Assessment of the Possible Inhibitory Effect of PFAS-Containing Aqueous Wastes on Aerobic Biomasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxygen Uptake Rate Test
2.1.1. Single-OUR Test
2.1.2. Multi-OUR Test
2.2. Tested Biomasses
- Biomass B-1: conventional activated sludge (CAS) of a municipal WWTP which treats municipal sewage only (namely: WWTP-1), with a mainly domestic contribution. WWTP-1 capacity: 100,000 PE (Population Equivalent);
- Biomass B-2: CAS of a municipal WWTP which is authorized to receive industrial AWs (namely: WWTP-2). WWTP-2 capacity: 60,000 PE.
2.3. Tested Substrates
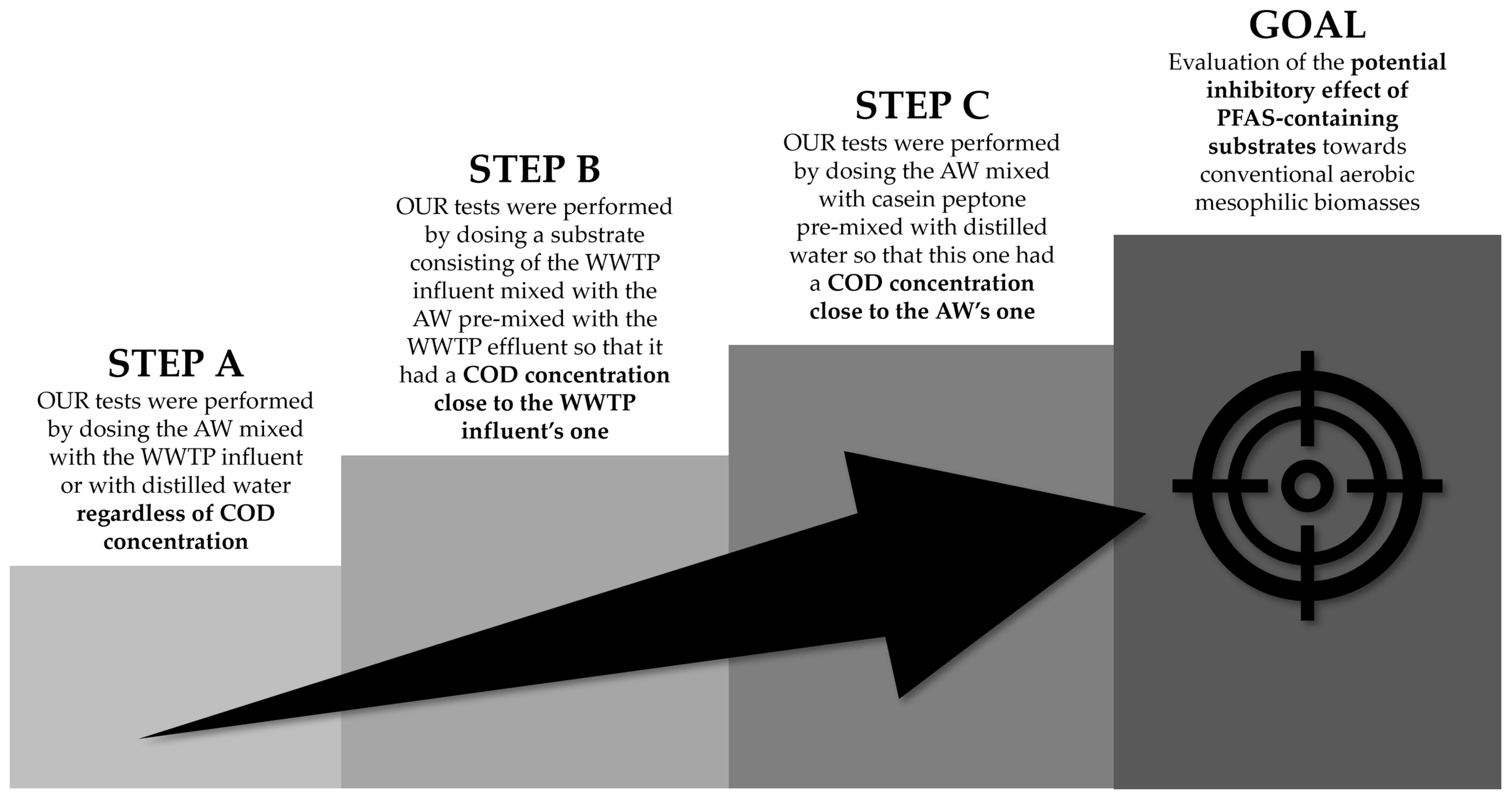
2.4. Methodological Approach
3. Results and Discussion
3.1. Step A
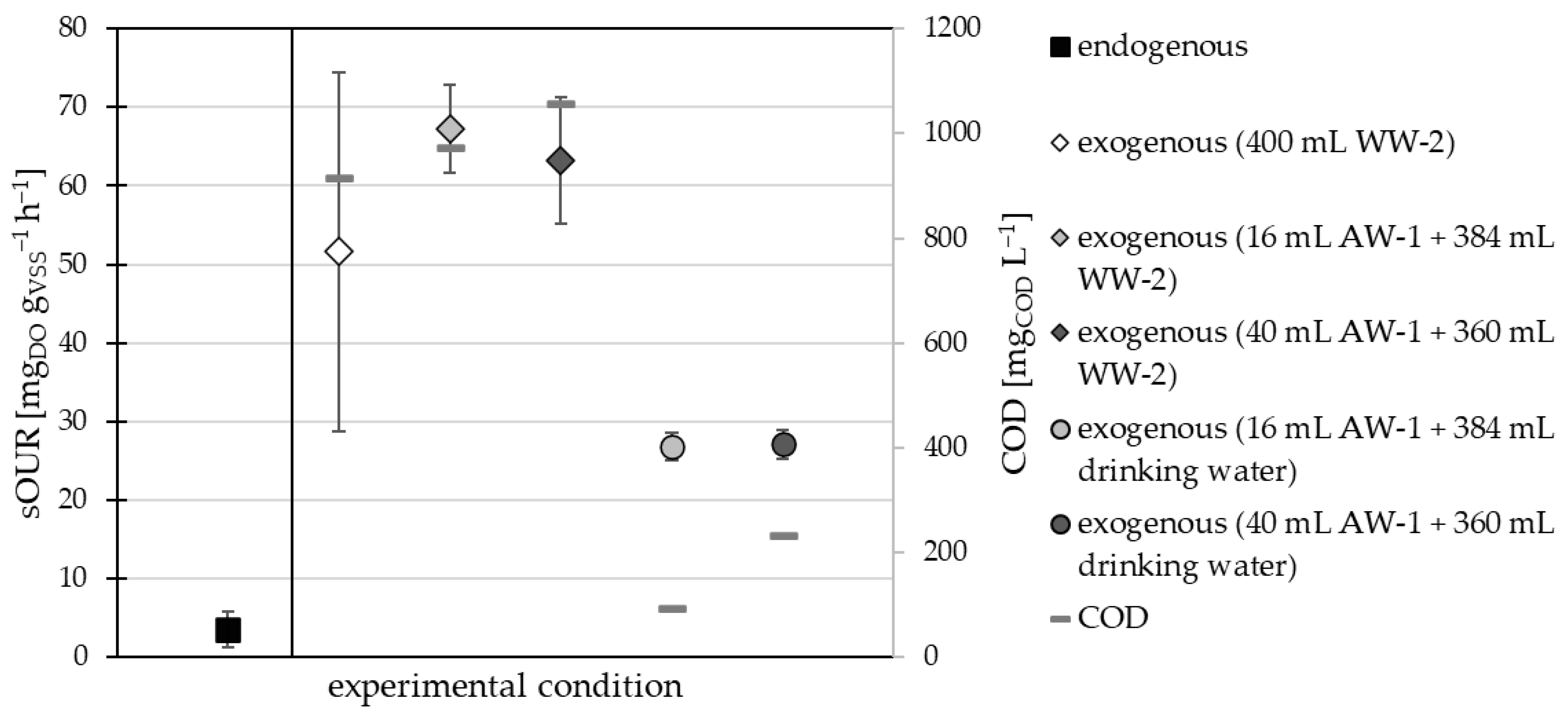
3.1.1. Sub-Step A(I)
3.1.2. Sub-Step A(II)
3.2. Step B
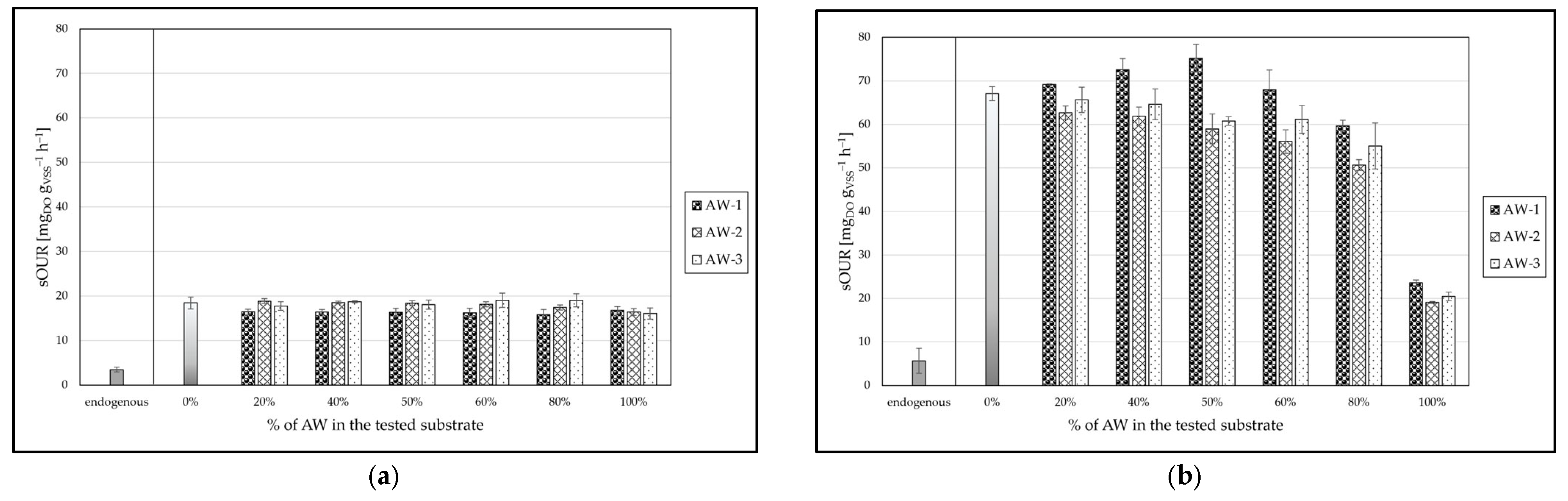
3.2.1. Sub-Step B(I)
