In Vivo Comparison of Resin-Modified and Pure Calcium-Silicate Cements for Direct Pulp Capping
Abstract
1. Introduction
| Material | Composition | Advantages | Disadvantages | Setting Type/Time |
|---|---|---|---|---|
| Dycal Pulpdent Pulpcal | Base: Zinc oxide, calcium phosphate, calcium tungstate Catalyst: Calcium hydroxide, zinc oxide, and titanium dioxide | Easy to work with, sets quickly, cost-effective [20] | Limited antibacterial activity over time [54] Poor cohesive strength [55] Greater solubility and marginal leakage [56,57] Tunnel defect in formed reparative dentin [21,22] | Self-curing 2–3 min |
| Cavit-G Zinogen | Zinc oxide eugenol | Reduces inflammation [58] | Releases eugenol, which even at low concentrations can be cytotoxic to dental pulp cells [59,60] | Self-curing 5–10 min setting, with curing continuing for 1–2 h |
| GIC Fuji | Glass ionomer/resin-modified glass ionomer | Excellent bacterial seal [61] Fluoride release [62,63] Bonds to both enamel and dentin Good biocompatibility [64] | Cytotoxic when in direct contact with cells, and does not lead to dentin bridge formation [41,65] | Self-curing 3–5 min |
| Mineral trioxide aggregate (MTA) | Tricalcium and dicalcium silicate, tricalcium aluminate, tricalcium and silicate oxides, and bismuth oxide | Good biocompatibility [7,27] More predictable hard tissue barrier formation than CH [66] Antibacterial properties [67] Osteoinductive [68] | Poor handling characteristics [69] Long setting time [70] | Self-curing 30 min–2 h |
| Biodentine | Powder: tricalcium silicate, calcium carbonate, and zirconium oxide Liquid: water and calcium chloride | Biocompatible [71] Bonds to deep moist dentin [32,72] Induces mineralized bridge formation [48] Faster setting time and good physiochemical properties [28] | Shorter working time and limited radiopacity [73] Difficulty in achieving the desired consistency Poor bonding with overlying restorations [74] | Self-curing 12 min |
| Theracal LC | Calcium silicate, barium sulphate, barium zirconate, fumed silica, and Bis-GMA and PEGDMA resin | Low solubility and good mechanical properties [75] Easy handling, convenient, and short setting time [76] Induced dentin bridge formation [77] | Long-term efficacy is limited [47] Higher cytotoxic effects and reduced cell viability [71] Reduced radiopacity | Light-cured 10 s |
| Premixed Bioceramics (EndoSequence BC, TotalFill BC RRM, iRoot BP Plus) | Calcium silicate, zirconium oxide, calcium phosphate, calcium sulphate, and fillers. | Superior handling, uniform capping consistency and convenient manipulation [78] Biocompatible [71] Ability to induce mineralization and odontoblast differentiation [37] | Limited long-term data [40] Tooth discoloration [42] | Self-curing 10–20 min |
2. Materials and Methods
2.1. Animals
2.2. Direct Pulp Capping Procedure
2.3. Immunofluorescence Labeling
2.4. Histological Examination
2.5. Micro-Computed Tomography (µCT)
2.6. Statistical Analysis
3. Results
3.1. Theracal-LC Led to Reduced Monocyte and Neutrophil Recruitment Compared with EndoSequence RRM Putty and Biodentine at 24 H
3.2. Mineralized Bridge Formation Induced by Calcium-Silicate-Based Pulp Capping Agents
3.3. All Calcium-Silicate-Based Pulp Capping Agents Showed Comparable Marginal Adaptation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagramian, R.A.; Garcia-Godoy, F.; Volpe, A.R. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009, 22, 3–8. [Google Scholar]
