforming Performance of Ni/SiO2 Catalysts. Front. Chem. 2022, 10, 993691. [Google Scholar] [CrossRef]
- Hong Phuong, P.; Cam Anh, H.; Tri, N.; Phung Anh, N.; Cam Loc, L. Effect of Support on Stability and Coke Resistance of Ni-Based Catalyst in Combined Steam and CO2 Reforming of CH4. ACS Omega 2022, 7, 20092–20103. [Google Scholar] [CrossRef]
- Visser, N.L.; Daoura, O.; Plessow, P.N.; Smulders, L.C.J.; De Rijk, J.W.; Stewart, J.A.; Vandegehuchte, B.D.; Studt, F.; Van Der Hoeven, J.E.S.; De Jongh, P.E. Particle Size Effects of Carbon Supported Nickel Nanoparticles for High Pressure CO2 Methanation. ChemCatChem 2022, 14, e202200665. [Google Scholar] [CrossRef]
- Alli, R.D.; Zhou, R.; Mohamedali, M.; Mahinpey, N. Effect of Thermal Treatment Conditions on the Stability of MOF-Derived Ni/CeO2 Catalyst for Dry Reforming of Methane. Chem. Eng. J. 2023, 466, 143242. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Highly Carbon-Resistant Ni–Co/SiO2 Catalysts Derived from Phyllosilicates for Dry Reforming of Methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Manabayeva, A.M.; Mäki-Arvela, P.; Vajglová, Z.; Martinéz-Klimov, M.; Tirri, T.; Baizhumanova, T.S.; Grigor’eva, V.P.; Zhumabek, M.; Aubakirov, Y.A.; Simakova, I.L.; et al. Dry Reforming of Methane over Ni–Fe–Al Catalysts Prepared by Solution Combustion Synthesis. Ind. Eng. Chem. Res. 2023, 62, 11439–11455. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Wazeer, I.; Bentalib, A.; Siva Kumar, N.; Abu-Dahrieh, J.K.; Al-Fatesh, A.S. The Effectiveness of Ni-Based Bimetallic Catalysts Supported by MgO-Modified Alumina in Dry Methane Reforming. Catalysts 2023, 13, 1420. [Google Scholar] [CrossRef]
- Álvarez Moreno, A.; Ramirez-Reina, T.; Ivanova, S.; Roger, A.-C.; Centeno, M.Á.; Odriozola, J.A. Bimetallic Ni–Ru and Ni–Re Catalysts for Dry Reforming of Methane: Understanding the Synergies of the Selected Promoters. Front. Chem. 2021, 9, 694976. [Google Scholar] [CrossRef] [PubMed]
- Abasaeed, A.E.; Ibrahim, A.A.; Fakeeha, A.H.; Bayazed, M.O.; Amer, M.S.; Abu-Dahrieh, J.K.; Al-Fatesh, A.S. Ni-Co Bimetallic Catalysts Supported on Mixed Oxides (Sc-Ce-Zr) for Enhanced Methane Dry Reforming. ChemistryOpen 2024, 13, e202400086. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, K.; Jiang, Y.; Li, M.; Tan, X.; Yang, Y.; Zhao, X.; Wang, L.; Wang, Y.; Wang, X.; et al. Photoinducing Different Mechanisms on a Co-Ni Bimetallic Alloy in Catalytic Dry Reforming of Methane. ACS Catal. 2023, 13, 10855–10865. [Google Scholar] [CrossRef]
- Cao, D.; Luo, C.; Tan, Z.; Luo, T.; Shi, Z.; Wu, F.; Li, X.; Zheng, Y.; Zhang, L. Carbon Deposition Properties and Regeneration Performance of La2NiO4 Perovskite Oxide for Dry Reforming of Methane. J. Environ. Chem. Eng. 2023, 11, 111022. [Google Scholar] [CrossRef]
- Du, Z.; Petru, C.; Yang, X.; Chen, F.; Fang, S.; Pan, F.; Gang, Y.; Zhou, H.-C.; Hu, Y.H.; Li, Y. Development of Stable La0.9Ce0.1NiO3 Perovskite Catalyst for Enhanced Photothermochemical Dry Reforming of Methane. J. CO2 Util. 2023, 67, 102317. [Google Scholar] [CrossRef]
- Jiang, Q.; Xin, Y.; Xing, J.; Cao, Y.; Sun, F.; Xing, X.; Hong, H.; Xu, C.; Jin, H. Enhanced Oxygen Migration in Tailored Lanthanum-Based Perovskite for Solar-Driven Dry Reforming of Methane. Fuel 2024, 363, 130852. [Google Scholar] [CrossRef]
