Formation and Study of Bismuth Sulphide Thin Films on Textiles of Different Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermo-Oxidative Acid Treatment of GF/PTFE and PET/PVC Samples
2.3. Deposition of the Bi2S3 Thin Films
2.4. Analytical Techniques
3. Results and Discussion
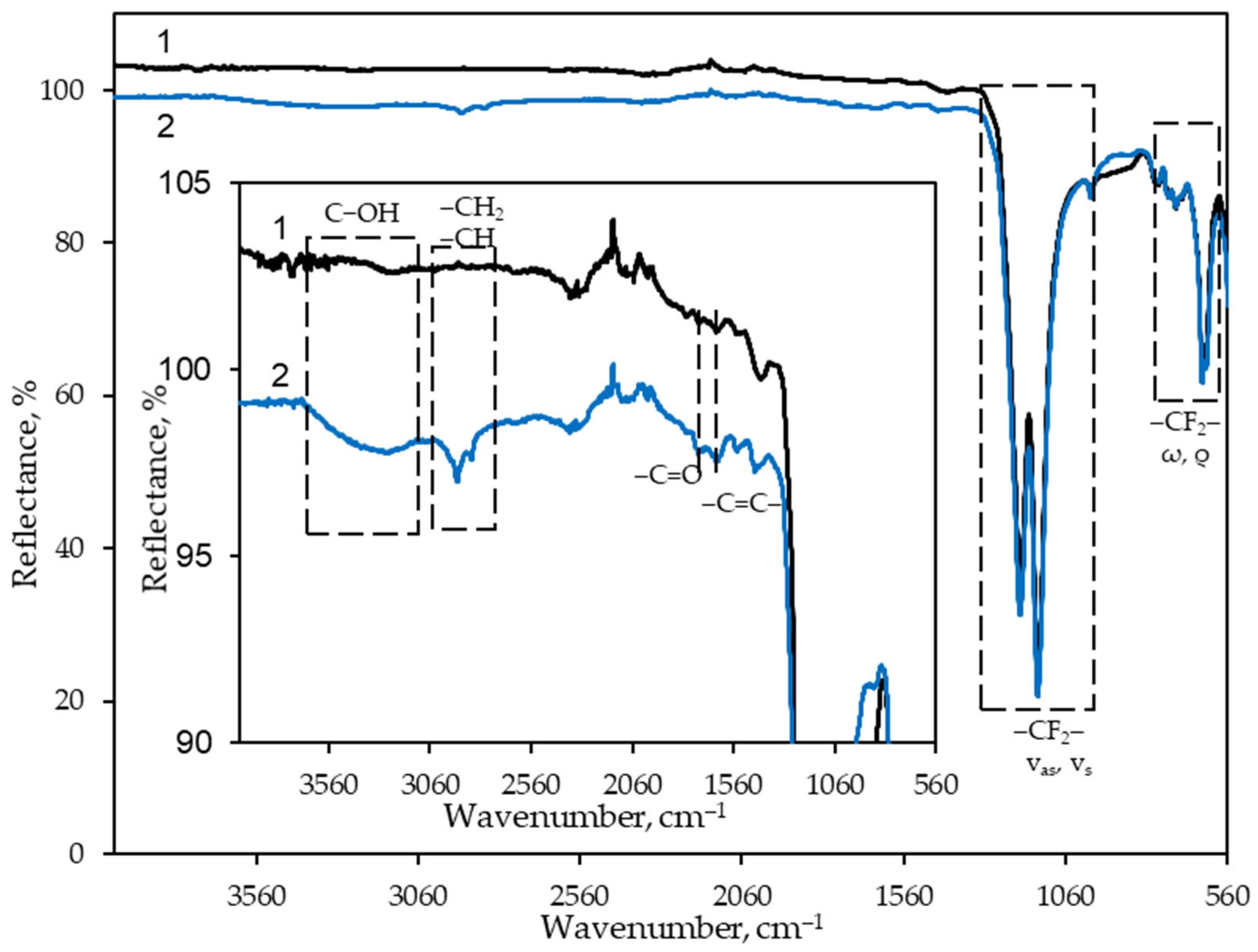
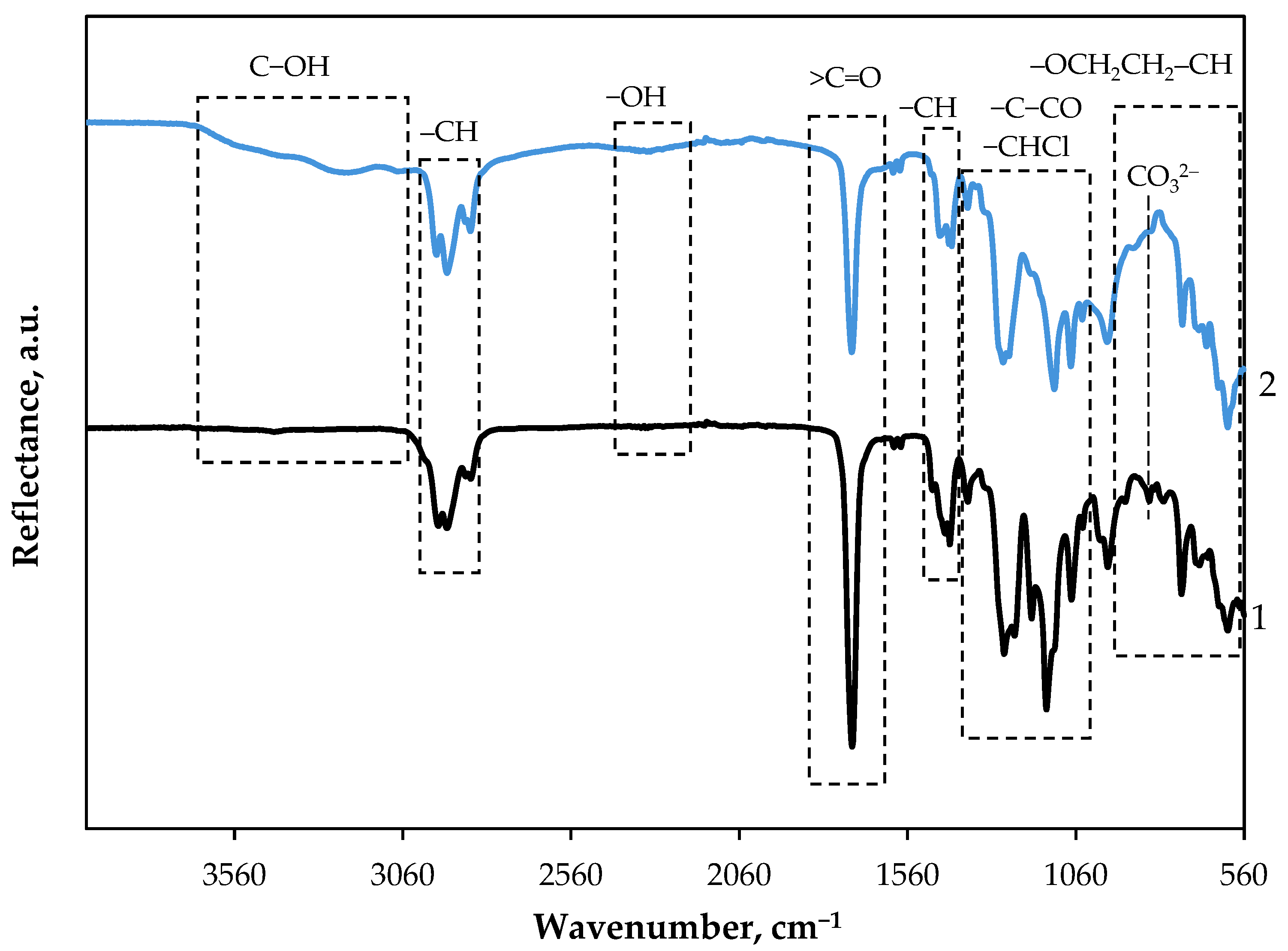
3.1. ATR-FTIR Spectroscopy of GF/PTFE and PET/PVC Samples
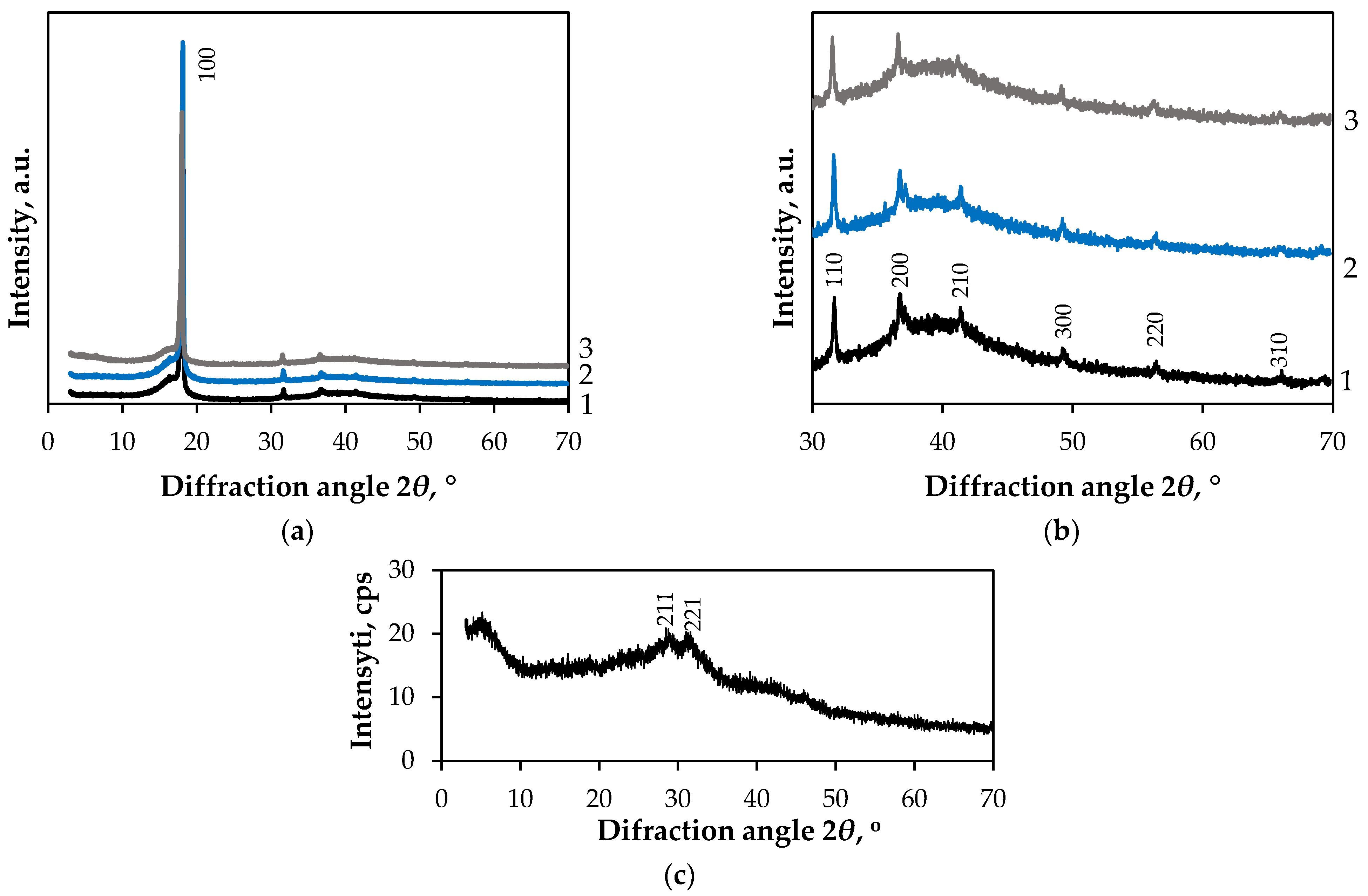
3.2. Structural, Morphological and Optical Characterisation of Obtained Composites
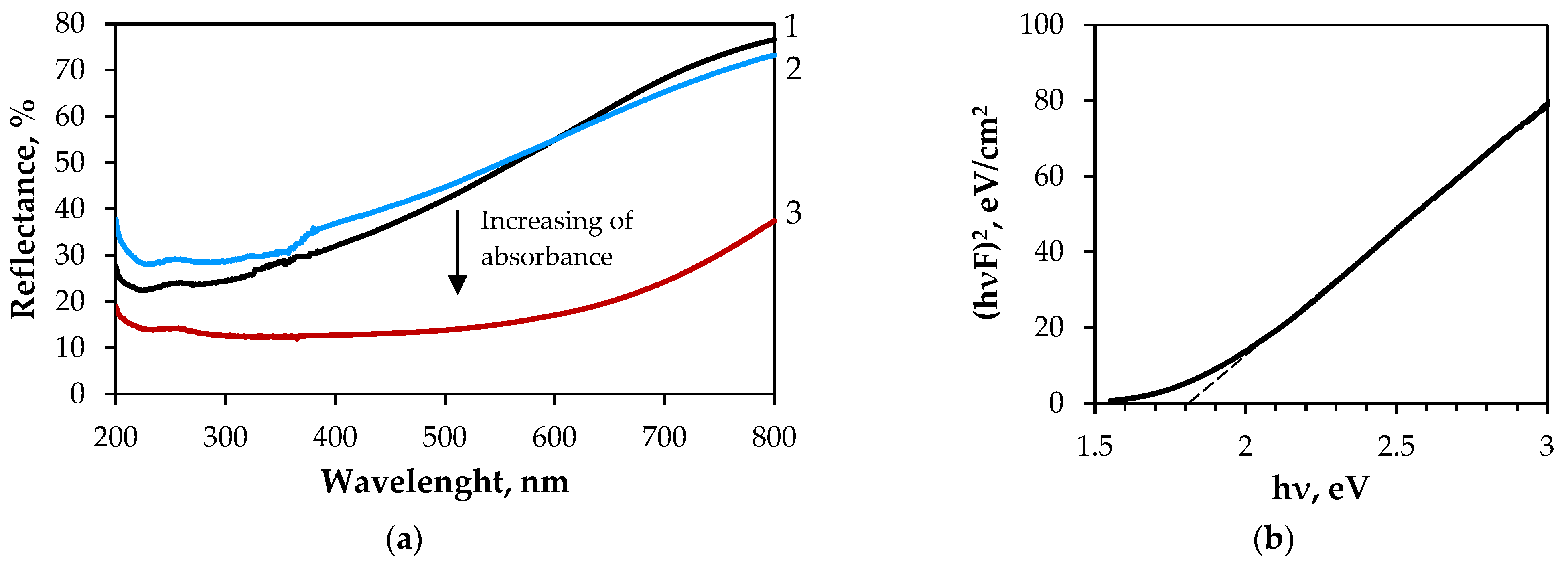
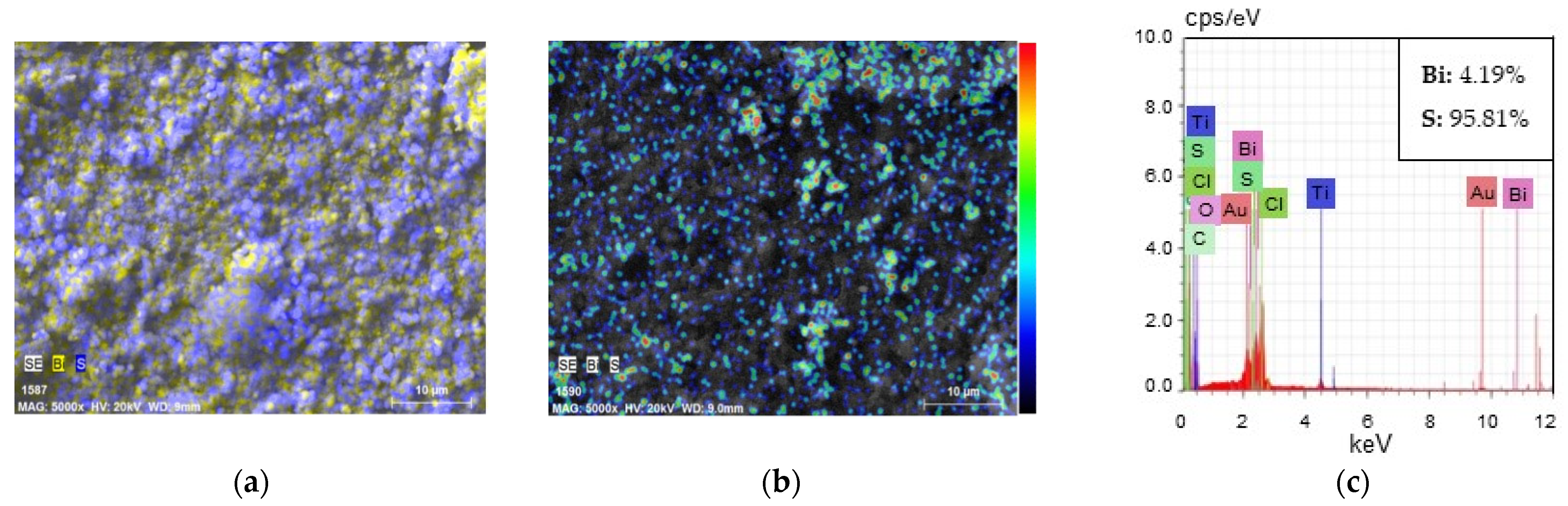
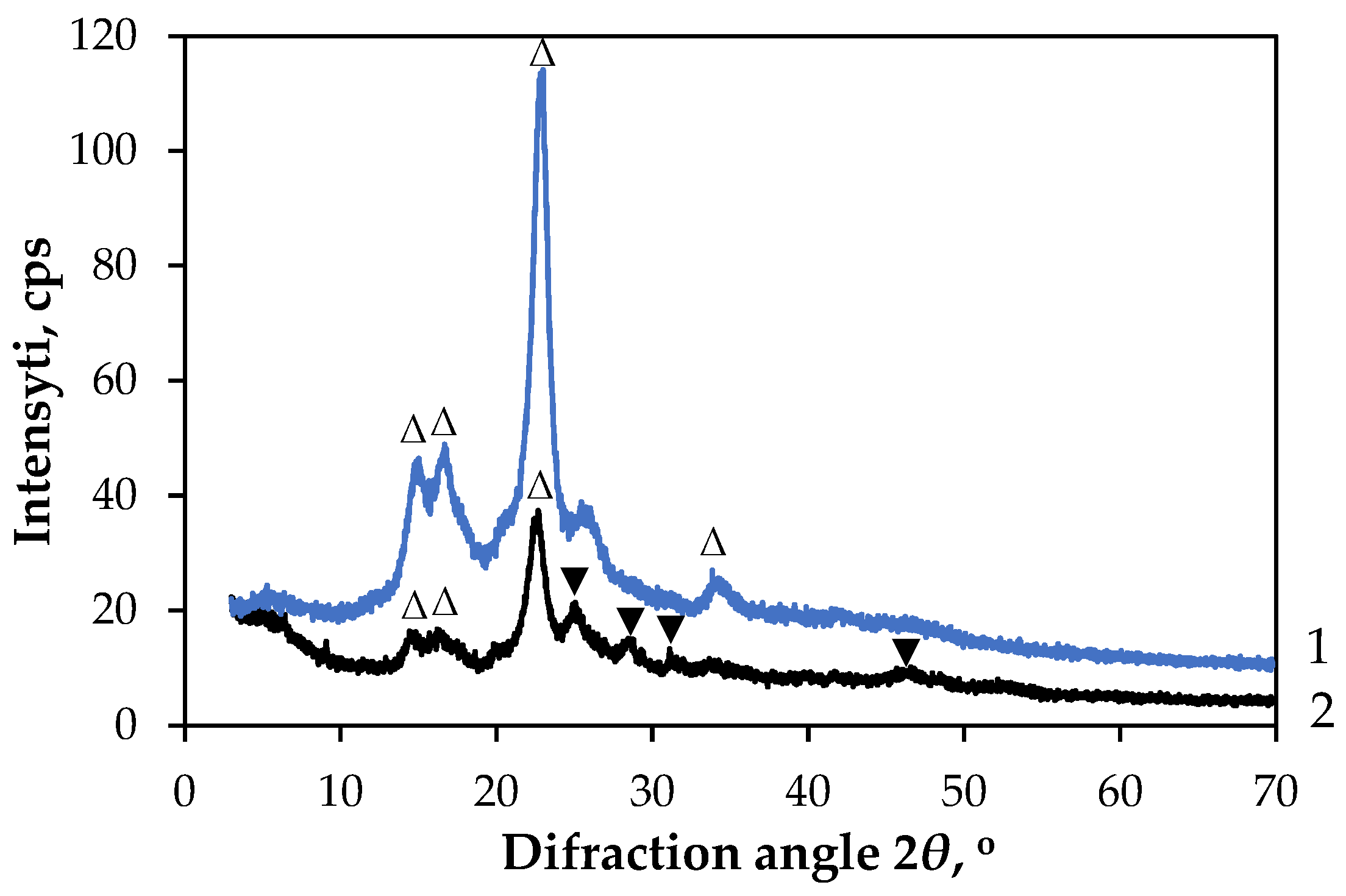
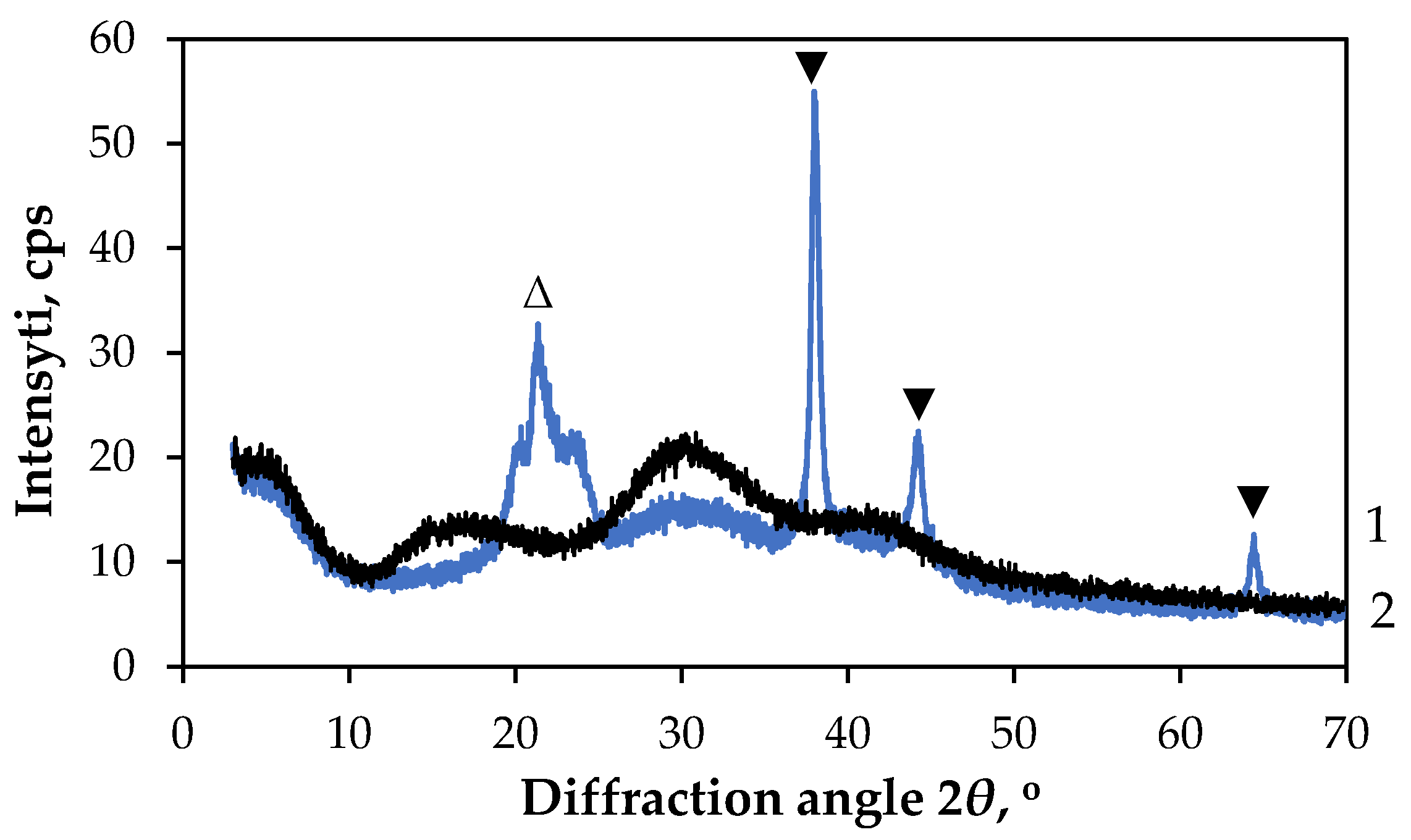
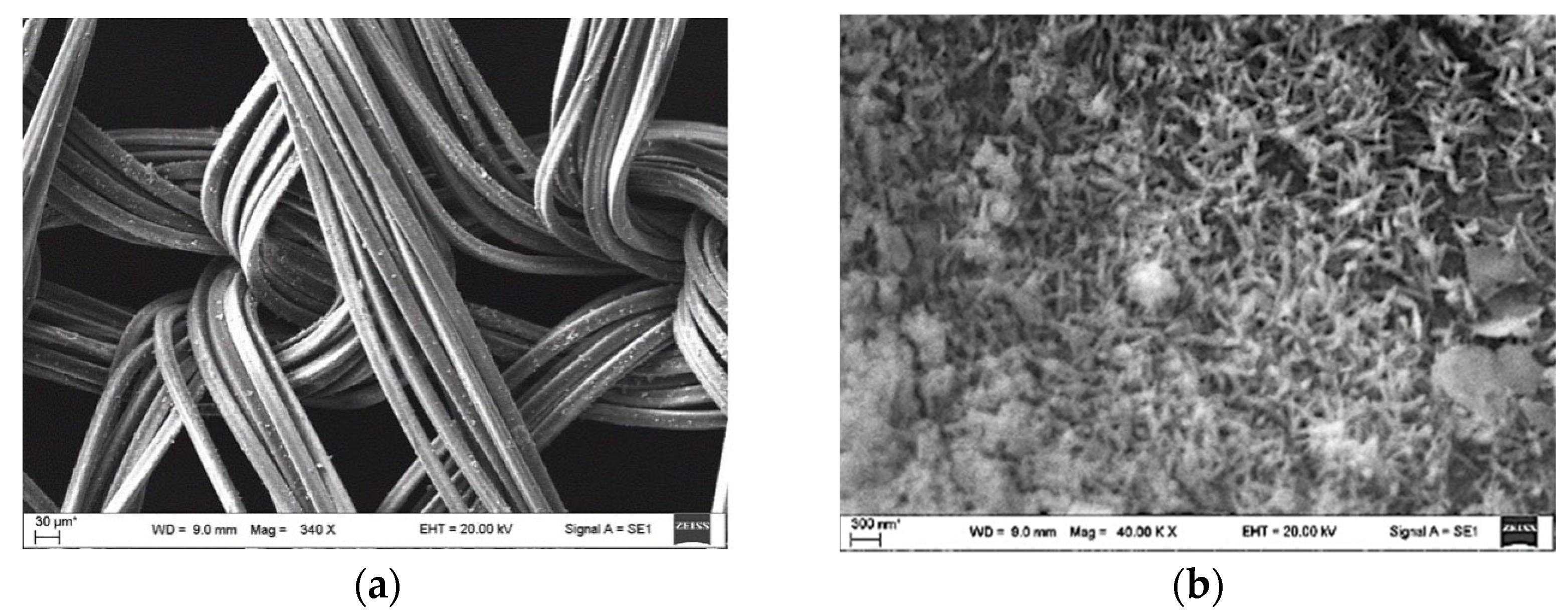
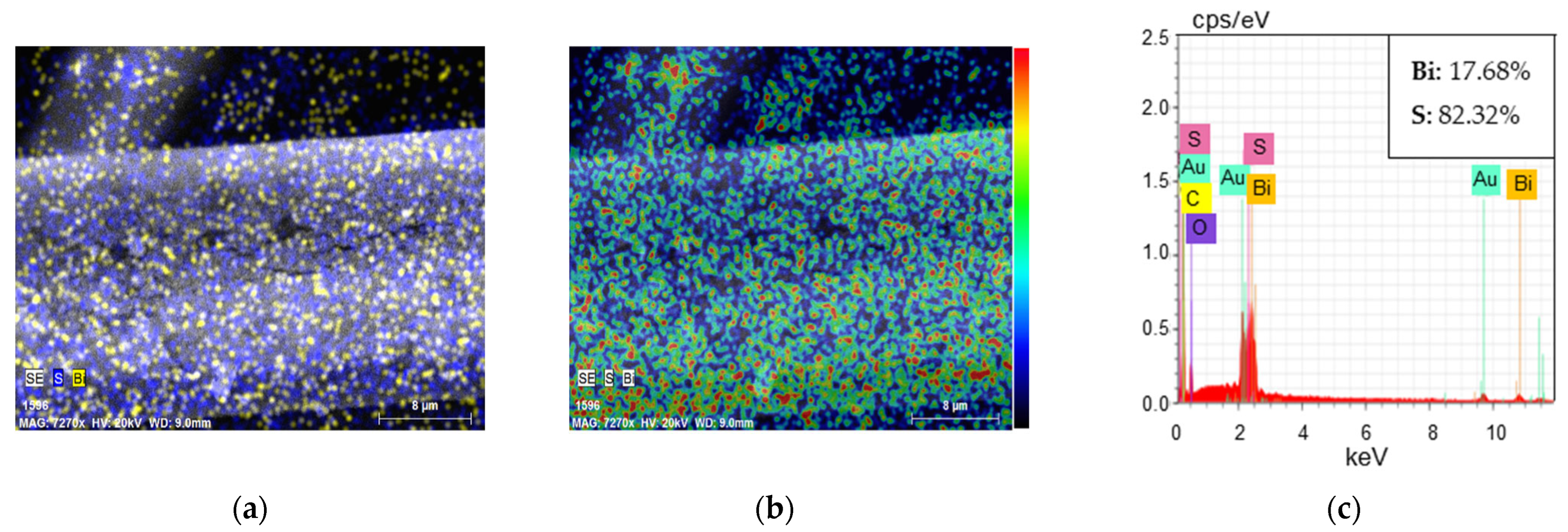
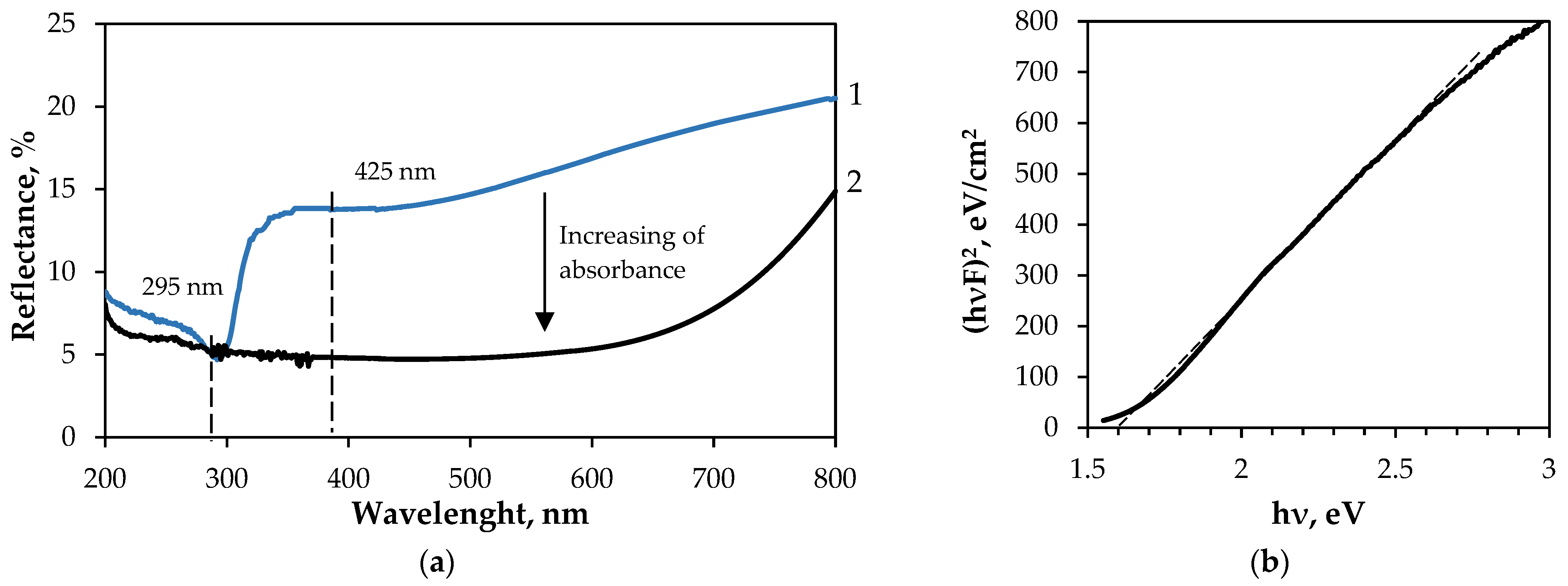
3.2.1. Characterisation of GF/PTFE/Bi-S Samples
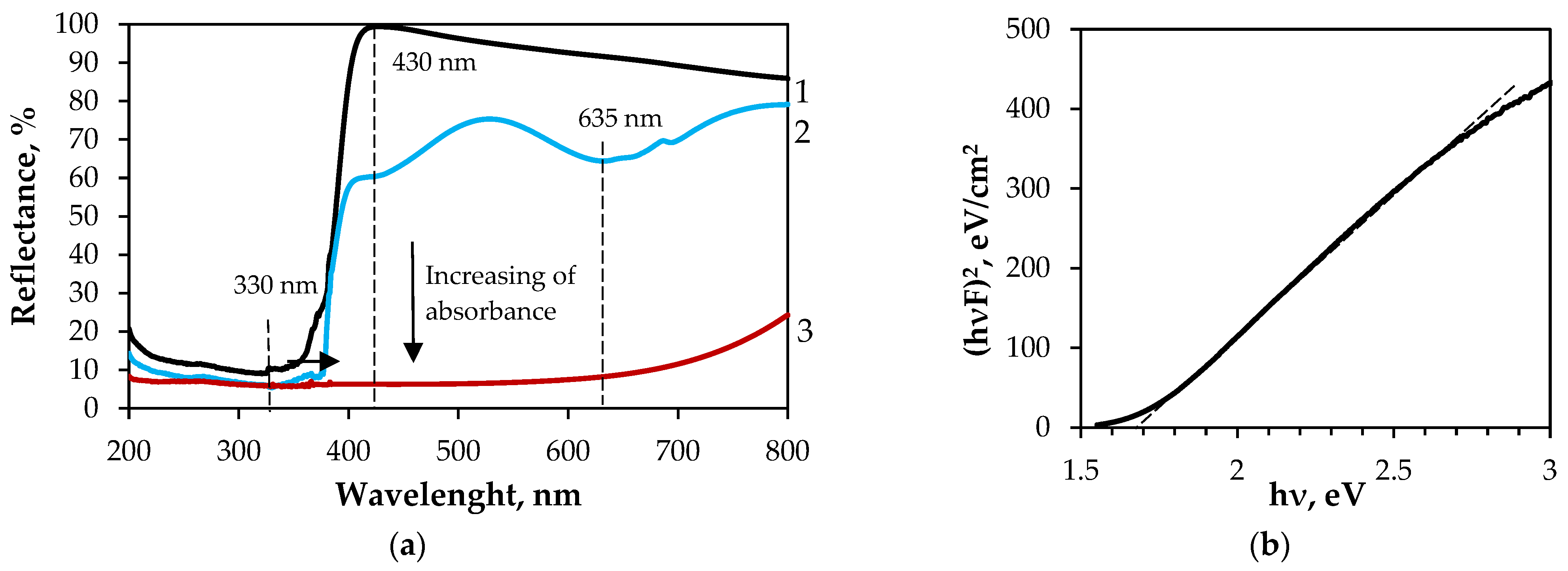
3.2.2. Characterisation of PET/PVC/Bi-S Samples
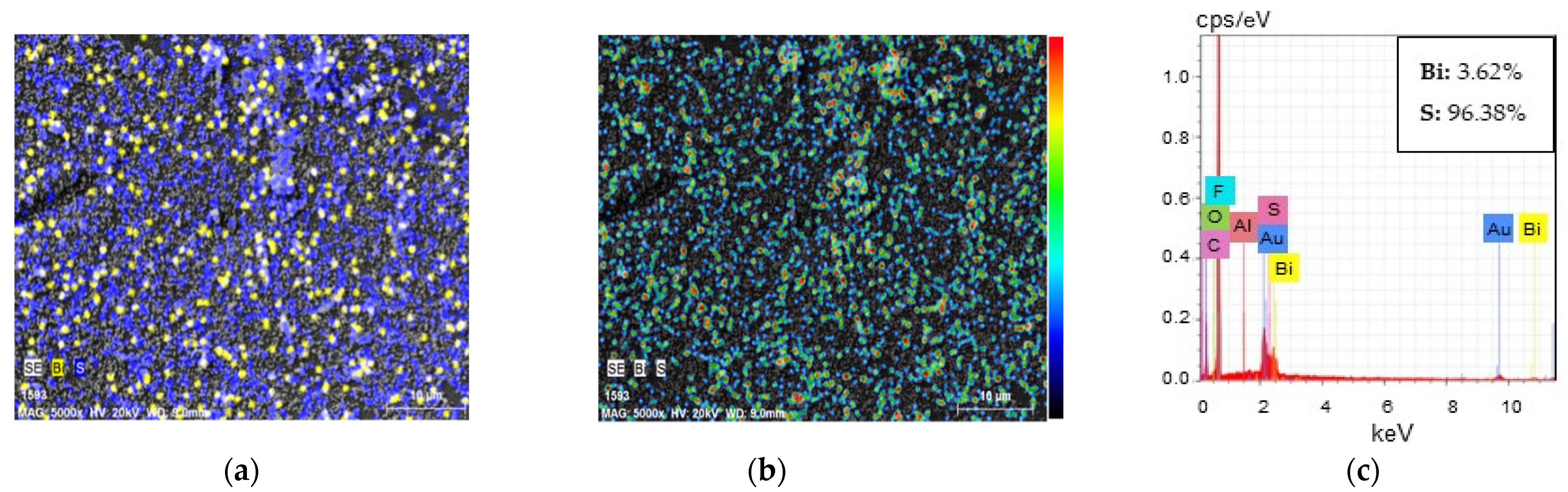
3.2.3. Characterisation of C/PES/S/Bi-S Samples
3.2.4. Characterisation of PA/Cu/Bi-S Samples
3.2.5. Characterisation of PA/Ag/Bi-S Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojstršek, A.; Plohl, O.; Gorgieva, S.; Kurečič, M.; Jančič, U.; Hribernik, S.; Fakin, D. Metallisation of textiles and protection of conductive layers: An overview of application techniques. Sensors 2021, 21, 3508. [Google Scholar] [CrossRef]
- Zhong, H.; Bai, Z.; Zou, B. Tuning the luminescence properties of colloidal I-III-VI semiconductor nanocrystals for optoelectronics and biotechnology applications. J. Phys. Chem. Lett. 2012, 3, 3167–3175. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Manimegalai, D.K.; Manikandan, A.; Moortheswaran, S.; Antony, S.A. One-pot microwave irradiation synthesis and characterization studies of nanocrystalline CdS photocatalysts. Adv. Sci. Eng. Med. 2015, 7, 722–727. [Google Scholar] [CrossRef]
- Manikandan, A.; Antony, S.A. A novel approach for the synthesis and characterization studies of Mn2+ -Doped CdS nano-crystals by a facile microwave-assisted combustion method. J. Supercond. Nov. Magn. 2014, 27, 2725–2733. [Google Scholar] [CrossRef]