3.2.2. Sub-Step B(II)
3.3. Step C
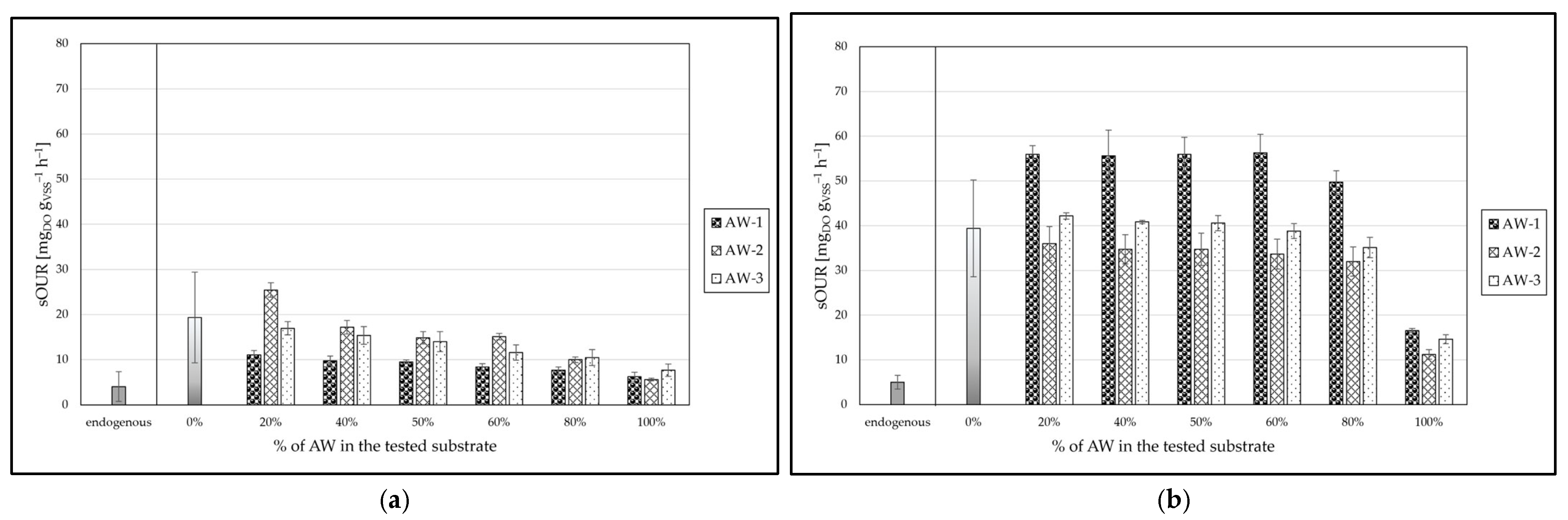
3.3.1. Sub-Step C(I)
- Long-chain PFASs include (I) perfluorocarboxylic acids (PFCAs) and their precursors with carbon chain lengths ≥ C7 (including perfluorooctanoic acid (PFOA)) and (II) perfluoroalkane sulfonic acids (PFSAs) and their precursors with carbon chain lengths ≥ C6 (including perfluorohexane sulfonic acid (PFHxS) and perfluorooctane sulfonic acid (PFOS)).
