Abstract
Droplet-based microfluidics has rapidly advanced applications in chemistry, biology, materials science, medicine, food science, and cosmetics. Using this technology, various oils have been employed for fluid encapsulation. This study is the first to investigate the use of an animal-based unsaturated fatty acid oil—emu oil—for microdroplet formation. We characterized droplet generation in the presence and absence of a non-fluorinated surfactant at a defined concentration and examined the influence of geometrical parameters using T-junction microchannels with two different central channel widths. The results were compared with those obtained from a plant-based oil (olive oil) under parallel experimental conditions. Given the growing concerns regarding the environmental and health risks of fluorocarbon oils combined with fluorinated surfactants, which are widely used in microfluidics, emu oil represents a potentially safer alternative for microdroplet-based technologies across multiple fields.
1. Introduction
In microfluidic techniques, two fluid phases are directed into the junction of microchannels where, at the point of contact—and when interfacial forces are properly balanced—microscale droplets are generated. Droplet-based microfluidic system-associated devices have rapidly found applications in controlling chemical reactions, regulating mass transport in biological studies, and enabling drug screening, among others [1,2,3,4,5,6].
Microfluidics technology can generally be categorized into two main areas: fluid-handling platforms (such as continuous-flow or droplet-based systems) and fabrication methods that enable the creation of these platforms. Among the fabrication methods, soft lithography using poly (dimethyl siloxane) (PDMS) is the most widely used, particularly in studies involving biological samples [7,8,9]. Within fluid-handling platforms, this study focuses on flow-focusing techniques, a broadly used method in microfluidics devices. Flow-focusing enables droplet formation in liquid–liquid systems, when droplet size is determined by the flow rates of each liquid and the flow rate ratio between the two employed liquids [10,11,12].
The two immiscible media, referred to as the continuous and dispersed phases, are the fundamental components of droplet-based microfluidics. At the junction, these phases may flow in parallel, or, under specific experimental conditions, one phase may encapsulate the other to form droplets [13]. The resulting microdroplets function as biochemical reactors for analyzing mixtures of materials and are widely used in single-cell and molecular analysis [14,15,16,17].
Among the most critical parameters in droplet formation and size are the flow rates of the two phases and the geometry of the microchannels in which droplets are produced [18]. One of the earliest microchannel designs was the T-junction, where the two phases meet perpendicularly and droplet generation occurs as one phase shears the other [19,20,21,22]. Additional factors that contribute to droplet formation and stabilization include the viscosity of the phases, which affects the shear rate, and the surface tension, which can be changed using surfactants [23,24].
Numerous studies have examined the relationship between droplet formation and different oils, including plant-based, mineral, silicone, and fluorinated oils [25,26,27,28,29,30,31]. However, research on microdroplet encapsulation using animal-derived oils is still limited. Among these oils, fluorinated types are considered favorable candidates for microdroplet formation in industries such as healthcare, food, and cosmetics due to their biocompatibility and high stability [29,32,33,34].
Animal-derived oils, typically composed of fatty acids, are often solid at room temperature. Some, such as fish oil, remain liquid but are prone to oxidation, which reduces their stability. Emu oil, however, is notable for its stability at room temperature and its biocompatibility. Its additional anti-inflammatory and antioxidant properties further enhance its potential for microdroplet applications [35,36].
Given growing concerns about the health and environmental risks associated with perfluoroalkyl substances (PFASs) [37,38], fatty acid-based oils may be promising alternatives for developing a new generation of non-fluorinated oils suitable for microdroplet production.
In this study, motivated by the need to identify effective yet safer oils for microdroplet formation, we investigate and report the formation and characterization of water-in-oil microdroplets in a microfluidic-focusing chip using emu oil and compare it with olive oil.
The following sections first describe the materials and methods used for microdroplet formation. We then present the characteristics observed with emu oil as an example of animal-derived oil and olive oil as an example of a plant-derived oil. The results are then discussed, followed by the conclusion.
2. Materials and Methods
2.1. Fluid Phases
The focus of this study is on the formation of water-in-oil microdroplets. To this point two phases considered: water as the dispersed phase and the two types of oils as the continuous phase.