- Renuka Devee, D.; Sivanesan, T.; Muthukrishnan, R.M.; Pourkodee, D.; Mohammed Yusuf Ansari, P.; Abdul Kader, S.M.; Raniani, R.A. A novel photocatalytic activity of Bi2S3 nanoparticles for pharmaceutical and organic pollution removal in water remediation. Chem. Phys. Impact 2024, 8, 100605. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Nkwe, V.M. Morphological variations in Bi2S3 nanoparticles synthesized by using single source precursor. Heliyon 2020, 6, e04505. [Google Scholar] [CrossRef]
- Razavi, F.S.; Mahdi, M.A.; Ghanbari, D.; Dawi, E.A.; Abed, M.J.; Ganduh, S.H.; Jasmin, L.S.; Salavati-Niasari, M. Fabrication and design of four-component Bi2S3/CuFe2O4/Cu2O nanocomposite as new active materials for high performance electrochemical hydrogen storage application. J. Energy Storage 2024, 94, 113493. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Z.; Lien, S.Y.; Yu, Y.; Gao, P. Self-powered thermoelectric hydrogen sensors based on low-cost bismuth sulphide thin films: Quick response at room temperature. ACS Appl. Mater. Interfaces 2022, 14, 47696–47705. [Google Scholar] [CrossRef]
- Kan, H.; Yang, W.; Guo, Z.; Li, M. Highly sensitive room-temperature NO2 gas sensor based on Bi2S3 nanorods. J. Mater. Sci. Mater. Electron. 2024, 35, 331. [Google Scholar] [CrossRef]
- Rong, P.; Gao, S.; Ren, S.; Lu, H.; Yan, J.; Li, L.; Zhang, M.; Han, Y.; Jiao, S.; Wang, J. Large-area freestanding Bi2S3 nanofibrous membranes for fast photoresponse flexible IR imaging photodetector. Adv. Funct. Mater. 2023, 33, 2300159. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, P.; Verma, A.; Yadav, B.C. Interfacial engineering enables polyaniline-decorated bismuth sulphide nanorods towards ultrafast metal-semiconductor-metal UV-Vis broad spectra photodetector. Adv. Compos. Hybrid Mater. 2024, 7, 88. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Tang, Y.; Lu, Z.; Hu, J.; Wang, Y.; Cao, Y. Oxygen self-doping Bi2S3@C spheric successfully enhanced long-term performance in lithium-ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 52423–52431. [Google Scholar] [CrossRef] [PubMed]
- Terdalkar, P.; Kumbhar, D.D.; Pawar, S.D.; Nirmal, K.A.; Kim, T.G.; Mukherjee, S.; Khot, K.V.; Dongale, T.D. Revealing switching statistics and artificial synaptic properties of Bi2S3 memristor. Solid State Electron. 2025, 225, 109076. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, Y.; Huang, Q.; Huang, J.; Kuang, J.; Gu, R.; Zeng, P.; Li, H.-Y.; Liang, H.; Liu, H. Electrochemical Biosensor Employing Bi2S3 Nanocrystals-Modified Electrode for Bladder Cancer Biomarker Detection. Chemosensors 2022, 10, 48. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Zhang, J.; Liu, J.; Yu, X. Application of bismuth sulphide based nanomaterials in cancer diagnosis and treatment. Nano Today 2023, 49, 101799. [Google Scholar] [CrossRef]