- Short-chain PFASs include (I) PFCAs with carbon chain lengths < C7 and (II) PFSAs with carbon chain lengths < C6.
3.3.2. Sub-Step C(II)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFAS/PFASs | Per- and Polyfluoroalkyl Substances |
| WWTP/WWTPs | Wastewater Treatment Plant(s) |
| AW/AWs | Aqueous Waste(s) |
| OUR | Oxygen Uptake Rate |
| sOUR | specific Oxygen Uptake Rate |
| VSS | Volatile Suspended Solids |
| DO | Dissolved Oxygen |
| ASP/CAS | Activated Sludge Process/Conventional Activated Sludge |
| PE | Population Equivalent |
| WW/WWs | Wastewater(s) |
| DW | Distilled Water |
| EDA | Effect-Directed Analysis |
| COD | Chemical Oxygen Demand |
| BOD5 | Biochemical Oxygen Demand after 5 days |
| ΔDO | Change in Dissolved Oxygen |
| POPs | Persistent Organic Pollutants |
| OECD | Organisation for Economic Co-operation and Development |
| PFCAs | Perfluorocarboxylic Acids |
| PFSAs | Perfluoroalkane Sulfonic Acids |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctanesulfonic Acid |
| PFHxS | Perfluorohexane Sulfonic Acid |
| PFBS | Perfluorobutanesulfonic Acid |
| HFPO-DA | Hexafluoropropylene Oxide Dimer Acid |
| AFFF/AFFFs | Aqueous Film-Forming Foam(s) |
| ASP | Activated Sludge Process |
References
- Wang, Z.; Lubick, N.; Roberts, J. An Assessment Report on Issues of Concern: Chemicals and Waste Issues Posing Risks to Human Health and the Environment; ETH Zurich: Zürich, Switzerland, 2020. [Google Scholar]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and Polyfluoroalkyl Substances in Water and Wastewater: A Critical Review of Their Global Occurrence and Distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A New OECD Definition for Per- and Polyfluoroalkyl Substances. Environ. Sci. Technol. 2021, 55, 15575–15578. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Bellazzi, S.; Caccamo, F.M.; Calatroni, S.; Milanese, C.; Baldi, M.; Abbà, A.; Sorlini, S.; Bertanza, G. Removal of Per- and Polyfluoroalkyl Substances by Adsorption on Innovative Adsorbent Materials. Sustainability 2023, 15, 13056. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental Impacts, Exposure Pathways, and Health Effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef]
- Lukić Bilela, L.; Matijošytė, I.; Krutkevičius, J.; Alexandrino, D.A.M.; Safarik, I.; Burlakovs, J.; Gaudêncio, S.P.; Carvalho, M.F. Impact of Per- and Polyfluorinated Alkyl Substances (PFAS) on the Marine Environment: Raising Awareness, Challenges, Legislation, and Mitigation Approaches under the One Health Concept. Mar. Pollut. Bull. 2023, 194, 115309. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, S.; Yang, J.; Zheng, S.; Sun, B. Per- and Polyfluoroalkyl Substances (PFAS) and Cancer: Detection Methodologies, Epidemiological Insights, Potential Carcinogenic Mechanisms, and Future Perspectives. Sci. Total Environ. 2024, 953, 176158. [Google Scholar] [CrossRef]
- Bline, A.P.; DeWitt, J.C.; Kwiatkowski, C.F.; Pelch, K.E.; Reade, A.; Varshavsky, J.R. Public Health Risks of PFAS-Related Immunotoxicity Are Real. Curr. Environ. Health Rep. 2024, 11, 118–127. [Google Scholar] [CrossRef]
- Solan, M.E.; Park, J.-A. Per- and Poly-Fluoroalkyl Substances (PFAS) Effects on Lung Health: A Perspective on the Current Literature and Future Recommendations. Front. Toxicol. 2024, 6, 1423449. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef] [PubMed]