- Gil-Muñoz, G.; Alcañiz-Monge, J. Examining the Effect of Zirconium Doping in Lanthanum Nickelate Perovskites on Their Performance as Catalysts for Dry Methane Reforming. J. Environ. Chem. Eng. 2025, 13, 115387. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Zhan, Y.; Liu, C.; Tang, Y.; Yan, D.; Li, J.; Jia, L. An Engineering Approach for Active and Stable Dry Methane Reforming within Solid Oxide Fuel Cell Stacks Using La0.6Sr0.2Cr0.85Ni0.15O3-δ as a Catalyst. Int. J. Hydrog. Energy 2025, 98, 1079–1086. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 Perovskites as Precursors for the Preparation of Coke-Resistant Dry Reforming Catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Liu, C.; Tang, W.; Zhong, Y.; Abuelgasim, S.; Xu, C.; Abdalazeez, A. Dry Reforming of Methane by LaNiO3 Perovskite Oxide: Cu-Substituted Improving Reactivity and Stability. Int. J. Hydrogen Energy 2025, 101, 617–626. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Korili, S.A.; Gil, A. Surface Tuning of a Highly Crystalline Ni/LaAlO3 Perovskite Catalyst Obtained from Aluminum Saline Slags Using Various Synthesis Methods for the Dry Reforming of Methane. Catal. Today 2025, 447, 115158. [Google Scholar] [CrossRef]
- Sellam, D.; Ikkour, K.; Dekkar, S.; Messaoudi, H.; Belaid, T.; Roger, A.-C. CO2 Reforming of Methane over LaNiO3 Perovskite Supported Catalysts: Influence of Silica Support. Bull. Chem. React. Eng. Catal. 2019, 14, 568–578. [Google Scholar] [CrossRef]
- Oni, B.A.; Tomomewo, O.S.; Sanni, S.E.; Ojo, V.O. Dry Reforming of Methane with CO2 over Co-La1−xCaxNiO3 Perovskite-Type Oxides Supported on ZrO2. Mater. Today Commun. 2023, 36, 106802. [Google Scholar] [CrossRef]
- Winterstein, T.F.; Malleier, C.; Mohammadi, A.; Krüger, H.; Kahlenberg, V.; Venter, A.M.; Bekheet, M.F.; Müller, J.T.; Gurlo, A.; Heggen, M.; et al. Lanthanum Nickel Titanate Perovskites as Model Systems for Ni-Perovskite Interfacial Engineering in Methane Dry Reforming. Mater. Today Chem. 2025, 45, 102620. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A Review on Perovskite Catalysts for Reforming of Methane to Hydrogen Production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Qiu, P.; Yuan, J.; Cheng, K.; Xiong, C.; Wu, S. Enhanced Catalytic Activity for Methane Dry Reforming of Ce-Doped Ba0.9Zr0.7Y0.2Ni0.1O3-δ Perovskite Catalyst. Mol. Catal. 2023, 549, 113543. [Google Scholar] [CrossRef]
- Osti, A.; Costa, S.; Rizzato, L.; Senoner, B.; Glisenti, A. Photothermal Activation of Methane Dry Reforming on Perovskite-Supported Ni-Catalysts: Impact of Support Composition and Ni Loading Method. Catal. Today 2025, 449, 115200. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J.; Vohs, J.M.; Han, J.W.; Kim, G. Highly Active Dry Methane Reforming Catalysts with Boosted in Situ Grown Ni-Fe Nanoparticles on Perovskite via Atomic Layer Deposition. Sci. Adv. 2020, 6, eabb1573. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, A.G.; Tsiotsias, A.I.; Siakavelas, G.I.; Charisiou, N.D.; Ehrhardt, B.; Wang, W.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Mascotto, S.; et al. An Experimental and Theoretical Approach for the Biogas Dry Reforming Reaction Using Perovskite-Derived La0.8X0.2NiO3-δ Catalysts (X = Sm, Pr, Ce). Renew. Energy 2024, 227, 120511. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A Review on Dry Reforming of Methane over Perovskite Derived Catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Korili, S.A.; Gil, A. Facile Synthesis of an Ni/LaAlO3—Perovskite via an MOF Gel Precursor for the Dry Reforming of Methane. Catal. Today 2024, 429, 114487. [Google Scholar] [CrossRef]
