1. Introduction
Modern titanium dental implants have proven remarkably successful for replacing missing teeth, consistently demonstrating longevity rates exceeding 95% for many years [
1]. Still, mechanical failures—specifically those caused by structural wear and tear over time—remain a problem, especially when implants experience repetitive chewing forces for lengthy periods [
2]. Titanium and its alloys stay the materials of choice for dental implants owing to their superb blend of resilience, resistance to degradation, and compatibility with human biology [
3]. That being said, titanium alloys (with an elasticity modulus approximately 110 GPa) and human bone (ranging from 3 to 10 GPa for trabecular bone and to 15 to 30 GPa for cortical bone) differ noticeably in stiffness, which can lead to stress shielding, peri-implant bone resorption, and ultimately implant failure [
4].
These biomechanical challenges really highlight how crucial it is to conduct thorough analyses when designing and evaluating implants. Finite element analysis (FEA) has emerged as a vital computational tool in dental biomechanics. It enables researchers to explore stress distributions, anticipate where failures might occur, and simulate various clinical scenarios that would be tough, expensive, or even unethical to test in real life [
5].
For instance, Van Staden et al. [
6] provided an early review highlighting FEA’s role in advancing implant design. More recently, Ziaie and Khalili [
7] employed FEA to predict abutment screw fatigue under cyclic loading, identifying probable failure after approximately 3·10
5 cycles. Meanwhile, Zieliński et al. [
8] took it a step further by combining FEA with physical fatigue testing, uncovering critical areas where cracks might start, all influenced by different loading angles.