- Chiavola, A.; Di Marcantonio, C.; Boni, M.R.; Biagioli, S.; Frugis, A.; Cecchini, G. Experimental Investigation on the Perfluorooctanoic and Perfluorooctane Sulfonic Acids Fate and Behaviour in the Activated Sludge Reactor. Process Saf. Environ. Prot. 2020, 134, 406–415. [Google Scholar] [CrossRef]
- Yu, X.; Nishimura, F.; Hidaka, T. Impact of Long-Term Perfluorooctanoic Acid (PFOA) Exposure on Activated Sludge Process. Water Air Soil Pollut. 2018, 229, 134. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, S.; Zhang, Q.; Fan, Q.; Huang, J.; Yu, G. Sorption of Perfluorooctane Sulfonate and Perfluorooctanoate on Activated Sludge. Chemosphere 2010, 81, 453–458. [Google Scholar] [CrossRef]
- Lee, H.; Tevlin, A.G.; Mabury, S.A.; Mabury, S.A. Fate of Polyfluoroalkyl Phosphate Diesters and Their Metabolites in Biosolids-Applied Soil: Biodegradation and Plant Uptake in Greenhouse and Field Experiments. Environ. Sci. Technol. 2014, 48, 340–349. [Google Scholar] [CrossRef]
- Weidemann, E.; Lämmer, R.; Göckener, B.; Bücking, M.; Gassmann, M. Transformation, Leaching and Plant Uptake Simulations of 6:2 and 8:2 Polyfluoroalkyl Phosphate Diesters (DiPAPs) and Related Transformation Products under near-Natural Conditions. Environ. Sci. Eur. 2024, 36, 63. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Hang Nguyen, T.A. Poly-and Perfluoroalkyl Substances in Water and Wastewater: A Comprehensive Review from Sources to Remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- Lu, B.; Qian, J.; He, F.; Wang, P.; He, Y.; Tang, S.; Tian, X. Effects of Long-Term Perfluorooctane Sulfonate (PFOS) Exposure on Activated Sludge Performance, Composition, and Its Microbial Community. Environ. Pollut. 2022, 295, 118684. [Google Scholar] [CrossRef]
- Sheng, S.; Chen, F.; Li, H.; Qian, J.; Li, K.; Tang, S.; Tian, X. Acute Bio-Augmentation Effect of Perfluorooctane Sulfonic Acid (PFOS) on Activated Sludge in Biological Denitrification Processes and Related Stress Mechanisms. Environ. Sci. Water Res. Technol. 2021, 7, 405–416. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.A.; Habib, S.; Sarker, J. Measuring Biodegradability of Industrial Wastewater by a Low-Cost Differential Respirometer. Res. J. Eng. Sci. 2013, 2, 1–4. [Google Scholar]
- Oppenheimer, C.H.; Stern, K.G. Biological Oxidation; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- HagMan, M.; la Cour Jansen, J. Oxygen Uptake Rate Measurements for Application at Wastewater Treatment Plants. Vatten 2007, 63, 131. [Google Scholar]
- Prokkola, H.; Heponiemi, A.; Pesonen, J.; Kuokkanen, T.; Lassi, U. Reliability of Biodegradation Measurements for Inhibitive Industrial Wastewaters. ChemEngineering 2022, 6, 15. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Caccamo, F.M.; Bellazzi, S.; Abbà, A.; Bertanza, G. Assessment of the Impact of a New Industrial Discharge on an Urban Wastewater Treatment Plant: Proposal for an Experimental Protocol. Environments 2023, 10, 108. [Google Scholar] [CrossRef]
- Bellazzi, S.; Collivignarelli, M.C.; Baldi, M.; Abbà, A. An Approach to Compute Respirometric Parameters from Continuous-Time Oxygen Uptake Rate Curves. 2024. Available online: https://ssrn.com/abstract=4944396 (accessed on 22 September 2025).