- Rudolph, B.; Tsiotsias, A.I.; Ehrhardt, B.; Dolcet, P.; Gross, S.; Haas, S.; Charisou, N.D.; Goula, M.A.; Mascotto, S. Nanoparticle Exsolution from Nanoporous Perovskites for Highly Active and Stable Catalysts. Adv. Sci. 2023, 10, 2205890. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Sun, Y.; Yue, X.; Yang, J.; Fu, L.; Deng, Q.; Zhao, H.; Yin, C.; Wu, K. Tuning Exsolution of Nanoparticles in Defect Engineered Layered Perovskite Oxides for Efficient CO2 Electrolysis. J. Energy Chem. 2023, 84, 219–227. [Google Scholar] [CrossRef]
- Piazzolla, F.; Moraes, T.S.; Figueiredo, S.S.; De Paula, D.F.; Dos Santos Veiga, E.L.; Rodella, C.B.; Fonseca, F.C. Exsolution of Ni Nanoparticles from La0.4Sr0.4Ti0.8Ni0.2O3-δ Perovskite for Ethanol Steam Reforming. Catal. Today 2025, 444, 115011. [Google Scholar] [CrossRef]
- Shah, S.; Xu, M.; Pan, X.; Gilliard-Abdulaziz, K.L. Exsolution of Embedded Ni–Fe–Co Nanoparticles: Implications for Dry Reforming of Methane. ACS Appl. Nano Mater. 2021, 4, 8626–8636. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Ng, K.H. The Roles of Oxygen Mobility and Oxygen Vacancy in Metallic Catalysts-Prompted Dry Reforming of Methane: A Review. Renew. Energy 2025, 256, 124074. [Google Scholar] [CrossRef]
- Yang, C.; Grimaud, A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalysts 2017, 7, 149. [Google Scholar] [CrossRef]
- Yu, K.; Lou, L.; Liu, S.; Zhou, W. Asymmetric Oxygen Vacancies: The Intrinsic Redox Active Sites in Metal Oxide Catalysts. Adv. Sci. 2020, 7, 1901970. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Ma, Y.; Han, X.; Schröder, M.; Yang, S. Activation and Catalysis of Methane over Metal–Organic Framework Materials. Acc. Mater. Res. 2025, 6, 77–88. [Google Scholar] [CrossRef]
- Ong, J.L.; Loy, A.C.M.; Teng, S.Y.; How, B.S. Future Paradigm of 3D Printed Ni-Based Metal Organic Framework Catalysts for Dry Methane Reforming: Techno-Economic and Environmental Analyses. ACS Omega 2022, 7, 15369–15384. [Google Scholar] [CrossRef]
- Zheng, K.; Gao, X.; Xie, Y.; He, Z.; Ma, Y.; Hou, S.; Su, D.; Ma, X. Free-Standing Bimetallic Co/Ni-MOF Foams toward Enhanced Methane Dry Reforming under Non-Thermal Plasma Catalysis. J. Colloid Interface Sci. 2025, 683, 564–573. [Google Scholar] [CrossRef]
- Wang, J.; Qi, T.; Li, G.; Zhang, Y.; Chen, H.; Li, W. Elucidating the Promoting Mechanism of Coordination-Driven Self-Assembly MOFs/SiO2 Composite Derived Catalyst for Dry Reforming of Methane with CO2. Fuel 2022, 330, 125569. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, H.; Cai, W.; Liang, W.; Xia, H.; Dang, C. Immobilization of Ni on MOF-Derived CeO2 for Promoting Low-Temperature Dry Reforming of Methane. Fuel 2024, 363, 130998. [Google Scholar] [CrossRef]
- Liu, H.; Dong, M.; Xiong, J.; Huang, Z.; Hou, H.; Liang, Y.; Lu, J. Study on Ce-MOF-Derived Oxides as Morphology-Tunable Catalyst Supports for Dry Reforming of Methane. Appl. Surf. Sci. 2025, 679, 161167. [Google Scholar] [CrossRef]
- Figueredo, G.P.; Medeiros, R.L.B.A.; Macedo, H.P.; De Oliveira, Â.A.S.; Braga, R.M.; Mercury, J.M.R.; Melo, M.A.F.; Melo, D.M.A. A Comparative Study of Dry Reforming of Methane over Nickel Catalysts Supported on Perovskite-Type LaAlO3 and Commercial α-Al2O3. Int. J. Hydrogen Energy 2018, 43, 11022–11037. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-Containing Ni-Based Catalysts for Dry Reforming of Methane: A Review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Shao, J.; Li, C.; Fei, Z.; Liu, Y.; Zhang, J.; Li, L. MOFs-Derived Ni@ZrO2 Catalyst for Dry Reforming of Methane: Tunable Metal-Support Interaction. Mol. Catal. 2024, 558, 114028. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Fung, W.-Y. Syngas Production via Dry Reforming of Methane over CeO2 Modified Ni/Al2O3 Catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Kung, H.-Y.; Wang, C.-B. Highly Stable and Active Ni-Doped Ordered Mesoporous Carbon Catalyst on the Steam Reforming of Ethanol Application. J. Saudi Chem. Soc. 2017, 21, 205–209. [Google Scholar] [CrossRef]
