Quantification of Caffeic Acid as Well as Antioxidant and Cytotoxic Activities of Ucuuba (Virola surinamensis) Co-Product Extract to Obtain New Functional and Nutraceutical Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents and Standards
2.2. Obtainment and Treatment of the Ucuuba Co-Product
2.3. Proximate Composition and Water Activity of the Co-Product
2.4. Extract Preparation
2.5. Characterization of the Ucuuba Extract and Co-Product
2.5.1. Thermal Behavior (TG/DTG)
2.5.2. Fourier-Transform Infrared (FTIR) Spectroscopy Profiles
2.5.3. Total Polyphenol Content of the Extract
2.5.4. Total Flavonoid Content of the Extract
2.5.5. Quantification of Majority Compounds in the Extract by High-Performance Liquid Chromatography (HPLC)
2.6. Validation of the Caffeic Acid Quantification Method
2.7. Antioxidant Activity of the Extract
2.7.1. Capture of the ABTS+ Radical Cation
2.7.2. Capture of the DPPH• Radical
2.8. In Vitro Cytotoxicity Assay
2.8.1. Obtaining and Cultivating Cells
2.8.2. Cell Viability
2.9. Statistical Analysis
3. Results
3.1. Proximate Composition, Moisture Content, and Water Activity of the Co-Product
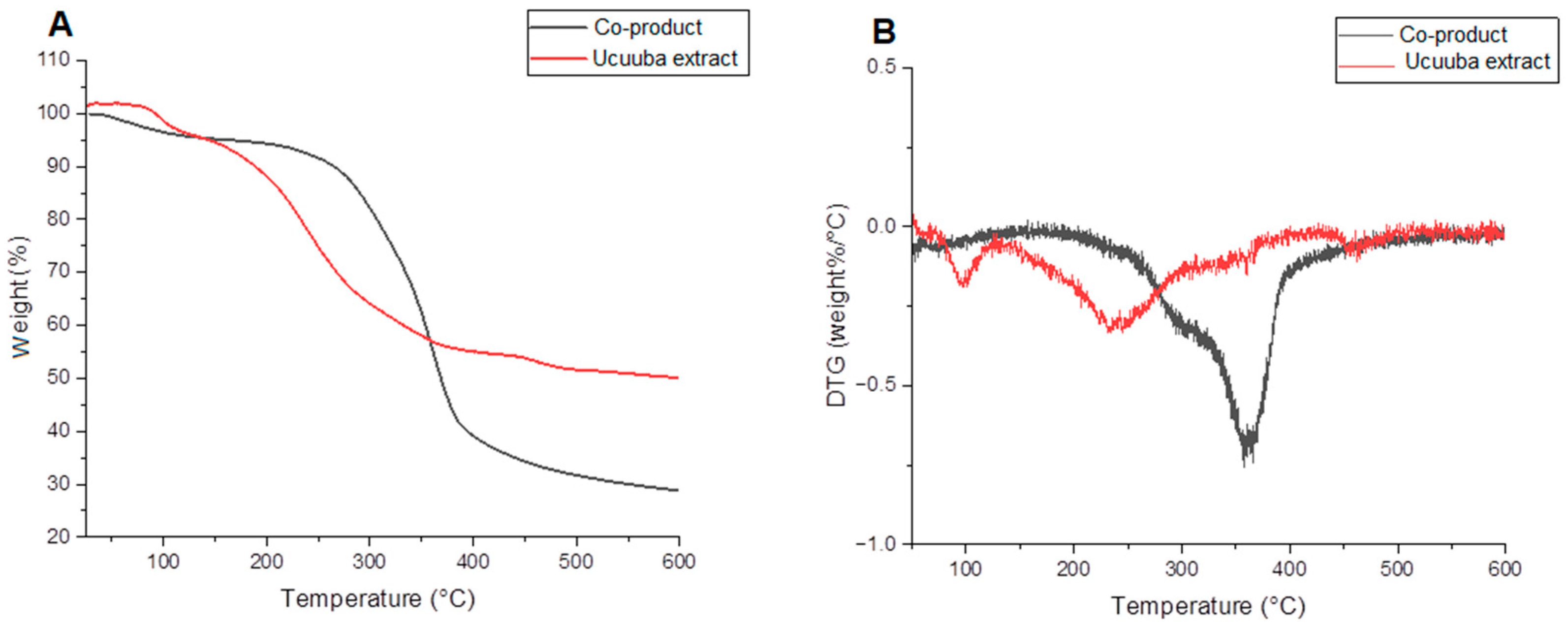
3.2. Thermogravimetric Analysis (TG/DTG) of the Ucuuba Co-Product and Its Extract
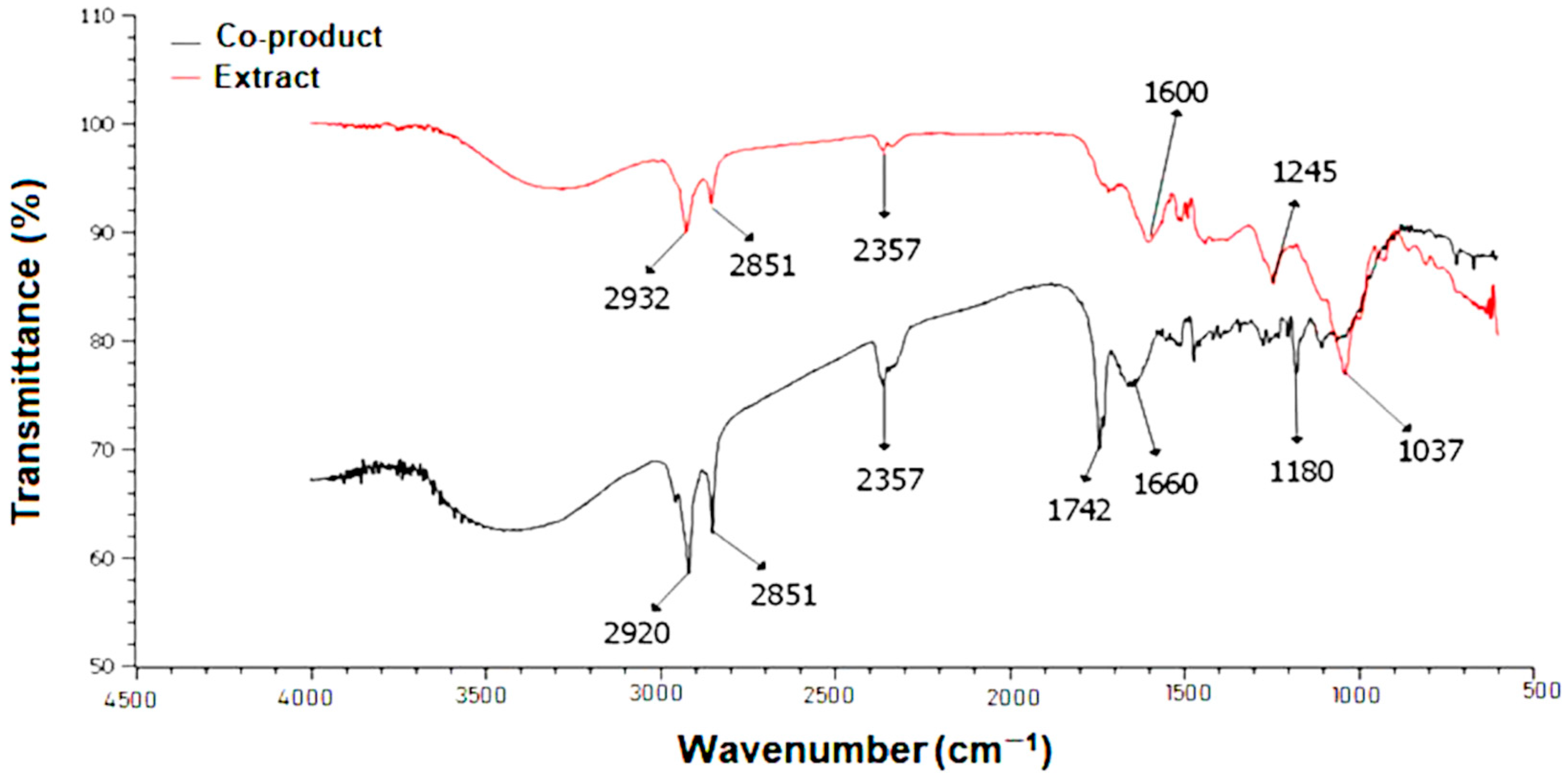
3.3. Fourier-Transform Infrared (FTIR) Spectroscopic Profile of Ucuuba Co-Product and Its Extract
3.4. Total Polyphenol and Total Flavonoid Contents of the Extract
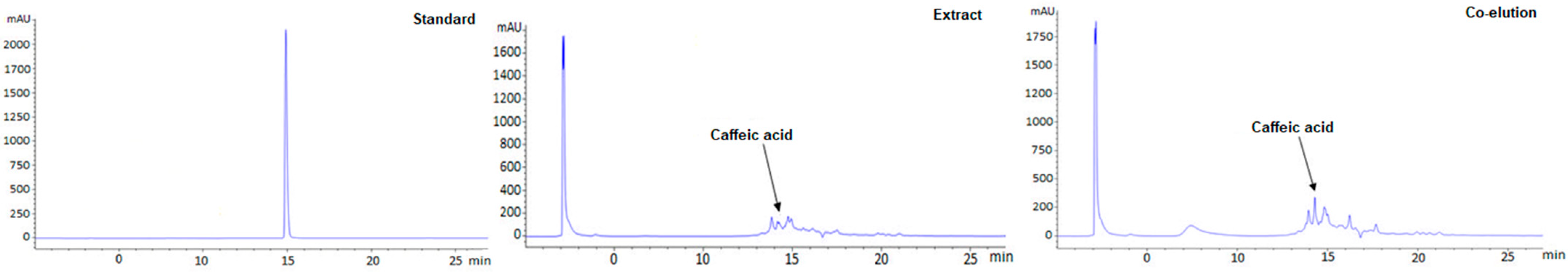
3.5. Quantification of Caffeic Acid in the Extract by HPLC
3.6. Total Antioxidant Activity of the Extract
