Hypoxia-Induced Extracellular Matrix Deposition in Human Mesenchymal Stem Cells: Insights from Atomic Force, Scanning Electron, and Confocal Laser Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Pharmaceutical Hypoxia Conditioning
2.3. Viability Assay
2.4. Quantitative ECM Analysis
2.5. Immunofluorescence Protocol: Confocal Microscopy
2.6. Scanning Electron Microscopy (SEM)
2.7. AFM Imaging
2.8. Statistical Analysis
3. Results
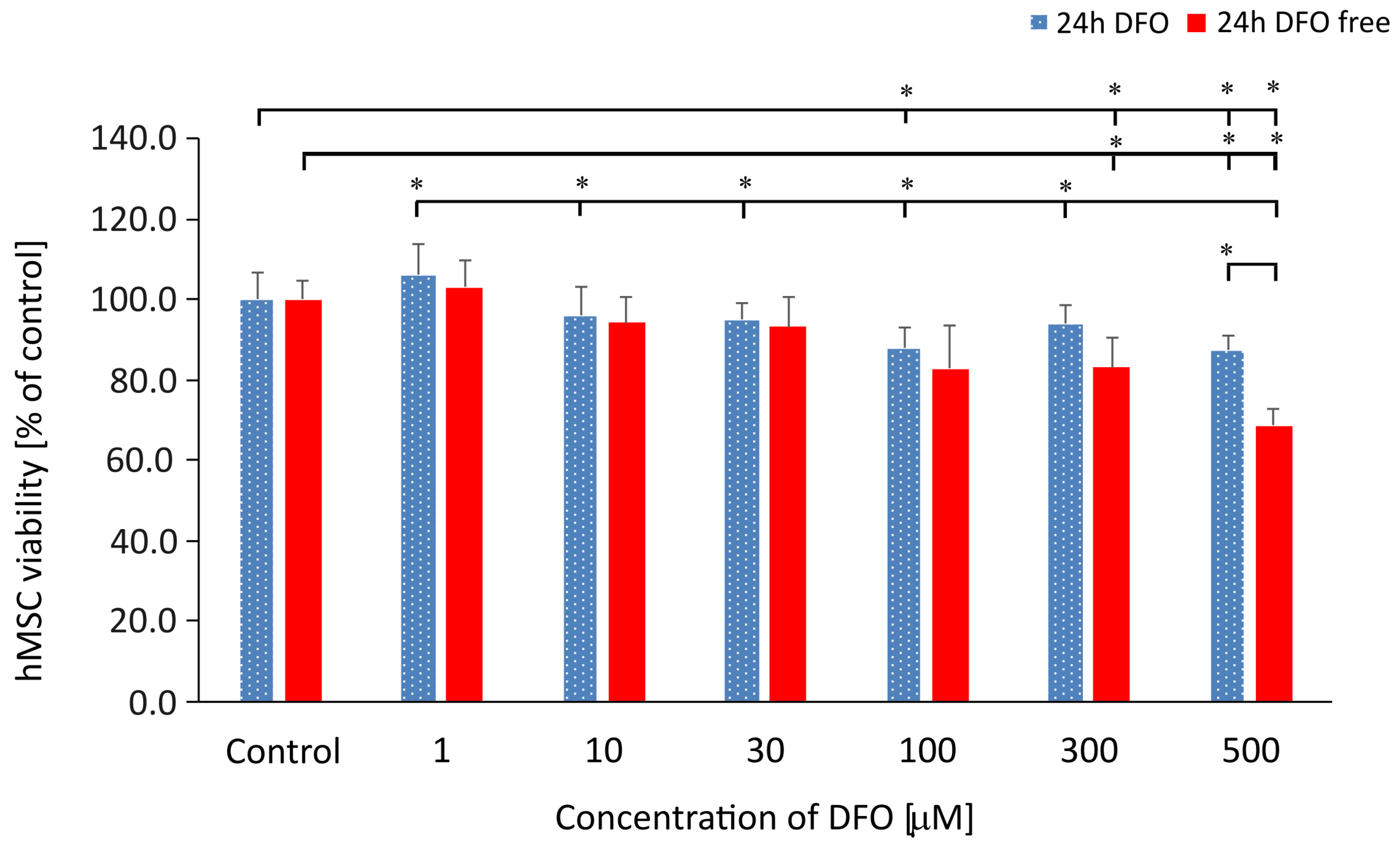
3.1. hMSC Viability Analysis Under the DFO Treatment
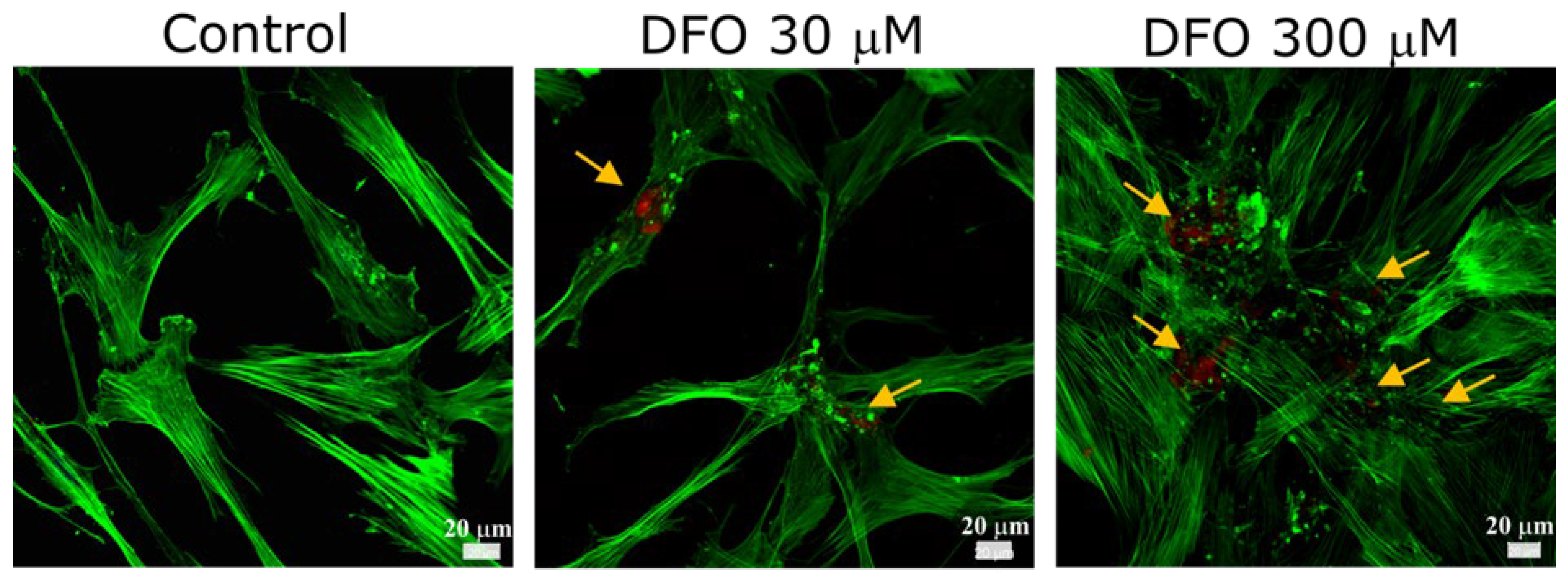
3.2. Identification of Collagen Type I in hMSC-Derived ECM Under Hypoxia with CLSM
3.3. Quantitative Evaluation of Collagen Type I in hMSC-Derived ECM Under Various Culture Conditions
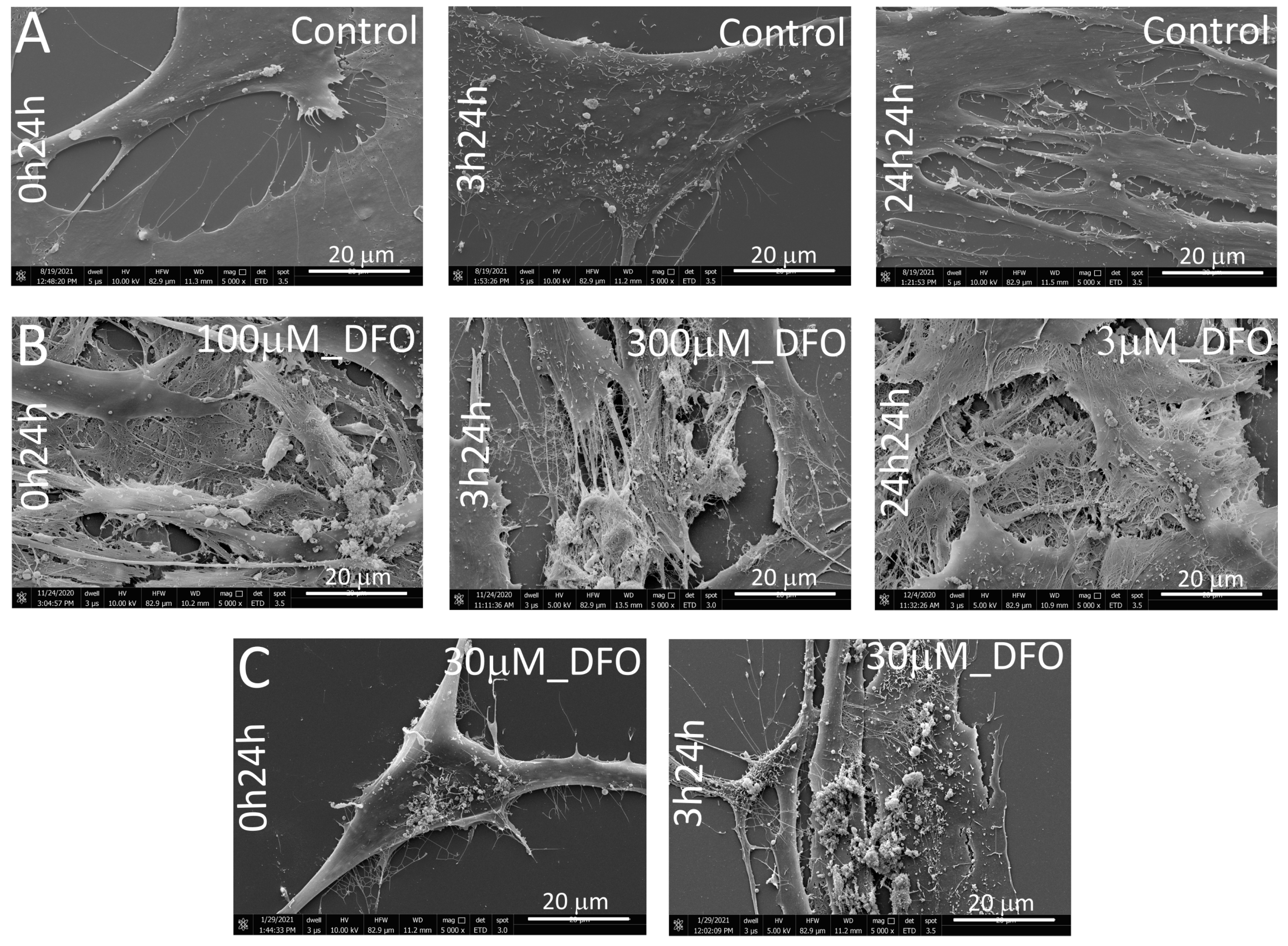
3.4. SEM Morphological Assessment of hMSC-Derived ECM Under DFO-Induced Hypoxia
3.5. Collagen Distribution Analysis in hMSC-Derived ECM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| hMSC | Human mesenchymal stem cells |
| DFO | Deferoxamine |
| Col1 | Collagen type I |
| α2β1 integrin | Collagen binding integrin |
| MMC | Macromolecular crowding |
| CoCl2 | Cobalt (II) chloride |
| VEGF | Vascular endothelial growth factor |
| bFGF | Fibroblast growth factor-2 |
| TGF-β | Transforming growth factor-β |
| HIF-1α | Hypoxia inducible factor-1α |
| LDHA | Lactate dehydrogenase A |
| HK2 | Hexokinase-2 |
| PDK1 | Pyruvate dehydrogenase kinase-1 |
| ETD | Everhart-Thornley detector |
References
- Mangani, S.; Vetoulas, M.; Mineschou, K.; Spanopoulos, K.; Vivanco, M.d.; Piperigkou, Z.; Karamanos, N.K. Design and Applications of Extracellular Matrix Scaffolds in Tissue Engineering and Regeneration. Cells 2025, 14, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xie, S.; Zhang, W.; Zhu, Y.; Xu, D.; Xian, S.; Sun, H.; Guo, X.; Li, Y.; Lu, J.; et al. Current application of tissue-engineered dermal scaffolds mimicking the extracellular matrix microenvironment in wound healing. Regen. Ther. 