Reactive Oxygen Species-Mediated Oral Cancer Cells Treatment Using Photosensitizer-Combined Carbon Dots via Apoptosis–Ferroptosis Synergistic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CDs
2.2. Cell Culture
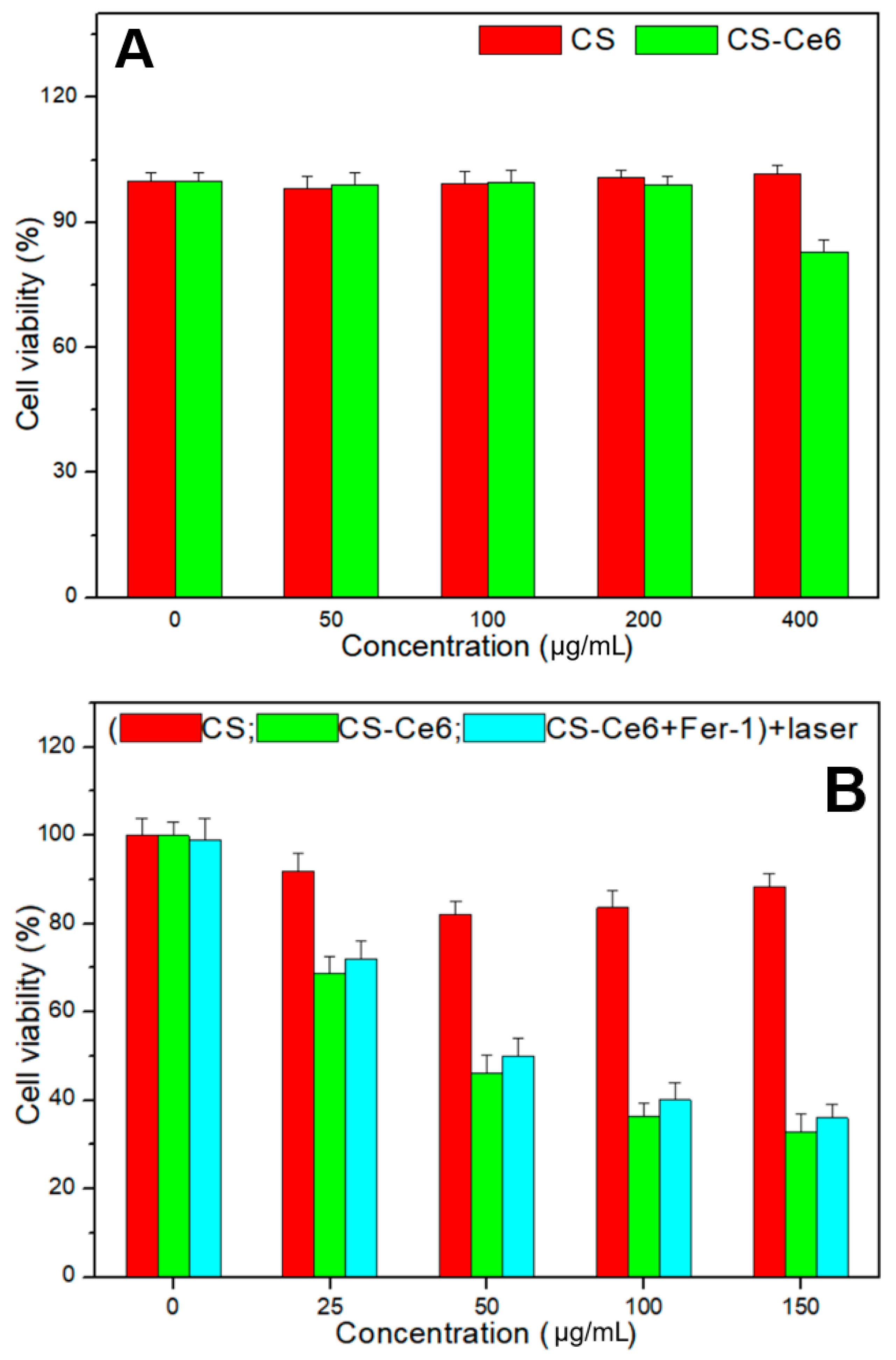
2.3. Cell Viability Test
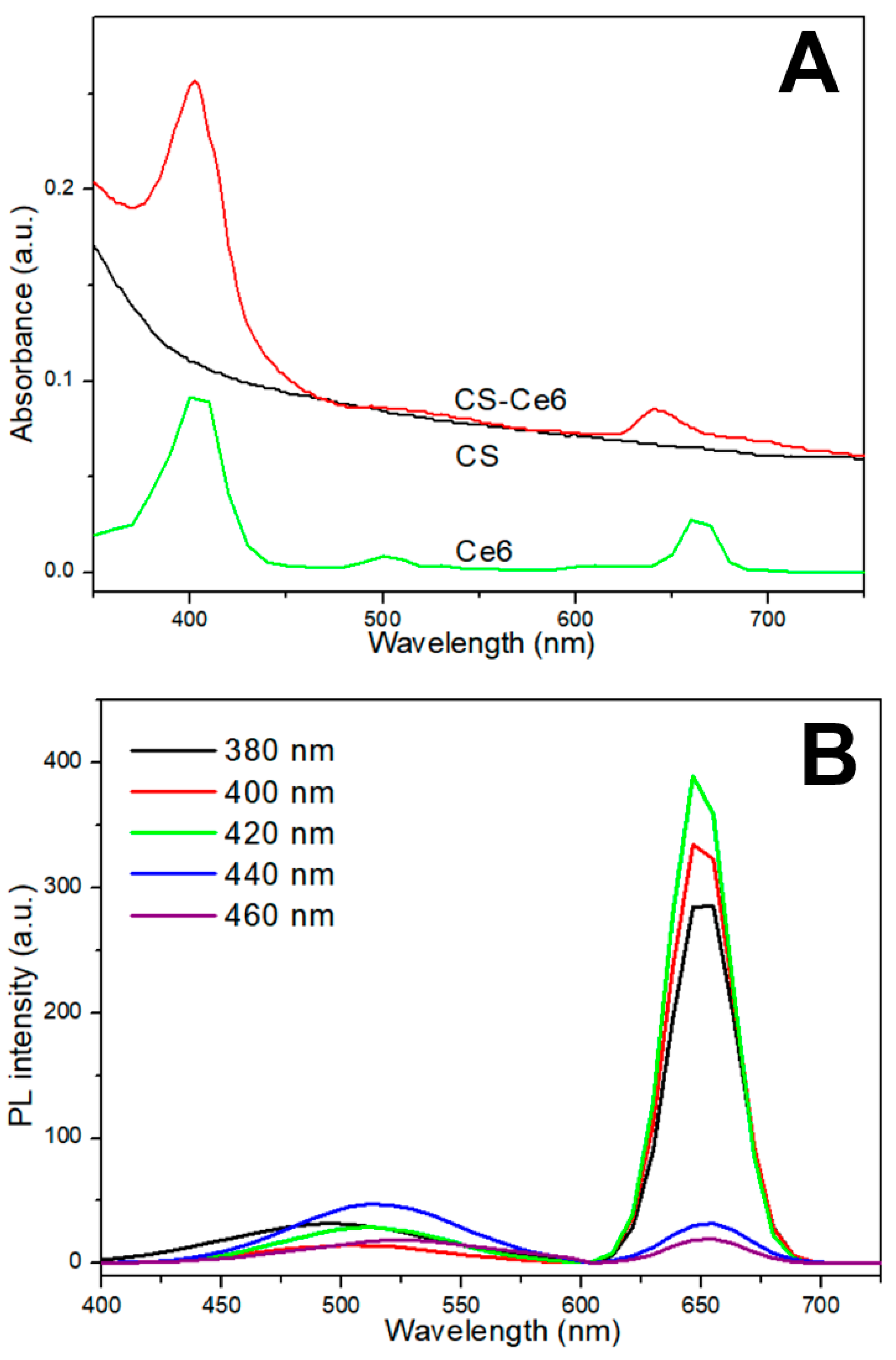
2.4. Characteristics of CS-Ce6
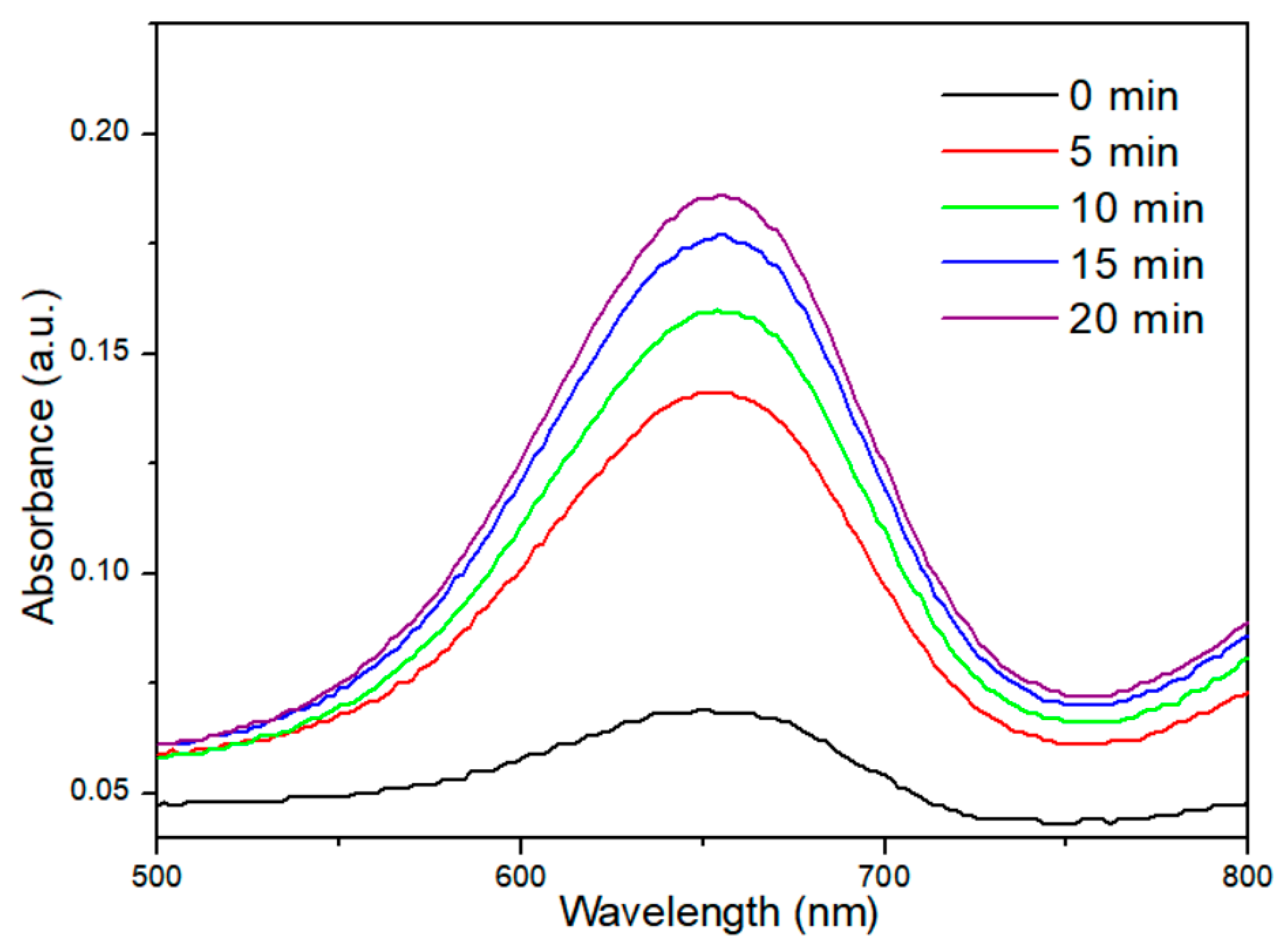
2.5. Evaluation of ROS Generation
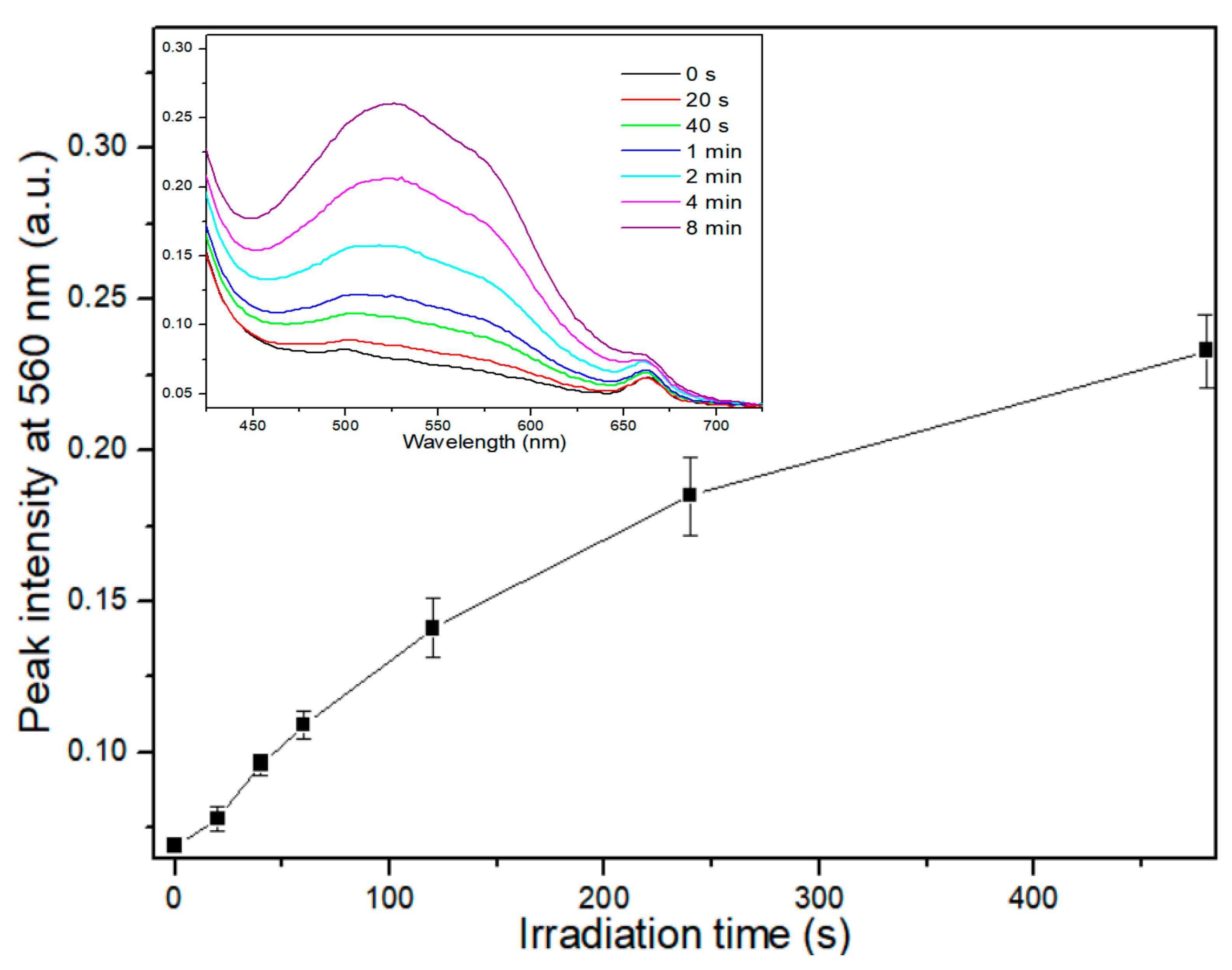
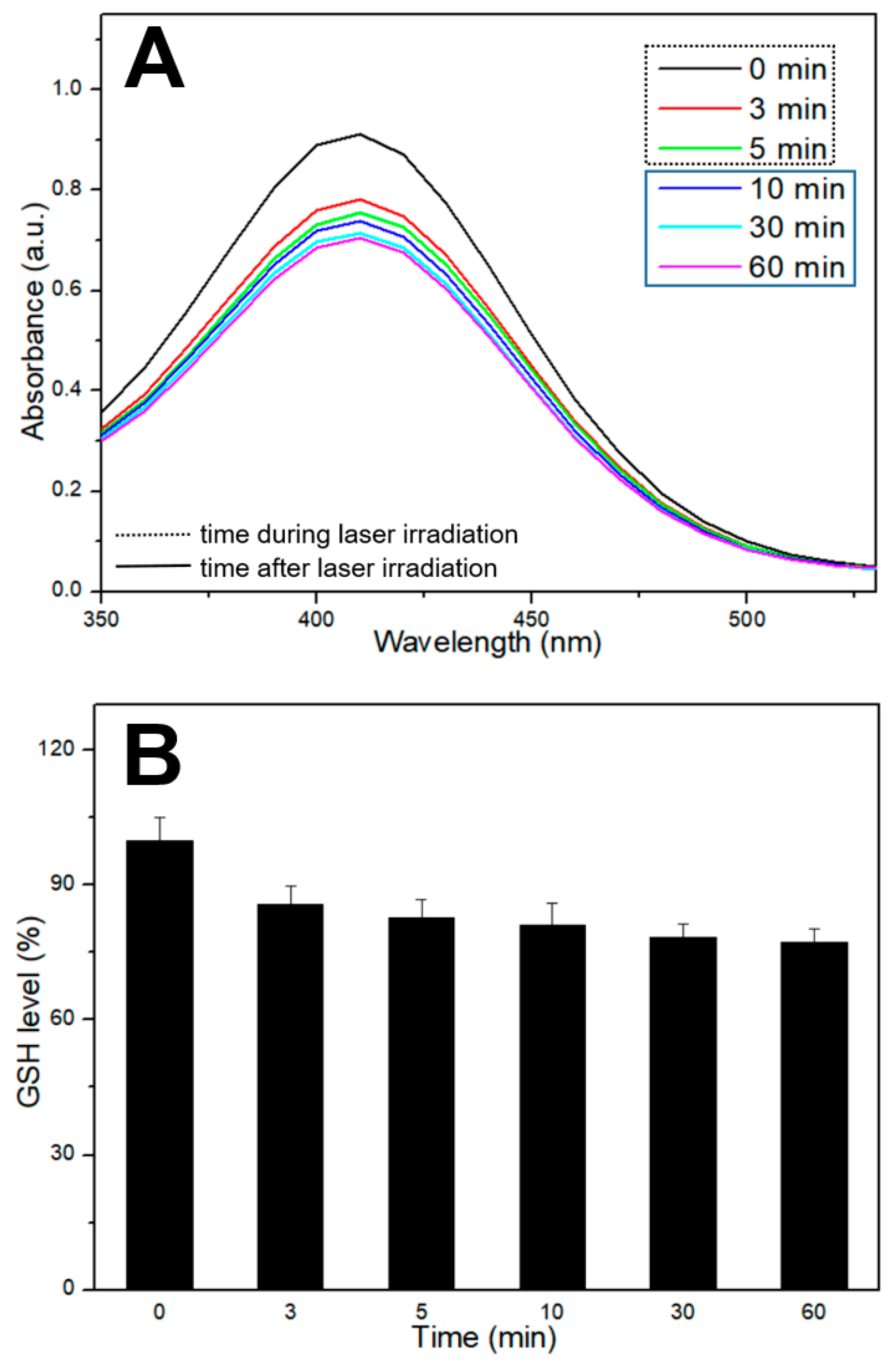
2.6. Evaluation of GSH Depletion
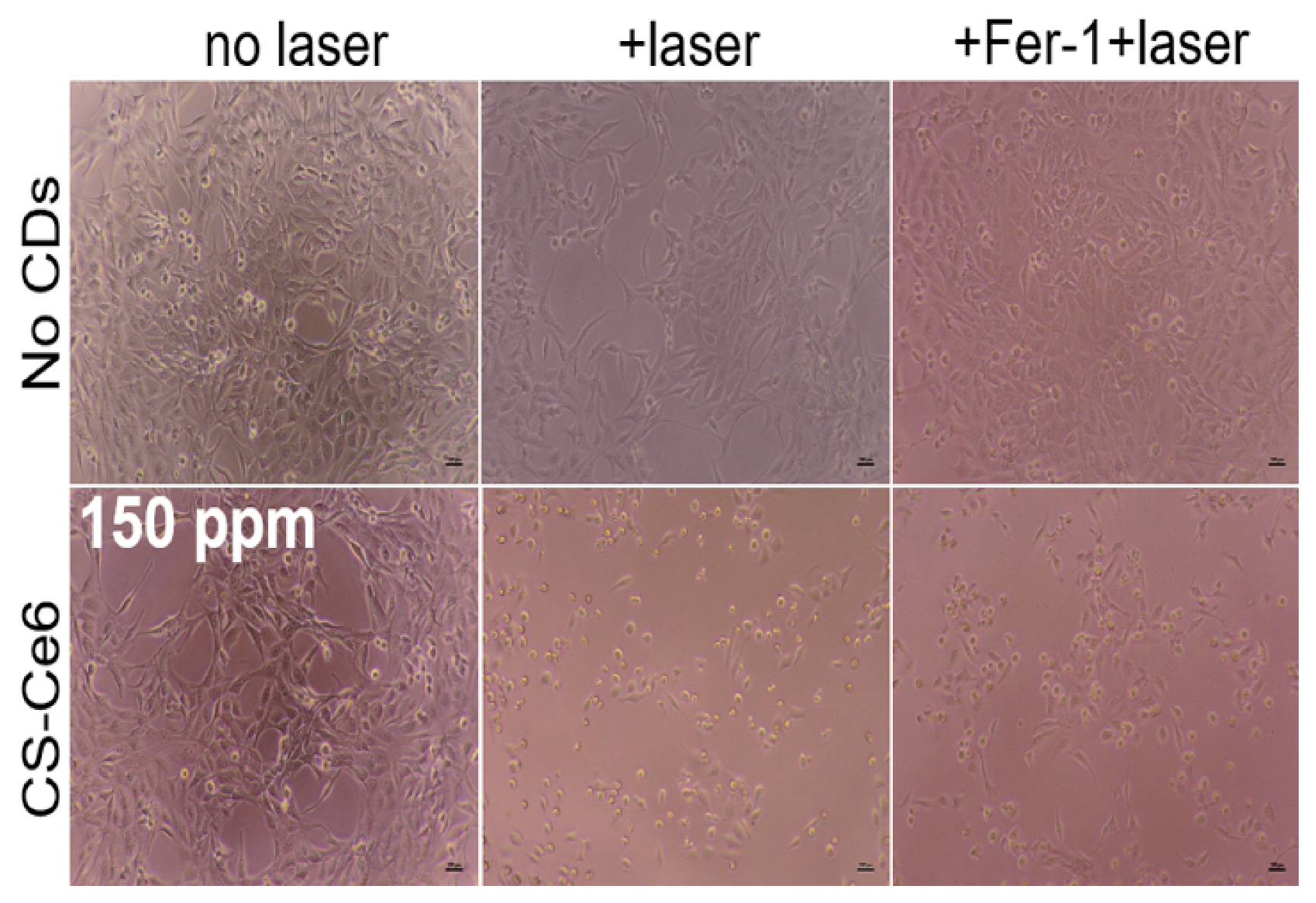
2.7. Live/Dead Cell Staining Assay
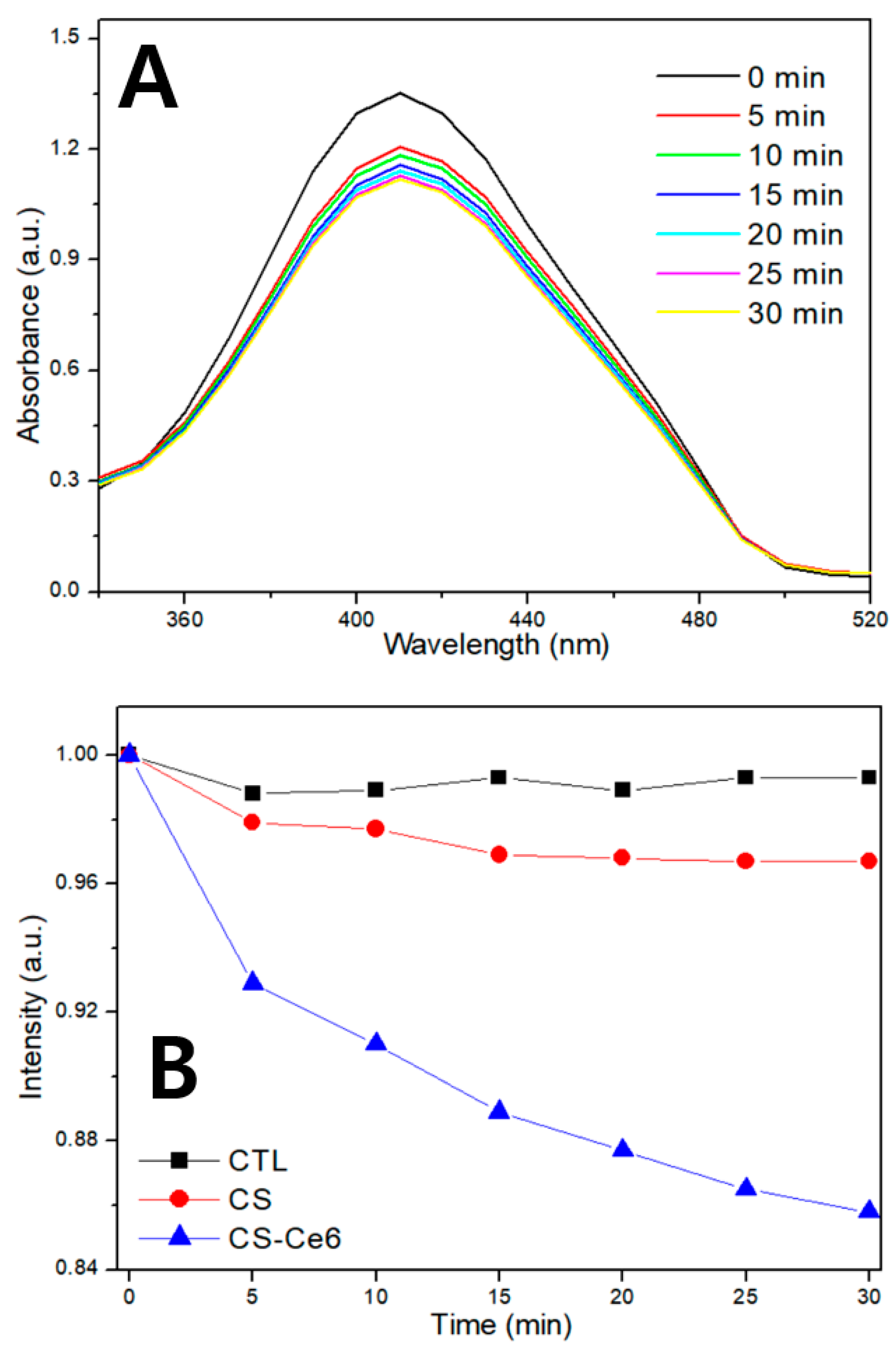
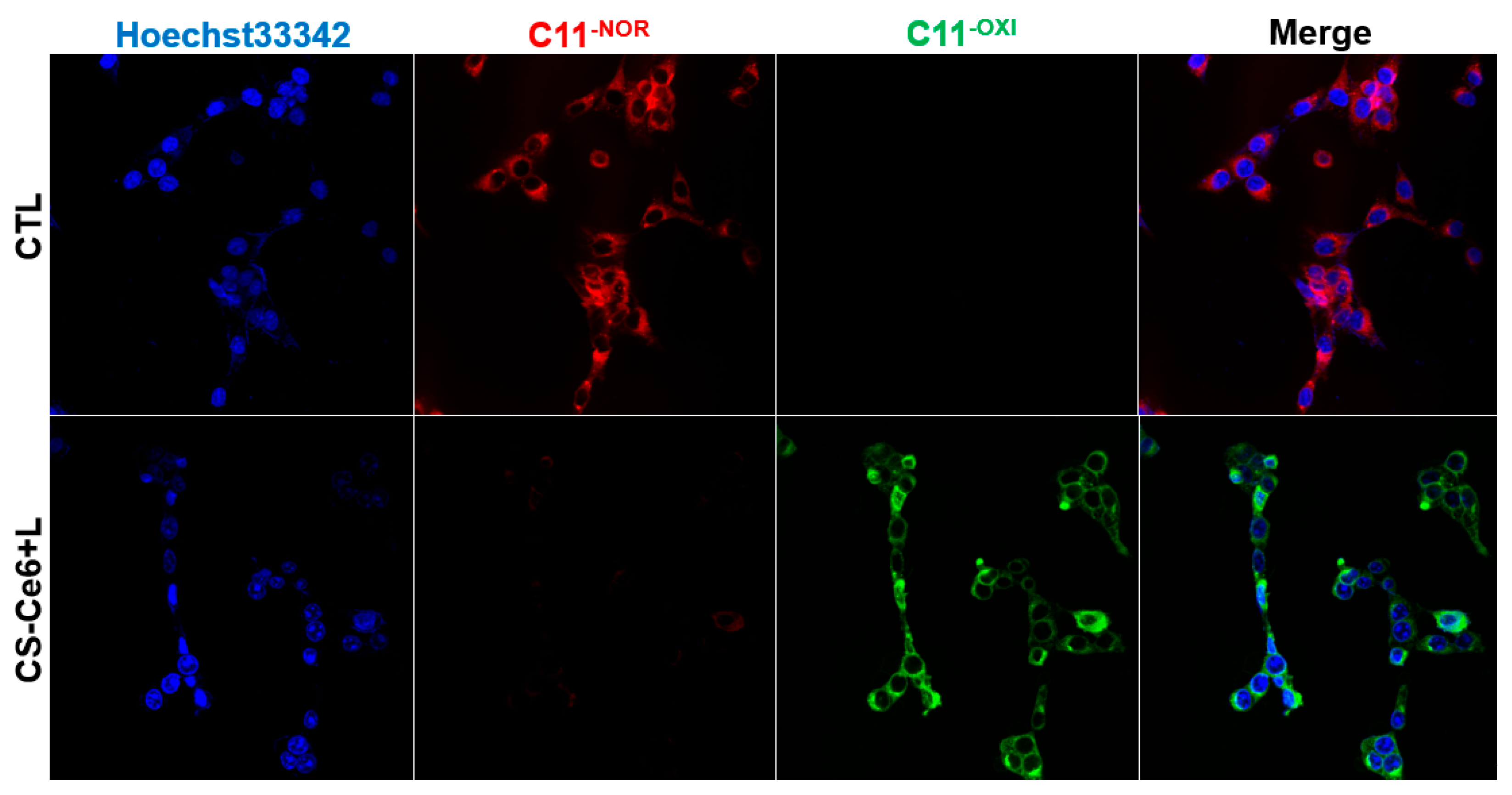
2.8. Intracellular LPO Detection