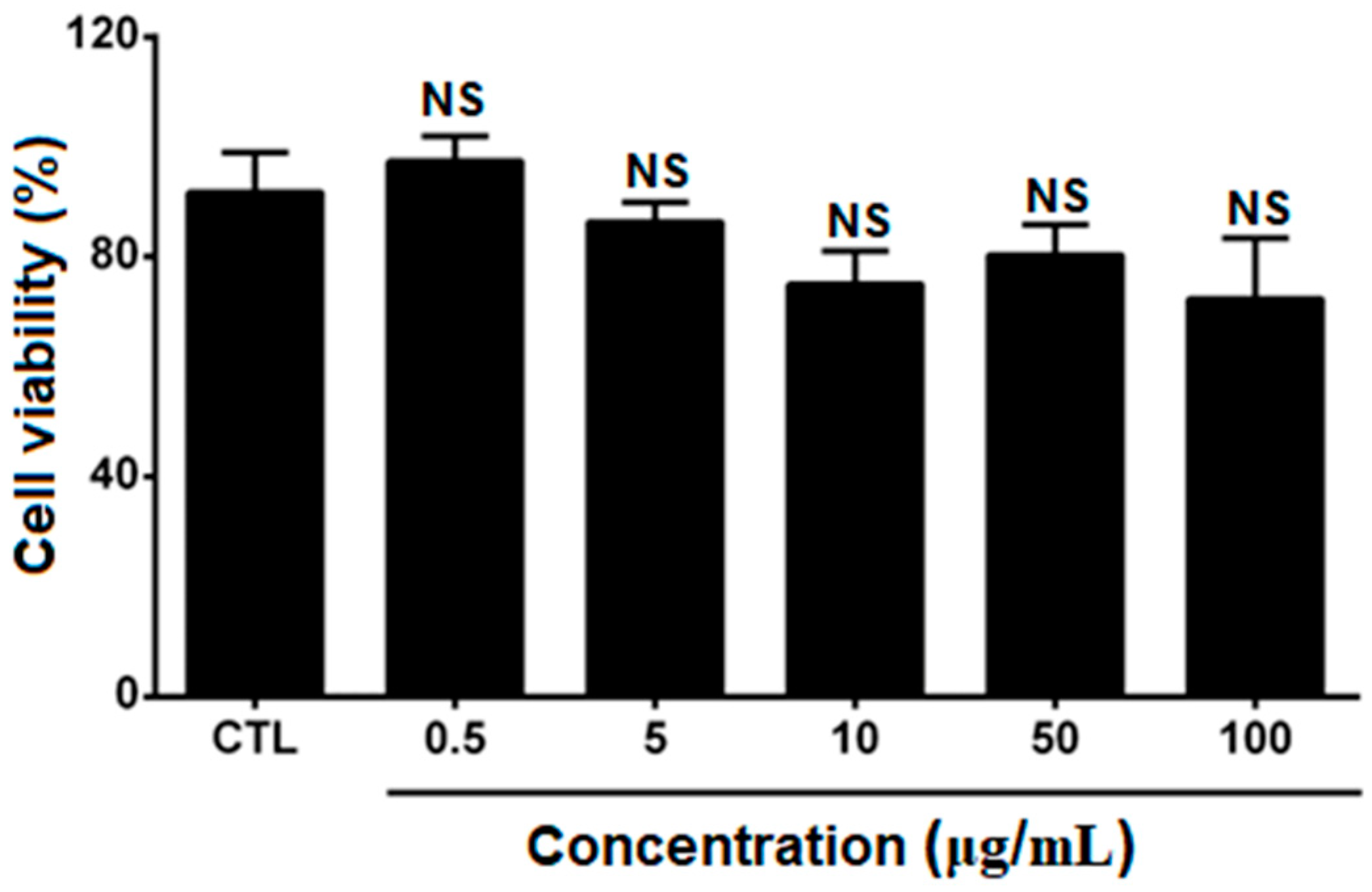
3.7. In Vitro Cytotoxicity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morais, L.R.B.; Gutjahr, E.; Gutjahr, E. Chemistry of Vegetable Oils: Valorization of the Amazon Biodiversity, 1st ed.; Brazilian Book Chamber: Belém, Brazil, 2011; pp. 68–69. [Google Scholar]
- Pereira, R.R.; Testi, M.; Rossi, F.; Silva Junior, J.O.C.; Ribeiro-Costa, R.M.; Bettini, R.; Santi, P.; Padula, C.; Sonvico, F. Ucuuba (Virola surinamensis) fat-based nanostructured lipid carriers for nail drug delivery of ketoconazole: Development and optimization using Box-Behnken design. Pharmaceutics 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet activity of natural bioactive extracts from mango (Mangifera indica L.) and its by-products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Sato, H.H. Synergistic effects of agroindustrial wastes on simultaneous production of protease and α-amylase under solid state fermentation using a simplex centroid mixture design. Ind. Crops Prod. 2013, 49, 813–821. [Google Scholar] [CrossRef]
- Wenger, J.; Pichler, S.; Näyhä, A.; Stern, T. Practitioners’ perceptions of co-product allocation methods in biorefinery development—A case study of the austrian pulp and paper industry. Sustainability 2022, 14, 2619. [Google Scholar] [CrossRef]
- Weng, Z.; Guo, S.; Qian, D.; Zhu, Z.; Zhang, S.; Li, A.; Lei, Z.; Duan, J. Sophora flavescens seed as a promising high potential by-product: Phytochemical characterization and bioactivity evaluation. Ind. Crops Prod. 2017, 109, 19–26. [Google Scholar] [CrossRef]