2025, 16, 371–382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanai, H.; Jacob, G.; Nakagawa, S.; Tuan, R.S.; Nakamura, N.; Shimomura, K. Potential of Soluble Decellularized Extracellular Matrix for Musculoskeletal Tissue Engineering—Comparison of Various Mesenchymal Tissues. Front. Cell Dev. Biol. 2020, 8, 581972. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J. Special Issue: Application of Extracellular Matrix in Regenerative Medicine. Appl. Sci. 2021, 11, 3262. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Ahmad, M.A.; Ao, Q.; Yu, Y.; Shao, D.; Yu, T. Engineering cell-derived extracellular matrix for peripheral nerve regeneration, Mater. Today Bio 2024, 27, 101125. [Google Scholar] [CrossRef]
- Gołębiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mat. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Bourget, J.M.; Gauvin, R.; Larouche, D.; Lavoie, A.; Labbé, R.; Auger, F.A. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials 2012, 33, 9205–9213. [Google Scholar] [CrossRef]
- Hoshiba, T. Cultured cell-derived decellularized matrices: A review towards the next decade. R. Soc. Chem. 2017, 5, 4322–4331. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and b-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2010, 7, 463–477. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Dusevich, V.; Feng, J.Q.; Manolagas, S.C.; Jilka, R.L. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 2007, 22, 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Decaris, M.L.; Leach, J.K. Design of Experiments Approach to Engineer Cell-Secreted Matrices for Directing Osteogenic Differentiation. Annals Biomed. Eng. 2011, 39, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.; Fichou, Y.; Huber, K.; Weiss, M.; Spruijt, E.; Ebbinghaus, S.; De Luca, G.; Morando, M.A.; Vetri, V.; Temussi, P.A.; et al. Molecular Crowding: The History and Development of a Scientific Paradigm. Chem. Rev. 2024, 124, 3186–3219. [Google Scholar] [CrossRef]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Del. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef]