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peltanova, B.; Raudensk, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral squamous cell carcinoma and concomitant primary tumors, what do we know? A review of the literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Z.; Xu, Z.; Liu, T.; Liu, W. A scientometric study of tobacco and alcohol use as risk factors for oral cavity health. J. Dent. Sci. 2023, 18, 1883–1888. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Wang, J.; Huang, R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob. Induc. Dis. 2019, 17, 29. [Google Scholar] [CrossRef]
- D’souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Novel tumor-targeting nanoparticles for cancer treatment—A review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Tiwary, S.K.; Sonker, M.; Joshi, A.; Gupta, V.; Kumar, Y.; Shreyash, N.; Biswas, S. Recent advances in nanoparticle-based cancer treatment: A review. ACS Appl. Nano Mater. 2021, 4, 6441–6470. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Q.; Liu, Z.; Sun, M.; Dong, X. Nano-ROS-generating approaches to cancer dynamic therapy: Lessons from nanoparticles. Chem. Eng. J. 2023, 457, 141225. [Google Scholar] [CrossRef]
- Sanati, M.; Afshari, A.R.; Kesharwani, P.; Sukhorukov, V.N.; Sahebkar, A. Recent trends in the application of nanoparticles in cancer therapy: The involvement of oxidative stress. J. Control. Release 2022, 348, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Vanden Berghe, T.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Ai, Y.; Meng, Y.; Yan, B.; Zhou, Q.; Wang, X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 2024, 84, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Sig. Transduct. Target Ther. 2020, 5, 108. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics 2023, 11, 262. [Google Scholar] [CrossRef]
