Supercritical Fluids as Alternative Insulation and Arc-Quenching Medium
Abstract
Featured Application
Abstract
1. Introduction
1.1. Motivation
1.2. Early Arc Quenching—A Brief History of Pre-SF6 High-Voltage Circuit Breakers
1.3. Review Methodology
- –
- Step 1: Database Search. We used Google Scholar as the primary academic search engine, supplemented with IEEE Xplore, ScienceDirect, and NIST technical reports.
- –
- Step 2: Screening. Articles were filtered based on relevance.
- –
- Step 3: Thematic Clustering. Relevant works were categorized into themes: (i) transport properties of SCFs, (ii) arc quenching in dense fluids, (iii) SF6 environmental alternatives, and (iv) high-voltage applications of SCFs.
- –
- Step 4: AI-Assisted Synthesis. ChatGPT was used not as a primary source of information, but as a tool for searching, structuring, and synthesis. Specifically, the model was employed to:
- Compare and summarize overlapping findings from multiple papers.
- Suggest gaps in the literature that align with our experimental work.
- Identify older sources that did not surface in standard search queries but proved relevant to the historical and theoretical context of SCFs.
2. Properties of Supercritical Carbon Dioxide
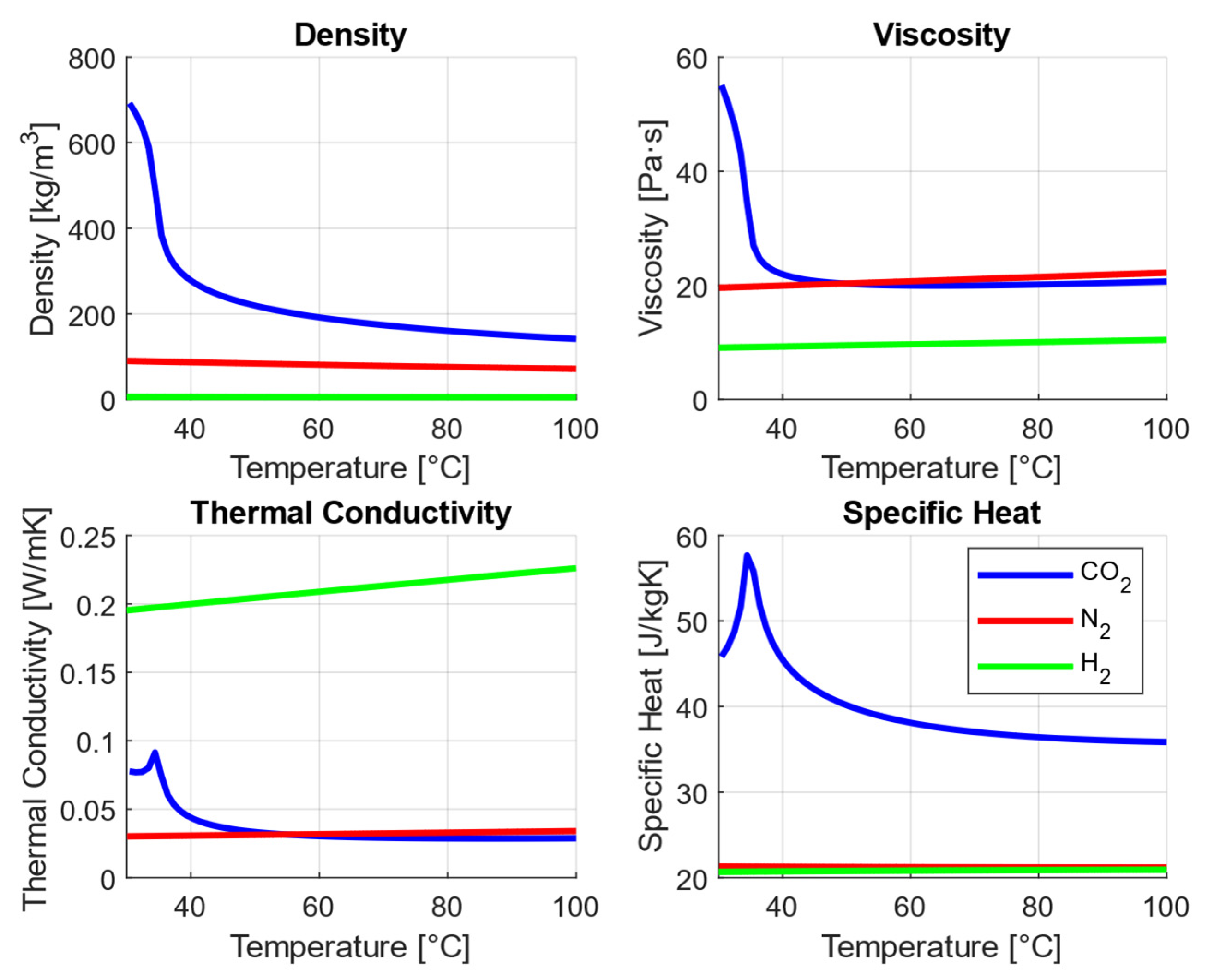
2.1. Transport Properties
2.2. Unique Structural Characteristics
2.3. Breakdown Voltage
2.4. Conductivity and Permittivity
3. Ionization in Supercritical Carbon Dioxide
3.1. Thermal Ionization
3.2. Ionization by Collision
3.3. Formation of Negative Ions
4. Supercritical CO2 as the Optimal Dielectric and Arc-Quenching Medium
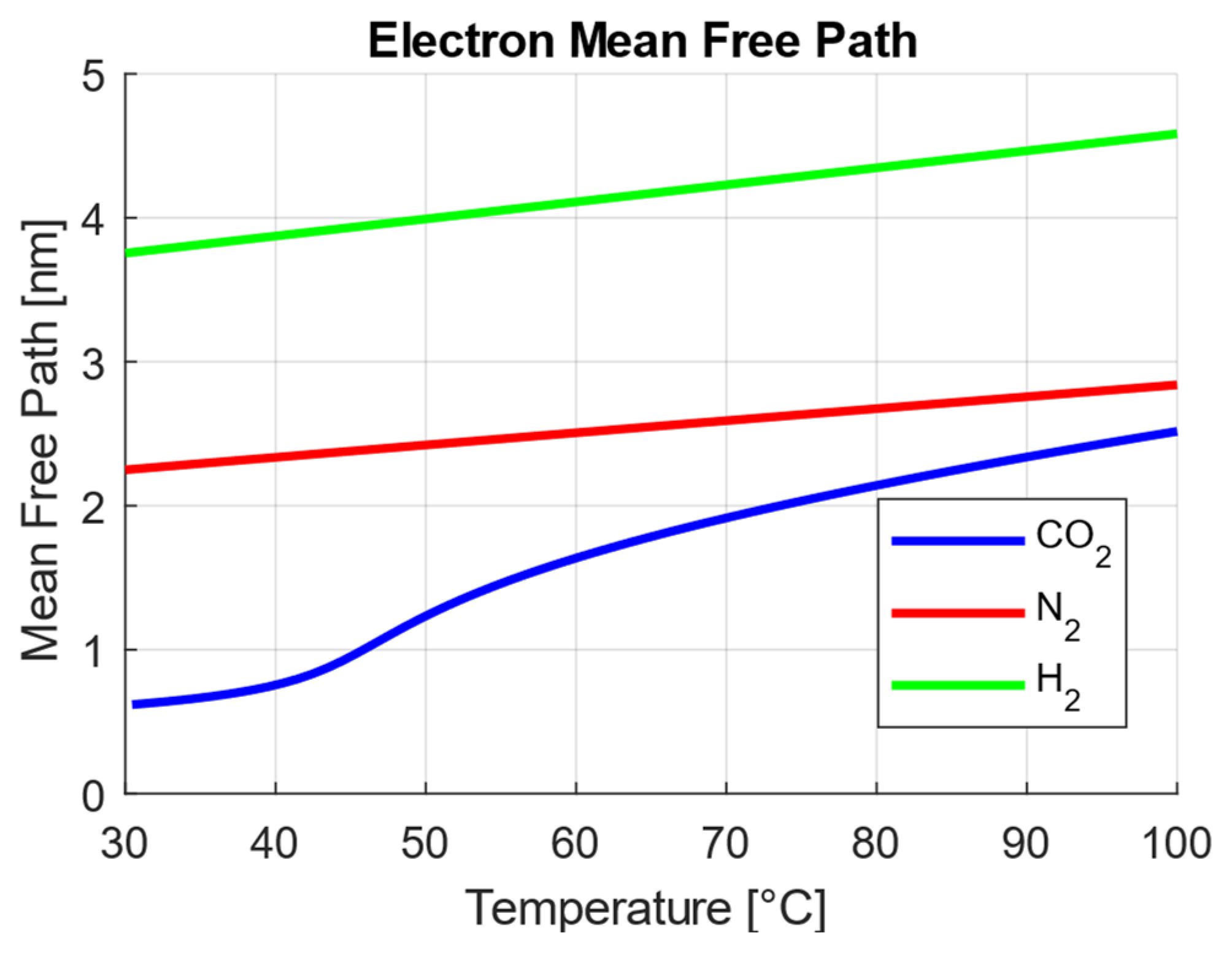