- Romo Sánchez, S.; Gil Sánchez, I.; Arévalo-Villena, M.; Briones Pérez, A. Production and immobilization of enzymes by solid-state fermentation of agroindustrial waste. Bioprocess. Biosyst. Eng. 2015, 38, 587–593. [Google Scholar] [CrossRef]
- Lima, S.K.R.; Durazzo, A.; Lucarini, M.; de Oliveira, J.J.A.; da Silva, R.A.; Arcanjo, D.D.R. Valorization of fruit co-product flours for human nutrition: Challenges, applications, and perspectives. Sustainability 2023, 15, 13665. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and biofunctional properties with emphasis on recent trends and advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar] [CrossRef]
- Milani, L.P.G.; Garcia, N.O.S.; Morais, M.C.; Dias, A.L.S.; Oliveira, N.L.; Conceição, E.C. Extract from byproduct Psidium guajava standardized in ellagic acid: Additivation of the in vitro photoprotective efficacy of a cosmetic formulation. Braz. J. Pharm. 2018, 28, 692–696. [Google Scholar] [CrossRef]
- Abdalla, A.E.M.; Darwish, S.M.; Ayad, E.H.E.; El-Hamahmy, R.M. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem. 2007, 103, 1134–1140. [Google Scholar] [CrossRef]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef]
- Mokhtarpour, A. Extraction of phenolic compounds and tannins from pistachio by-products. Annu. Res. Rev. Biol. 2014, 4, 1330–1338. [Google Scholar] [CrossRef]
- Rao, J.; Decker, E.A.; Xiao, H.; McClements, D.J. Nutraceutical nanoemulsions: Influence of carrier oil composition (digestible versus indigestible oil) on β-carotene bioavailability. J. Sci. Food Agric. 2013, 93, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Peixoto Araujo, N.M.; Arruda, H.S.; Marques, D.R.P.; de Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and nutritional properties of selected amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [PubMed]