- Singh, H.; Razzaghi, M.; Ghorbanpoor, H.; Ebrahimi, A.; Avci, H.; Akbari, M.; Hassan, S. Carbon dots in drug delivery and therapeutic applications. Adv. Drug Deliv. Rev. 2025, 224, 115644. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Sig. Transduct. Target Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Yang, N.; Song, X.; Dong, X. Recent advances of cancer chemodynamic therapy based on Fenton/Fenton-like chemistry. Chem. Sci. 2022, 13, 863–889. [Google Scholar] [CrossRef]
- Cao, W.; Jin, M.; Yang, K.; Chen, B.; Xiong, M.; Li, X.; Cao, G. Fenton/Fenton-like metal-based nanomaterials combine with oxidase for synergistic tumor therapy. J. Nanobiotechnol. 2021, 19, 325. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, Q.; Zhang, R.; Xing, D.; Zhang, T. Eradication of solid tumors by chemodynamic theranostics with H2O2-catalyzed hydroxyl radical burst. Theranostics 2021, 11, 2334–2348. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.K.; Chudal, L.; Phan, J.; Lin, L.; Johnson, O.; Xing, M.; Liu, J.P.; Li, H.; Huang, X.; Shu, Y.; et al. A facile method for the synthesis of copper-cysteamine nanoparticles and study of ROS production for cancer treatment. J. Mater. Chem. B 2019, 7, 6630–6642. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, C.; Lyu, M.; Lyu, S.; Hu, L.; Xiao, E.; Xu, P. Novel albumin-binding photodynamic agent EB-Ppa for targeted fluorescent imaging guided tumour photodynamic therapy. RSC Adv. 2023, 13, 3534–3540. [Google Scholar] [CrossRef]