- Liang, K.; Wang, C. Capacitive and photocatalytic performance of Bi2S3 nanostructures synthesized by solvothermal method. Phys. Lett. A 2017, 38, 652–657. [Google Scholar] [CrossRef]
- Supekar, A.T.; Bhujbal, P.K.; Salunke, S.A.; Rathod, S.M.; Patole, S.P.; Pathan, H.M. Bismuth sulphide and antimony sulphide-based solar cells: A review. ES Energy Environ. 2023, 19, 848. [Google Scholar] [CrossRef]
- Yu, X.L.; Cao, C.B. Photoresponse and field-emission properties of bismuth sulphide nanoflowers. Cryst. Growth Des. 2008, 8, 3951–3955. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, C.H.; Wang, R.H.; Chen, Q.; Peng, L.M. High-quality ultralong Bi2S3 nanowires: Structure, growth, and properties. J. Phys. Chem. 2005, 109, 18772–18776. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Bismuth sulphide based compounds: Properties, synthesis and applications. Results Chem. 2021, 3, 100151. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Oyewo, O.A.; Atamtürk, U.; Ojelere, O.; Mathur, S. Photocatalytic reduction of Cr(VI) using star-shaped Bi2S3 obtained from microwave irradiation of bismuth complex. J. Environ. Chem. Eng. 2020, 8, 103816. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, D.J.; Wang, R.Z.; Feng, J.J.; Bai, Z.; Wang, A.J. D-penicillanime assited hydrothermal synthesis of Bi2S3 nanoflowers and their electrochemical application. Mater. Sci. Eng. 2013, 33, 3980–3985. [Google Scholar] [CrossRef]
- Linhart, W.M.; Zelewski, S.J.; Scharoch, P.; Dybała, F.; Kudrawiec, R. Nesting-like band gap in bismuth sulphide Bi2S3. J. Mater. Chem. C 2021, 9, 13733–13738. [Google Scholar] [CrossRef]
- Ran, Y.; Song, Y.; Jia, X.; Gu, P.; Cheng, Z.; Zhu, Y.; Wang, Q.; Pan, Y.; Li, Y.; Gao, Y.; et al. Large-Scale Vertically Interconnected Complementary Field-Effect Transistors Based on Thermal Evaporation. Small 2024, 20, 2309953. [Google Scholar] [CrossRef] [PubMed]
- Atamturk, U.; Jung, E.; Fischer, T.; Mathur, S. Tale of Two Bismuth Alkylthiolate Precursors’ Bifurcating Paths in Chemical Vapor Deposition. Chem. Mater. 2022, 34, 7344–7356. [Google Scholar] [CrossRef]