- Peres, M.A. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Kazeminia, M.; Abdi, A.; Shohaimi, S.; Jalali, R.; Vaisi-Raygani, A.; Salari, N.; Mohammadi, M. Dental caries in primary and permanent teeth in children’s worldwide, 1995 to 2019: A systematic review and meta-analysis. Head Face Med. 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Lasfargues, J.J. Pulpo-dentinal complex revisited. J. Dent. 1995, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.S.; Galicia, J.C.; Ruparel, N.B. American Association of Endodontics (AAE) Position Statement on Vital Pulp Therapy. J. Endod. 2021, 47, 1340–1344. [Google Scholar]
- Bjørndal, L.; Kidd, E.A. The treatment of deep dentine caries lesions. Dent. Update 2005, 32, 402–404+407–410+413. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F.; Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef]
- Duncan, H.F.; Kirkevang, L.L.; Peters, O.A.; El-Karim, I.; Krastl, G.; Del Fabbro, M.; Chong, B.S.; Galler, K.M.; Segura-Egea, J.J.; Kebschull, M.; et al. Treatment of pulpal and apical disease: The European Society of Endodontology (ESE) S3-level clinical practice guideline. Int. Endod. J. 2023, 56 (Suppl. 3), 238–295. [Google Scholar] [CrossRef]
- Tavares, P.B.; Bonte, E.; Boukpessi, T.; Siqueira, J.F.; Lasfargues, J.J. Prevalence of apical periodontitis in root canal-treated teeth from an urban French population: Influence of the quality of root canal fillings and coronal restorations. J. Endod. 2009, 35, 810–813. [Google Scholar] [CrossRef]
- Fadhil, N.H.; Ali, A.H.; Al Hashimi, R.A.; Sabri Al-Qathi, O.; Foschi, F. Assessment of Treatment Quality Risk Factors Influencing the Radiographic Detection of Apical Periodontitis in Root-Filled Teeth: A Retrospective CBCT Analysis. Eur. Endod. J. 2024, 9, 252–259. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal intervention dentistry for managing dental caries—A review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243. [Google Scholar] [CrossRef]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- (AAE) AAoE. Glossary of Endodontic Terms; American Association of Endodontists: Chicago, IL, USA, 2019. [Google Scholar]
- American Assoication of Endodontists. Guide to Clinical Endodontics, 6th ed.; American Association of Endodontists: Chicago, IL, USA, 2013. [Google Scholar]
- Maltz, M.; Alves, L.S. Pulp capping material is an important prognostic factor for direct pulp capping in permanent teeth. J. Evid. Based Dent. Pract. 2013, 13, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Seo, D.G.; Lee, S.J.; Lee, J.; Jung, I.Y. Prognostic factors for clinical outcomes according to time after direct pulp capping. J. Endod. 2013, 39, 327–331. [Google Scholar] [CrossRef]
- Cohen, B.D.; Combe, E.C. Development of new adhesive pulp capping materials. Dent. Update 1994, 21, 57–62. [Google Scholar] [PubMed]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef]
- Zhu, C.; Ju, B.; Ni, R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17055–17060. [Google Scholar]
- Cox, C.F.; Sübay, R.K.; Ostro, E.; Suzuki, S.; Suzuki, S.H. Tunnel defects in dentin bridges: Their formation following direct pulp capping. Oper. Dent. 1996, 21, 4–11. [Google Scholar]
- Amin, L.E.; Montaser, M. Comparative evaluation of pulpal repair after direct pulp capping using stem cell therapy and biodentine: An animal study. Aust. Endod. J. 2021, 47, 11–19. [Google Scholar] [CrossRef]