Even though finite element analysis is incredibly powerful, it does have its drawbacks when it comes to being used in iterative design or clinical decision-making processes. Creating high-fidelity, patient-specific simulations often involves complex geometry preparation, meshing, and tuning the solver, which can take anywhere from hours to days of computational time. This really limits its usefulness in fast-paced clinical settings. To address this issue, surrogate modeling techniques that leverage machine learning (ML) have started to show great promise. Liang et al. implemented a deep learning model that predicts aortic wall stress distributions based on input geometries, achieving less than 1% error compared to traditional FEA results [
9]. Similarly, Lu et al. created a neural network to estimate cartilage stress in the knee during multibody dynamics, significantly cutting down computation time [
10]. These examples highlight how artificial neural networks (ANNs) can deliver FEA results with impressive accuracy and speed.
In the field of dental implant biomechanics, researchers are diving into the use of neural networks for various applications, including design optimization, stress prediction, and estimating fatigue life. Griggs introduced a framework based on artificial neural networks (ANN) aimed at optimizing implant geometry to enhance fatigue resistance, taking into account multiaxial loading and material properties [
11]. Additionally, physics-informed neural networks are becoming increasingly popular because they can integrate fundamental physical equations into the learning process, which boosts the model’s generalizability and interpretability.
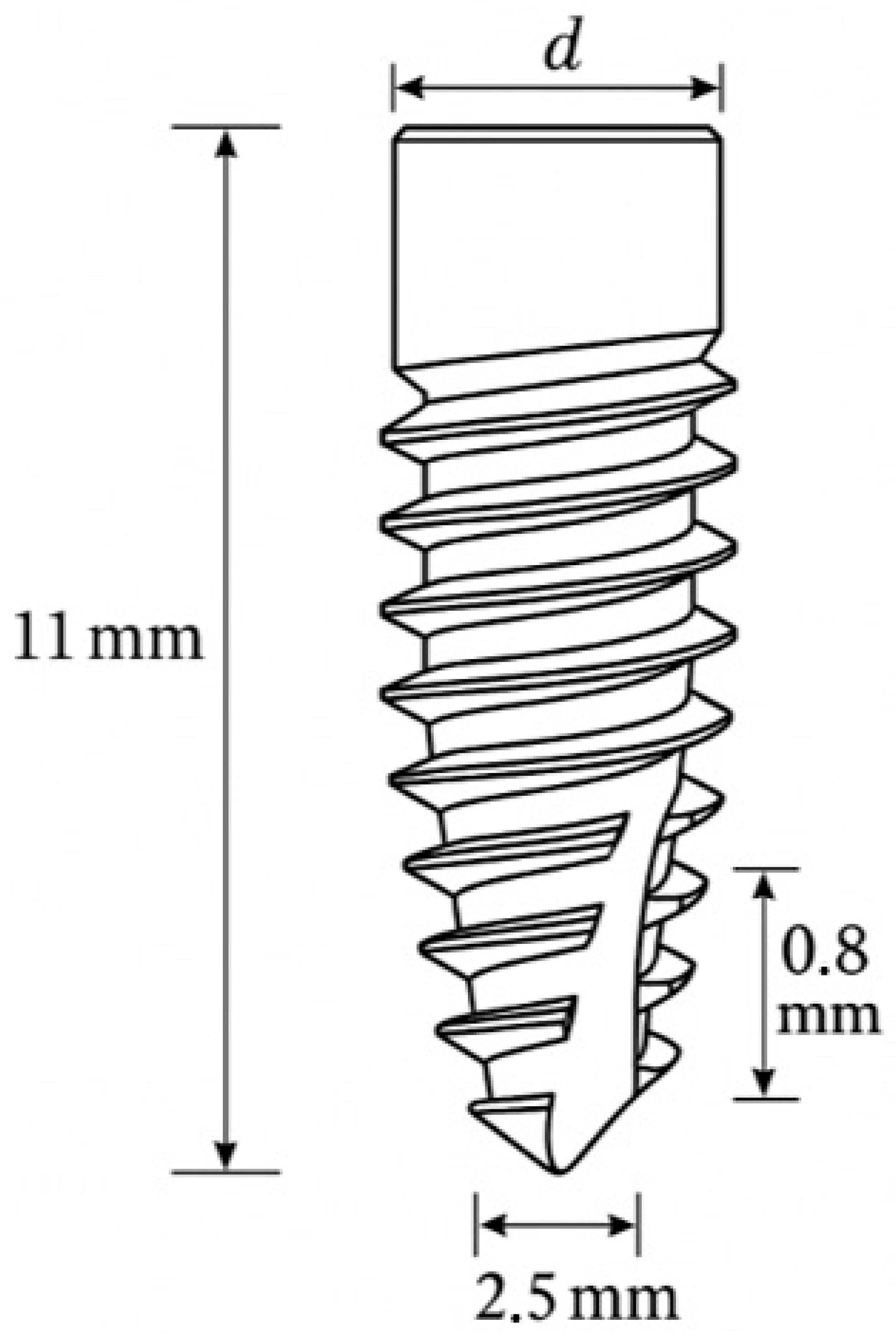
This study showcases a proof-of-concept that combines FEA data with neural networks to predict three crucial biomechanical indicators related to dental implant fatigue behavior: von Mises stress, deformation, and the fatigue safety factor. By focusing on a Grade 4 titanium implant system, the present study explores various geometric and loading scenarios to train and validate an ANN that can accurately estimate FEA results. Therefore, the goal is to speed up the implant development process, lessen the reliance on repetitive simulations, and establish a foundation for real-time, AI-driven biomechanical assessments in clinical environments.