- Huang, J.Y.C.; Cheng, M.-D. Measurement and New Applications of Oxygen Uptake Rates in Activated Sludge Processes. Water Pollut. Control. Fed. 1984, 56, 259–265. [Google Scholar]
- Borzooei, S.; Simonetti, M.; Scibilia, G.; Zanetti, M.C. Critical Evaluation of Respirometric and Physicochemical Methods for Characterization of Municipal Wastewater during Wet-Weather Events. J. Environ. Chem. Eng. 2021, 9, 105238. [Google Scholar] [CrossRef]
- Capodici, M.; Fabio Corsino, S.; Di Pippo, F.; Di Trapani, D.; Torregrossa, M. An Innovative Respirometric Method to Assess the Autotrophic Active Fraction: Application to an Alternate Oxic–Anoxic MBR Pilot Plant. Chem. Eng. J. 2016, 300, 367–375. [Google Scholar] [CrossRef]
- Andreottola, G.; Foladori, P.; Ziglio, G.; Cantaloni, C.; Bruni, L.; Cadonna, M. Methods for Toxicity Testing of Xenobiotics in Wastewater Treatment Plants and in Receiving Water Bodies. In Dangerous Pollutants (Xenobiotics) in Urban Water Cycle; Springer: Dordrecht, The Netherlands, 2008; pp. 191–206. [Google Scholar]
- Bertanza, G.; Collivignarelli, C. Impianti Di Trattamento Acque: Verifiche Di Funzionalità e Collaudo. Manuale Operativo; Hoepli, Ed.; Hoepli: Milan, Italy, 2012. [Google Scholar]
- Ziglio, G.; Andreottola, G.; Foladori, P.; Ragazzi, M. Experimental Validation of a Single-OUR Method for Wastewater RBCOD Characterisation. Water Sci. Technol. 2001, 43, 119–126. [Google Scholar] [CrossRef]
- Environment Directorate. Report on Per-and Polyfluoroalkyl Substances and Alternatives in Coating, Paints and Vernishes (CPVs): Hazard Profile. 2023. Available online: https://www.oecd.org/content/dam/oecd/en/publications/reports/2023/12/per-and-polyfluoroalkyl-substances-and-alternatives-in-coatings-paints-and-varnishes-cpvs-hazard-profile_916f7fe8/c60c42d5-en.pdf (accessed on 22 September 2025).
- O’Carroll, D.M.; Jeffries, T.C.; Lee, M.J.; Le, S.T.; Yeung, A.; Wallace, S.; Battye, N.; Patch, D.J.; Manefield, M.J.; Weber, K.P. Developing a Roadmap to Determine Per- and Polyfluoroalkyl Substances-Microbial Population Interactions. Sci. Total Environ. 2020, 712, 135994. [Google Scholar] [CrossRef]


| PFAS-Containing Aqueous Wastes | Influent Wastewaters | |||||
|---|---|---|---|---|---|---|
| Parameter | U.M. 1 | AW-1 | AW-2 | AW-3 | WW-1 | WW-2 |
| COD | mgCOD L−1 | 2333 | 3300 | 1350 | 100–200 | 800–1500 |
| BOD5 | mgBOD5 L−1 | 400 | 1730 | 460 | 80–100 | 600–1100 |
| pH | - | 8.00 | 7.80 | 8.09 | 6.50–7.50 | 6.50–7.50 |
| PFBS 2 | μg L−1 | 2.191 × 101 | 3.757 × 101 | 1.587 × 101 | <0.05 | <0.05 |
| PFOA 3 | μg L−1 | 2.870 × 103 | 2.414 × 101 | 2.340 × 100 | <0.05 | <0.05 |
| PFOS 4 | μg L−1 | 1.484 × 102 | 7.880 × 10−1 | 8.400 × 10−2 | <0.05 | <0.05 |
| Sum of PFASs | μg L−1 | 3.268 × 103 | 7.870 × 101 | 2.638 × 101 | <0.05 | <0.05 |
| STEP | SUB-STEP | Amount of Performed Tests | Type of Performed Tests | Tested Biomasses | Tested Substrates |
