Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes
Abstract
1. Introduction
2. Connective Tissue Substitutes: Properties and Applications
2.1. Acellular Dermal Matrices
2.2. Collagen Matrices
3. Clinical Applications of CTGS in Peri-Implant Soft Tissue Augmentation
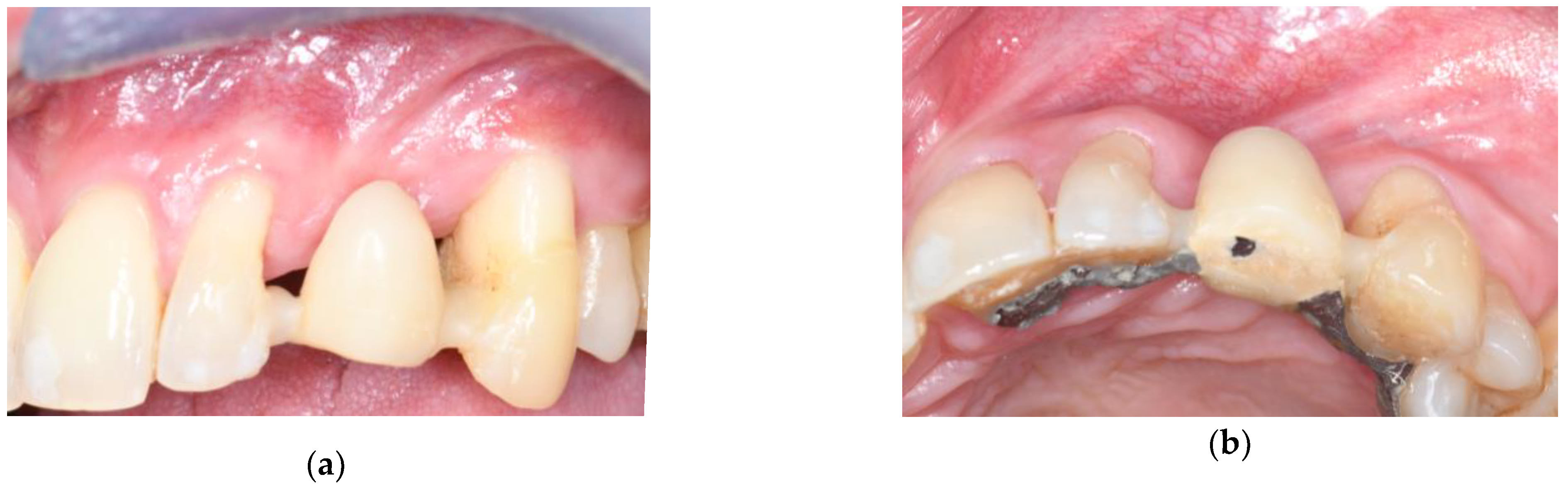
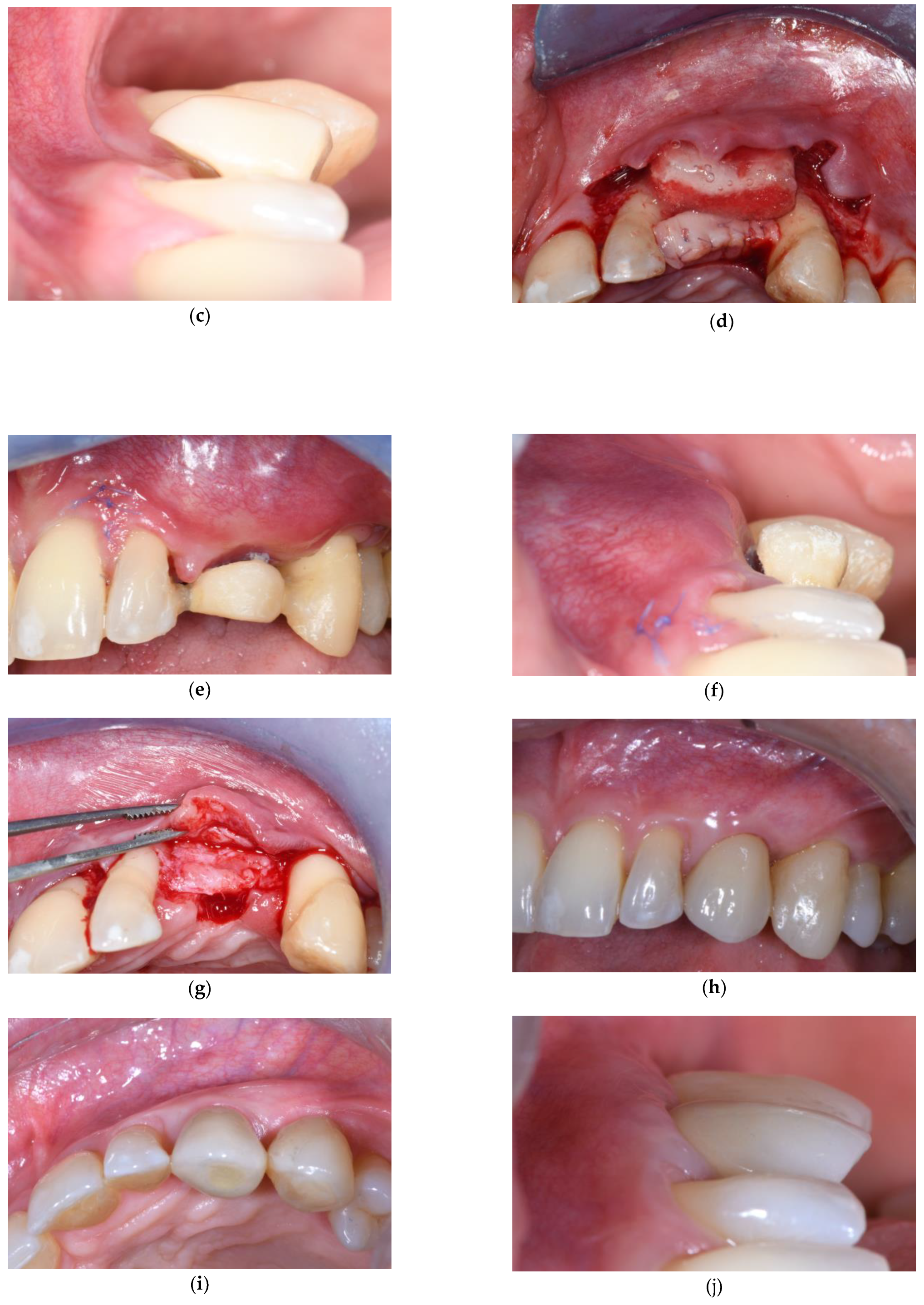