- de Meneses Costa Ferreira, L.M.; Pereira, R.R.; de Carvalho-Guimaraes, F.B.; do Nascimento Remigio, M.S.; Ramos Barbosa, W.L.; Ribeiro-Costa, R.M.; Carrera Silva-Junior, J.O. Microencapsulation by spray drying and antioxidant activity of phenolic compounds from tucuma coproduct (Astrocaryum vulgare Mart.) almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef]
- Alves, T.V.G.; da Costa, R.S.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Arruda, M.S.P.; Silva-Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat. Prod. Res. 2019, 33, 589–592. [Google Scholar] [CrossRef]
- da Costa, R.S.; Teixeira, C.B.; Gabbay Alves, T.V.; Ribeiro-Costa, R.M.; Casazza, A.A.; Aliakbarian, B.; Converti, A.; Silva Júnior, J.O.C.; Perego, P. Optimization of spray drying conditions to microencapsulate cupuassu (Theobroma grandiflorum) seed by-product extract. Nat. Prod. Res. 2018, 33, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.L.; Ferreira, L.M.; Silva-Júnior, J.O.; Converti, A.; Ribeiro-Costa, R.M. Co-product of pracaxi seeds: Quantification of epicatechin by HPLC-DAD and microencapsulation of the extract by spray drying. Processes 2024, 12, 997. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos, 1st ed.; Instituto Adolfo Lutz: São Paulo, SP, Brazil, 2008; p. 1020.
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Ministério da Saúde; Agência Nacional de Vigilância Sanitária—Anvisa. Farmacopeia Brasileira, v. 1, 6th ed. 2019. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/farmacopeia-brasileira (accessed on 2 July 2024).