4.1. Mean Free Path and Heat Dissipation
4.2. Arcing Time Constant−Projected Performance for scCO2
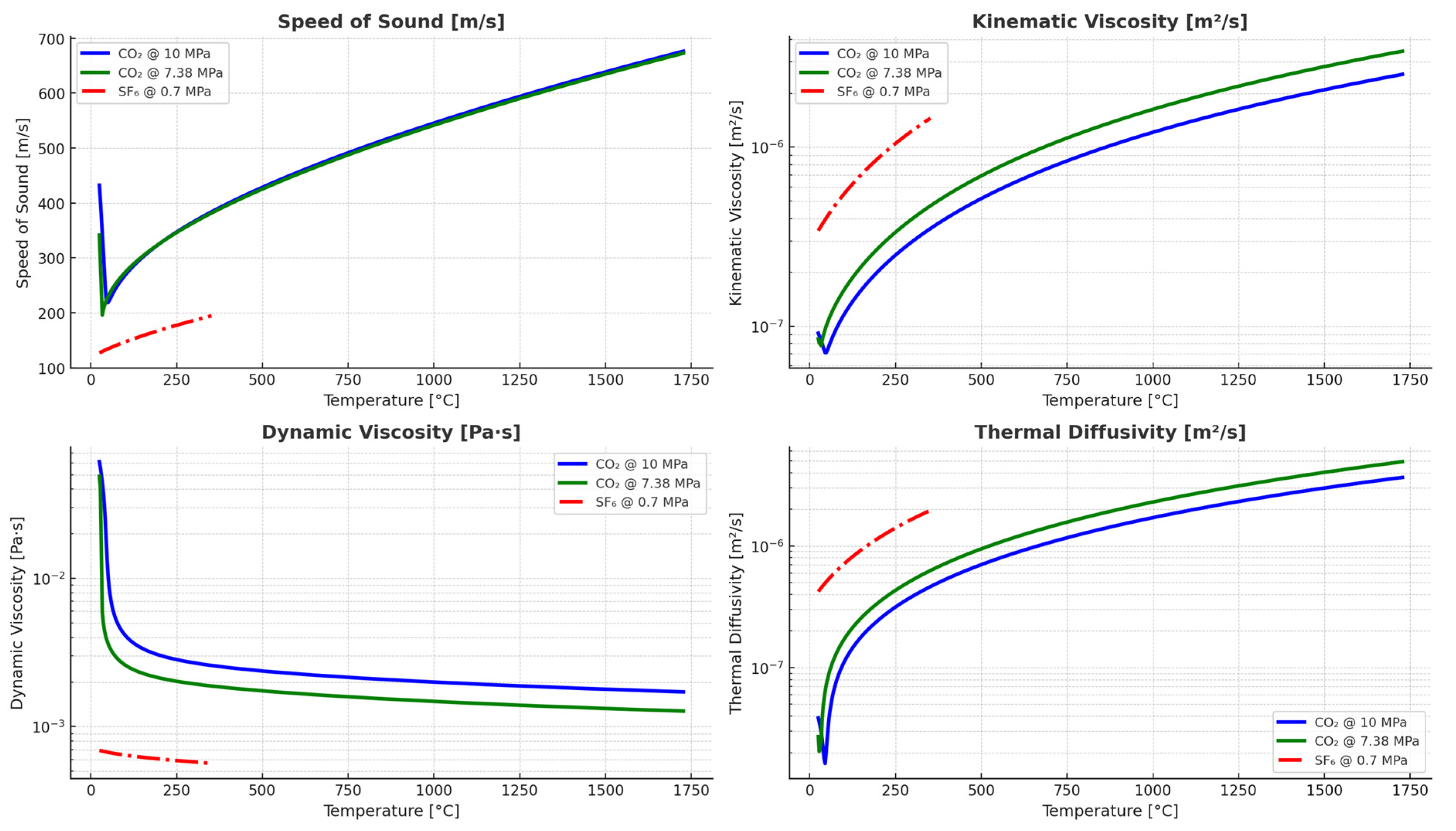
4.3. Thermophysical Properties Relevant to Arc-Quenching Performance
4.3.1. Speed of Sound
4.3.2. Kinematic Viscosity
4.3.3. Dynamic Viscosity
4.3.4. Thermal Diffusivity
4.4. Limitations of Alternative Supercritical Fluids
5. Supercritical CO2 Mixtures
6. Supercritical CO2 Dielectric Life Expectancy
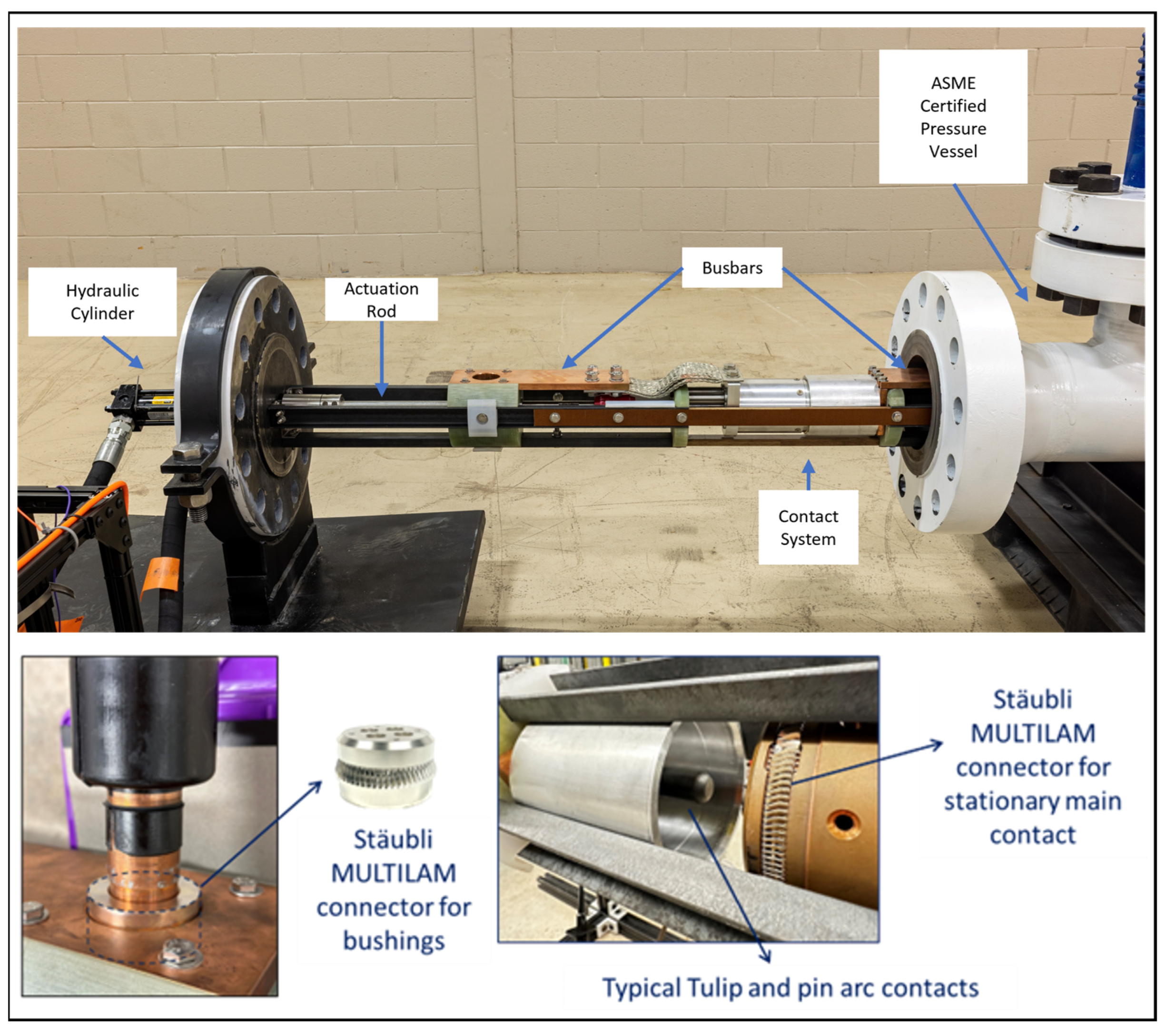
7. Tough and Ecological Supercritical Line Breaker for AC (TESLA)
7.1. Development of SF6-Free High-Voltage Circuit Breaker
7.2. Economic Tradeoffs in Circuit Breaker Design
7.2.1. Raw Material Cost
7.2.2. Fluid and Inventory Management Cost
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. Environmental Protection Agency. Sulfur Hexafluoride (SF6) Basics. Available online: https://www.epa.gov/eps-partnership/sulfur-hexafluoride-sf6-basics (accessed on 2 November 2024).
- U.S. Environmental Protection Agency. Byproducts of Sulfur Hexafluoride (SF6) Use in the Electric Power Industry; ICF Consulting: Fairfax, VA, USA, 2002.
- European Environment Agency. Hydrofluorocarbon Phase-Down in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/hydrofluorocarbon-phase-down-in-europe (accessed on 2 November 2024).
- U.S. Department of Energy. G7 Energy Ministers Achieve Breakthroughs on Unabated Coal Phaseout, Global Energy Storage, and Phasing Out Harmful Non-CO2 Pollutants. Available online: https://www.energy.gov/articles/g7-energy-ministers-achieve-breakthroughs-unabated-coal-phaseout-global-energy-storage-and (accessed on 2 November 2024).