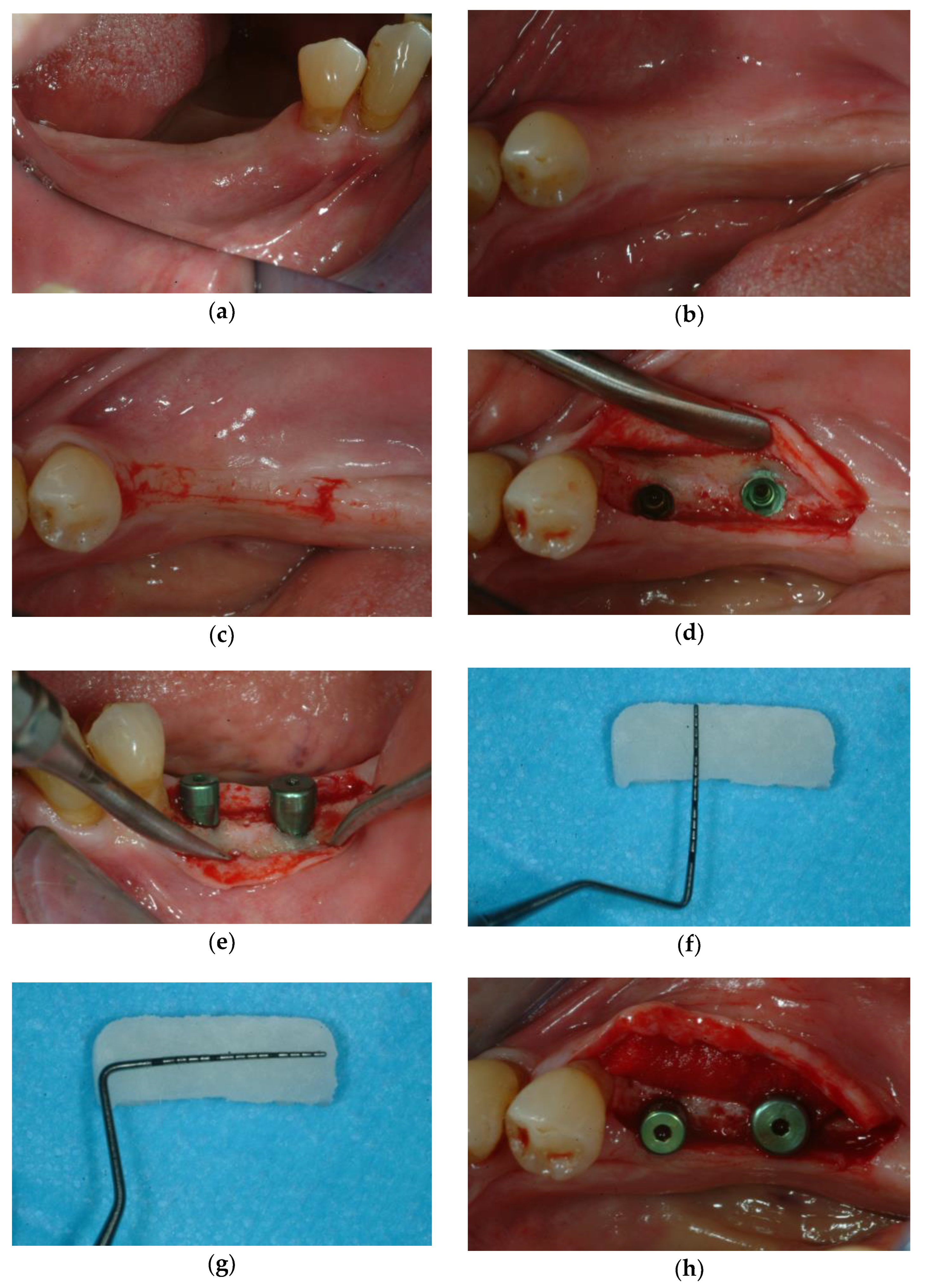
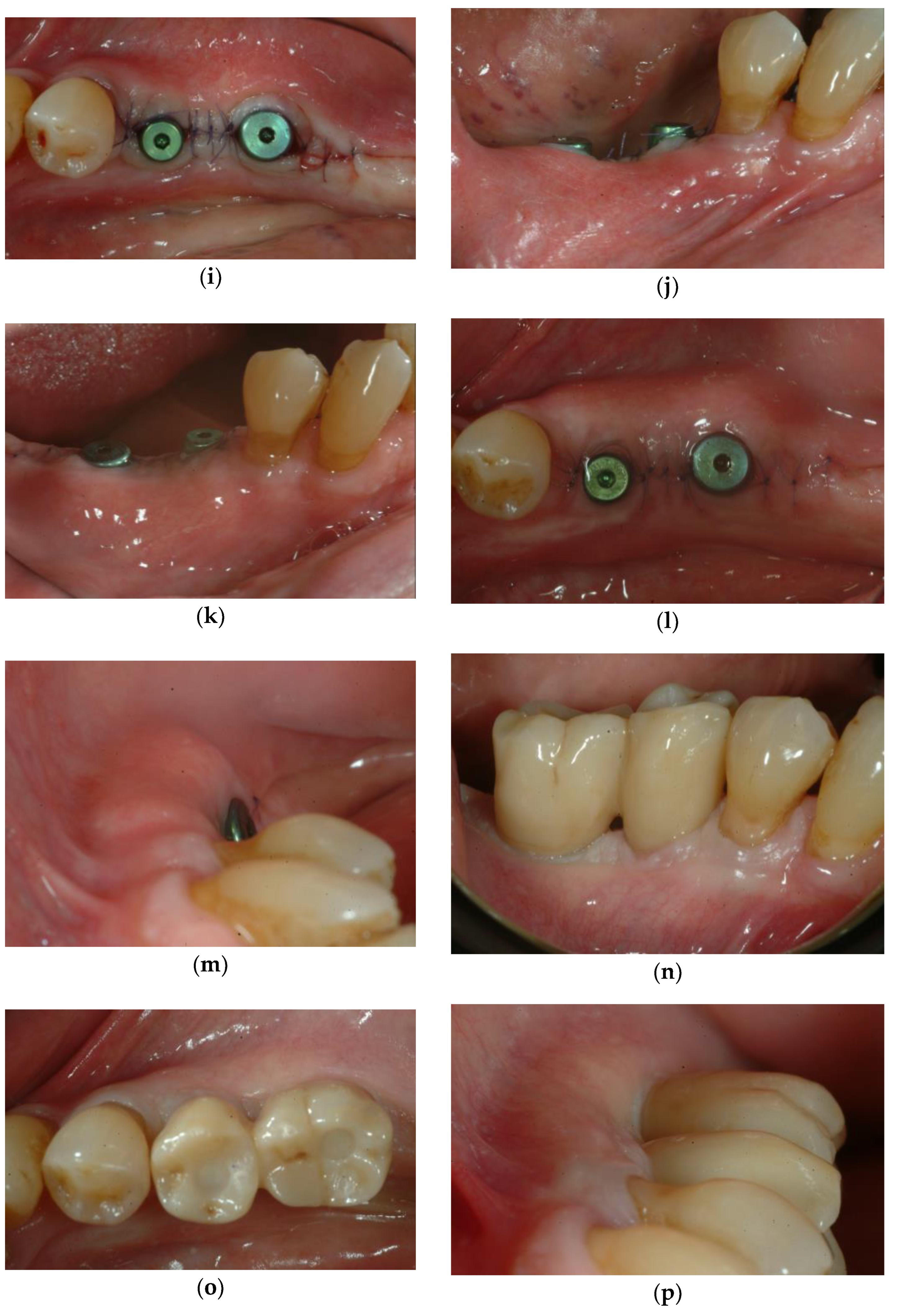