- Lareu, R.R.; Arsianti, I.; Subramhanya, H.K.; Peng, Y.; Raghunath, M. In Vitro Enhancement of Collagen Matrix Formation and Crosslinking for Applications in Tissue Engineering: A Preliminary Study. Tissue Eng. 2007, 13, 385–391. [Google Scholar] [CrossRef]
- Blocki, A.; Wang, Y.; Koch, M.; Goralczyk, A.; Beyer, S.; Agarwal, N. Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications. Mol. Ther. J. 2015, 23, 510–522. [Google Scholar] [CrossRef]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Koda, I.; Song, M.; Chen, G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 9658–9666. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Zeugolis, D.I. It is time to crowd your cell culture media—Physicochemical considerations with biological consequences. Biomaterials 2021, 275, 120943. [Google Scholar] [CrossRef]
- Wana, H.Y.; Shina, R.L.; Chend, J.C.H.; Assunçãoa, M.; Wanga, D.; Nilssone, S.K.; Tuana, R.R.; Blocki, A. Dextran sulfate-amplified extracellular matrix deposition promotes osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2022, 140, 163–177. [Google Scholar] [CrossRef]
- Gaspar, D.; Fuller, K.P.; Zeugolis, D.I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 2019, 88, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Ryana, C.N.M.; Pugliese, E.; Shologua, N.; Gaspar, D.; Rooney, P.; Islamb, N.; O’Riordane, A.; Biggs, M.J.; Griffinb, M.D.; Zeugolis, D.I. Physicochemical cues are not potent regulators of human dermal fibroblast trans-differentiation. Biomater. Biosys. 2023, 11, 100079. [Google Scholar] [CrossRef] [PubMed]
- Satyam, A.; Kumar, P.; Cigognini, D.; Pandit, A.; Zeugolis, D.A. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 2016, 15, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Du, H.C.; Jiang, L.; Geng, W.X.; Li, J.; Zhang, R.; Dang, J.G.; Shu, M.G.; Li, L.W. Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity. Hindawi BioMed Res. Intern. 2017, 2017, 2578017. [Google Scholar] [CrossRef]