- Owens, J.; Xiao, A.; Bonk, J.; DeLorme, M.; Zhang, A. Recent Development of Two Alternative Gases to SF6 for High Voltage Electrical Power Applications. Energies 2021, 14, 5051. [Google Scholar] [CrossRef]
- Simka, P.; Doiron, C.B.; Scheel, S.; Di-Gianni, A. Decomposition of Alternative Gaseous Insulation under Partial Discharge. In Proceedings of the 20th International Symposium on High Voltage Engineering (ISH 2017), Buenos Aires, Argentina, 27 August–1 September 2017. [Google Scholar]
- Seeger, M.; Smeets, R.; Yan, J.; Ito, H.; Claessens, M.; Dullni, E.; Franck, C.M.; Gentils, F.; Hartmann, W.; Kieffel, Y.; et al. Recent Development of Alternative Gases to SF6 for Switching Applications. Electra 2017, 291, 26–29. [Google Scholar]
- Yedinak, E.; Lentijo, K.; Kizilyalli, I.C. Eliminating SF6 from Switchgear. In Direct Current Fault Protection; Kizilyalli, I.C., Shen, Z.J., Cunningham, D.W., Eds.; Power Systems; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cruz Feliciano, A.J.; Neall, S.M.; Al Hossain, M.S.; Shabani, H.; Guo, N.A.; Jin, Z.; Park, C.; Graber, L. Preliminary Investigation of Arc Quenching in Supercritical CO2. In Proceedings of the 2024 IEEE Electrical Insulation Conference (EIC), Minneapolis, MN, USA, 2–5 June 2024; pp. 255–258. [Google Scholar] [CrossRef]
- Graber, L.; Steurer, M.M.; Saeedifard, M.; Jin, Z.; Yang, Q.; Tousi, M. Efficient DC Interrupter with Surge Protection (EDISON). In Direct Current Fault Protection; Kizilyalli, I.C., Shen, Z.J., Cunningham, D.W., Eds.; Power Systems; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Yeckley, R.; Perulfi, J. Oil Circuit Breakers: A Look at the Earlier Generation [History]. IEEE Power Energy Mag. 2018, 16, 86–97. [Google Scholar] [CrossRef]