Deionized water was used for the dispersed phase. To make a distinction between the gas-in oil droplet produced in the system and the water-in-oil droplets during the visualization under the microscope, 0.5% v/v black dye was added to the water. The density and dynamic viscosity of dispersed phase were measured to be 986 kg/m3 and 0.001 Pa·s, respectively (Rheometric Scientific-ARES, Rheometric Scientific, Inc., Piscataway, NJ, USA).
For the continuous phase we employed Emu oil (commercially purchased from Botanical Beauty—Purchased through Amazon Inc., Seattle, WA, USA). We used the pure oils first and then added 0.5% span 80 in separate and parallel experiments to identify the effects of non-fluorinated surfactants and the role of surface chemistry in droplet formation.
Emu oil was initially partially unsaturated at room temperature. To extract the unsaturated portion of the oil, it was first centrifuged (Denville, 240 Microcentrifuge, Denville Scientific, Inc., Metuchen, NJ, USA) for 5 min, after which the liquid phase was pipetted out and filtered using (Syringe Cellulose Acetate, Sigma-Aldrich Corporation, St. Louis, MI, USA—0.45 µm). The resulting transparent, fully liquid extract was used as the continuous phase for droplet generation at room temperature of 25 °C. Since all experiments were consistently conducted at this temperature, investigating the potential solidification of the processed oil under varying temperature was beyond the scope of this study while the effects of the temperature on microdroplet formation with this oil are currently being investigated by our research group.
The viscosity of the processed oil was measured to be 0.058 Pa·s (Rheometric Scientific-ARES- Rheometric Scientific, Inc., Piscataway, NJ, USA) with a density of 980 kg/m3. Olive oil (commercially purchased from a local grocery store) had measured viscosity of 0.0745 Pa·s. and the value of the surface tension, , was 0.033 N/m which was also used in parallel experiments in this study.
In this study, two syringe pumps (InfusionONE, New Era Pump Systems, Inc., Farmingdale, New York, NY, USA) were used to introduce the fluids inside the channels of the chip and to control the flow rates of fluid phases as they were connected to the micro channels in the microfluidic chip.
2.2. Microfluidic Chip
A T-junction microchannel chip was created for droplet generation in this microfluidic experiment. The microfluidic 285 chip (microfluidics ChipShop GmbH, Jena, Germany) was employed for this purpose. This chip has several designed channels [39]. We specifically used channel D and C on this microchip. The two micro-channels are similar except that the central channel of the microchannel “D” is wider in length than the corresponding one in microchannels “C”. The central microchannel was considered for the continuous fluid phase (oil inlet), while the first cross flowing microchannel was used for the disperse fluid phase (water inlet). In this chip, the second cross flowing channel was blocked, as its presence was negligible for the objectives of this study. The design of the microchannel is shown in Figure 1.
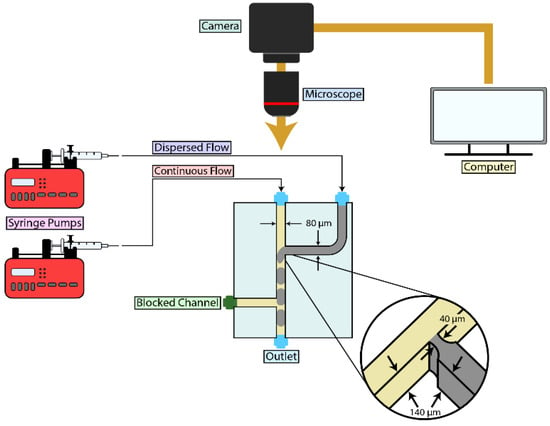
Figure 1.
Schematic Overview of the Experimental Setup for Microdroplet Generation Using a Syringe Pump: The experimental setup for generating microdroplets involved a syringe pump connected to the main inlet of a T-junction chip to inject oil. A second syringe was connected to the side inlet to inject water. The T-junction chip featured a channel width of 0.08 mm for both the central and side inlets, with a channel depth of 0.140 mm. The nozzle at the junction had a channel diameter of 0.04 mm. Droplet formation was monitored and recorded using a high-speed camera.