- Rao, Q.; Kuang, J.; Mao, C.; Dai, J.; Hu, L.; Lei, Z.; Song, G.; Yuan, G. Comparison of iRoot BP Plus and Calcium Hydroxide as Pulpotomy Materials in Permanent Incisors with Complicated Crown Fractures: A Retrospective Study. J. Endod. 2020, 46, 352–357. [Google Scholar] [CrossRef]
- Holland, R.; de Souza, V.; de Mello, W.; Nery, M.J.; Bernabé, P.F.; Otoboni Filho, J.A. Permeability of the hard tissue bridge formed after pulpotomy with calcium hydroxide: A histologic study. J. Am. Dent. Assoc. 1979, 99, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Carti, O.; Oznurhan, F. Evaluation and comparison of mineral trioxide aggregate and biodentine in primary tooth pulpotomy: Clinical and radiographic study. Niger. J. Clin. Pract. 2017, 20, 1604–1609. [Google Scholar]
- Andrei, M.; Vacaru, R.P.; Coricovac, A.; Ilinca, R.; Didilescu, A.C.; Demetrescu, I. The Effect of Calcium-Silicate Cements on Reparative Dentinogenesis Following Direct Pulp Capping on Animal Models. Molecules 2021, 26, 2725. [Google Scholar] [CrossRef] [PubMed]
- Alnour, A.; Almohammad, G.; Abdo, A.; Layous, K. Evaluation of the pulp response following direct pulp capping with exogenous nitric oxide and Mineral Trioxide Aggregate (MTA) a histologic study. Heliyon 2023, 9, e17458. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Karapinar Kazandağ, M.; Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. BioMed Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Gasner, N.S.; Brizuela, M. Endodontic Materials Used To Fill Root Canals. In StatPearls; Updated 19 March 2023; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587367/ (accessed on 30 April 2025).
- About, I. Biodentine: From biochemical and bioactive properties to clinical applications Biodentine: Dalle proprietà biochimiche e bioattive alle applicazioni cliniche. G. Ital. Endod. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Chae, Y.K.; Ye, J.R.; Nam, O.H. Evaluation of biocompatibility and bioactive potential of Well-Root PT by comparison with ProRoot MTA and Biodentine. J. Dent. Sci. 2024, 19, 2218–2225. [Google Scholar] [CrossRef]
- Gangishetti, S.; Kolluri, A.; Raj, K.A.; Kamsani, D.; Manchala, S.; Jarupula, D. Bioactivity of Calcium Silicate-Based Endodontic Materials: A Comparative. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 2), S1716–S1720. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Al-Saudi, K.W. A paradigm shift from calcium hydroxide to bioceramics in direct pulp capping: A narrative review. J. Conserv. Dent. Endod. 2024, 27, 2–10. [Google Scholar] [CrossRef]
- Cavenago, B.C.; Pereira, T.C.; Duarte, M.A.; Ordinola-Zapata, R.; Marciano, M.A.; Bramante, C.M.; Bernardineli, N. Influence of powder-to-water ratio on radiopacity, setting time, pH, calcium ion release and a micro-CT volumetric solubility of white mineral trioxide aggregate. Int. Endod. J. 2014, 47, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Domingos Pires, M.; Cordeiro, J.; Vasconcelos, I.; Alves, M.; Quaresma, S.A.; Ginjeira, A.; Camilleri, J. Effect of different manipulations on the physical, chemical and microstructural characteristics of Biodentine. Dent. Mater. 2021, 37, e399–e406. [Google Scholar] [CrossRef]
- Mahgoub, N.; Alqadasi, B.; Aldhorae, K.; Assiry, A.; Altawili, Z.M.; Hong, T. Comparison between iRoot BP Plus (EndoSequence Root Repair Material) and Mineral Trioxide Aggregate as Pulp-capping Agents: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 542–552. [Google Scholar]