4. Discussion
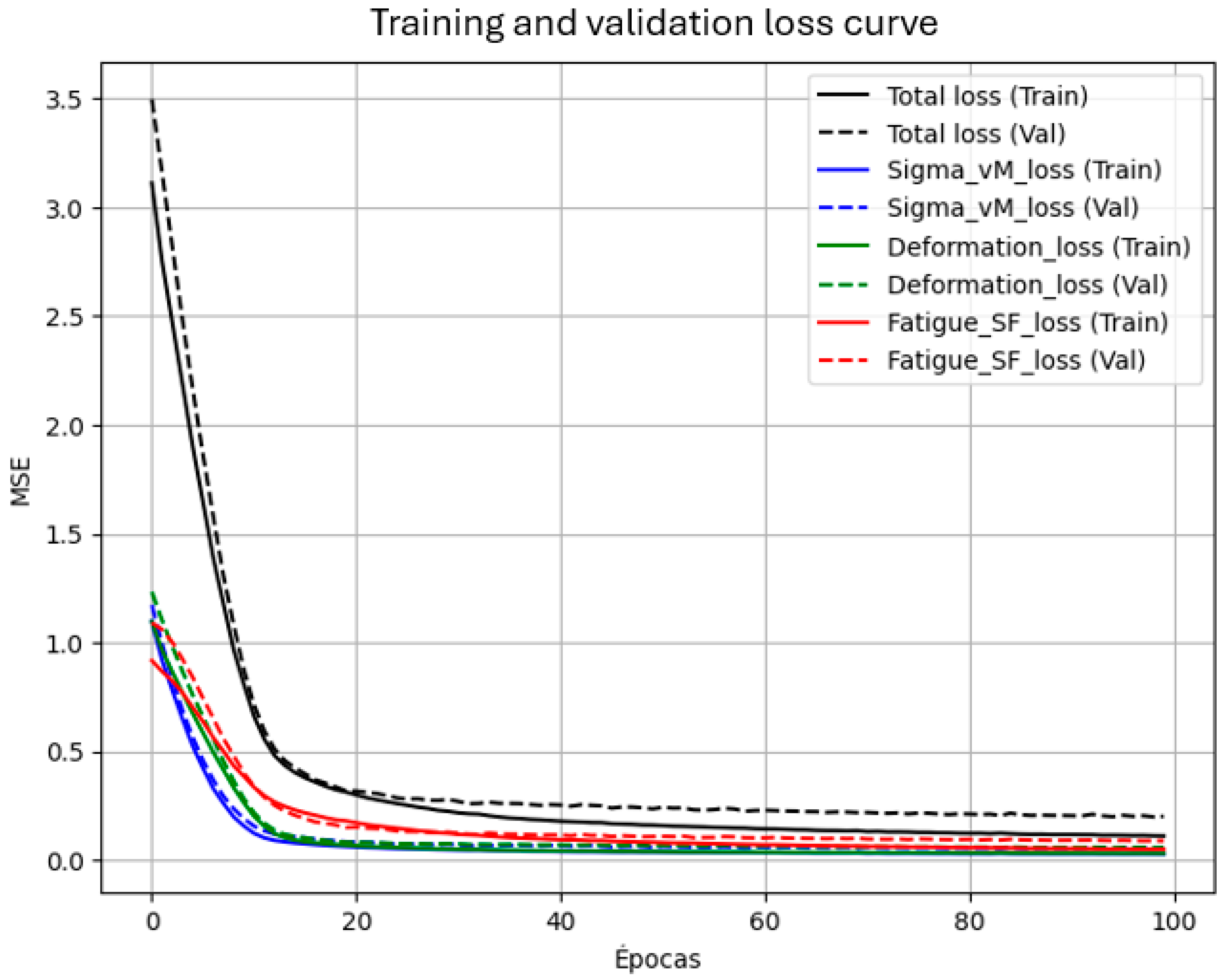
The primary goal of the current work is to develop and validate an artificial neural network model able to predict with precision important biomechanical parameters such as von Mises stress, deformation, and fatigue safety factor from data derived from finite element analysis (FEA) with the ultimate goal to optimize dental implant design.
Outcomes corroborate the viability of using neural networks as surrogates to bridge FEA data to predict dental implant fatigue behavior. High R
2 (≥0.92) values in all outputs confirm that even a relatively straightforward feed-forward ANN is capable of modeling complex biomechanical correlations in the dataset. This matches the trend in the literature where ANNs have effectively extracted patterns from FEA or experimental data in biomechanical contexts [
14,
15]. In the current work, the three input parameters (Force, Angle, Diameter) were adequate to account for the majority variance in outcomes, as these were the main variables under variation. Further input parameters (e.g., implant length, thread characteristics, or material properties) can be added in the future to encompass wider implant design spaces.
The data integration strategy applied in this study is particularly noteworthy. Instead of approaching the task purely as a black-box machine learning problem, domain knowledge was incorporated into the development of both the FEA dataset and the ANN architecture. For example, the inclusion of the loading angle as an input variable was based on its established influence on implant stress distribution. By training the model on FEA outputs, the ANN inherently reflects the physical principles embedded in those simulations, at least within the bounds of the training data. Once trained, the ANN functions as a rapid physics-based predictor [
16,
17].