2.3. Visualization and Analysis
Fluids were introduced into the microfluidic chip (microfluidics ChipShop 285, Jena, Germany) via Teflon microtubes with an internal diameter of 0.5 mm (microfluidics ChipShop, Jena, Germany). The chip was mounted on the stage of an upright brightfield microscope (AmScope Microscopes, Irvine, CA, USA) and observed using a 4× objective lens. A high-speed camera (FLIR-Teledyne Imaging—with frame rate of 55 fps, Wilsonville, OR, USA) attached to the microscope was employed to capture fluid behavior and droplet generation. The droplet formation process was recorded using video microscopy with SpinView software [Computer software, version 4.2.0.88, Teledyne DALSA Inc., Waterloo, ON, Canada).
The recorded videos were analyzed using ImageJ software (version number 1.54p, Rasband, W.S., ImageJ, National Institutes of Health, Bethesda, MD, USA). As the droplets formed in the squeezed regime (with diameters smaller than the microchannel width), their lengths of droplets were measured post-formation. Data were collected from a large number of droplets following the complete breakup and formation of each droplet. The mean ± standard deviation (SD) was calculated, and a t-test was performed for statistical analysis. A significance p-value threshold of 0.05 was applied for the statistical comparisons.
3. Results
We conducted experiments on microdroplet formation using partially saturated animal-based fatty acid oil, specifically Emu oil and compared the results with those obtained using plant-based oil, olive oil. The experiments were performed in a T-junction microchannel. The central channel carried the continuous fluid phase (Emu oil or olive oil) while the side channel carried the dispersed phase (water). Both channels had a width of 0.08 mm and a depth of 0.14 mm. To study the geometrical effects on droplet generation, the experiment was repeated using another microchannel of similar design but with the wider central channel of 0.5 mm. The nozzle of the dispersed-phase microchannel at the junction had a diameter of 0.04 mm. A schematic view of the experimental setup is shown in Figure 1.
The experiment was repeated with the addition of 0.5% v/v Span 80, a non-fluorinated surfactant, to the continuous phase (oil). In this study, water-in-oil (W/O) droplets were generated. The focus was on evaluating the characteristics of jetting regime as well as droplet formation in the droplet-generation regime. Key factors investigated included the relationship between flow conditions and droplet size, the effect of surfactants and surface chemistry on droplet formation, and the geometry of the microchannel [40].
To evaluate the experimental conditions under which microdroplets can be generated as well as the relation between droplet size and flow rates, the flow rate of the dispersed phase was held constant while the flow rate of the continuous phase was varied. Data was collected after the system reached equilibrium.
In experiments using Emu oil as the continuous phase, the droplet generated regime was observed when the flow rate of the dispersed phase was 900 µL/h and the flew rate of the continuous phase was set to be 30 µL/h. As the flow rate of the continuous phase increased from 30 to 200 µL/h, the size of the droplets generated in the microchannel decreased. The measured size of the droplets in different flow rates is expressed in Table 1A and the trend of droplet size versus the flow rate ratio (Q = Qc/Qd) is shown in Figure 2A.

Table 1.
Droplet Formation Parameters.
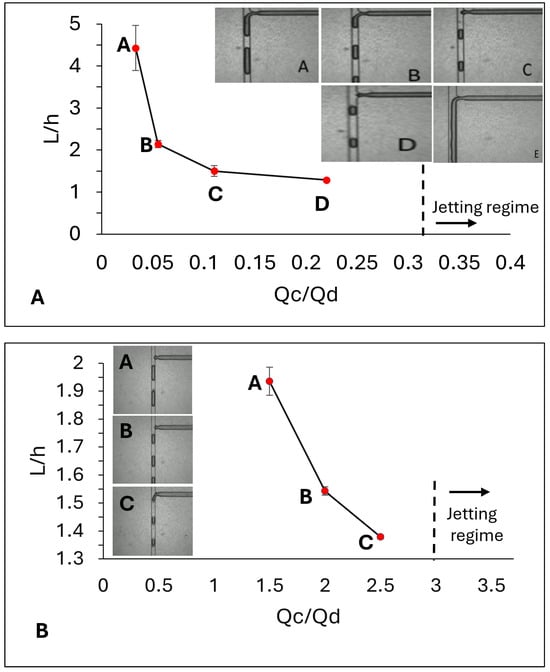
Figure 2.