- Ferreira, L.M.; Souza, P.D.; Pereira, R.R.; da Silva, E.O.; Barbosa, W.L.; Silva-Júnior, J.O.; Converti, A.; Ribeiro-Costa, R.M. Preliminary study on the chemical and biological properties of propolis extract from stingless bees from the northern region of Brazil. Processes 2024, 12, 700. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Sampaio, F.C.; de Faria, J.T.; Pitangui, C.G.; Lovaglio, F.; Casazza, A.A.; Converti, A.; Perego, P. Optimization of spray drying microencapsulation of olive pomace polyphenols using response surface methodology and artificial neural. LWT-Food Sci. Technol. 2018, 93, 220–228. [Google Scholar] [CrossRef]
- Bioanalytical Method Validation Guidance for Industry. Biopharmaceutics. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 10 October 2024).
- Li, J.E.; Fan, S.T.; Qiu, Z.H.; Li, C.; Nie, S.P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT-Food Sci. Technol. 2015, 64, 1022–1027. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- ANVISA (National Health Surveillance Agency). Resolution RE 166. Validation of Analytical Methods; ANVISA (National Health Surveillance Agency): Brasilia, Brazil, 2017. Available online: https://antigo.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401 (accessed on 20 July 2024).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- ICH–Harmonised Tripartite Guidance. Validation of Analytical Procedures: Text and Methodology Q2(R1). In International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 30 August 2024).