- Liu, S.; Wang, S.; Dong, Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 2015, 41, 652–657. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Motwani, N.; Ikhar, A.; Nikhade, P.; Chandak, M.; Rathi, S.; Dugar, M.; Rajnekar, R. Premixed bioceramics: A novel pulp capping agent. J. Conserv. Dent. 2021, 24, 124–129. [Google Scholar] [CrossRef]
- Šimundić Munitić, M.; Poklepović Peričić, T.; Utrobičić, A.; Bago, I.; Puljak, L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: A systematic review. PLoS ONE 2019, 14, e0223575. [Google Scholar] [CrossRef] [PubMed]
- Prasad Kumara, P.A.A.S.; Cooper, P.R.; Cathro, P.; Gould, M.; Dias, G.; Ratnayake, J. Bioceramics in Endodontics: Limitations and Future Innovations—A Review. Dent. J. 2025, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Hebling, J.; Lessa, F.C.; Nogueira, I.; Carvalho, R.M.; Costa, C.A. Cytotoxicity of resin-based light-cured liners. Am. J. Dent. 2009, 22, 137–142. [Google Scholar]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Naghi Moosavi, F.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V.; et al. Human Pulp Responses to Partial Pulpotomy Treatment with TheraCal as Compared with Biodentine and ProRoot MTA: A Clinical Trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.Z.; Rabi, T. TheraCal LC: From Biochemical and Bioactive Properties to Clinical Applications. Int. J. Dent. 2018, 2018, 3484653. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.S.; Chandak, M.G.; Jaiswal, A.A.; Mankar, N.P.; Paul, P. A Breakthrough in the Era of Calcium Silicate-Based Cements: A Critical Review. Cureus 2022, 14, e28562. [Google Scholar] [CrossRef]
- Mahapatra, J.; Nikhade, P.P.; Patel, A.; Mankar, N.; Taori, P. Comparative Evaluation of the Efficacy of TheraCal LC, Mineral Trioxide Aggregate, and Biodentine as Direct Pulp Capping Materials in Patients with Pulpal Exposure in Posterior Teeth: A Triple-Blinded Randomized Parallel Group Clinical Trial. Cureus 2024, 16, e55022. [Google Scholar] [CrossRef]
- Minic, S.; Florimond, M.; Sadoine, J.; Valot-Salengro, A.; Chaussain, C.; Renard, E.; Boukpessi, T. Evaluation of Pulp Repair after Biodentine. Biomedicines 2021, 9, 784. [Google Scholar] [CrossRef]
- Bui, A.H.; Pham, K.V. Evaluation of Reparative Dentine Bridge Formation after Direct Pulp Capping with Biodentine. J. Int. Soc. Prev. Community Dent. 2021, 11, 77–82. [Google Scholar] [CrossRef]
- De Rossi, A.; Silva, L.A.; Gatón-Hernández, P.; Sousa-Neto, M.D.; Nelson-Filho, P.; Silva, R.A.; de Queiroz, A.M. Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J. Endod. 2014, 40, 1362–1369. [Google Scholar] [CrossRef]
- Al Tuwirqi, A.A.; El Ashiry, E.A.; Alzahrani, A.Y.; Bamashmous, N.; Bakhsh, T.A. Tomographic Evaluation of the Internal Adaptation for Recent Calcium Silicate-Based Pulp Capping Materials in Primary Teeth. BioMed Res. Int. 2021, 2021, 5523145. [Google Scholar] [CrossRef]
- Patel, C.; Patel, M.; Panchal, M.; Bhatt, R.; Patel, F.; Makwani, D. Evaluation of Microleakage and Shear Bond Strength of Two Different Calcium-based Silicate Indirect Pulp Capping Agents When Restored with Glass Ionomer Cement and Composite Resin Restoration: A Comparative. Int. J. Clin. Pediatr. Dent. 2024, 17, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Muliyar, S.; Shameem, K.A.; Thankachan, R.P.; Francis, P.G.; Jayapalan, C.S.; Hafiz, K.A. Microleakage in endodontics. J. Int. Oral Health 2014, 6, 99–104. [Google Scholar]