The relationship between droplet size, normalized by channel depth, and the flow rate ratio in a microchannel with a central channel width of 0.08 mm. Droplet size decreases for both Emu oil (A) and Olive oil (B) as the flow rate ratio increases (the value of the ratio is expressed in Table 1). During the experiment, the flow rate of the dispersed phase was maintained constant, while the flow rate of the continuous phase was increased. At a flow rate ratio of
= 0.33 the jetting regime was observed for the Emu oil. This threshold for the Olive oil was observed to be = 3. In above figures, error bars represent +/− standard deviation from average measurement. The inset images show snapshots of the squeezed droplets within the central channel after their formation. In each droplet, the darker margin corresponds to the oil phase, while the encapsulated colored water appears in a lighter shade than the surrounding margin.
The flow rates of the continuous and dispersed phases in a T-junction microchannel with a central channel width of 0.08 mm are presented above. The differences in the mean droplet sizes corresponding to different flow rate sets were statistically analyzed using the t-test method. The calculated p-values for each set of comparisons were less than 0.0001, indicating that the differences between the averages were statistically significant. The other droplet size measurements with significantly higher p-value have not been included in the table below. For example, for the Emu Oil, when the flow rate of the continuous oil was set to be 10 mL/h and the flow rate of the dispersed phase was 900 mu/h (Qc/Qd = 0.01), the measured droplet size was 0.581 ± 0.069 (n = 20). In the t-test evaluation, the p-value between this measurement and the next level when the flow rate of the continuous phase was 30 mL/h, was calculated to be 0.03, which was significantly larger than 0.001. Therefore, those rates when the measurement of the droplet size with the next flow rate set were not obviously significantly different from each other were not included in the table below.
Another droplet formation parameter is Capillary number. The Capillary number of the continuous phase which compares the viscous force to capillary force is where is the viscosity of the continuous phase, is the surface tension, and , when is the flow rates of the continuous phase reflected in the table and A is the cross section of the central channel (1.12 × 10−8 m2). For Emu oil = 0.058 Pa·s, is the surface tension of the oleic oil—used for this study as Emu oil is majorly consisting of oleic oil [41]. For Olive oil = 0.0745 Pa·s, . The flow rates at which jetting was observed have also been expressed above.
In this experiment the jetting regime—where two fluid phases flowed adjacent without mixing-was observed at the threshold of Q = 0.33, when the flow rate of dispersed phase and continuous phase were 600 µL/h and 200 µL/h consequently. Below this threshold, the system was in the droplet generating stage and dripping regime.
In parallel experiments using olive oil as the continuous phase, the droplets formed when the flow rate of the dispersed phase was held constant at 200 µL/h, while the continuous phase varied from 300 µL/h to 600 µL/h.
At the threshold of Q = 3, the system transitioned into the jetting regime. The average droplet size ± SD at different flow rates along with other droplet formation parameters including Capillary number, which compares the viscous force to capillary force, and the trend of droplet size versus the flow rate ratio for olive oil are shown in Table 1B and Figure 2B.
The comparison of average microdroplet lengths is presented in the histograms of Figure 3. The difference between the averages was found to be statistically significant.
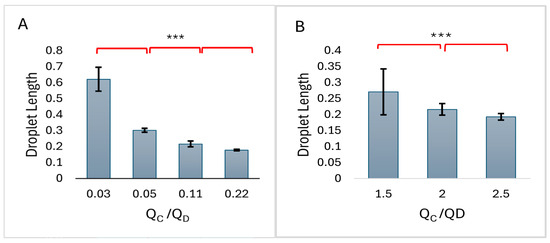
Figure 3.
The average between the droplet lengths for different flow rate ratios for (A) Emu oil, (B) Olive oil. *** represents that the mean values are significantly different as determined by t-test. Droplet lengths are millimeters.