- Tezcan, F. A new synthesis route of Bi2S3 with solvothermal deposition in photoelectrochemical hydrogen production. J. Mol. Struct. 2024, 1301, 137418. [Google Scholar] [CrossRef]
- Parra-Arciniega, S.M.; Garcia-Gomez, N.A.; Garza-Toyar, L.L.; García-Gutiérrez, D.I.; Sánchez, E.M. Ultrasonic irradiation-assisted synthesis of Bi2S3 nanoparticles in aqueous ionic liquid at ambient condition. Ultrason. Sonochemistry 2017, 36, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rosales, K.; Cruz-Gomez, J.; Cruz, J.S.; Guillen-Cervantes, A.; de Moure-Flores, F.; Villagran-Muniz, M. Plasma emission spectroscopy for studying Bi2S3 produced by pulsed laser deposition and effects of substrate temperature on structural, morphological, and optical properties of thin films. Mater. Sci. Eng. B 2025, 312, 117867. [Google Scholar] [CrossRef]
- Bouachri, M.; Oubakalla, M.; El Farri, H.; Diaz-Guerra, C.; Mhalla, J.; Zimou, J.; El-Habib, A.; Beraich, M.; Nouneh, K.; Fahoume, M.; et al. Substrate temperature effects on the structural, morphological and optical properties of Bi2S3 thin films deposited by spray pyrolysis: An experimental and first-principles study. Opt. Mater. 2023, 135, 113215. [Google Scholar] [CrossRef]
- Grubač, Z.; Metikoš-Huković, M. Electrodeposition of thin sulphide films: Nucleation and growth observed for Bi2S3. Thin Solid Films 2002, 412, 248–256. [Google Scholar] [CrossRef]
- Castillo-Carillo, A.; Rivas-Valles, B.G.; Castillo, S.J.; Ramirez, M.M.; Luque-Morales, P.A. New formulation to synthesize semiconductor Bi2S3 thin films using chemical bath deposition for optoelectronic applications. Chem. Sym. 2022, 14, 2487. [Google Scholar] [CrossRef]
- Raut, S.S.; Dhobale, J.A.; Sankapal, B.R. SILAR deposited Bi2S3 thin film toward electrochemical supercapacitor. Phys. E 2017, 87, 209–212. [Google Scholar] [CrossRef]
- Selmane, N.; Eddine, H. Effect of complexing agent on the structural properties of bismuth sulphide Bi2S3 thins films prepared by chemical bath deposition technique. Rev. Sci. Sci. L’ingénieur 2010, 1, 22–26. [Google Scholar]
- Fazal, T.; Iqbal, S.; Shah, M.; Ismail, B.; Shaheen, N.; Alharthi, A.I.; Awwad, N.S.; Ibrahium, H.A. Correlation between structural, morphological and optical properties of Bi2S3 thin films deposited by various aqueous and non-aqueous chemical bath deposition methods. Results Phys. 2022, 40, 105817. [Google Scholar] [CrossRef]