- Zhao, S.; Cai, W.; Li, Y.; Yu, H.; Zhang, S.; Cui, L. Syngas Production from Ethanol Dry Reforming over Rh/CeO2 Catalyst. J. Saudi Chem. Soc. 2018, 22, 58–65. [Google Scholar] [CrossRef]
- Cao, D.; Zeng, F.; Zhao, Z.; Cai, W.; Li, Y.; Yu, H.; Zhang, S.; Qu, F. Cu Based Catalysts for Syngas Production from Ethanol Dry Reforming: Effect of Oxide Supports. Fuel 2018, 219, 406–416. [Google Scholar] [CrossRef]
- Dewa, M.; Elharati, M.A.; Hussain, A.M.; Miura, Y.; Song, D.; Fukuyama, Y.; Furuya, Y.; Dale, N.; Zhang, X.; Marin-Flores, O.G.; et al. Metal-Supported Solid Oxide Fuel Cell System with Infiltrated Reforming Catalyst Layer for Direct Ethanol Feed Operation. J. Power Sources 2022, 541, 231625. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Gao, J.; Wang, J.; Ma, G.; Wen, X.; Yang, Y.; Li, Y.; Ding, M. A Hydrophobic FeMn@Si Catalyst Increases Olefins from Syngas by Suppressing C1 By-Products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef]
- Senapati, S.; Ray, K.; Pradhan, N.C. An Energy-Efficient Aspen plus Model for H2-Rich Syngas Production via Dry Reforming of Ethanol: A Thermodynamic Analysis. Int. J. Hydrogen Energy 2025, 98, 1107–1118. [Google Scholar] [CrossRef]
- Attili, R.; Viet Nguyen Vo, D.; Sabri Mahmud, M. Kinetic Study of Ethanol Dry Reforming Using Lanthanum Copper Perovskite. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Drif, A.; Bion, N.; Brahmi, R.; Ojala, S.; Pirault-Roy, L.; Turpeinen, E.; Seelam, P.K.; Keiski, R.L.; Epron, F. Study of the Dry Reforming of Methane and Ethanol Using Rh Catalysts Supported on Doped Alumina. Appl. Catal. Gen. 2015, 504, 576–584. [Google Scholar] [CrossRef]
- Hou, T.; Lei, Y.; Zhang, S.; Zhang, J.; Cai, W. Ethanol Dry Reforming for Syngas Production over Ir/CeO2 Catalyst. J. Rare Earths 2015, 33, 42–45. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, W.; Liu, Y.; Zhang, S.; Li, Y.; Huang, D.; Wang, T.; Li, Y. Synthesis of Cu-Ce0.8Zr0.2O2 Catalyst by Ball Milling for CO2 Reforming of Ethanol. J. Saudi Chem. Soc. 2019, 23, 111–117. [Google Scholar] [CrossRef]
- Ramkiran, A.; Vo, D.-V.N.; Mahmud, M.S. Syngas Production from Ethanol Dry Reforming Using Cu-Based Perovskite Catalysts Promoted with Rare Earth Metals. Int. J. Hydrogen Energy 2021, 46, 24845–24854. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, W.; Deng, S.; Li, Z.; Yu, H.; Zhang, S.; Yu, Z.; Cui, L.; Qu, F. Efficient Syngas Production via Dry Reforming of Renewable Ethanol over Ni/KIT-6 Nanocatalysts. Renew. Energy 2020, 145, 1507–1516. [Google Scholar] [CrossRef]
- Zhukova, A.; Fionov, Y.; Chuklina, S.; Mikhalenko, I.; Fionov, A.V.; Isaikina, O.; Zhukov, D.Y.; De Lima, A.M. CO2 Reforming of Ethanol over Ni/Al2 O3 -(Zr–Yb)O2 Catalysts: The Effect of Zr:Al Ratio on Nickel Activity and Carbon Formation. Energy Fuels 2024, 38, 482–498. [Google Scholar] [CrossRef]
- Cao, D.; Cai, W.; Li, Y.; Li, C.; Yu, H.; Zhang, S.; Qu, F. Syngas Production from Ethanol Dry Reforming over Cu/Ce0.8Zr0.2O2 Catalyst. Catal. Lett. 2017, 147, 2929–2939. [Google Scholar] [CrossRef]