The effect of surfactant was evaluated by adding 0.5% v/v Span 80 to Emu oil and measuring droplet size at certain flow rates. For Emu oil this assessment was conducted both in the present and absence of Span 80 with droplets generated under a dispersed-phase flow rate of 900 µL/h and continuous-phase flow rate of 200 µL/h (Q = 0.22). The average droplet size for pure oil was 0.178 ± 0.005 mm (n = 73), whereas in the presence of Span 80, the size decreased to 0.137 ± 0.016 mm (n = 50). A t-test confirmed that this difference was statistically significant (p < 0.0001).
For olive oil, the effect of Span 80 was evaluated at the dispersed-phase flow rate of 200 µL/h and continues phase flow rate of 300 µL/h (Q = 1.5). In the absence of the surfactant, the average droplet was measured to be 0.271 ± 0.072 mm (n = 100). This value dropped to 0.136 ± 0.006 mm (n = 20). t-test confirmed that this difference was statistically significant (p < 0.0001).
In the final stage, to evaluate the effect of microchannel geometry on droplet formation, the same microfluidic chip (Microfluidics 285, ChipShop) was used, but with a T-junction microchannel with wider continuous-phase inlet (width = 0.5 mm). In this wider channel, the flow rates required to achieve the dripping regime and droplet generation differed significantly from those in the channel with a narrower central inlet.
For Emu oil, the change in microchannel geometry resulted in droplet formation at different flow rates. To make the experiment as comparable as possible to the narrow channel case, the dispersed flow rate was set at 900 µL/h. Under this condition, droplets were generated when the continuous-phase flow rate was 750 µL/h (Q = 0.8), with an average droplet length of 0.584 ± 0.071 mm (n = 21). Notably, under these same flow rates, the system in the narrow channel was not even in the droplet-generation regime.
For olive oil, the flow rates required for droplet formation also differed from those in the narrow channel case. The droplet generated regime at Q = 1.5 occurred when the dispersed-phase flow rate was 800 µL/h and continuous-phase flow rate was 1200 µL/h. Under these conditions, the average droplet size measured to be 0.240 ± 0.012 mm (n = 20) which was not significantly different from the size measured in microchannel with narrow central inlet for this type of oil.
4. Discussion
In this study, we introduce, for the first time, a fatty acid-based oil and method suitable for microfluidics and droplet generation and compare it with a plant-based oil. Emu oil, which contains more than 60% unsaturated fatty acids, including oleic and linoleic acids, offers antioxidant and anti-inflammatory properties along with demonstrated toxicological safety [35,36]. These characteristics make it a promising candidate for microdroplet formation, particularly in applications involving human-related research.
Given the differences in viscosity and surface tension between emu oil and olive oil, we first achieved the experimental conditions for droplet generation using a T-junction microchannel. The size of the droplets was then investigated. Both oils exhibited similar behavior in terms of droplet size when flow rates were systematically varied or when the non-fluorinated surfactant Span 80, at a specific concentration, was added. In addition, both oils showed that droplet formation can be influenced by the geometry of the microchannel.
This study demonstrates the feasibility of utilizing emu oil in droplet-based research and technologies and compares the results with those obtained from a plant-based oil such as olive oil. Furthermore, although fluorosurfactant-stabilized water-in-fluorinated-oil (w/o) droplets have been extensively studied and widely applied across research and industry, growing concerns have emerged regarding the environmental safety and health risks of exposure to perfluoroalkyl and polyfluoroalkyl substances (PFASs). As the demand for safer alternatives increases, emu oil may serve as a reliable candidate for producing microdroplets.
This work provides a point of departure for future investigations into the experimental, numerical, and clinical aspects of using this unsaturated fatty acid-based animal oil. Areas for further study include biocompatibility, droplet stability, the effects of surfactants at different concentrations, and droplet formation in microchannels with different architecture.
Author Contributions
Conceptualization, M.S.F.; methodology, M.S.F.; software, investigation, R.A., A.A. and M.S.F.; resources, M.S.F.; writing—original draft preparation, M.S.F.; writing—review and editing, M.S.F.; visualization, R.A. and A.A.; supervision, project administration, M.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Seton Hall University, College of Arts & Sciences, and Physics Department.