- Alaburdaitė, R.; Krylova, V. Polypropylene film surface modification for improving its hydrophilicity for innovative applications. Polym. Degrad. Stab. 2023, 211, 110334. [Google Scholar] [CrossRef]
- Nasr, M.; Viter, R.; Eid, C.; Warmont, F.; Habchi, R.; Miele, P.; Bechelany, M. Synthesis of novel ZnO/ZnAl2O4 multi co-centric nanotubes and their long-term stability in photocatalytic application. RSC Adv. 2016, 6, 103692–103699. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Asrafali, S.P.; Periyasamy, T.; Kim, S.C. Hydrophilic Nature of Polytetrafluoroethylene through Modification with Perfluorosulfonic Acid-Based Polymers. Sustainability 2023, 15, 16479. [Google Scholar] [CrossRef]
- Pugmire, D.L.; Wetteland, C.J.; Duncan, W.S.; Lakis, R.E.; Schwartz, D.S. Cross-linking of polytetrafluoroethylene during room-temperature irradiation. Polym. Degrad. Stab. 2009, 94, 1533–1541. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew. Chem. Int. Ed. 2011, 50, 5913–5917. [Google Scholar] [CrossRef]
- Krylova, V.; Dukštienė, N.; Lelis, M.; Tučkutė, S. PES/PVC textile surface modification by thermo-chemical treatment for improving its hydrophilicity. Surf. Interfaces 2021, 25, 101184. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Q.; Zhuang, X.; Zhang, S.; Duan, C.; Wang, X.; Cheng, B. Hot-pressed wet-laid polyethylene terephthalate nonwoven as support for separation membranes. Polymers 2019, 11, 1547. [Google Scholar] [CrossRef]
- Kormunda, M.; Pavlik, J. Characterization of oxygen and argon ion flux interaction with PET surfaces by in-situ XPS and ex-situ FTIR. Polym. Degrad. Stab. 2010, 95, 1783–1788. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Mao, N.; Sun, R.; Xu, J. Fabrication of magnetized polyester fabric grafted with β-cyclodextrin for controlled release of menthol. J. Ind. Text. 2016, 47, 1060–1082. [Google Scholar] [CrossRef]
- Moros, J.; Cassella, R.J.; Barciela-Alonso, M.C.; Moreda-Pineiro, A.; Herbello-Hermelo, P.; Bermejo-Barrera, P. Estuarine sediment quality assesment by Fourier-transform infrared spectroscopy. Vib. Spectrosc. 2010, 53, 204–213. [Google Scholar] [CrossRef]
- Mina, M.S.; Kabir, H.; Rahman, M.M.; Kabir, M.A.; Rahaman, M.; Bashar, M.S.; Islam, M.S.; Sharmin, A.; Ahmed, F. Optical and Morphological Characterization of BaSe Thin Films Synthesized via Chemical Bath Deposition. IOSR J. Appl. Phys. 2013, 4, 30–35. [Google Scholar] [CrossRef]