- Shi, G.; Wang, Y.; Cao, Y.; Cai, W.; Tan, F. Synthesis of High-Performance Ni/Ce0.8Zr0.2O2 Catalyst via Co-Nanocasting Method for Ethanol Dry Reforming. Korean J. Chem. Eng. 2020, 37, 2143–2151. [Google Scholar] [CrossRef]
- Li, F.; Dong, J.; Wang, M.; Lin, X.; Cai, W.; Liu, X. Ethanol Dry Reforming over Ordered Mesoporous Co-Zn Composite Oxide for Syngas Production. Korean J. Chem. Eng. 2022, 39, 1744–1752. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Nginyo, J.; Cai, W.; Yu, B. Syngas Production through Dry Reforming of Ethanol over Co@SiO2 Catalysts: Effect of SiO2 Shell Thickness. Mol. Catal. 2023, 547, 113307. [Google Scholar] [CrossRef]
- Cai, W.; Dong, J.; Chen, Q.; Xu, T.; Zhai, S.; Liu, X.; Cui, L.; Zhang, S. One-Pot Microwave-Assisted Synthesis of Cu-Ce0.8Zr0.2O2 with Flower-like Morphology: Enhanced Stability for Ethanol Dry Reforming. Adv. Powder Technol. 2020, 31, 3874–3881. [Google Scholar] [CrossRef]
- Fionov, Y.; Khlusova, K.; Chuklina, S.; Mushtakov, A.; Fionov, A.; Zhukov, D.; Averin, A.; Zhukova, A. High-Performance Ni/Al2O3-(Zr + Ce)O2 Catalysts for Syngas Production via Ethanol Dry Reforming. Fuel 2024, 376, 132685. [Google Scholar] [CrossRef]
- Fedorova, V.; Bespalko, Y.; Arapova, M.; Smal, E.; Valeev, K.; Prosvirin, I.; Sadykov, V.; Parkhomenko, K.; Roger, A.; Simonov, M. Ethanol Dry Reforming over Bimetallic Ni-Containing Catalysts Based on Ceria-Zirconia for Hydrogen Production. ChemCatChem 2023, 15, e202201491. [Google Scholar] [CrossRef]
- Zhukova, A.; Fionov, Y.; Semenova, S.; Khaibullin, S.; Chuklina, S.; Maslakov, K.; Zhukov, D.; Isaikina, O.; Mushtakov, A.; Fionov, A. Ethanol Dry Reforming for Hydrogen-Rich Syngas Production over Cu-Promoted Ni/Al2 O3 –ZrO2 Catalysts. J. Phys. Chem. C 2024, 128, 20177–20194. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, D.; Kim, S.-I.; Kim, Y.J.; Park, J.H.; Heo, I.; Chang, T.S.; Lee, J.H. Effects of Alkali Metals on Nickel/Alumina Catalyzed Ethanol Dry Reforming. Catalysts 2021, 11, 260. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Li, T.; Tian, Y.; Wang, M.; Cai, W. Effect of Calcination Temperature on the Performance of SiO2@Co@CeO2 Catalyst in CO2 Reforming With Ethanol. Catal. Lett. 2023, 153, 3712–3723. [Google Scholar] [CrossRef]
- Labhasetwar, N.; Saravanan, G.; Kumar Megarajan, S.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-Type Catalytic Materials for Environmental Applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Eremeev, N.F.; Hanna, S.A.; Sadovskaya, E.M.; Leonova, A.A.; Bulavchenko, O.A.; Ishchenko, A.V.; Prosvirin, I.P.; Sadykov, V.A.; Bespalko, Y.N. Catalysts for Ethanol Dry Reforming Based on High-Entropy Perovskites. J. Catal. 2025, 445, 116028. [Google Scholar] [CrossRef]
- Tian, Y.; Li, T.; Cai, W. Ethanol Reforming with CO2 over MOF-Derived CoMOx (M:Ce,Zr,Zn): Impact of Support on Activity/Stability. J. Mater. Sci. 2023, 58, 16033–16045. [Google Scholar] [CrossRef]
- Wang, M.; Li, T.; Tian, Y.; Zhang, J.; Cai, W. CO2 Reforming with Ethanol Over Bimetallic Co(Ni)/ZnO Catalyst with Enhanced Activity: Synergistic Effect of Ni and Co. Catal. Lett. 2024, 154, 3829–3838. [Google Scholar] [CrossRef]
- Rao, Z.; Cao, Y.; Huang, Z.; Yin, Z.; Wan, W.; Ma, M.; Wu, Y.; Wang, J.; Yang, G.; Cui, Y.; et al. Insights into the Nonthermal Effects of Light in Dry Reforming of Methane to Enhance the H2/CO Ratio Near Unity over Ni/Ga2 O3. ACS Catal. 2021, 11, 4730–4738. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Cao, D.; Liu, Q.; Cai, W. Photothermal Dry Reforming of Ethanol over Rod-like Ni/ZrO2 Catalysts. Mol. Catal. 2024, 564, 114308. [Google Scholar] [CrossRef]