- Mirnaghi, F.S.; Goryński, K.; Rodriguez-Lafuente, A.; Boyacı, E.; Bojko, B.; Pawliszyn, J. Microextraction versus exhaustive extraction approaches for simultaneous analysis of compounds in wide range of polarity. J. Chromatogr. 2013, 1316, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.P.; de Píccolo, M.B.F.; de Ribeiro, J.P.A.; Ferreira, E.B.; Ciabotti, S. Characterization of food bars manufactured with agroindustrial by-products and waste. Food Sci. Technol. 2012, 36, 333–340. [Google Scholar] [CrossRef][Green Version]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-industrial fruit byproducts as health-promoting ingredients used to supplement baked food products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit wastes as a valuable source of value-added compounds: A collaborative perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Marcal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- Mikropoulou, E.V.; Petrakis, E.A.; Argyropoulou, A.; Mitakou, S.; Halabalaki, M.; Skaltsounis, L.A. Quantification of bioactive lignans in sesame seeds using HPTLC densitometry: Comparative evaluation by HPLC-PDA. Food Chem. 2019, 288, 1–7. [Google Scholar] [CrossRef]
- Lopes, N.P.; De Almeida Blumenthal, E.E.; Cavalheiro, A.J.; Katot, J.; Yosh1da, M. Lignans, y-lactones and propiophenones of Virola surinamensis. Phytochemistry 1996, 43, 1089–1092. [Google Scholar] [CrossRef]
- Pereira, E.; Ferreira, M.C.; Sampaio, K.A.; Grimaldi, R.; de Almeida Meirelles, A.J.; Maximo, G.J. Physical properties of Amazonian fats and oils and their blends. Food Chem. 2019, 278, 208–215. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca Cechin, C.; Carvalho, G.G.; Kabuki, D.Y. Occurrence, genetic characterization, and antibiotic susceptibility of Cronobacter spp. isolated from low water activity functional foods in Brazil. Food Microbiol. 2024, 122, 104570. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT-Food Sci. Technol. 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Soria, A.C.; Villamiel, M.; Montilla, A. Quality parameters in convective dehydrated carrots blanched by ultrasound and conventional treatment. Food Chem. 2013, 141, 616–624. [Google Scholar] [CrossRef]
- Sandulachi, E. Water activity concept and its role in food preservation. Meridian Ing. 2012, 4, 40–48. [Google Scholar]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the beneficial effects of phenolic compounds: A review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Ghonime, M.; Emara, M.; Shawky, R.; Soliman, H.; El-Domany, R.; Abdelaziz, A. Immunomodulation of RAW 264.7 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunol. Invest. 2015, 44, 237–252. [Google Scholar] [CrossRef] [PubMed]
| Proximate Composition | Mean Value ± SD |
|---|---|
| Fibers | 41.42 ± 0.73% |
| Lipids | 15.54 ± 0.36% |
| Proteins | 11.07 ± 0.19% |
| Carbohydrates * | 20.61% |
| Ash | 4.66 ± 0.02% |
| Moisture | 6.73 ± 0.05% |
| Water activity | 0.403 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Pereira, R.R.; Oliveira, K.d.A.; Converti, A.; da Silva, E.O.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Quantification of Caffeic Acid as Well as Antioxidant and Cytotoxic Activities of Ucuuba (Virola surinamensis) Co-Product Extract to Obtain New Functional and Nutraceutical Foods. Appl. Sci. 2025, 15, 10291. https://doi.org/10.3390/app151810291
Ferreira LMdMC, Pereira RR, Oliveira KdA, Converti A, da Silva EO, Silva-Júnior JOC, Ribeiro-Costa RM. Quantification of Caffeic Acid as Well as Antioxidant and Cytotoxic Activities of Ucuuba (Virola surinamensis) Co-Product Extract to Obtain New Functional and Nutraceutical Foods. Applied Sciences. 2025; 15(18):10291. https://doi.org/10.3390/app151810291
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Rayanne Rocha Pereira, Kalene de Almeida Oliveira, Attilio Converti, Edilene Oliveira da Silva, José Otávio Carréra Silva-Júnior, and Roseane Maria Ribeiro-Costa. 2025. "Quantification of Caffeic Acid as Well as Antioxidant and Cytotoxic Activities of Ucuuba (Virola surinamensis) Co-Product Extract to Obtain New Functional and Nutraceutical Foods" Applied Sciences 15, no. 18: 10291. https://doi.org/10.3390/app151810291
APA StyleFerreira, L. M. d. M. C., Pereira, R. R., Oliveira, K. d. A., Converti, A., da Silva, E. O., Silva-Júnior, J. O. C., & Ribeiro-Costa, R. M. (2025). Quantification of Caffeic Acid as Well as Antioxidant and Cytotoxic Activities of Ucuuba (Virola surinamensis) Co-Product Extract to Obtain New Functional and Nutraceutical Foods. Applied Sciences, 15(18), 10291. https://doi.org/10.3390/app151810291