- Si, J.; Ma, R.; Wu, Y.; Dong, Y. Microstructure and magnetic properties of novel powder cores composed of iron-based amorphous alloy and PTFE. J. Mater. Sci. 2022, 57, 8154–8166. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Yu, H.; Zhao, D.; Dong, S.; Zhou, Q. Quantitative analysis of naturally colored cotton and white cotton blends by UVvis diffuse reflectance spectroscopy. J. Appl. Spect. 2015, 81, 949–955. [Google Scholar] [CrossRef]
- Essaidia, H.; Gantassi, A.; Touihria, S.; Ouerfelli, J. Tuning the structural, optical and electrical properties of AgInSe2 thin films prepared by sequentially deposited silver and indium nano-films under vacuum. Optik 2019, 182, 866–875. [Google Scholar] [CrossRef]
- Rincón, M.E.; Campos, J.; Suárez, R. A comparison of the various thermal treatments of chemically deposited bismuth sulfide thin films and the effect on the structural and electrical properties. J. Phys. Chem. Solids 1999, 60, 385–392. [Google Scholar] [CrossRef]
- Gao, C.; Shen, H.; Sun, L.; Shen, Z. Chemical bath deposition of Bi2S3 films by a novel deposition system. Appl. Surf. Sci. 2011, 257, 7529–7533. [Google Scholar] [CrossRef]
- Li, Z.; Si, G.; Ning, Z.; Liu, J.; Fang, Y.; Si, B.; Cheng, Z.; Yang, C. Highly Sensitive Sphere-Tube Coupled Photoacoustic Cell Suitable for Detection of a Variety of Trace Gases: NO2 as an Example. Sensors 2022, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Maji, S.K.; Mitra, K.; Sarkar, A.; Saha, N.; Ghosh, A.B.; Adhikary, B. Single source precursor approach to the synthesis of Bi2S3 nanoparticles: A new amperometric hydrogen peroxide biosensor. Sens. Actuators B Chem. 2024, 192, 578–585. [Google Scholar] [CrossRef]
- Achwal, W.B. Sun protection properties of textile substrates. Colorage 1997, 44, 31–32. [Google Scholar]
- Abdel-Fattah, E.; Alharthi, A.I.; Fahmy, T. Spectroscopic, optical and thermal characterization of polyvinyl chloride-based plasma-functionalized MWCNTs composite thin films. Appl. Phys. A. 2019, 125, 475. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Hou, X. Preparation of CaCO3-TiO2 composite particles and their pigment properties. Materials 2018, 11, 1131. [Google Scholar] [CrossRef]
- da Silva, D.J.; Escote, M.T.; Cruz, S.A.; Simião, D.F.; Zenatti, A.; Curvello, M.S. Polycarbonate/TiO2 nanofibers nanocomposite: Preparation and properties. Polym. Compos. 2018, 39, 780–790. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Abidi, N. Cellulose Dissolution in Ionic Liquid under Mild Conditions: Effect of Hydrolysis and Temperature. Fibers 2021, 9, 5. [Google Scholar] [CrossRef]