- Li, T.; Tian, Y.; Nginyo, J.; Difuma Luis, D.I.; Cai, W. Light-Assisted Ethanol Dry Reforming over NiZnOx Hollow Microspheres with Enhanced Activity and Stability. Renew. Energy 2024, 227, 120514. [Google Scholar] [CrossRef]
- Shafiqah, M.-N.N.; Tran, H.N.; Nguyen, T.D.; Phuong, P.T.T.; Abdullah, B.; Lam, S.S.; Nguyen-Tri, P.; Kumar, R.; Nanda, S.; Vo, D.-V.N. Ethanol CO2 Reforming on La2O3 and CeO2-Promoted Cu/Al2O3 Catalysts for Enhanced Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 18398–18410. [Google Scholar] [CrossRef]
- Wang, M.; Li, F.; Chen, Q.; Cai, W. Ethanol Dry Reforming over Mn-Doped Co/CeO2 Catalysts with Enhanced Activity and Stability. Energy Fuels 2021, 35, 13945–13954. [Google Scholar] [CrossRef]
- Arapova, M.; Smal, E.; Bespalko, Y.; Fedorova, V.; Valeev, K.; Cherepanova, S.; Ischenko, A.; Sadykov, V.; Simonov, M. Ethanol Dry Reforming over Ni Supported on Modified Ceria-Zirconia Catalysts– the Effect of Ti and Nb Dopants. Int. J. Hydrogen Energy 2021, 46, 39236–39250. [Google Scholar] [CrossRef]
- Wang, M.; Li, F.; Dong, J.; Lin, X.; Liu, X.; Wang, D.; Cai, W. MOF-Derived CoCeOx Nanocomposite Catalyst with Enhanced Anti-Coking Property for Ethanol Reforming with CO2. J. Environ. Chem. Eng. 2022, 10, 107892. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, N.Y.; Yoo, E.; Kim, J.; Joo, J.B. Enhanced Coke Resistant Ni/SiO2@SiO2 Core–Shell Nanostructured Catalysts for Dry Reforming of Methane: Effect of Metal-Support Interaction and SiO2 Shell. Chem. Eng. Sci. 2024, 299, 120480. [Google Scholar] [CrossRef]
- Moradi, M.J.; Iranshahi, D. Investigating the Performance of Ni/Al2O3-ZrO2 Thin Film Nanocatalysts Synthesized by Pvd Method for Methane Dry Reforming. Results Eng. 2025, 27, 106625. [Google Scholar] [CrossRef]

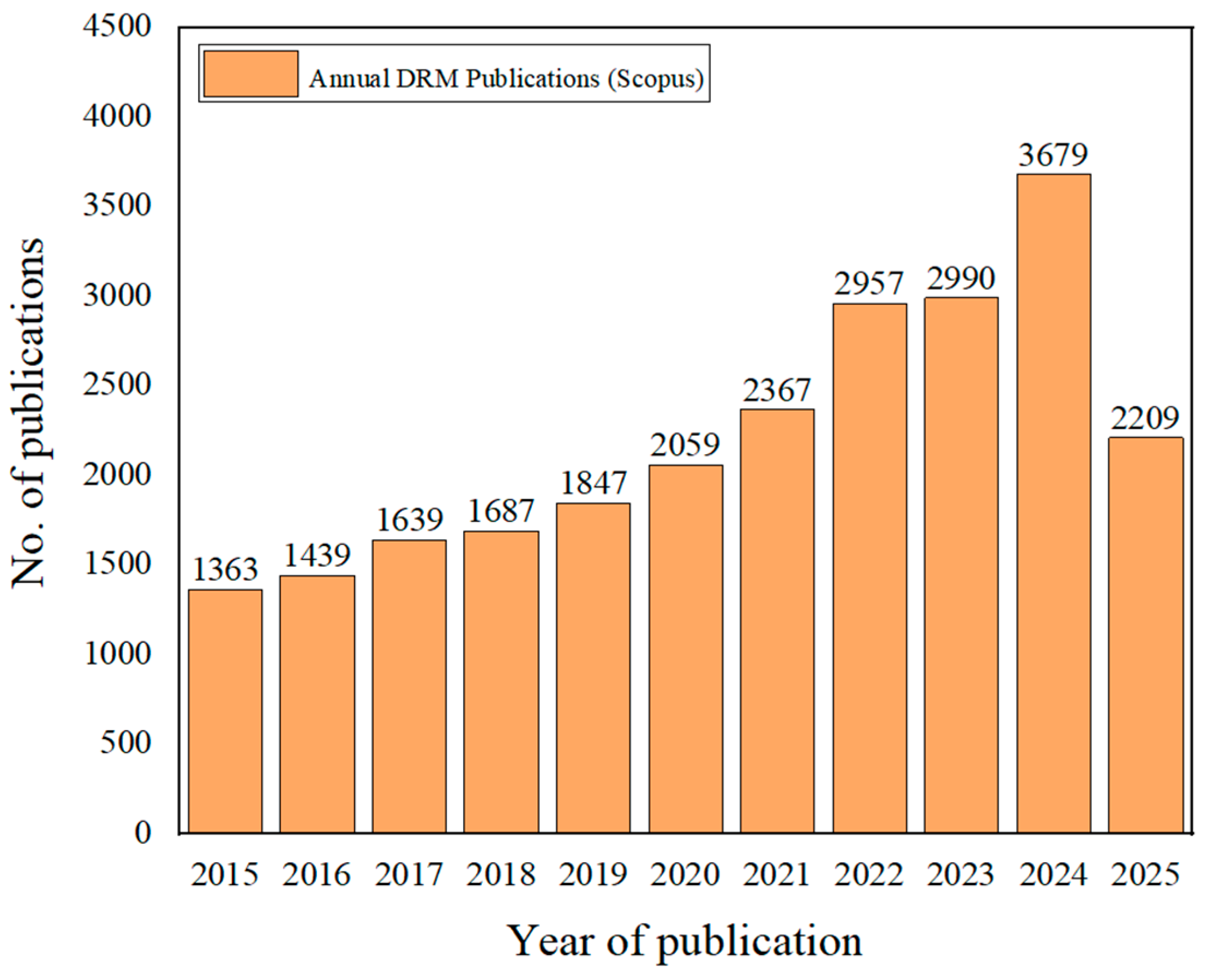
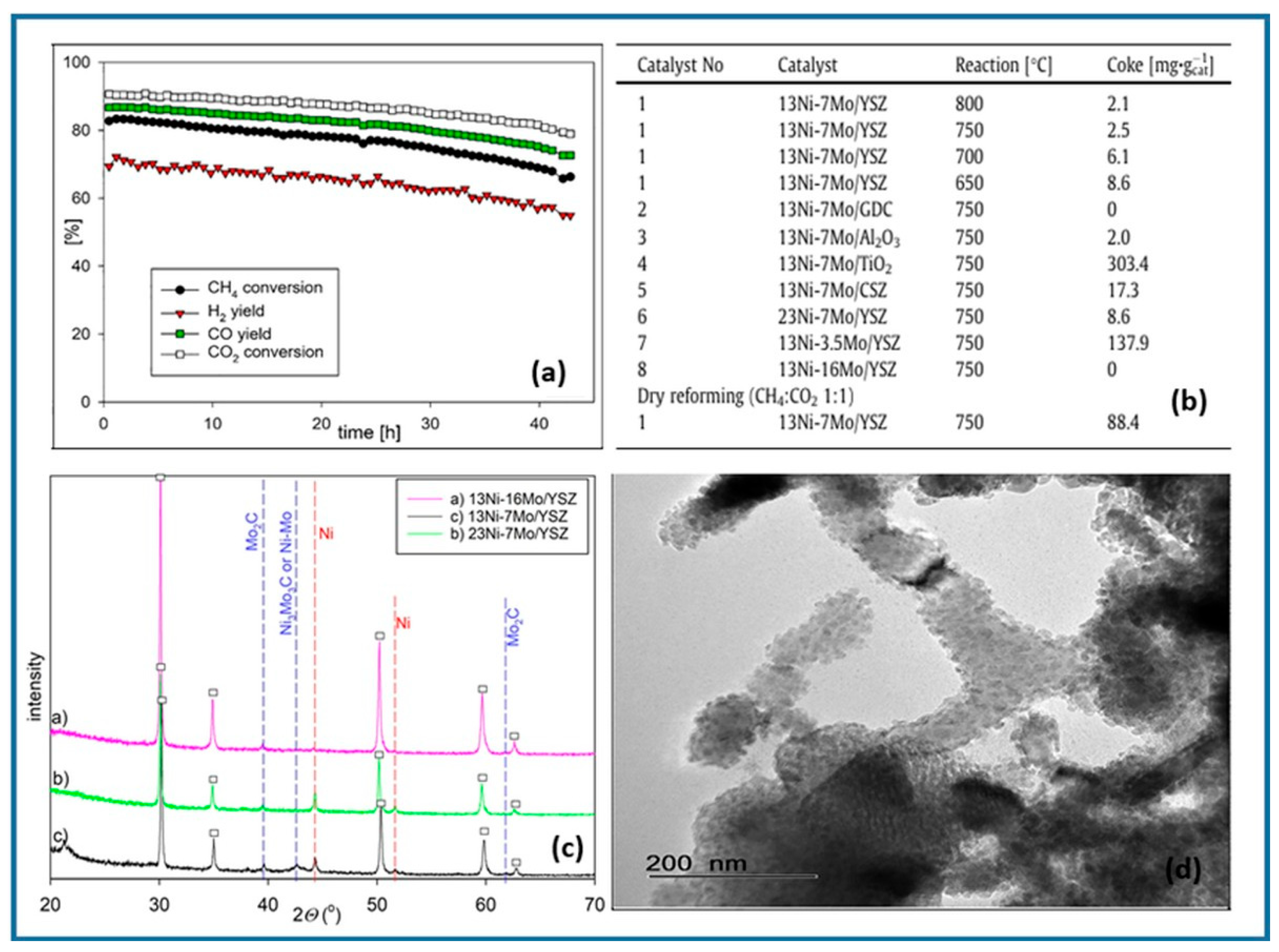

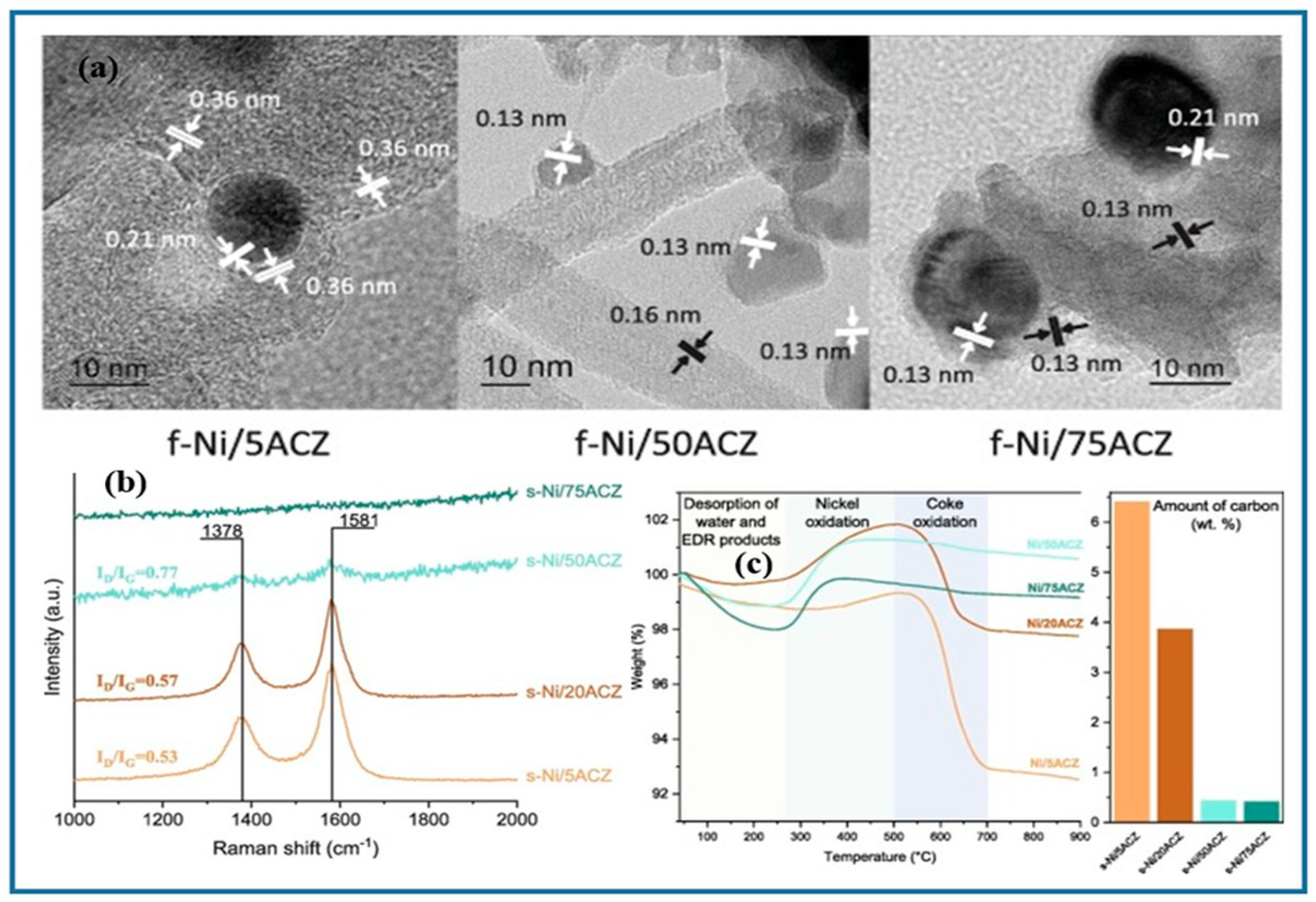
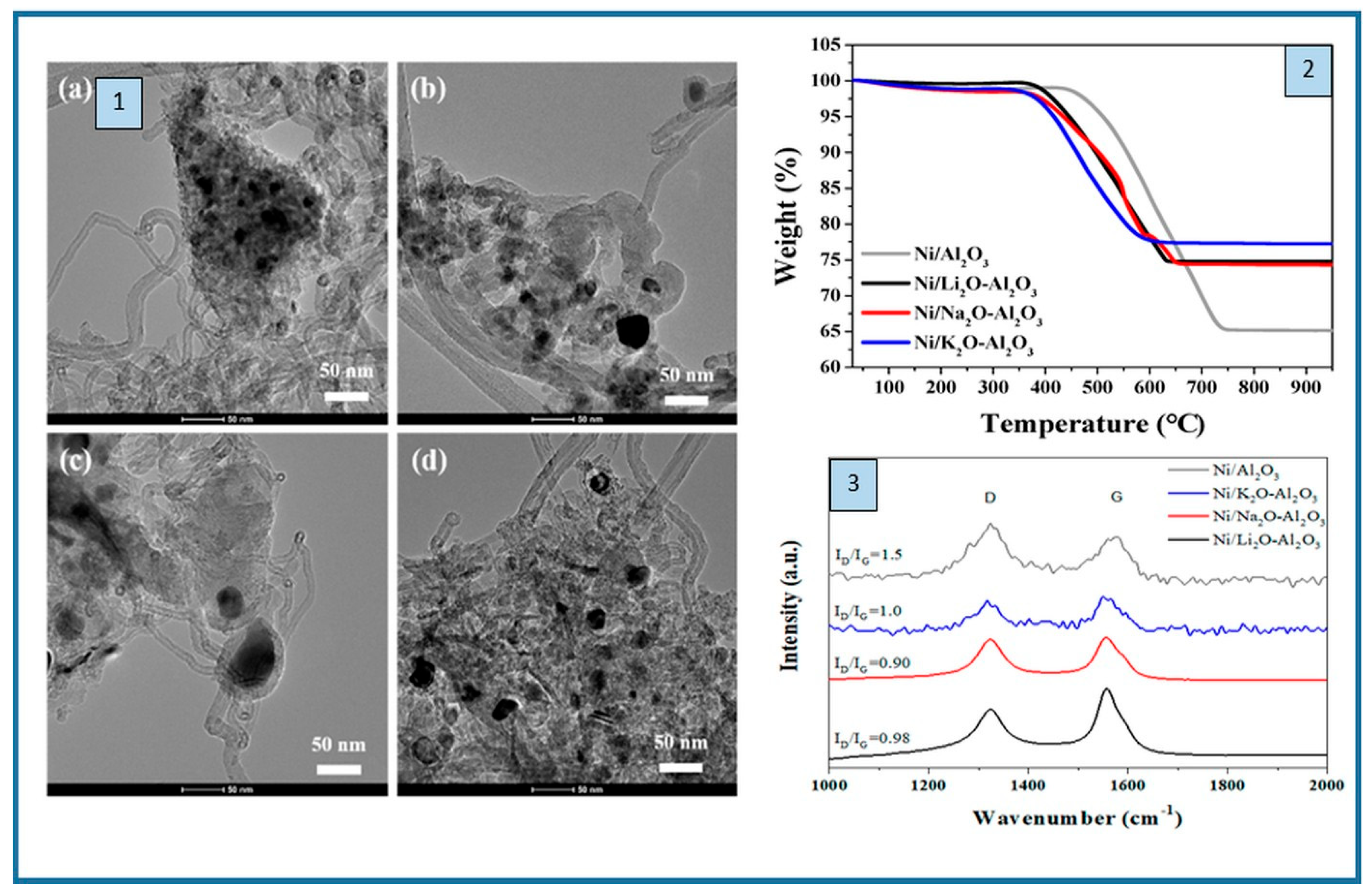
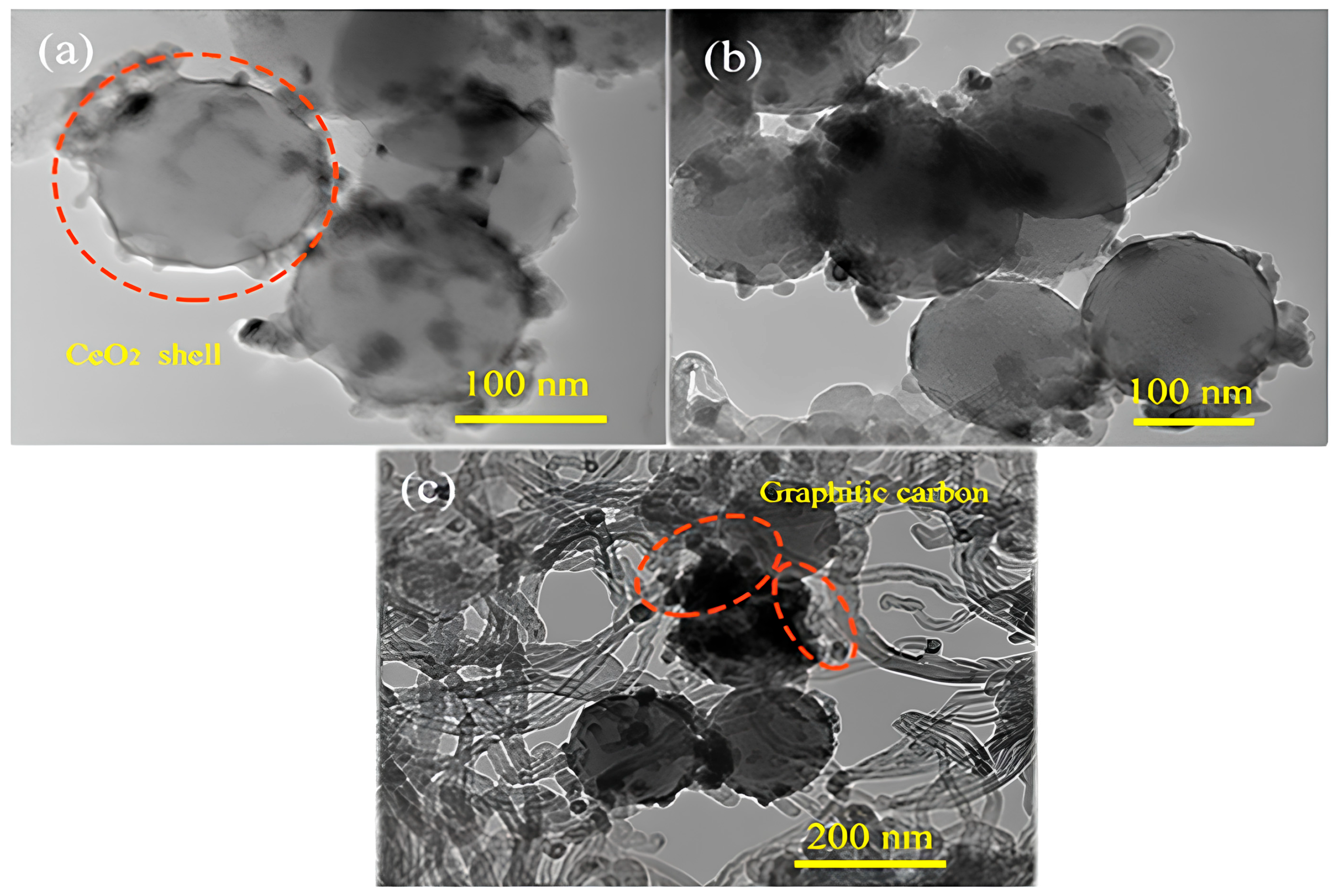
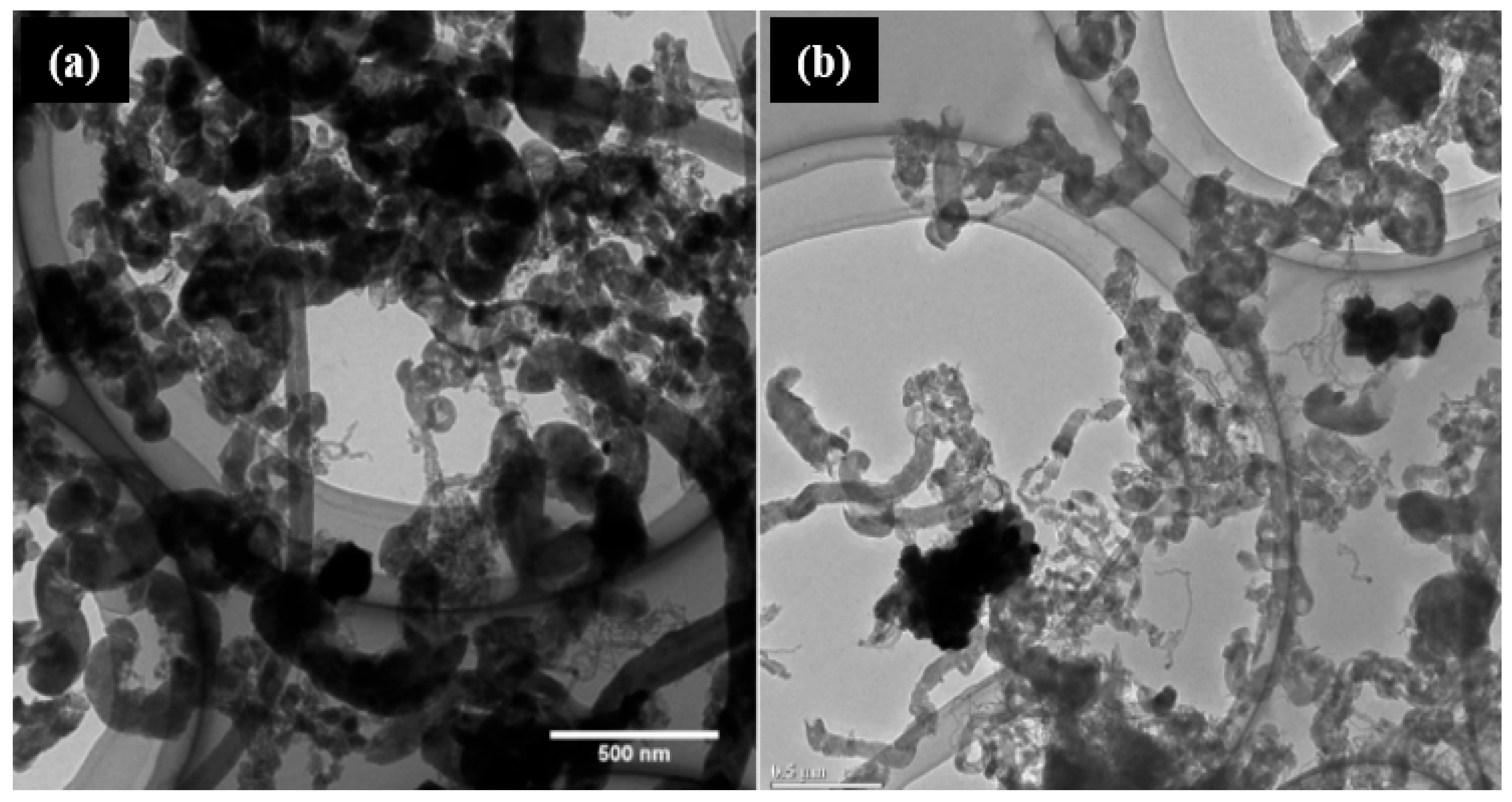
| No | Reaction Name | Equation | ΔH° 298k (kJ/mol) |
|---|---|---|---|
| 1 | Dry reforming of methane | CO2 + CH4 → 2CO + 2H2 | +247.0 |
| 2 | Reverse Water-Gas Shift reaction | CO2 + H2 → CO + H2O | 41.0 |
| 3 | Boudouard reaction | 2CO → C + CO2 | −171.0 |
| 4 | Decomposition of methane | CH4 → C + 2H2 | 75.0 |
| 5 | Carbon monoxide reduction | CO + H2 → C + H2O | −131.3 |
| 6 | Hydrogenation of CO2 | CO2 + 2H2 → C + 2H2O | −90.0 |
| Catalyst | Method of Synthesis | Process Conditions (T, GHSV) | Feed Gas Ratio | Conversion, % (CH4/CO2) | Key Features | Ref |
|---|---|---|---|---|---|---|
| La2NiO4 | Sol–gel | 850 °C, 24,000 h−1 | CH2:CO2 = 1:1 | 83/92 | High dispersion metallic Ni0, coke reduction | [72] |
| La0.9Ce0.1NiO3 | Pechini method | 700 °C, 300 l·h−1·gcat−1 | 10% CO2/10% CH4/80% Ar | 80/71 | Oxygen vacancies and electron-hole pairs | [73] |
| LaNiO3 | Sol–gel | 800 °C, 15 l·h−1·gcat−1 | CH2:CO2 = 1:1 | 97/95 | High resistance to coke, Ni dispersion | [74] |
| LaZrxNi1-xO3 | Citrate method | 700 °C, 58L/(g.h.) | 25/25/50 CH4/CO2/He) | 77/86 | Resistance to sintering, activity at extreme temperatures | [75] |
| LSCrN@NF | Self-combustion method | 750 °C, 10 mL min−1 | 45% CH4–45% CO2–10% N2 | 80/83 | Optimized basicity, increased CO2 adsorption | [76] |