This approach has promising applications in both implant design and clinical settings. During the design process, engineers can quickly assess the fatigue implications of different implant geometries under varying loads. The ANN enables rapid screening of design options before refinement with detailed FEA. This process is comparable to an optimization loop, where the ANN acts as an estimator of the objective function. Previous studies have proposed the use of neural networks for implant optimization, aiming to enhance fatigue life and reduce undesirable stress shielding. The findings presented here provide concrete validation that such a surrogate modeling approach can be both practical and accurate [
18].
Clinically, while the ultimate decision will be not depend solely on stress, the availability of an in-real-time predictive tool whether or not an existing implant (of known diameter) in a particular patient context (estimated biting force and inclination) is in risk of failure due to mechanics would be beneficial. It might, for instance, warn the clinician that an existing 3.3 mm implant under high inclination in a bruxism patient has relatively low fatigue margin, leading to consideration of an implant with higher diameter or the option to opt for an alternative treatment. Incorporation into treatment planning software could further add value to risk assessment on an individual basis. This is part of the overall trend towards personalized medicine and the application of computational tools to surgical planning.
The performance outside of the precise training distribution was also quantified. For instance, a test scenario where the load angle is 60°—just beyond the 45° maximum encountered during training—was considered. The model extrapolated to give large σvM (~900 MPa) and low FSF (~0.4) for an 800 N, 4.0 mm diameter implant for this loading case. This outcome is qualitatively reasonable, since larger angle tends to induce more bending stress.
The surrogate is valid within a constrained domain: Grade 4 titanium, 3.0–5.0 mm implant diameter, 100–800 N static loads at 0–45°, and rigid fixation. Extrapolation (e.g., dynamic loading, other materials, partial osseointegration) requires retraining, validation, and uncertainty quantification. The 60° example is illustrative and not evidence of generalization.
However, such extensions are made cautiously, for even the accuracy for those values outside the training span cannot be guaranteed. The model remains strong within the constraints of the original dataset. The performance suffers slightly for very high-stress and low-FSF values. This limitation can be mitigated through augmentation using additional training examples for that region or through adding physical constraints, e.g., specifying the yield stress that is empirically known to be an upper bound [
19,
20].
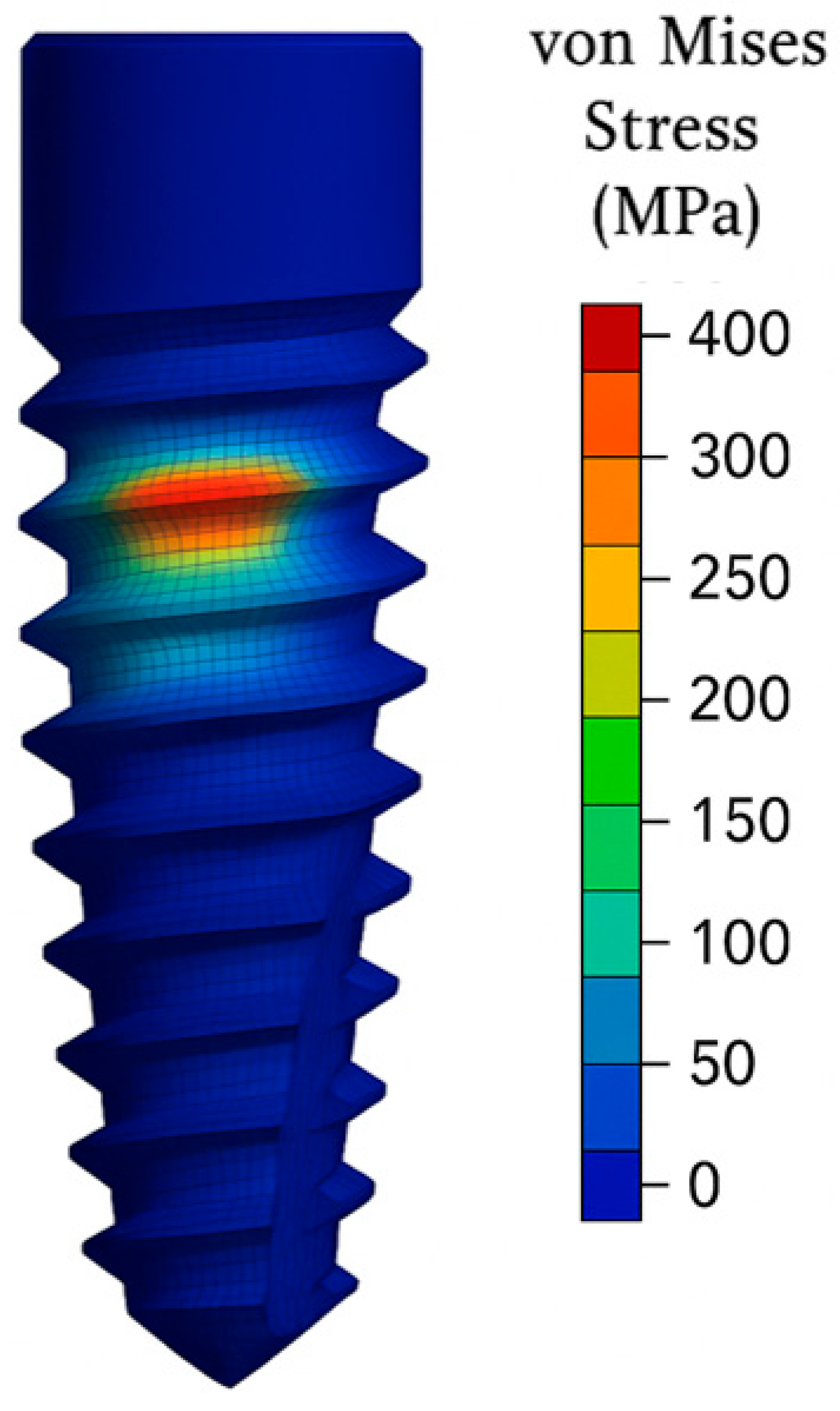