- Coogan, M.M.; Creaven, P.J. Antibacterial properties of eight dental cements. Int. Endod. J. 1993, 26, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.F.; Hafez, A.A.; Akimoto, N.; Otsuki, M.; Suzuki, S.; Tarim, B. Biocompatibility of primer, adhesive and resin composite systems on non-exposed and exposed pulps of non-human primate teeth. Am. J. Dent. 1998, 11, S55–S63. [Google Scholar]
- Goldberg, M.; Njeh, A.; Uzunoglu, E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediat. Inflamm. 2015, 2015, 347649. [Google Scholar] [CrossRef]
- Taira, Y.; Shinkai, K.; Suzuki, M.; Kato, C.; Katoh, Y. Direct pulp capping effect with experimentally developed adhesive resin systems containing reparative dentin-promoting agents on rat pulp: Mixed amounts of additives and their effect on wound healing. Odontology 2011, 99, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 557–561. [Google Scholar] [CrossRef]
- Hume, W.R. The pharmacologic and toxicological properties of zinc oxide-eugenol. J. Am. Dent. Assoc. 1986, 113, 789–791. [Google Scholar] [CrossRef]
- Escobar-García, M.; Rodríguez-Contreras, K.; Ruiz-Rodríguez, S.; Pierdant-Pérez, M.; Cerda-Cristerna, B.; Pozos-Guillén, A. Eugenol Toxicity in Human Dental Pulp Fibroblasts of Primary Teeth. J. Clin. Pediatr. Dent. 2016, 40, 312–318. [Google Scholar] [CrossRef]
- Alomari, Q.D.; Reinhardt, J.W.; Boyer, D.B. Effect of liners on cusp deflection and gap formation in composite restorations. Oper. Dent. 2001, 26, 406–411. [Google Scholar]
- Nakade, O.; Koyama, H.; Arai, J.; Ariji, H.; Takada, J.; Kaku, T. Stimulation by low concentrations of fluoride of the proliferation and alkaline phosphatase activity of human dental pulp cells in vitro. Arch. Oral. Biol. 1999, 44, 89–92. [Google Scholar] [CrossRef]
- Thaweboon, S.; Thaweboon, B.; Chunhabundit, P.; Suppukpatana, P. Effect of fluoride on human dental pulp cells in vitro. Southeast Asian J. Trop. Med. Public Health 2003, 34, 915–918. [Google Scholar] [PubMed]
- Mitra, S.B.; Lee, C.Y.; Bui, H.T.; Tantbirojn, D.; Rusin, R.P. Long-term adhesion and mechanism of bonding of a paste-liquid resin-modified glass-ionomer. Dent. Mater. 2009, 25, 459–466. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances released from resin-based dental restorative materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [PubMed]
- Suhag, K.; Duhan, J.; Tewari, S.; Sangwan, P. Success of Direct Pulp Capping Using Mineral Trioxide Aggregate and Calcium Hydroxide in Mature Permanent Molars with Pulps Exposed during Carious Tissue Removal: 1-year Follow-up. J. Endod. 2019, 45, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Okiji, T.; Yoshiba, K. Reparative dentinogenesis induced by mineral trioxide aggregate: A review from the biological and physicochemical points of view. Int. J. Dent. 2009, 2009, 464280. [Google Scholar] [CrossRef]
- Johnson, B.R. Considerations in the selection of a root-end filling material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1999, 87, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Shahabi, S.; Jafarzadeh, T.; Amini, S.; Kheirieh, S. The properties of a new endodontic material. J. Endod. 2008, 34, 990–993. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Dagna, A.; Beltrami, R.; Colombo, M.; Chiesa, M. In vitro cytotoxicity evaluation of different pulp capping materials: A comparative study. Arh. Hig. Rada Toksikol. 2015, 66, 181–188. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef]