- Wilkins, R.; Crellin, E.A. High Voltage Oil Circuit Breakers; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1930. [Google Scholar]
- Leeds, W.M. A high-power oilless circuit interrupter using water. Electr. Eng. 1941, 60, 85–88. [Google Scholar] [CrossRef]
- Slepian, J.; Denault, C.L.; Strom, A.P. Dielectric Strength of Water in Relation to Use in Circuit Interrupters. Trans. Am. Inst. Electr. Eng. 1941, 60, 389–395. [Google Scholar] [CrossRef]
- Smeets, R.; van der Sluis, L.; Kapetanovic, M.; Peelo, D.F.; Janssen, A. Switching in Electrical Transmission and Distribution Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yeckley, R.N.; Colclaser, R.G. First SF6 Breaker Design: Westinghouse Engineers Tell the Inside Story [History]. IEEE Power Energy Mag. 2016, 14, 80–95. [Google Scholar] [CrossRef]
- Lemmon, E.; McLinden, M.; Friend, D.; Linstrom, P.; Mallard, W. NIST Chemistry WebBook, Nist Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011.
- Stauss, S.; Muneoka, H.; Urabe, K.; Terashima, K. Review of Electric Discharge Microplasmas Generated in Highly Fluctuating Fluids: Characteristics and Application to Nanomaterials Synthesis. Phys. Plasmas 2015, 22, 057103. [Google Scholar] [CrossRef]
- Shon, C.-H.; Song, K.-D.; Oh, Y.-H.; Oh, H.-S. Investigation of the Supercritical Fluids as an Insulating Medium for High Speed Switching. J. Electr. Eng. Technol. 2016, 11, 1783–1786. [Google Scholar] [CrossRef][Green Version]