The network learned well the force, angle, and diameter interactions. For instance, keeping force constant, the model captures well more stress and deflection for larger values of angle (due to effects of bending increasing stress). And it captures larger diameter reducing stress nonlinearly, though with diminishing returns for very large diameters.
One such restriction we encountered was handling categorical variation in failure modes–for some simulations, where position of max stress differed (e.g., between abutment and thread). While our own results (=max values) do not offer location, a next-generation version of our model could potentially encompass failure location classification. This information our current ANN does not provide, since it was intended for regression.
Another factor is the absence of bone in our FEA analyses. Practically, bone presence would alter stress distribution differently and could incur results such as bone microstrain or implant-bone interface failure. Our ANN, modeled for in-air simulations of the implant, would need to be reconnected or undergo transfer learning if mechanics for bone were incorporated [
5,
21].
Our proof of concept utilized a simplified scenario (single implant, no bone, static loading) and subsequent models could potentially include bone and perhaps dynamic loading patterns mimicking actual chewing cycles. The fatigue safety factor which we computed using high-cycle fatigue theory’s Goodman criterion is a simplification and one could favor a more superior one to account for low-cycle fatigue, different failure criteria (e.g., Soderberg, Gerber, etc.) or even directly output cycles to failure. Such models could be trained through data derived from FEA supplemented with fatigue solvers or actual physical fatigue tests corresponding to simulation results [
22].
Expanding the surrogate’s scope requires integrating orthotropic bone domains, contact-based osseointegration, and fatigue under multi-axial loading. These additions will be phased through a roadmap combining high-fidelity FEA, ANN retraining with calibrated UQ, and eventual clinical integration—bridging static implant modeling with real-world complexity.
Also, even though our focus was regression, classification models could predict categorical variables (fail or no fail for selected cycles) that might be more clinically interpretable for chosen cases. A third alternative, physics-informed neural networks (PINNs) offer a way for inclusion of known physical constraints (like stress equilibrium or S-N curves) during learning. For example, a PINN could be designed to impose Basquin’s fatigue equation automatically during data fitting and enhance reliability in extrapolations [
23,
24].
Another potential future direction is expanding the input parameter space. In addition to geometry and loading, one could include surface treatment (roughness), material choices (titanium versus new alloys or PEEK), and even bone quality (D1 dense versus D4 soft bone) and others. Each would necessitate more data, but active learning methods might be able to reduce needed FEA simulations through recognition of most informative cases.