Data Availability Statement
The dataset is available on request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Qingrong Huang from the Department of Food Science at Rutgers University for providing access to his laboratory and the assistance in measuring the viscosity of emu oil. Additionally, we thank Cosimo Antonacci from the Department of Chemistry and Biochemistry at Seton Hall University for his invaluable assistance in filtering the emu oil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitesides, G. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Hang, L.; Cheng, Y.; Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Fedorets, A.A.; Bormashenko, E.; Dombrovsky, L.A.; Nosonovsky, M. Droplet clusters: Nature-inspired biological reactors and aerosols. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20190121. [Google Scholar] [CrossRef]
- Park, S.Y.; Kalim, S.; Callahan, C.; Teitell, M.A.; Chiou, E.P. A light-induced dielectrophoretic droplet manipulation platform. Lab Chip 2009, 9, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.A.; Wu, M.; Ren, W.; Hesselink, L. Expanding medium compatibility with lateral-field optoelectronic tweezers. Appl. Phys. Lett. 2025, 127, 101101. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Wu, M.; Ren, W.; Jensen, M.A.; Davis, R.W.; Hesselink, L. Controlled transport of individual microparticles using dielectrophoresis. Langmuir 2023, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Velve-Casquillas, G.; Le Berre, M.; Piel, M.; Tran, P.T. Microfluidic tools for cell biological research. Nano Today 2010, 5, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, S.; Chen, W.; Zhang, Q.; Chai, Y. Self-assembly of nanoparticles with stimulated responses at liquid interfaces. Nano Today 2024, 54, 102073. [Google Scholar] [CrossRef]
- Saeki, D.; Sugiura, S.; Kanamori, T.; Sato, S.; Ichikawa, S. Microfluidic preparation of water-in-oil-in-water emulsions with an ultra-thin oil phase layer. Lab Chip 2010, 10, 357–362. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Guillot, P.; Colin, A. Stability of parallel flows in a microchannel after a T junction. Phys. Rev. E 2005, 72, 066301. [Google Scholar] [CrossRef]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Zheng, F.Y.; Fu, F.F.; Cheng, Y.; Wang, C.; Zhao, Y.; Gu, Z.Z. Organ-on-a-chip systems: Microengineering to biomimic living systems. Small 2016, 12, 2253–2282. [Google Scholar] [CrossRef]
- Cerdeira, A.T.S.; Campos, J.B.L.M.; Miranda, J.M.; Araújo, J.D.P. Review on microbubbles and microdroplets flowing through microfluidic geometrical elements. Micromachines 2020, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L. Experimental investigation of bubble formation in a microfluidic T-shaped junction. Nanoscale Microscale Thermophys. Eng. 2009, 13, 228–242. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic flow phenomena. J. Phys. D Appl. Phys. 2007, 40, R319. [Google Scholar] [CrossRef]
- Li, X.-B.; Li, F.-C.; Yang, J.-C.; Kinoshita, H.; Oishi, M.; Oshima, M. Study on the mechanism of droplet formation in T-junction microchannel. Chem. Eng. Sci. 2012, 69, 340–351. [Google Scholar] [CrossRef]
- Sivasamy, J.; Wong, T.-N.; Nguyen, N.-T.; Kao, L.T.-H. An investigation on the mechanism of droplet formation in a microfluidic T-junction. Microfluid. Nanofluidics 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Wacker, V.K.; Parashar, M.A.M.; Gijs, M. Influence of oil type and viscosity on droplet size in a flow focusing microfluidic device. Procedia Chem. 2009, 1, 1083–1086. [Google Scholar] [CrossRef]
- Mazutis, L.; Griffiths, A.D. Selective droplet coalescence using microfluidic systems. Lab Chip 2012, 12, 1800–1806. [Google Scholar] [CrossRef]