- Landino, L.M.; Mall, C.B.; Nicklay, J.J.; Dutcher, S.K.; Moynihan, K.L. Oxidation of 5-thio-2-nitrobenzoic acid, by the biologically-relevant oxidants peroxynitrite anion, hydrogen peroxide and hypochlorous acid. Nitric Oxide 2007, 18, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. Enhanced singlet oxygen production over a photocatalytic stable metal organic framework composed of porphyrin and Ag. J. Colloid Interface Sci. 2021, 602, 300–306. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Roeck, B.F.; Nasudivar, S.L.; Vorndran, M.R.H.; Schueller, L.; Yapici, F.I.; Rübsam, M.; von Karstedt, S.; Niessen, C.M.; Garcia-Saez, A.J. Ferroptosis spreads to neighboring cells via plasma membrane contacts. Nat. Commun. 2025, 16, 2951. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Chen, Y.; Sun, H.; Yang, L. Targeting ferroptosis as a potential prevention and treatment strategy for aging-related diseases. Pharmacol. Res. 2024, 208, 107370. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, M.M.; Luo, Q.W.; Zhang, Y.C.; Liu, T.T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Lv, X.; Zhang, P.; Xiao, C.; Chen, X. DNA-Free Guanosine-Based Polymer Nanoreactors with Multienzyme Activities for Ferroptosis-Apoptosis Combined Antitumor Therapy. ACS Nano 2024, 18, 3531–33544. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Ryu, M.-H.; Kim, W.; Garcia-Godoy, F.; Kwon, Y.H.; Jang, H.-O. Reactive Oxygen Species-Mediated Oral Cancer Cells Treatment Using Photosensitizer-Combined Carbon Dots via Apoptosis–Ferroptosis Synergistic Therapy. Appl. Sci. 2025, 15, 10446. https://doi.org/10.3390/app151910446