- Tanalp, J.; Karapınar-Kazandağ, M.; Dölekoğlu, S.; Kayahan, M.B. Comparison of the radiopacities of different root-end filling and repair materials. Sci. World J. 2013, 2013, 594950. [Google Scholar] [CrossRef]
- Arandi, N.Z.; Thabet, M. Minimal Intervention in Dentistry: A Literature Review on Biodentine as a Bioactive Pulp Capping Material. BioMed Res. Int. 2021, 2021, 5569313. [Google Scholar] [CrossRef]
- Bakir, E.P.; Yildirim, Z.S.; Bakir, Ş.; Ketani, A. Are resin-containing pulp capping materials as reliable as traditional ones in terms of local and systemic biological effects? Dent. Mater. J. 2022, 41, 78–86. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wu, J.; Wang, X.; Huang, J.; Li, Q. Clinical influencing factors of vital pulp therapy on pulpitis permanent teeth with 2 calcium silicate-based materials: A randomized clinical trial. Medicine 2024, 103, e38015. [Google Scholar] [CrossRef]
- Cannon, M.; Gerodias, N.; Viera, A.; Percinoto, C.; Jurado, R. Primate pulpal healing after exposure and TheraCal application. J. Clin. Pediatr. Dent. 2014, 38, 333–337. [Google Scholar] [CrossRef]
- Song, M.; Lee, S.M.; Bang, J.Y.; Kim, R.H.; Kwak, S.W.; Kim, H.C. Chemomechanical Properties and Biocompatibility of Various Premixed Putty-type Bioactive Ceramic Cements. J. Endod. 2023, 49, 1713–1721. [Google Scholar] [CrossRef]
- Nagendrababu, V.; HDummer, P.M. Preferred Reporting Items for study Designs in Endodontology (PRIDE): Guiding authors to produce high-quality manuscripts. J. Conserv. Dent. 2020, 23, 320–324. [Google Scholar]
- Nagendrababu, V.; Kishen, A.; Murray, P.E.; Nekoofar, M.H.; de Figueiredo, J.A.P.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Jakovljevic, A.; Dummer, P.M.H. PRIASE 2021 guidelines for reporting animal studies in Endodontology: Explanation and elaboration. Int. Endod. J. 2021, 54, 858–886. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartilage 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Solidum, J.G.N.; Park, D. Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration. J. Bone Metab. 2023, 30, 231–244. [Google Scholar] [CrossRef]
- Byers, M.R.; Närhi, M.V. Dental injury models: Experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit. Rev. Oral. Biol. Med. 1999, 10, 4–39. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.B.; Chu, E.Y.; Kram, V.; de Castro, L.F.; Somerman, M.J.; Foster, B.L. Guidelines for Micro-Computed Tomography Analysis of Rodent Dentoalveolar Tissues. JBMR Plus 2021, 5, e10474. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.V.; Faizuddin, U.; Karunakar, P.; Deepthi Sarvani, G.; Sree Soumya, S. Coronal Pulpotomy Technique Analysis as an Alternative to Pulpectomy for Preserving the Tooth Vitality, in the Context of Tissue Regeneration: A Correlated Clinical Study across 4 Adult Permanent Molars. Case Rep. Dent. 2015, 2015, 916060. [Google Scholar] [CrossRef]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Fazlyab, M.; Baghban, A.A.; Ghoddusi, J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: A non-inferiority multicenter randomized clinical trial. Clin. Oral. Investig. 2015, 19, 335–341. [Google Scholar] [CrossRef]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Tomson, P.L.; Lundy, F.T.; Cooper, P.; Clarke, M.; El Karim, I.A. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: A systematic review. J. Dent. 2019, 88, 103158. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B. Minimally invasive endodontics: A new era for pulpotomy in mature permanent teeth. Br. Dent. J. 2022, 233, 1035–1041. [Google Scholar] [CrossRef]