- Gerecsei, T.; Ungai-Salánki, R.; Saftics, A.; Derényi, I.; Horvath, R.; Szabó, B. Characterization of the dissolution of water microdroplets in oil. Colloids Interfaces 2022, 6, 14. [Google Scholar] [CrossRef]
- Mohamadzade Sani, H.; Falahi, M.; Aieneh, K.; Hosseinalipour, S.M.; Salehi, S.; Asiaei, S. Performance optimization of droplet formation and break up within a microfluidic device—Numerical and experimental evaluation. Int. J. Heat Fluid Flow 2024, 106, 109266. [Google Scholar] [CrossRef]
- Zhu, B.; Du, Z.; Dai, Y.; Kitaguchi, T.; Behrens, S.; Seelig, B. Nanodroplet-based reagent delivery into water-in-fluorinated-oil droplets. Biosensors 2023, 13, 768. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Zheng, W.; Kumari, S.; Heyman, J.; Zhang, X.; Dey, P.; Weitz, D.A.; Haag, R. Dendronized fluorosurfactant for highly stable water-in-fluorinated-oil emulsions. Nat. Commun. 2019, 10, 4546. [Google Scholar] [CrossRef] [PubMed]
- Holtze, C.; Rowat, A.C.; Agresti, J.J.; Hutchison, J.B.; Angilè, F.E.; Schmitz, C.H.J.; Köster, S.; Duan, H.; Humphry, K.J.; Scanga, R.A.; et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 2008, 8, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Scanga, R.; Chrastecka, L.; Mohammad, R.; Meadows, A.; Quan, P.-L.; Brouzes, E. Click-chemistry approaches to expand PEG-based fluorinated surfactants for droplet microfluidics. RSC Adv. 2018, 8, 12960–12974, Erratum in RSC Adv. 2019, 9, 27625–27639. [Google Scholar] [CrossRef]
- Laos, R.; Benner, S. Fluorinated oil–surfactant mixtures with the density of water. PLoS ONE 2022, 17, e0252361. [Google Scholar] [CrossRef]
- DeJournette, C.J.; Kim, J.; Medlen, H.; Li, X.; Vincent, L.J.; Easley, C.J. Creating biocompatible oil-water interfaces without synthesis: Direct interactions between primary amines and carboxylated perfluorocarbon surfactants. Anal. Chem. 2013, 85, 10556–10564. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Chan, H.F.; Phua, K.K.L.; Zhang, Y.; Juul, S.; Knudsen, B.R.; Ho, Y.-P.; Leong, K.W. Synthesis of fluorosurfactants for emulsion-based biological applications. ACS Nano 2014, 8, 3913–3920. [Google Scholar] [CrossRef]
- Prastowo, A.; Feuerborn, A.; Cook, P.R.; Walsh, E.J. Biocompatibility of fluids for multiphase drops-in-drops microfluidics. Biomed. Microdevices 2016, 18, 114. [Google Scholar] [CrossRef]
- Bucław, M.; Majewska, D.; Szczerbińska, D.; Ligocki, M. The influence of age and gender on emu (Dromaius novaehollandiae) fat. Sci. Rep. 2020, 10, 11082. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Li, L.; Luo, S.; Chen, J.; Yi, X.; Zhang, X.; Li, B.; Chen, Z. Chemical characterization and in vivo toxicological safety evaluation of emu oil. Nutrients 2022, 14, 2238. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X. Research progress of perfluoroalkyl substances in edible oil: A review. Foods 2023, 12, 2624. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Our Current Understanding of the Human Health and Environmental Risks of PFAS. 2024. Available online: https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas (accessed on 1 September 2025).
- Microfluidic Chipshop. Droplet Generator Chips. Available online: https://www.microfluidic-chipshop.com/catalogue/microfluidic-chips/polymer-chips/droplet-generator-chips/droplet-generator-chips-multi-channel-design-fluidic-285/ (accessed on 1 September 2025).
- Nunes, J.K.; Tsai, S.S.H.; Wan, J.; Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 2013, 46, 114002. [Google Scholar] [CrossRef] [PubMed]
- Chumpitaz, L.D.D.; Coutinho, L.F.; Meirelles, A.J.A. Surface tension of fatty acids and triglycerides. J. Am. Oil Chem. Soc. 1999, 76, 379–382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).