- Beroual, A.; Khaled, U.; Coulibaly, M.-L. Experimental Investigation of the Breakdown Voltage of CO2, N2, and SF6 Gases, and CO2–SF6 and N2–SF6 Mixtures under Different Voltage Waveforms. Energies 2018, 11, 902. [Google Scholar] [CrossRef]
- Goldfarb, D.L.; Fernández, D.P.; Corti, H.R. Dielectric and Volumetric Properties of Supercritical Carbon Dioxide(1)+Methanol(2) Mixtures at 323.15 K. Fluid Phase Equilibria 1999, 158–160, 1011–1019. [Google Scholar] [CrossRef]
- Nishikawa, M.; Holroyd, R.A.; Itoh, K. Behavior of excess electrons in supercritical fluids-electron attachment. In Proceedings of the 1999 IEEE 13th International Conference on Dielectric Liquids (ICDL’99) (Cat. No.99CH36213), Nara, Japan, 25 July 1999; pp. 9–12. [Google Scholar] [CrossRef]
- Nishikawa, M.; Itoh, K.; Holroyd, R.A. Electron Attachment to CO2 in Supercritical Ethane. J. Phys. Chem. A 1999, 103, 550–556. [Google Scholar] [CrossRef]
- Wu, D.; Wei, M.; Tian, R.; Zheng, S.; He, J. A Review of Flow and Heat Transfer Characteristics of Supercritical Carbon Dioxide under Cooling Conditions in Energy and Power Systems. Energies 2022, 15, 8785. [Google Scholar] [CrossRef]
- Liao, G.; Du, Y.; Zhang, F.; Jiaqiang, E. Comprehensive review on physical properties of supercritical carbon dioxide calculated by molecular simulation. Korean J. Chem. Eng. 2023, 40, 11–36. [Google Scholar] [CrossRef]
- Reece, M.P. Physics of Circuit-Breaker Arcs. In Power Circuit Breaker Theory and Design; Flurscheim, C.H., Ed.; Institution of Electrical Engineers: London, UK, 1982; pp. 20–63. [Google Scholar] [CrossRef]