The idea of replacing traditional finite element analysis with machine learning is no longer a speculative detour—it is becoming a credible alternative in computational biomechanics. A striking illustration comes from the work of Liang et al., who demonstrated that a deep neural network could predict full-field aortic wall stresses with less than 1% error [
9]. Their approach, fueled by imaging-based geometry and leveraging a sophisticated convolutional neural network (CNN), tackled a problem of considerable anatomical and computational complexity.
In contrast, our study deals with a more structured input-output relationship, where a simpler artificial neural network was not only sufficient but remarkably effective. This simplicity, however, does not diminish the significance of the task. It underscores the importance of architectural alignment between model complexity and problem structure.
Lu et al. previously explored the synergy between multibody dynamics and ANN for estimating cartilage stress in the knee joint. While their results indicated improved computational speed and acceptable accuracy, a closer reading suggests they focused on predicting stress at discrete locations rather than global maxima—an important distinction in biomechanical applications [
10].
Liang et al. developed a framework for full-field stress prediction using convolutional neural networks (CNNs) trained on geometry encodings derived from medical imaging. Their method optimizes pixel- or voxel-wise loss functions to replicate the spatial distribution of stress, enabling detailed field-level outputs. However, this approach typically demands larger model capacity and substantially more data to capture fine-grained patterns across spatial domains [
10].
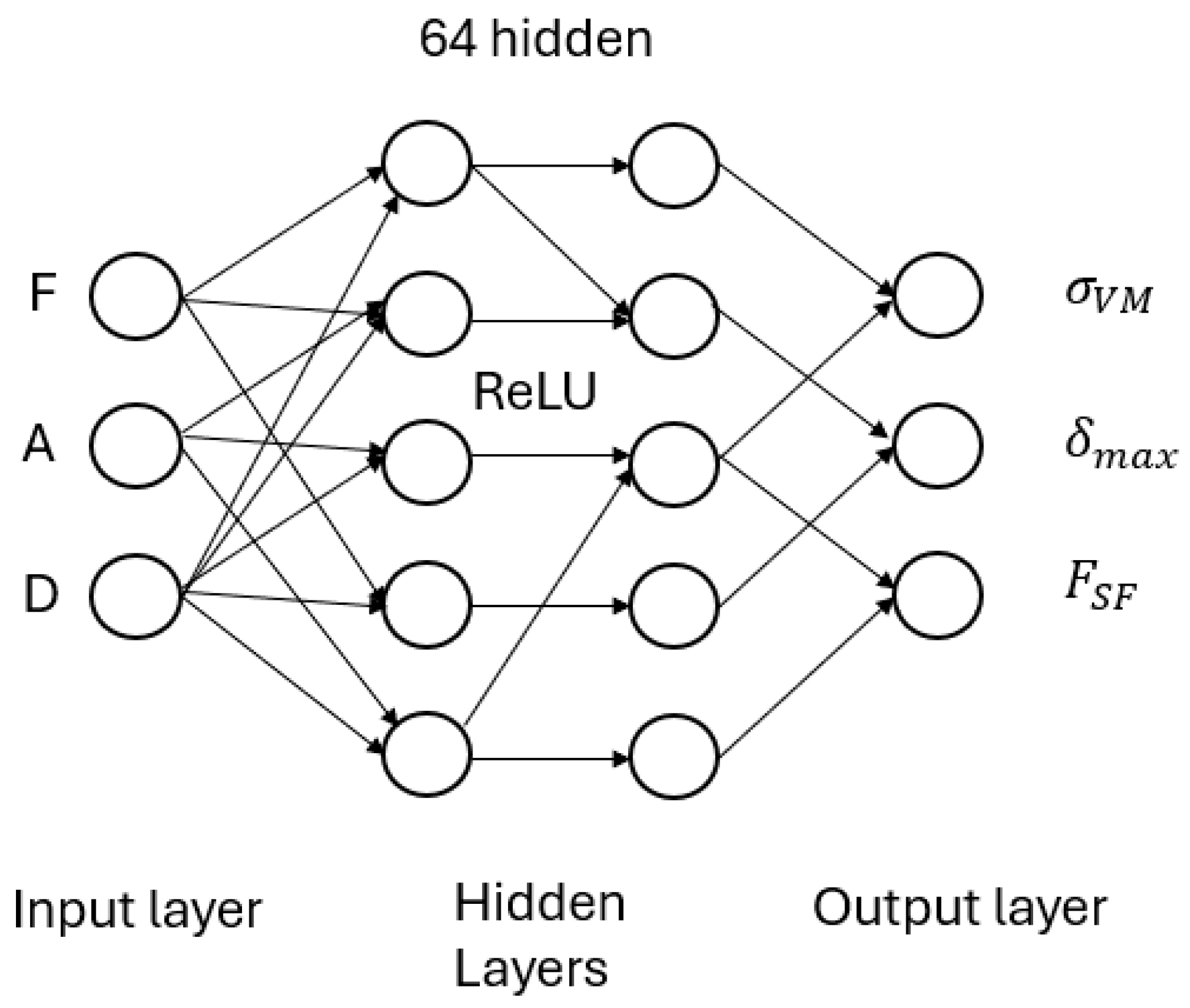
In contrast, our problem formulation is inherently low-dimensional, with inputs comprising only force magnitude (F), insertion angle (A), and implant diameter (D), and outputs limited to scalar quantities—namely, von Mises stress (σᵥᴹ), maximum displacement (δₘₐₓ), and the failure safety factor (FSF). Given this structure, a compact multilayer perceptron (MLP) is more appropriate, prioritizing computational efficiency and data economy over spatial resolution.
A middle-ground approach is presented by Lu et al. [
10], whose ANN functions as an emulator within a multibody dynamics framework, predicting location-specific cartilage stresses based on system-level kinematic inputs. This represents an intermediate level of model complexity—balancing local specificity with broader system interactions.
Our model, by contrast, is designed to directly map (F, A, D) to global stress extrema and FSF, enabling rapid, interactive “what-if” analyses during implant design or planning. Collectively, these comparisons illustrate a general design principle: the choice of model architecture should align with the nature of the prediction target (full-field vs. localized vs. global scalar), the dimensionality of the input data, and latency or data availability constraints.