3.1. Soft Tissue Augmentation Before Implant Installation
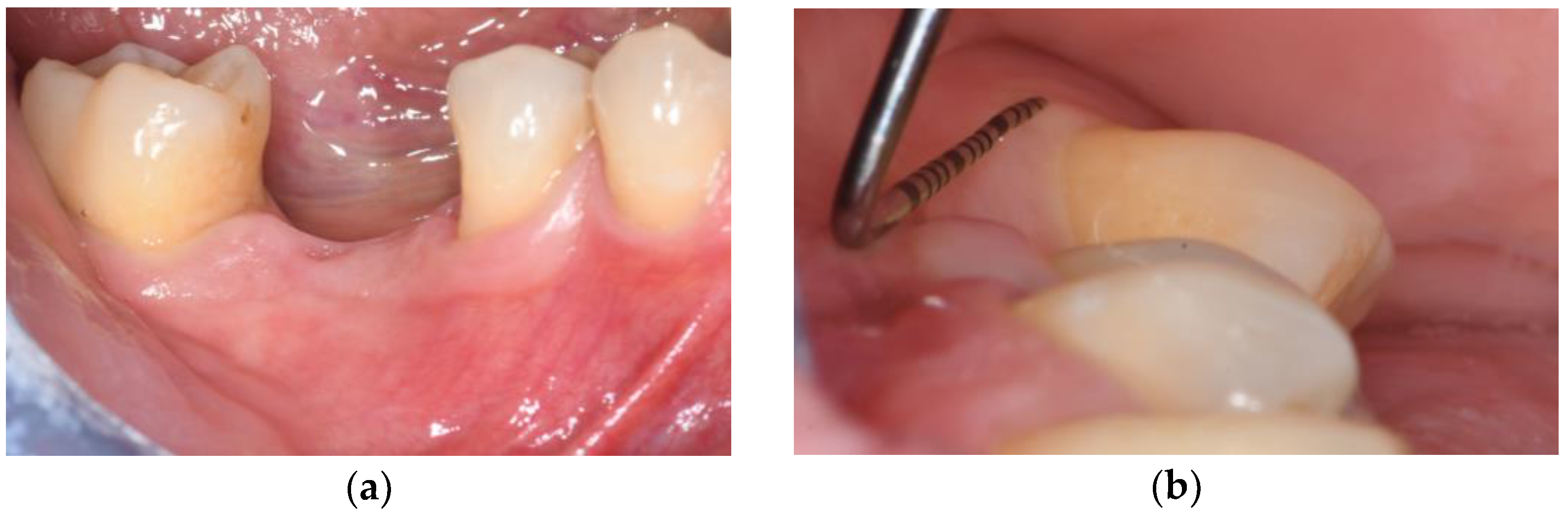
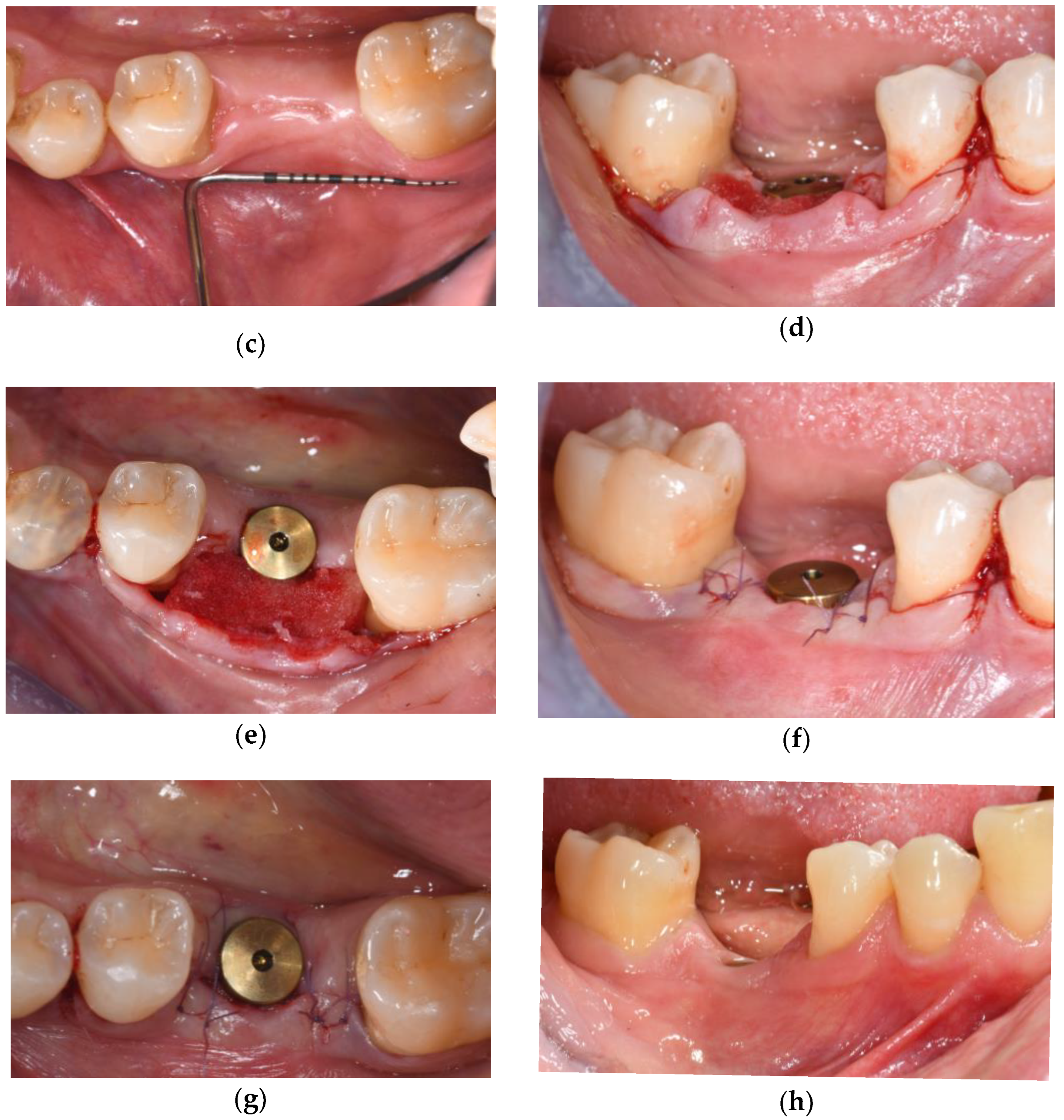