Park S-Y, Ryu M-H, Kim W, Garcia-Godoy F, Kwon YH, Jang H-O. Reactive Oxygen Species-Mediated Oral Cancer Cells Treatment Using Photosensitizer-Combined Carbon Dots via Apoptosis–Ferroptosis Synergistic Therapy. Applied Sciences. 2025; 15(19):10446. https://doi.org/10.3390/app151910446
Chicago/Turabian StylePark, So-Young, Mi-Heon Ryu, Wooil Kim, Franklin Garcia-Godoy, Yong Hoon Kwon, and Hye-Ock Jang. 2025. "Reactive Oxygen Species-Mediated Oral Cancer Cells Treatment Using Photosensitizer-Combined Carbon Dots via Apoptosis–Ferroptosis Synergistic Therapy" Applied Sciences 15, no. 19: 10446. https://doi.org/10.3390/app151910446
APA StylePark, S.-Y., Ryu, M.-H., Kim, W., Garcia-Godoy, F., Kwon, Y. H., & Jang, H.-O. (2025). Reactive Oxygen Species-Mediated Oral Cancer Cells Treatment Using Photosensitizer-Combined Carbon Dots via Apoptosis–Ferroptosis Synergistic Therapy. Applied Sciences, 15(19), 10446. https://doi.org/10.3390/app151910446