- Knobloch, H. The Comparison of Arc-Extinguishing Capability of Sulfur Hexafluoride (SF6) with Alternative Gases in High-Voltage Circuit-Breakers. In Gaseous Dielectrics VIII; Christophorou, L.G., Olthoff, J.K., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 565–571. [Google Scholar] [CrossRef]
- Yokomizu, Y.; Morooka, I.; Matsumura, T. Arc Parameters around Current Zero in CO2-Blast Quenching Chamber and Their Dependences on Filled Pressure. IEEJ Trans. PE 2007, 127, 699–704. [Google Scholar] [CrossRef][Green Version]
- Bini, R.; Basse, N.T.; Seeger, M. Arc-Induced Turbulent Mixing in an SF6 Circuit Breaker Model. J. Phys. D Appl. Phys. 2011, 44, 025203. [Google Scholar] [CrossRef]
- Popovtsev, V.V.; Khalyasmaa, A.I.; Patrakov, Y.V. Fluid Dynamics Calculation in SF6 Circuit Breaker during Breaking as a Prerequisite for the Digital Twin Creation. Axioms 2023, 12, 623. [Google Scholar] [CrossRef]
- Babrauskas, V. Electric Arc Explosions—A Review. Fire Saf. J. 2017, 89, 7–15. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Ghajar, A.J. Heat and Mass Transfer: Fundamentals and Applications, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2015; Chapter 9.5.8. [Google Scholar] [CrossRef]
- Wei, J.; Park, C.; Graber, L. Breakdown Characteristics of Carbon Dioxide–Ethane Azeotropic Mixtures Near the Critical Point. Phys. Fluids 2020, 32, 053305. [Google Scholar] [CrossRef]
- Wei, J.; Cruz, A.; Haque, F.; Park, C.; Graber, L. Investigation of the Dielectric Strength of Supercritical Carbon Dioxide–Trifluoroiodomethane Fluid Mixtures. Phys. Fluids 2020, 32, 103309. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, Y.; Liu, S.; Wang, Y.; Wang, H.; Jiang, J. A CO2/O2 Mixed Gas DC Circuit Breaker with Superconducting Fault Current-Limiting Technology. IEEE Trans. Power Deliv. 2020, 35, 1960–1967. [Google Scholar] [CrossRef]