To our knowledge, this is among the first studies to apply an ANN to FEA-derived data from dental implants, specifically targeting fatigue-related outputs. In doing so, it offers not only a proof of concept but a practical pathway toward AI-driven design methodologies. Our work aligns closely with the vision articulated by Griggs [
11], who proposed AI as a transformative tool in implant design. Here, we provide a tangible step in that direction: a functioning model that could be readily adapted for generative or optimization-based tasks in future implant development.
Although our current dataset of 200 finite element analysis (FEA) simulations suffices to demonstrate the feasibility of the proposed surrogate model, the development of a more robust and generalizable surrogate will benefit from the incorporation of a larger and more diverse dataset. Expanding the dataset will help reduce variance, improve generalization, and stabilize predictions—particularly near the boundaries of the design domain.
As part of our future work, we aim to extend the design space by adopting space-filling sampling techniques, such as Latin hypercube designs, alongside adaptive or active sampling strategies guided by model uncertainty or prediction error. This will enable targeted enrichment of the training dataset in regions where the surrogate model exhibits reduced predictive reliability or higher output sensitivity (e.g., under conditions of extreme obliquity or reduced geometrical dimensions). While we currently report point estimates derived from a held-out test set to provide clarity in performance metrics, small and structured datasets—such as ours—are better served by resampling-based validation strategies that more accurately reflect generalization performance. In future work, we intend to adopt K-fold cross-validation (e.g., K = 5) and repeated hold-out validation to quantify variability induced by data partitioning. Performance will be summarized using robust statistics, such as the median and interquartile ranges (IQRs) of R2, mean absolute error (MAE), and root mean squared error (RMSE) across folds.
Ultimately, if artificial neural networks (ANNs) are to inform safety-critical decisions, their reliability must be validated with the same rigor traditionally reserved for established engineering methods. We advocate for a hybrid approach: leveraging ANN predictions for rapid insights and early-stage design filtering, then applying targeted FEA simulations to the most critical or ambiguous cases for final verification. This strategy preserves the high standards of decision trustworthiness while capitalizing on the speed and efficiency that AI offers.