- Riahi, M.; Martinez-Tomas, C.; Agouram, S.; Boukhachem, A.; Maghraoui-Meherzi, H. The effects of thermal treatment on structural morphological and optical properties of electrochemically deposited Bi2S3 thin films. Thin Solid Films 2017, 626, 9–16. [Google Scholar] [CrossRef]
- Sonawane, P.S.; Patil, L.A. Effect of nonstoichiometry on structural and optical properties of nanostructured Bi2S3 thin films prepared chemically at room temperature. Mater. Chem. Phys. 2007, 105, 157–161. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.; Pinto, R.J.; Trindade, T.; Neto, C.P. Surface modification of cellulosic fibres for multipurpose TiO2 based nanocomposites. Compos. Sci. Technol. 2009, 69, 7–8. [Google Scholar] [CrossRef]
- Bai, C.; Yuan, X.; Cao, Y.; Hao, Z.; Pan, L.; Peng, J.; Liu, N.; Chen, S. Perdurable antimicrobial and ultraviolet-blocking cotton fabric by one-step eco-friendly strategy. Ind. Crop. Prod. 2025, 223, 120038. [Google Scholar] [CrossRef]
- Das, B.R. UV Radiation Protective Clothing. The Op. Text. J. 2010, 3, 14–21. [Google Scholar]
- Foadi, F.; Etminan, M.; Aghamir, F.M.; Mohammadizadeh, M.R. Role of surface morphological parameters on wettability of obliquely deposited Cu thin films in a plasma focus device. J. Mater. Res. 2023, 38, 3666–3676. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Reichenbach, J.; Klaas, W. Frustration vs Prenucleation: Understanding the Suprising Stability of Supersaturated Sodium Thiosulfate Solutions. J. Phys. Chem. B 2018, 122, 7590–7596. [Google Scholar] [CrossRef]
- Steudel, R. Homocyclic Sulfur Molecules. Top. Curr. Chem. 1982, 102, 149–176. [Google Scholar] [CrossRef]
- Fleck, N.; Amli, H.; Dhanak, V.; Ahmed, W. Characterization Techniques in Energy Generation and Storage, Emerging Nanotechnologies for Renewable Energy. In Micro and Nano Technologies; Ahmed, W., Booth, M., Nourafkan, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 11; pp. 259–285. ISBN 9780128213469. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, Y.-K. Monoclinic sulfur cathode utilizing carbon for high-performance lithium-sulfur batteries. J. Power Sources 2016, 325, 495–500. [Google Scholar] [CrossRef]