- Cvek, M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J. Endod. 1978, 4, 232–237. [Google Scholar] [CrossRef]
- Gudkina, J.; Mindere, A.; Locane, G.; Brinkmane, A. Review of the success of pulp exposure treatment of cariously and traumatically exposed pulps in immature permanent incisors and molars. Stomatologija 2012, 14, 71–80. [Google Scholar]
- Duncan, H.F. Present status and future directions-Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55 (Suppl. 3), 497–511. [Google Scholar] [CrossRef] [PubMed]
- Dummer, P.M.; Hicks, R.; Huws, D. Clinical signs and symptoms in pulp disease. Int. Endod. J. 1980, 13, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Dammaschke, T.; Lipski, M.; Schäfer, E. Effectiveness of diagnosing pulpitis: A systematic review. Int. Endod. J. 2023, 56 (Suppl. 3), 296–325. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine-Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.; Akamp, T.; Smeda, M.; Uderhardt, S.; Besold, D.; Krastl, G.; Galler, K.M.; Buchalla, W.; Widbiller, M. Understanding dental pulp inflammation: From signaling to structure. Front. Immunol. 2024, 15, 1474466. [Google Scholar] [CrossRef]
- Erdogan, O.; Xia, J.; Chiu, I.M.; Gibbs, J.L. Dynamics of Innate Immune Response in Bacteria-Induced Mouse Model of Pulpitis. J. Endod. 2023, 49, 1529–1536. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.; Ma, L.; Li, X.; Wei, C.; Tian, X.; Liu, K. Identification of the characteristics of infiltrating immune cells in pulpitis and its potential molecular regulation mechanism by bioinformatics method. BMC Oral Health 2023, 23, 287. [Google Scholar] [CrossRef]
- Gaudin, A.; Renard, E.; Hill, M.; Bouchet-Delbos, L.; Bienvenu-Louvet, G.; Farges, J.C.; Cuturi, M.C.; Alliot-Licht, B. Phenotypic analysis of immunocompetent cells in healthy human dental pulp. J. Endod. 2015, 41, 621–627. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Erdogan, O.; Michot, B.; Xia, J.; Alabdulaaly, L.; Yesares Rubi, P.; Ha, V.; Chiu, I.M.; Gibbs, J.L. Neuronal-immune axis alters pain and sensory afferent damage during dental pulp injury. Pain 2024, 165, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, B.W.; Jensen, E.; Örtengren, U.; Michelsen, V.B. Analysis of organic components in resin-modified pulp capping materials: Critical considerations. Eur. J. Oral. Sci. 2017, 125, 183–194. [Google Scholar] [CrossRef]
- Makkar, S.; Kaur, H.; Aggarwal, A.; Vashisht, R. A Confocal Laser Scanning Microscopic Study Evaluating the Sealing Ability of Mineral Trioxide Aggregate, Biodentine and Anew Pulp Capping Agent-Theracal. Dent. J. Adv. Stud. 2015, 3, 20–25. [Google Scholar] [CrossRef]
- Deepa, V.L.; Dhamaraju, B.; Bollu, I.P.; Balaji, T.S. Shear bond strength evaluation of resin composite bonded to three different liners: TheraCal LC, Biodentine, and resin-modified glass ionomer cement using universal adhesive: An in vitro study. J. Conserv. Dent. 2016, 19, 166–170. [Google Scholar] [CrossRef]
- Cantekin, K. Bond strength of different restorative materials to light-curable mineral trioxide aggregate. J. Clin. Pediatr. Dent. 2015, 39, 143–148. [Google Scholar] [CrossRef]