Looking ahead, the potential for continuous improvement is clear. As more data become available—including real-world clinical outcomes—the ANN can be iteratively retrained, gradually refining its accuracy and broadening its ability to capture patient- and design-specific variability.
Even at this preliminary stage, the ANN has performed exceptionally well within its defined scope: predicting key FEA outcomes for dental implants based on load and geometric parameters. It is, we believe, a promising first step toward integrating AI not as a replacement, but as a powerful partner in biomechanical design and evaluation.
The surrogate can be deployed as a REST API or SDK, embedded in clinical CAD platforms, accepting DICOM/CBCT and mesh inputs and returning FSF/σᵥᴹ/δₘₐₓ predictions with uncertainty and safety checks. Regulatory compliance requires alignment with IEC 62304, ISO 14971, IEC 62366, and GDPR [
25,
26,
27,
28]. Deployment includes model versioning, real-world validation, and drift monitoring to support reliable clinician use.
5. Conclusions
This study proposes a novel yet grounded integration of finite element analysis data with a feed-forward neural network, aimed at predicting fatigue-related mechanical responses in titanium dental implants. The approach reflects a growing paradigm in computational biomechanics—where artificial intelligence does not replace physics, but rather accelerates its practical application.
The neural network was trained on a triplet of input variables—applied force, angulation, and implant diameter—to estimate three biomechanical outputs derived from FEA: von Mises stress, total deformation, and the fatigue safety factor. Across all predicted variables, the model achieved a coefficient of determination (R2) exceeding 0.90, with average errors hovering around 5%. In other words, the network learned not just to interpolate data, but to approximate a highly nonlinear mechanical process with remarkable fidelity.
The advantage is not merely predictive accuracy, but speed. Traditional FEA, though robust, is computationally expensive—often prohibitively so for iterative design or real-time scenarios. In stark contrast, the trained network delivers near-instantaneous results, enabling large-scale parametric sweeps and unlocking new possibilities in clinical decision-making and design optimization workflows.
A crucial pillar of this framework lies in the thoughtful incorporation of domain knowledge: from the generation of simulation datasets to the careful selection of inputs. The fidelity of the results underscores the importance of interdisciplinary synergy—where engineering expertise informs machine learning, and vice versa. This foundation also allows for scalability: the methodology is readily extendable to more complex scenarios, including bone–implant interactions, time-dependent loading, or even physics-informed neural networks for enhanced generalizability.
This study presents several limitations that must be acknowledged before any clinical or industrial application. First, all simulations were performed under static loading conditions, without incorporating the dynamic, cyclic forces typical of mastication. This significantly limits the model’s applicability to real-world fatigue scenarios. Although the fatigue safety factor was estimated using the Goodman criterion, more comprehensive fatigue life predictions (e.g., number of cycles to failure) were not included. From a geometric perspective, only the implant diameter was varied, while other critical features such as implant length, thread profile, and neck geometry remained fixed. This reduces the surrogate model’s ability to generalize across a wider range of implant designs. Furthermore, the ANN was trained within a constrained input domain—loads from 100 to 800 N, angles from 0° to 45°, and diameters from 3.0 to 5.0 mm—which limits its reliability outside this region unless retraining and validation are performed. Another important limitation is that the network only predicts global output values (maximum stress, deformation, and FSF) without providing spatial information or localizing stress concentrations. As such, it does not distinguish whether critical stress points occur at the implant neck, abutment screw, or thread roots—locations clinically relevant for failure prediction and design optimization. Finally, the model assumes full osseointegration and excludes any representation of surrounding bone or patient-specific anatomy. Consequently, factors such as bone density, bruxism, and anatomical variability are not yet captured. These limitations should be carefully considered when interpreting the results, and future work must address them by incorporating dynamic fatigue models, expanding the geometric and clinical parameter space, and validating predictions with real patient data to ensure translational relevance.
In conclusion, this convergence between finite element modeling and artificial intelligence marks a significant step forward in implant biomechanics. By uniting the precision of traditional simulation with the speed and adaptability of AI, researchers are now better equipped to explore broader design spaces, anticipate mechanical failures, and ultimately improve clinical outcomes in dental implantology.