- Moon, S.; Jung, Y.H.; Jung, W.K.; Jung, D.S.; Choi, J.W.; Kim, D.K. Encapsulated Monoclinic Sulfur for Stable Cycling of Li–S Rechargeable Batteries. Adv. Mater. 2013, 25, 6547–6553. [Google Scholar] [CrossRef]





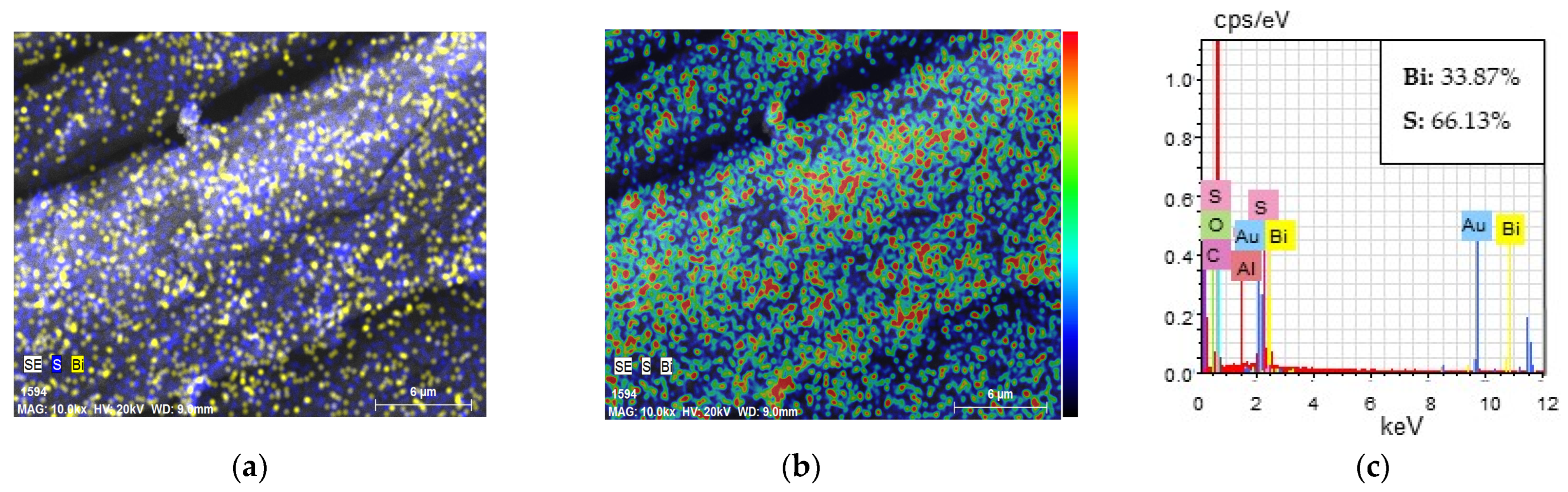
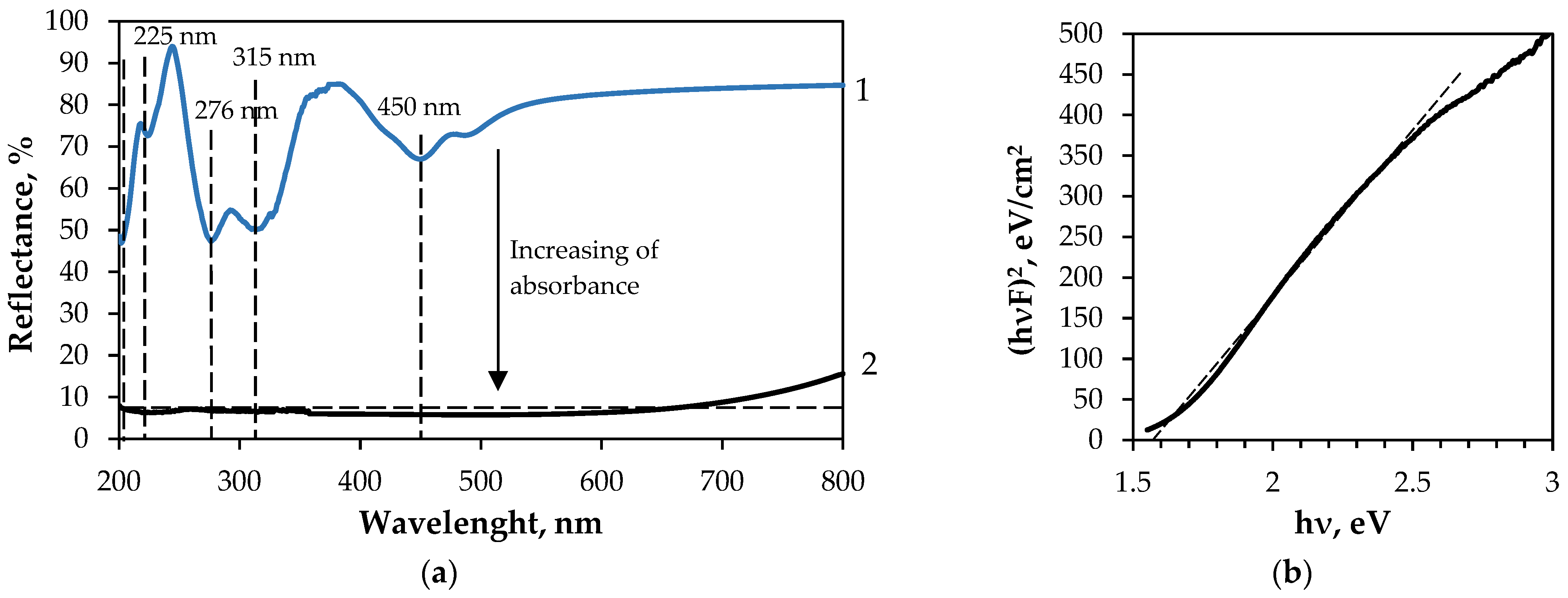
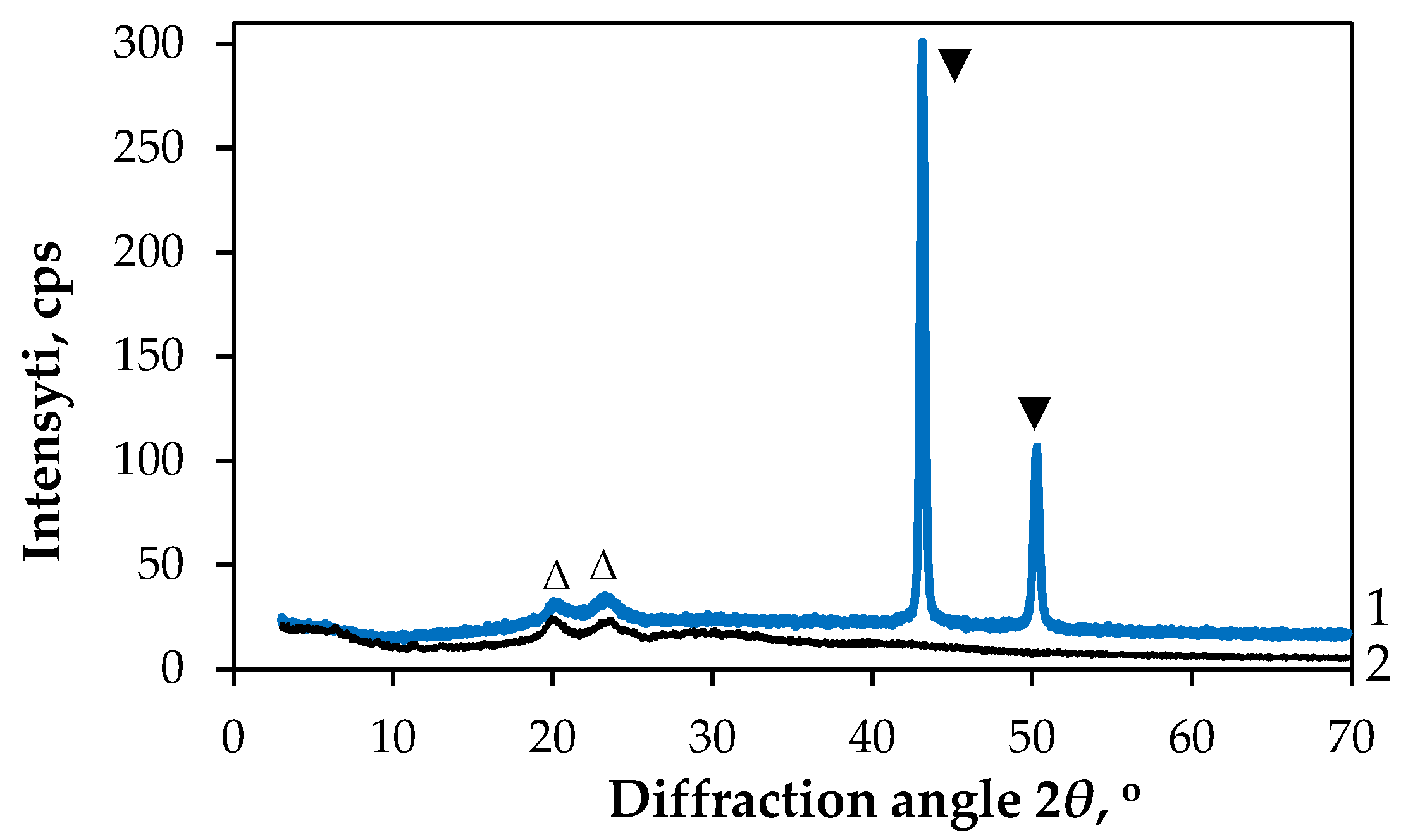

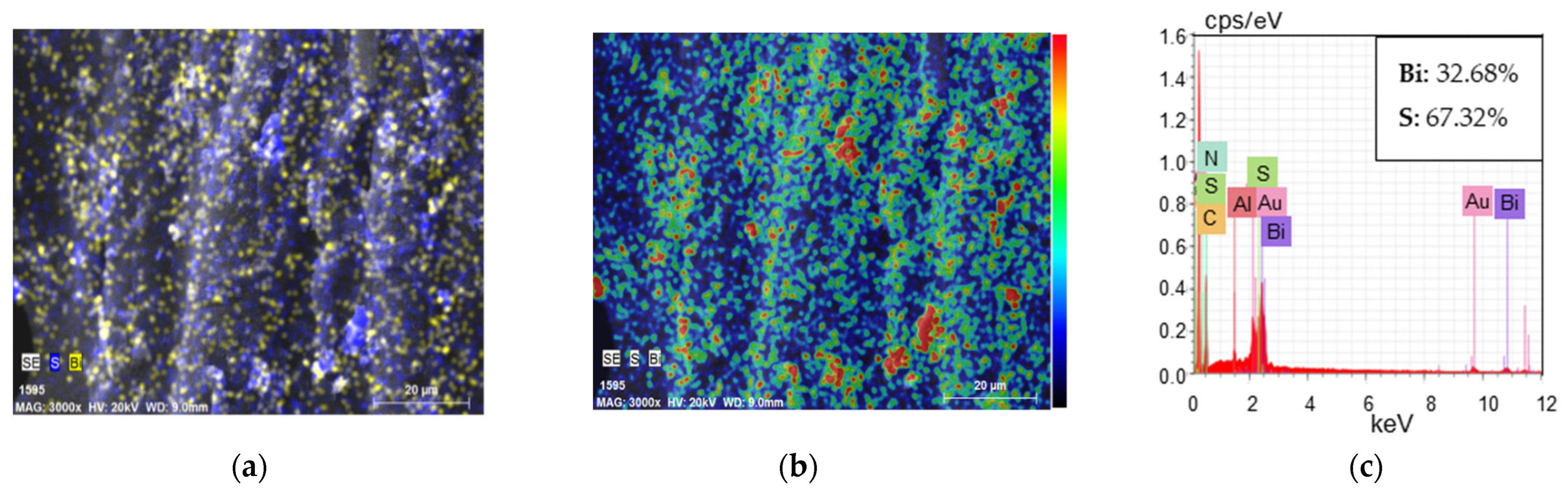

| Textile | GF/PTFE | PET/PVC | C/PES/S | PA/Cu | PA/Ag |
|---|---|---|---|---|---|
| Photographs |  |  |  |  |  |
| Composition | PTFE-coated fibreglass | PET yarn, coating PVC, filler 1 | 75% cotton (C), 24% polyester (PES), 1% spandex (S) 2 | 100% nylon (PA), metallisation copper (Cu) | 100% nylon (PA), metallisation silver (Ag) |
| Thickness, mm | 0.58 | 0.56 | 0.35 | 0.05 | 0.10 |
| Basis weight, g/m2 | 1100 | 850 | 290 | 52 | 34 |
| Heat resistance, ℃ | 260 | 70 | 80 | 30 | 30 |
| Cold resistance, ℃ | −73 | −30 | −30 | −30 | −30 |
| Solution Composition and Concentration, M | Sample | Etching Conditions | |
|---|---|---|---|
| Temperature, °C | Duration, h | ||
| H2SO4, 7.2 H3PO4, 6.6 CrO3, 0.36 | GF/PTFE | 100 ± 1 | 2 |
| PET/PVC | 68 ± 1 | 2 | |
| Composite | Bi/S Ratios 1 | Crystallinity | Eg, eV |
|---|---|---|---|
| GF/PTFE/Bi-S | 0.038 | Polycrystalline orthorhombic Bi2S3 | 1.8 |
| PET/PVC/Bi-S | 0.044 | Polycrystalline orthorhombic Bi2S3, monoclinic sulphur S8 | 1.68 |
| C/PES/S/Bi-S | 0.512 | Orthorhombic Bi2S3 | 1.58 |
| PA/Cu/Bi-S | 0.485 | Amorphous phase or very small crystallites of Bi2S3 | 1.68 |
| PA/Ag/Bi-S | 0.215 | Absence of a defined crystal structure, with only short-range order present | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sruogaite, V.; Krylova, V. Formation and Study of Bismuth Sulphide Thin Films on Textiles of Different Compositions. Appl. Sci. 2025, 15, 9904. https://doi.org/10.3390/app15189904
Sruogaite V, Krylova V. Formation and Study of Bismuth Sulphide Thin Films on Textiles of Different Compositions. Applied Sciences. 2025; 15(18):9904. https://doi.org/10.3390/app15189904
Chicago/Turabian StyleSruogaite, Veja, and Valentina Krylova. 2025. "Formation and Study of Bismuth Sulphide Thin Films on Textiles of Different Compositions" Applied Sciences 15, no. 18: 9904. https://doi.org/10.3390/app15189904
APA StyleSruogaite, V., & Krylova, V. (2025). Formation and Study of Bismuth Sulphide Thin Films on Textiles of Different Compositions. Applied Sciences, 15(18), 9904. https://doi.org/10.3390/app15189904