| LaNi0.8Cu0.2O3 | Sol–gel | 700 °C, 250 l·h−1·gcat−1 | CH4:CO2:Ar = 10:10:1 | 83/85 | Low activation energy, Cu-Ni synergy | [78] |
| 10 wt % Ni/LaAlO3 | Impregnation | 700 °C, 1.2 10−4 cm3/(h g) | CH2:CO2 = 1:1 | 87/86 | Strong interaction of active centers with the carrier | [79] |
| 2%Co- La0.2Ca0.8NiO3-ZrO2 | Co-precipitation method | 700 °C, 42,000 h−1 | CH4: CO2:N2(Ar) = 1:1:1 | 88/90 | Metal-carrier interaction and metal synergy | [81] |
| La0.8Sm0.2NiO3 | Citrate sol–gel method | 750 °C, 200,000 h−1 | Ar:CH4:CO2 = 2.5:1.5:1 | 40/78 | Reduced ordering/crystallinity of deposited coke | [87] |
| Catalyst | Ni Particle Size (nm) | Coke Formation Rate (mg·g−1·h−1) | CH4/CO2 Conversion at 700 °C (%) | Stability (Particle Growth, %) | Specific Surface Area (m2/g) | Key Features | Ref |
|---|---|---|---|---|---|---|---|
| 5.3 wt.%Ni-3.1 wt.% MgO@mSiO2 (MOF-derived) | 7.2 → 18.3 (60 h) | 1.25 (vs. 7.47 for analog) | 96 → 75/75 → 69 | +154% | n/a | SiO2 mesopores; OH− groups; Ni–O–Si anchoring (phyllosilicate interphase) | [100] |
| 20 wt.% Ni-Ce-BTC (N2-pyr) | 2.19 → 18.3 (120 h) | 2.88 (vs. 65.4 with CO2 treatment) | n/a | +740% | 245 | 37% Ce3+, protective carbon layer, high proportion of oxygen vacancies | [65] |
| 4.57 wt.% Ni/CeO2-M | 17.2 → 17.9 (10 h) | 1.25 (vs. 7.47 for Ni/CeO2-C) | 30.8/40.1 (550 °C) | +4% | 22.4 | CeO2 nanorods, ID/IF = 0.18 | [101] |
| 10 wt.% Ni/LaAlO3 | 5.03 → 18.3 (60 h) | 1.25 (vs. 7.47 for sol–gel analog) | 75/80 | +264% | 33 | Perovskite structure, oxygen vacancies, mesopores | [79] |
| 13.45 wt.% Ni/ZrO2-B | 20.47 → 16.6 (6 h) | 3.8 wt.% (vs. 6.9 wt.% for A; 0.7 wt.% for C) | n/a | −19% | 12.93 | Oxygen vacancies ZrO2, 25 nm mesopores, strong metal-support interaction | [105] |
| № | Reaction Name | Equation | ΔH° 298k (kJ/mol) |
|---|---|---|---|
| 1 | The main reaction of EDR | C2H5OH + CO2 → 3CO +3H2 | +296.7 |
| 2 | Dehydrogenation of ethanol | C2H5OH → CH3CHO + H2 | +68.5 |
| 3 | Decomposition of ethanol | C2H5OH → CO + CH4 + H2 | +49.6 |
| 4 | Acetaldehyde decomposition | CH3COH→ CH4 + CO | −18.9 |
| 5 | Dry reforming of acetaldehyde | CH3COH + CO2 → 2H2 + 3CO | −186.3 |
| 6 | Methanation of CO2 | CO2 + 4H2 → CH4 + 2H2O | −153.0 |
| 7 | Methanation of CO | CO2 + 3H2 → CH4 + H2O | −206.2 |
| 8 | Dry reforming of methane | CH4 + CO2 → 2CO + 2H2 | +247.0 |
| Catalyst | Method of Preparation | Reaction Conditions | Reduction Conditions | Activity | Stability | Ref |
|---|---|---|---|---|---|---|
| 15 wt.% Cu-Ce0.8Zr0.2O2 | co-precipitation | T = 700 °C, EtOH/CO2 = 1:1; volumetric velocity = 14,000 mL/gcat/h | in situ T = 500 °C for 1 h, 5 vol.% H2/N2 (30 mL/min) | XC2H5OH = 90%, yield CO = 37%, yield H2 = 22%, yield CH4 = 28% | 50 h | [125] |
| 3wt.%La–10 wt.% Cu/Al2O3 | wet impregnation | T = 750 °C, EtOH/CO2 = 1:1 | n/a- | XC2H5OH = 87.6% XCO2 = 55.1% yield CO = 27% yield H2 = 52% | - | [138] |
| Mn-15 wt.% Co/CeO2 | urea co-precipitation | T = 650 °C, EtOH/CO2/N2 = 1:1:2 volumetric velocity = 14,000 mLgcat−1 h−1). | n/a | XC2H5OH = 97% Yield H2 = 45 mol.% yield CO = 45 mol.% | 50 h | [139] |
| LaCuO3 (bulk perovskite) | citrate sol–gel | T = 750 °C volumetric velocity = 42 L/gcat/h | n/a | XC2H5OH = 93.7% yield H2 = 54%, yield CO = 50% | - | [118] |
| 5 wt.%Ni/Ce0.75Zr0.25- x(Nb,Ti)xO2-δ | solvothermal method | EtOH/CO2 =1:1 | T = 650 °C for 1 h, 5 vol.% H2/N2 (100 mL/min) | XCO2 = 60–80% | - | [140] |
| 8.09 wt.% CoCe-MOF | solvothermal method | EtOH/CO2/N2 = 1:1:1.5, GHSV = 27,700 mLgcat−1 h−1 | in situ T = 500 °C for 1 h, 5 vol.% H2/N2 (40 mL/min) | XC2H5OH = 97% | 40 h | [141] |