|---|---|---|---|---|---|
| A | A(I) | 68 | Single-OUR tests | B-2 | AW-1 WW-2 |
| A(II) | 4 | Multi-OUR tests | B-2 | AW-1 WW-2 | |
| B | B(I) | 144 | Single-OUR tests | B-1 B-2 | AW-1 AW-2 AW-3 WW-1 (only for B-1) WW-2 (only for B-2) |
| B(II) | 8 | Multi-OUR tests | B-1 B-2 | AW-1 AW-2 AW-3 WW-1 (only for B-1) WW-2 (only for B-2) | |
| C | C(I) | 144 | Single-OUR tests | B-1 B-2 | AW-1 AW-2 AW-3 Casein peptone |
| C(II) | 6 | Multi-OUR tests | B-1 B-2 | AW-1 AW-2 AW-3 Casein peptone |
| Tested Biomass | Tested Substrate | Overall ΔDO [mgDO gVSS−1] | ||
|---|---|---|---|---|
| Composition | BOD5/COD [-] | Sum of PFASs [mg L−1] | ||
| B-2 | 10% AW-1 + 90% WW-2 | 0.65 | 0.327 | 114.15 |
| 4% AW-1 + 96% WW-2 | 0.87 | 0.131 | 71.20 | |
| 10% AW-1 + 90% DW | 0.17 | 0.327 | 92.68 | |
| 4% AW-1 + 96% DW | 0.17 | 0.131 | 68.57 | |
| Tested Biomass | Tested Substrate | Overall ΔDO [mgDO gVSS−1] | ||
|---|---|---|---|---|
| Composition | BOD5/COD [-] | Sum of PFASs [mg L−1] | ||
| B-1 | WW-1 | 0.70 | <5 × 10–5 | 17.73 |
| AW-1 1 | 0.96 | 0.004 | 29.44 | |
| AW-2 1 | 0.90 | 0.004 | 36.07 | |
| AW-3 1 | 0.72 | 0.002 | 27.49 | |
| B-2 | WW-2 | 0.78 | <5 × 10–5 | 93.82 |
| AW-1 2 | 0.19 | 0.930 | 57.21 | |
| AW-2 2 | 0.22 | 0.020 | 76.48 | |
| AW-3 2 | 0.30 | 0.010 | 58.83 | |
| Tested Biomass | Tested Substrate | Overall ΔO2 [mgDO gVSS−1] | ||
|---|---|---|---|---|
| Composition | BOD5/COD [-] | Sum of PFASs [mg L−1] | ||
| B-1 | AW-1 3 | 0.17 | 3.268 | 151.28 |
| AW-2 3 | 0.52 | 0.079 | 227.00 | |
| AW-3 3 | 0.34 | 0.026 | 236.00 | |
| B-2 | AW-1 3 | 0.17 | 3.268 | 250.28 |
| AW-2 3 | 0.52 | 0.079 | 269.85 | |
| AW-3 3 | 0.34 | 0.026 | 369.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Pedrazzani, R.; Bellazzi, S.; Grecchi, G.; Baldi, M.; Abbà, A.; Bertanza, G. Assessment of the Possible Inhibitory Effect of PFAS-Containing Aqueous Wastes on Aerobic Biomasses. Appl. Sci. 2025, 15, 10448. https://doi.org/10.3390/app151910448
Collivignarelli MC, Pedrazzani R, Bellazzi S, Grecchi G, Baldi M, Abbà A, Bertanza G. Assessment of the Possible Inhibitory Effect of PFAS-Containing Aqueous Wastes on Aerobic Biomasses. Applied Sciences. 2025; 15(19):10448. https://doi.org/10.3390/app151910448
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Roberta Pedrazzani, Stefano Bellazzi, Giorgia Grecchi, Marco Baldi, Alessandro Abbà, and Giorgio Bertanza. 2025. "Assessment of the Possible Inhibitory Effect of PFAS-Containing Aqueous Wastes on Aerobic Biomasses" Applied Sciences 15, no. 19: 10448. https://doi.org/10.3390/app151910448
APA StyleCollivignarelli, M. C., Pedrazzani, R., Bellazzi, S., Grecchi, G., Baldi, M., Abbà, A., & Bertanza, G. (2025). Assessment of the Possible Inhibitory Effect of PFAS-Containing Aqueous Wastes on Aerobic Biomasses. Applied Sciences, 15(19), 10448. https://doi.org/10.3390/app151910448