- Uchii, T.; Shibata, T.; Watanabe, H.; Ito, H.; Takuma, T. Fundamental Research on SF6-Free Gas Insulated Switchgear Adopting CO2 Gas and Its Mixtures. In Proceedings of the International Symposium on EcoTopia Science (ISETS), Nagoya, Japan, 23–25 November 2007. [Google Scholar]
- Chen, B.; Li, H.; Diao, M.; Yan, J. Simulation of No-load Medium Recovery Characteristics of CO2 Circuit Breaker. In Proceedings of the 2021 IEEE/IAS Industrial and Commercial Power System Asia (I&CPS Asia), Chengdu, China, 18–21 July 2021; pp. 1121–1127. [Google Scholar] [CrossRef]
- Uchii, T.; Hoshina, Y.; Mori, T.; Kawano, H.; Nakamoto, T.; Mizoguchi, H. Investigations on SF6-Free Gas Circuit Breaker Adopting CO2 Gas as an Alternative Arc-Quenching and Insulating Medium. In Gaseous Dielectrics X; Christophorou, L.G., Olthoff, J.K., Vassiliou, P., Eds.; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Prévé, C.; Maladen, R.; Trichon, F.; Piccoz, D. Innovative SF6-Free Switch with Shunt Vacuum Interruption Technology. In Proceedings of the 25th International Conference on Electricity Distribution (CIRED), Madrid, Spain, 3–6 June 2019. Paper No. 770. [Google Scholar]
- Siemens. Siemens Receives Order for World’s First SF6-Free Gas-Insulated Switchgear with Clean Air and Vacuum Switching Technology. Siemens Press Release. 2020. Available online: https://press.siemens.com/global/en/pressrelease/siemens-receives-order-worlds-first-sf6-free-gas-insulated-switchgear-clean-air-and (accessed on 4 November 2024).