| 13.7 wt.% CoZnO-HT | hard template method | T = 650 °C, EtOH/CO2/N2 = 1:1:1 | T = 500 °C for 1 h, 5 vol.% H2/N2 | XC2H5OH = 100%, XCO2 = 25% yield H2 = 30 mol.% yield CO = 40 mol.% | 40 h | [123] |
| SiO2@Co@CeO2 | electrostatic adsorption | T = 500 °C, EtOH/CO2/N2 = 1:1:1.5, GHSV = 27,700 mLg−1 h−1 | T = 500 °C for 1 h, H2/N2 5 vol.% (40 mL/min) | XC2H5OH = 92% yield H2 = 55.1 mol.% yield CO = 17.8 mol.% | 15 h | [130] |
| 10 wt.% Ni/ACZ | sol–gel | T = 650 °C, EtOH:CO2 = 1:1.8 | n/a | XC2H5OH = 100%, XCO2 = 63% | 7 h | [126] |
| 9.3 wt.% NiZnOx-M | template method | EtOH/CO2/N2 = 1:1:3 | in situ T = 500 °C for 1 h, 5 vol.% H2/N2 | XC2H5OH = 80% | 100 h | [137] |
| 10 wt.% Ni/35AZ | sol–gel | T = 600 °C, CO2/C2H5OH = 1.4:1 | n/a | yield H2 = 47% yield = CO 34% | - | [120] |
| 12 wt.% Ni/CoZn-MOF | MOF precursor-based method | EtOH/CO2/N2 = 1:1:1, 14,000 mLg−1 h−1 | 5 vol.% H2/N2 (40 mL/min) | XC2H5OH = 100% yield H2 = 47% | - | [134] |
| Ni/ZrO2 | precipitation method | T = 450 °C, EtOH:CO2 = 1:1 | n/a | XC2H5OH = 70% | 40 h | [136] |
| 5 wt.% Ni/La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 (LCMFCN) | citrate method | T = 700 °C, EtOH/CO2 = 1:1 | T = 600 °C for 1 h, 5% H2+Ar 2 vol.% EtOH + 2 vol.% CO2 + Ar, T = 600–750 °C | XC2H5OH = 99.7% yield H2 = 50% yield CO = 87% | 5 h | [132] |
| Process | Advantages | Disadvantages |
|---|---|---|
| DRM | Utilizes two greenhouse gases simultaneously (CH4 and CO2); Produces syngas with H2/CO ≈ 1 (suitable for Fischer–Tropsch and oxygenate synthesis); Well-studied with extensive literature and pilot trials | Requires very high temperatures (≥700 °C); Severe coking via CH4 cracking and Boudouard reaction; Ni sintering at elevated temperatures; H2/CO ratio often needs adjustment for downstream use |
| EDR | Combines renewable ethanol with CO2 utilization (bio-based, sustainable); Operates at milder temperatures (300–600 °C); Produces syngas with H2/CO ≈ 1, closer to downstream optimum; Attractive integration into biorefinery concepts | Complex reaction network (dehydration, dehydrogenation, condensation); Formation of oxygenates and polymeric coke precursors; Catalyst deactivation through coke and side-products; Still an emerging technology with limited pilot-scale validation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambetova, M.; Anissova, M.; Myltykbayeva, L.; Makayeva, N.; Dossumov, K.; Yergaziyeva, G. Catalyst Development for Dry Reforming of Methane and Ethanol into Syngas: Recent Advances and Perspectives. Appl. Sci. 2025, 15, 10722. https://doi.org/10.3390/app151910722
Mambetova M, Anissova M, Myltykbayeva L, Makayeva N, Dossumov K, Yergaziyeva G. Catalyst Development for Dry Reforming of Methane and Ethanol into Syngas: Recent Advances and Perspectives. Applied Sciences. 2025; 15(19):10722. https://doi.org/10.3390/app151910722
Chicago/Turabian StyleMambetova, Manshuk, Moldir Anissova, Laura Myltykbayeva, Nursaya Makayeva, Kusman Dossumov, and Gaukhar Yergaziyeva. 2025. "Catalyst Development for Dry Reforming of Methane and Ethanol into Syngas: Recent Advances and Perspectives" Applied Sciences 15, no. 19: 10722. https://doi.org/10.3390/app151910722
APA StyleMambetova, M., Anissova, M., Myltykbayeva, L., Makayeva, N., Dossumov, K., & Yergaziyeva, G. (2025). Catalyst Development for Dry Reforming of Methane and Ethanol into Syngas: Recent Advances and Perspectives. Applied Sciences, 15(19), 10722. https://doi.org/10.3390/app151910722