- Noronha, N.D.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Shen, H.; Ma, Y.; Qiao, Y.; Zhang, C.; Chen, J.; Zhang, R. Application of Deferoxamine in tissue regeneration attributed to promoted angiogenesis. Molecules 2024, 29, 20250. [Google Scholar] [CrossRef]
- Gaeta, I.M.; Meenderink, L.M.; Postema, M.M.; Cencer, C.S.; Tyska, M.J. Direct visualization of epithelial microvilli biogenesis. Cur. Biol. 2021, 31, 2561–2575. [Google Scholar] [CrossRef]
- Matsunaga, K.; Fujisawa, K.; Takami, T.; Burganova, G.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Nupr1 acts as a pro-survival factor in human bone marrow-derived mesenchymal stem cells and is induced by the hypoxia mimetic reagent deferoxamine. J. Clin. Biochem. Nutr. 2019, 64, 209–216. [Google Scholar] [CrossRef]
- Peyvandi, A.A.; Abbaszadeh, H.A.; Roozbahany, N.A.; Pourbakht, A.; Khoshsirat, S.; Niri, H.H.; Peyvandi, H.; Niknazar, S. Deferoxamine promotes mesenchymal stem cell homing in noise-induced injured cochlea through PI3K/AKT pathway. Cell Prolif. 2018, 51, e12434. [Google Scholar] [CrossRef]
- Liu, G.S.; Peshavariya, H.M.; Higuchi, M. Pharmacological priming of adipose derived stem cells for paracrine VEGF production with deferoxamine. J. Tissue Eng. Reg. Med. 2016, 10, E167–E176. [Google Scholar] [CrossRef]
- Nowak-Stępniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Insight in hypoxia-mimetic agents as potential tools for mesenchymal stem cell priming in regenerative medicine. Hindawi Stem Cells Inter. 2022, 2022, 8775591. [Google Scholar] [CrossRef] [PubMed]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Li, Y.-C.E.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue adhesives: From research clinical translation. Nano Today 2021, 36, 1010492021. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.A.; Fernandes, A.V.; Sundararaghavan, H.G.; Shreiber, D.I. Positively and Negatively Modulating Cell Adhesion to Type I Collagen Via Peptide Grafting. Tissue Eng. Part A 2011, 17, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Rosell-García, T.; Rodriguez-Pascual, T.F. Techniques to Assess Collagen Synthesis, Deposition, and Cross-Linking In Vitro. Methods Mol. Biol. 2021, 2299, 115–122. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E. Collagen fibril formation in vitro and in vivo. Inter. Exper. Pathol. 2017, 98, 4–16. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef]
- Pickard, A.; Garva, R.; Adamson, A.; Calverley, B.C.; Hoyle, A.; Hayward, C.E.; Spiller, D.; Lu, Y.; Hodson, N.; Mandolfo, O.; et al. Collagen fibril formation at the plasma membrane occurs independently from collagen secretion. BioRxiv 2024. [Google Scholar] [CrossRef]
- Ambrock, K.; Grohe, B.; Mittler, S. Oriented Type I Collagen—A Review on Artificial Alignment Strategies. IJSEIMS 2021, 9, 96–123. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen fibril assembly and function. Cur. Top Devel. Biol. 2018, 130, 107–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Liu, Q.; Shen, M.; Liu, Y.; Zhang, X. Simultaneous co-assembly of collagen and glycosaminoglycans to build a biomimetic extracellular matrix for bone regeneration. Inter. J. Biol. Macromol. 2024, 279, 135535. [Google Scholar] [CrossRef]
- Ambati, J.; Adamis, A.P. Transscleral drug delivery to the retina and choroid. Prog. Retinal Eye Res. 2002, 21, 145–151. [Google Scholar] [CrossRef]
- Stearns, M.L. Studies on the development of connective tissue in transparent chambers in the rabbit’s ear. II. Am. J. Anatom. 1940, 67, 55–97. [Google Scholar] [CrossRef]
- Trelstad, R.L.; Kimiko, H. Tendon collagen fibrillogenesis: Intracellular subassemblies and cell surface changes associated with fibril growth. Develop. Biol. 1979, 71, 228–242. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, J.; Zhong, L.; Liu, P.; Li, T.; Yang, K.; Gao, W.; Zhang, G.; Sun, J.; Zou, X. Enhanced therapeutic effects of hypoxia-preconditioned mesenchymal stromal cell-derived extracellular vesicles in renal ischemic injury. Stem Cell Res. Ther. 2025, 16, 39. [Google Scholar] [CrossRef]