- Siemens Energy. Blue Products for Sustainable and Greenhouse Gas-Free Power Grids. Siemens Energy Press Release. 2021. Available online: https://www.siemens-energy.com/global/en/home/press-releases/path-zero-f-gas-free-power-transmission.html (accessed on 4 November 2024).
- Chen, Y.; Grijalva, S.; Jin, Z.; Graber, L. Lifecycle Analysis of Greenhouse Gas Emissions: Comparing SF6 and scCO2 Circuit Breakers. In Proceedings of the 2024 56th North American Power Symposium (NAPS), El Paso, TX, USA, 13–15 October 2024; pp. 1–6. [Google Scholar] [CrossRef]

| Property (Section Ref.) | Key Insights | Implication |
|---|---|---|
| Transport Properties (Section 2.1) | Liquid-like density with gas-like mobility enhances thermal conductivity and convective cooling. | Supports stable arc cooling and dielectric recovery. |
| Structural Characteristics (Section 2.2) | Unique fluid microstructures near the critical point reduce the mean free path. | Improves dielectric strength beyond conventional gases. |
| Breakdown Behavior (Section 2.3) | Breakdown strength can be tuned by adjusting the pressure–temperature condition. | Ensure reliable insulation under high-voltage stress. |
| Thermal Ionization (Section 3.1) | High density and heat capacity improve energy transport and cooling, limiting excessive ionization growth. | Prevents thermal runaway and supports stable post-arc dielectric recovery. |
| Collision Ionization (Section 3.2) | High density boosts collisions, limiting electron avalanches. | Stabilizes insulation under fault stress. |
| Negative Ion Formation (Section 3.3) | Transient CO2− ions capture electrons and accelerate recombination. | Reduce electron availability for avalanche growth, accelerating arc extinction. |
| Arc-Quenching Dynamics (Section 4.1, Section 4.2 and Section 4.3) | Short arcing time constants, strong convective cooling, and short MFP. | Enables reliable current interruption and reduced contact erosion. |
| Limitations of Other SCFs (Section 4.4) | N2, O2, and H2 have impractical critical points; NH3 faces toxicity and material issues. | Positions CO2 as uniquely suitable among SCFs. |
| CO2 Mixtures (Section 5) | Additives can tailor dielectric, thermal, and quenching behavior. | Offers an optimization pathway beyond pure CO2. |
| Applications and Case Study (Section 6) | The 72 kV prototype breaker demonstrates feasibility, with cost–performance tradeoffs being manageable. | Confirms technical viability and pathway for scaling. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz Feliciano, A.J.; Jin, Z.; Graber, L. Supercritical Fluids as Alternative Insulation and Arc-Quenching Medium. Appl. Sci. 2025, 15, 9986. https://doi.org/10.3390/app15189986
Cruz Feliciano AJ, Jin Z, Graber L. Supercritical Fluids as Alternative Insulation and Arc-Quenching Medium. Applied Sciences. 2025; 15(18):9986. https://doi.org/10.3390/app15189986
Chicago/Turabian StyleCruz Feliciano, Alfonso J., Zhiyang Jin, and Lukas Graber. 2025. "Supercritical Fluids as Alternative Insulation and Arc-Quenching Medium" Applied Sciences 15, no. 18: 9986. https://doi.org/10.3390/app15189986
APA StyleCruz Feliciano, A. J., Jin, Z., & Graber, L. (2025). Supercritical Fluids as Alternative Insulation and Arc-Quenching Medium. Applied Sciences, 15(18), 9986. https://doi.org/10.3390/app15189986