- Meraji, N.; Camilleri, J. Bonding over Dentin Replacement Materials. J. Endod. 2017, 43, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Karadas, M.; Cantekin, K.; Gumus, H.; Ateş, S.M.; Duymuş, Z.Y. Evaluation of the bond strength of different adhesive agents to a resin-modified calcium silicate material (TheraCal LC). Scanning 2016, 38, 403–411. [Google Scholar] [CrossRef]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Toia, C.C.; Teixeira, F.B.; Cucco, C.; Valera, M.C.; Cavalcanti, B.N. Filling ability of three bioceramic root-end filling materials: A micro-computed tomography analysis. Aust. Endod. J. 2020, 46, 424–431. [Google Scholar] [CrossRef]
- Jung, M.; Lommel, D.; Klimek, J. The imaging of root canal obturation using micro-CT. Int. Endod. J. 2005, 38, 617–626. [Google Scholar] [CrossRef]
- Aubeux, D.; Renard, E.; Pérez, F.; Tessier, S.; Geoffroy, V.; Gaudin, A. Review of Animal Models to Study Pulp Inflammation. Front. Dent. Med. 2021, 2, 673552. [Google Scholar] [CrossRef]
- Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab. Anim. 2010, 44, 1–6. [Google Scholar] [CrossRef]
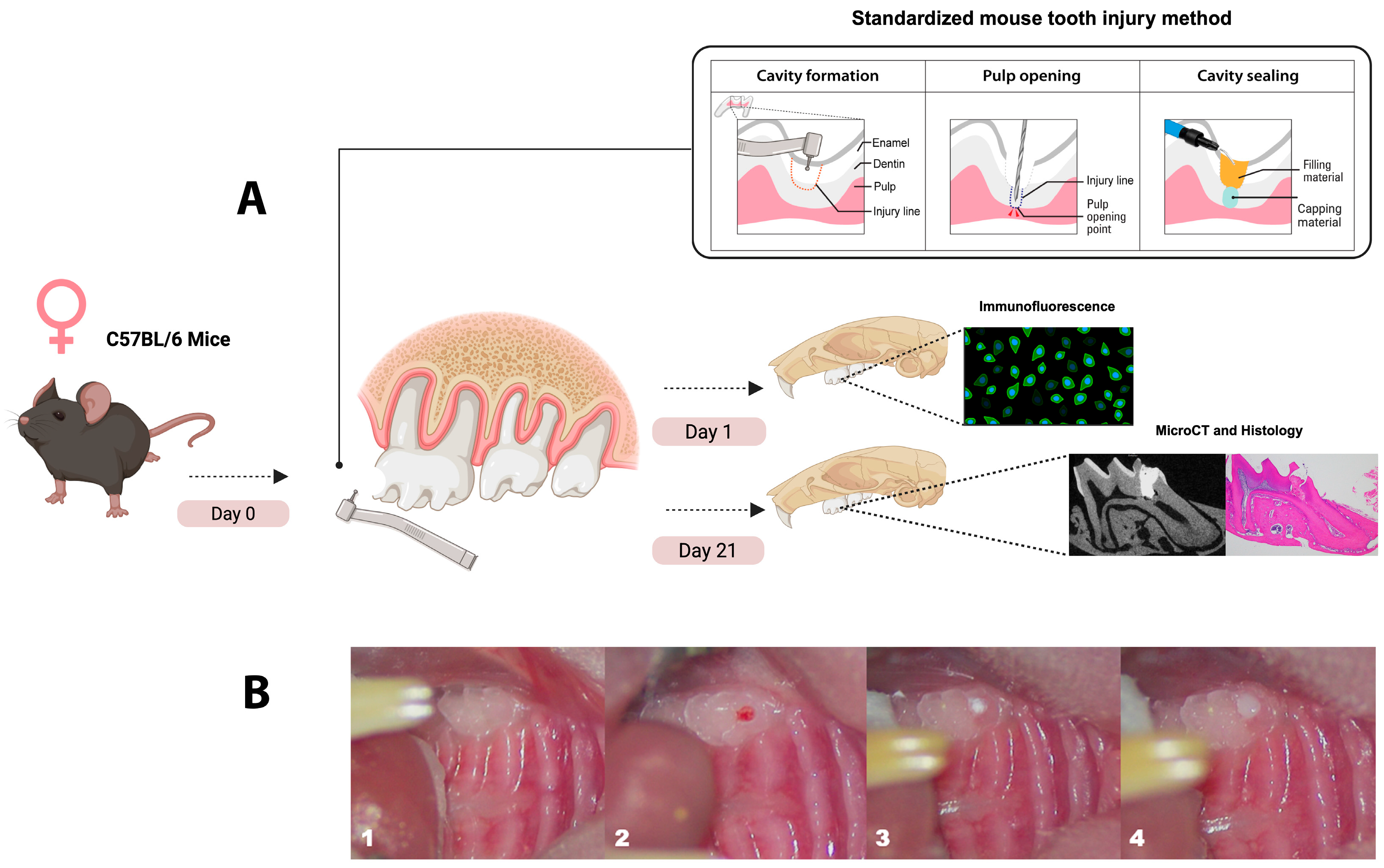

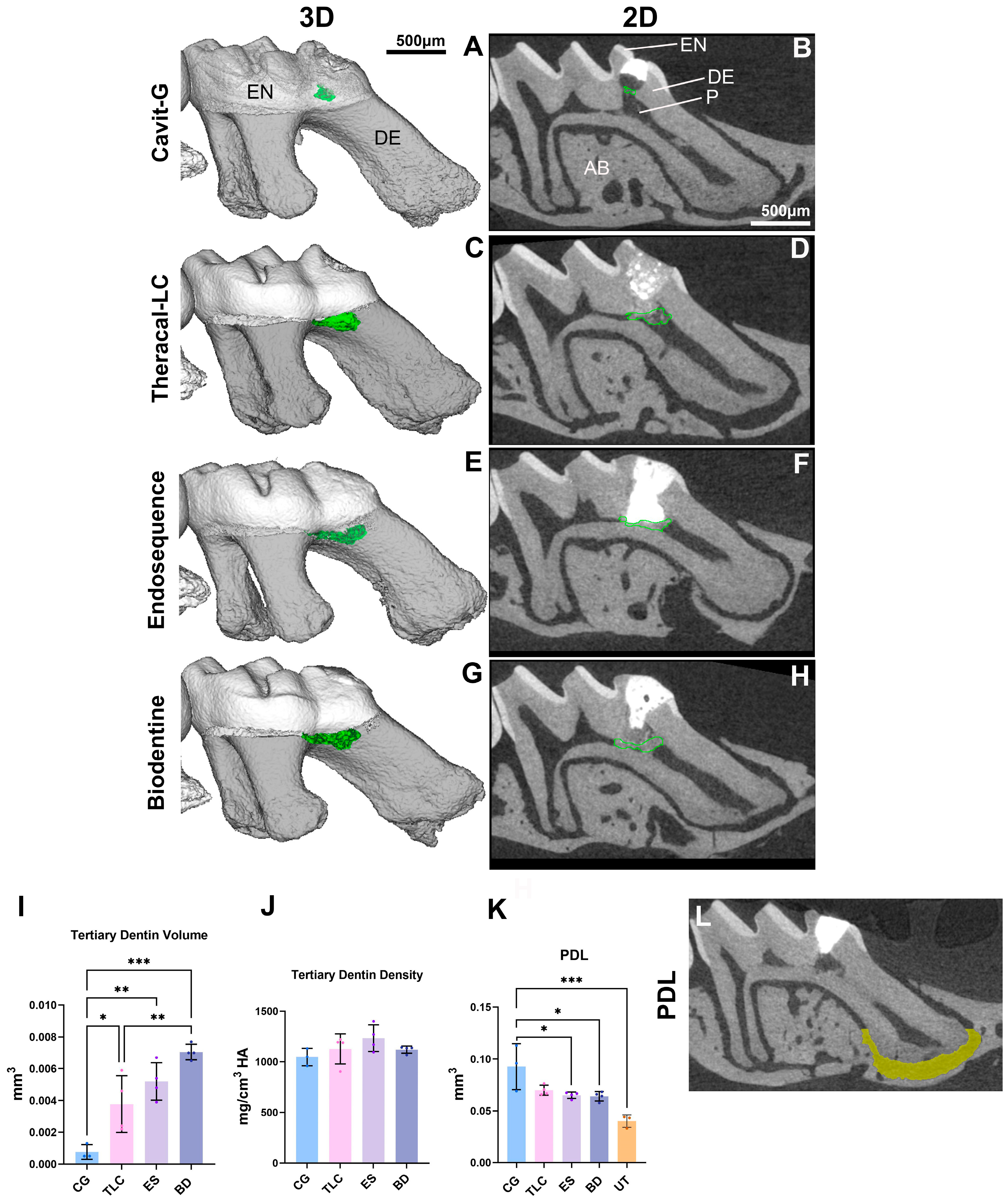
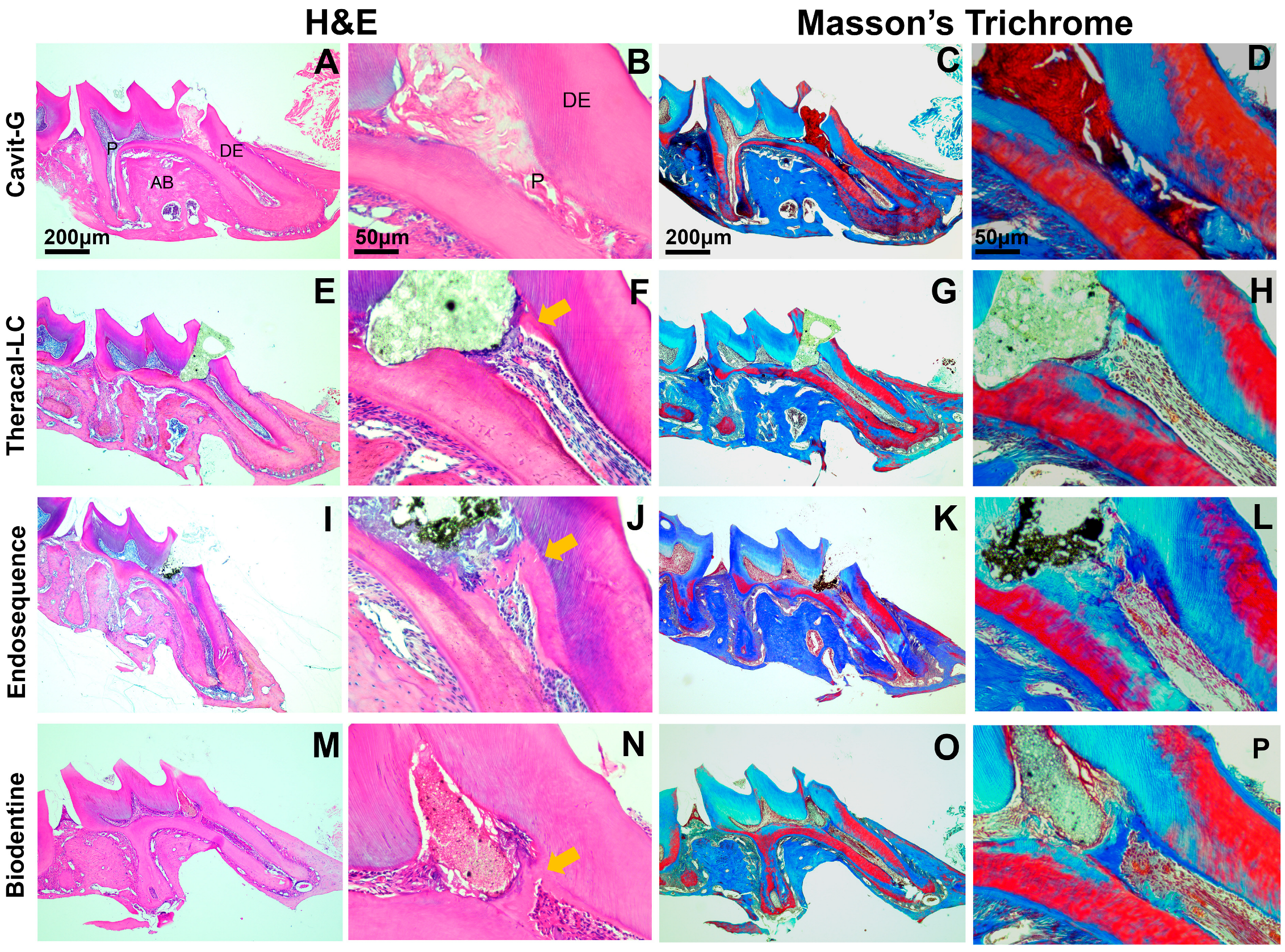
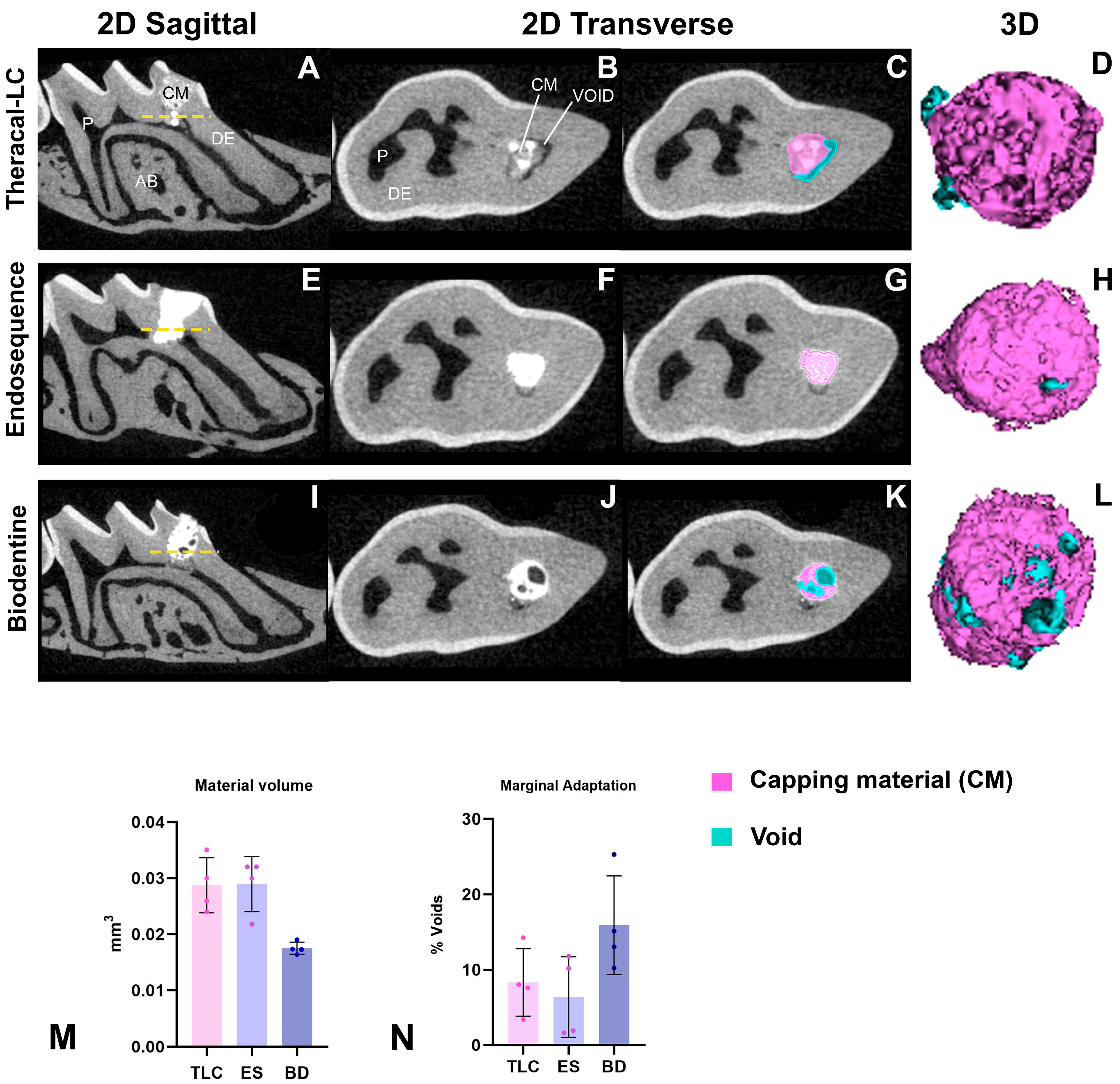
| Capping Material | Material Application | Setting Time |
|---|---|---|
| Biodentine (BD): | BD powder and liquid were mixed according to the manufacturer’s instructions to form a putty-like paste. The powder capsule was placed into the provided stand and 5 drops of liquid were added, after which the capsule was closed and placed into the 3M ESPE RotoMixer (3M ESPE, Solventum, Solventum, MN, USA) for 30 s at 4000 vibrations per minute. The mixed BD paste (<1 mm) was gently placed over the pulp with a micro-spatula and allowed to set before final restoration. | 12 min |
| Theracal-LC (TLC): | After achieving gentle hemostasias with a sterile paper point, a thin layer not exceeding 1 mm of TLC was applied directly to the pulp exposure site using a micro-spatula. Polymerization of TLC was performed using a two-LED-based curing blue light for 20 s with a wavelength ranging from 420 to 490 nm and an output intensity of 1200 mW/cm2 (Essentials, The Dentistry Supply Company, Inc., Melville, NY, USA), after which the final restoration was placed over the TLC to seal the cavity. | 20 s |
| EndoSequence RRM Putty (ES): | Leaving the dentin lightly moistened, 0.5–1 mm of ES putty was delivered with a sterile applicator and gently adapted to the pulp surface. As a moisture-setting material, it was allowed to harden before applying the final restoration. | 10 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenesha, F.; Phanrungsuwan, A.; Foster, B.L.; Diogenes, A.; Peters, S.B. In Vivo Comparison of Resin-Modified and Pure Calcium-Silicate Cements for Direct Pulp Capping. Appl. Sci. 2025, 15, 10639. https://doi.org/10.3390/app151910639
Fenesha F, Phanrungsuwan A, Foster BL, Diogenes A, Peters SB. In Vivo Comparison of Resin-Modified and Pure Calcium-Silicate Cements for Direct Pulp Capping. Applied Sciences. 2025; 15(19):10639. https://doi.org/10.3390/app151910639
Chicago/Turabian StyleFenesha, Fatma, Aonjittra Phanrungsuwan, Brian L. Foster, Anibal Diogenes, and Sarah B. Peters. 2025. "In Vivo Comparison of Resin-Modified and Pure Calcium-Silicate Cements for Direct Pulp Capping" Applied Sciences 15, no. 19: 10639. https://doi.org/10.3390/app151910639
APA StyleFenesha, F., Phanrungsuwan, A., Foster, B. L., Diogenes, A., & Peters, S. B. (2025). In Vivo Comparison of Resin-Modified and Pure Calcium-Silicate Cements for Direct Pulp Capping. Applied Sciences, 15(19), 10639. https://doi.org/10.3390/app151910639