- Wang, P.; Husch, J.; Amtz, O.A.; van der Kraan, P.; van de Loo, F.; van den Beucken, J.J. ECM-binding properties of extracellular vesicles: Advanced delivery strategies for therapeutic application in bone and joint diseases. Cell Commun. Sign. 2025, 23, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Head (Coming Out Collagen) (Red Arrow) [nm] | Thin Collagen (Green Arrow) [nm] | Thicker Collagen (Yellow Arrow) [nm] | |
|---|---|---|---|
| Structure diameter | 67 ± 7 | 55 ± 7 | 166 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak-Stępniowska, A.; Osuchowska, P.N.; Fiedorowicz, H.; Trafny, E.A. Hypoxia-Induced Extracellular Matrix Deposition in Human Mesenchymal Stem Cells: Insights from Atomic Force, Scanning Electron, and Confocal Laser Microscopy. Appl. Sci. 2025, 15, 10701. https://doi.org/10.3390/app151910701
Nowak-Stępniowska A, Osuchowska PN, Fiedorowicz H, Trafny EA. Hypoxia-Induced Extracellular Matrix Deposition in Human Mesenchymal Stem Cells: Insights from Atomic Force, Scanning Electron, and Confocal Laser Microscopy. Applied Sciences. 2025; 15(19):10701. https://doi.org/10.3390/app151910701
Chicago/Turabian StyleNowak-Stępniowska, Agata, Paulina Natalia Osuchowska, Henryk Fiedorowicz, and Elżbieta Anna Trafny. 2025. "Hypoxia-Induced Extracellular Matrix Deposition in Human Mesenchymal Stem Cells: Insights from Atomic Force, Scanning Electron, and Confocal Laser Microscopy" Applied Sciences 15, no. 19: 10701. https://doi.org/10.3390/app151910701
APA StyleNowak-Stępniowska, A., Osuchowska, P. N., Fiedorowicz, H., & Trafny, E. A. (2025). Hypoxia-Induced Extracellular Matrix Deposition in Human Mesenchymal Stem Cells: Insights from Atomic Force, Scanning Electron, and Confocal Laser Microscopy. Applied Sciences, 15(19), 10701. https://doi.org/10.3390/app151910701