3.2. Soft Tissue Augmentation During Implant Installation
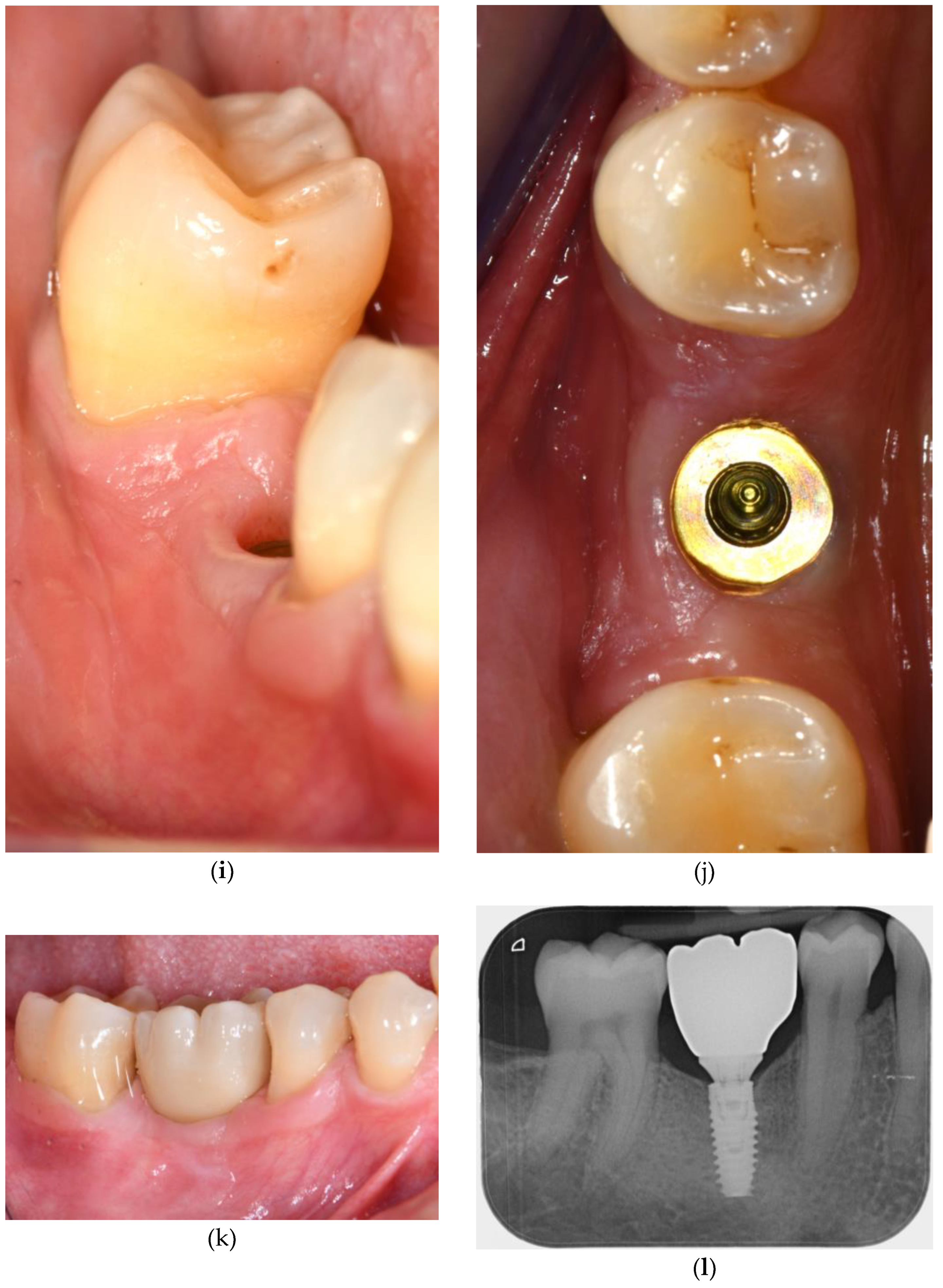
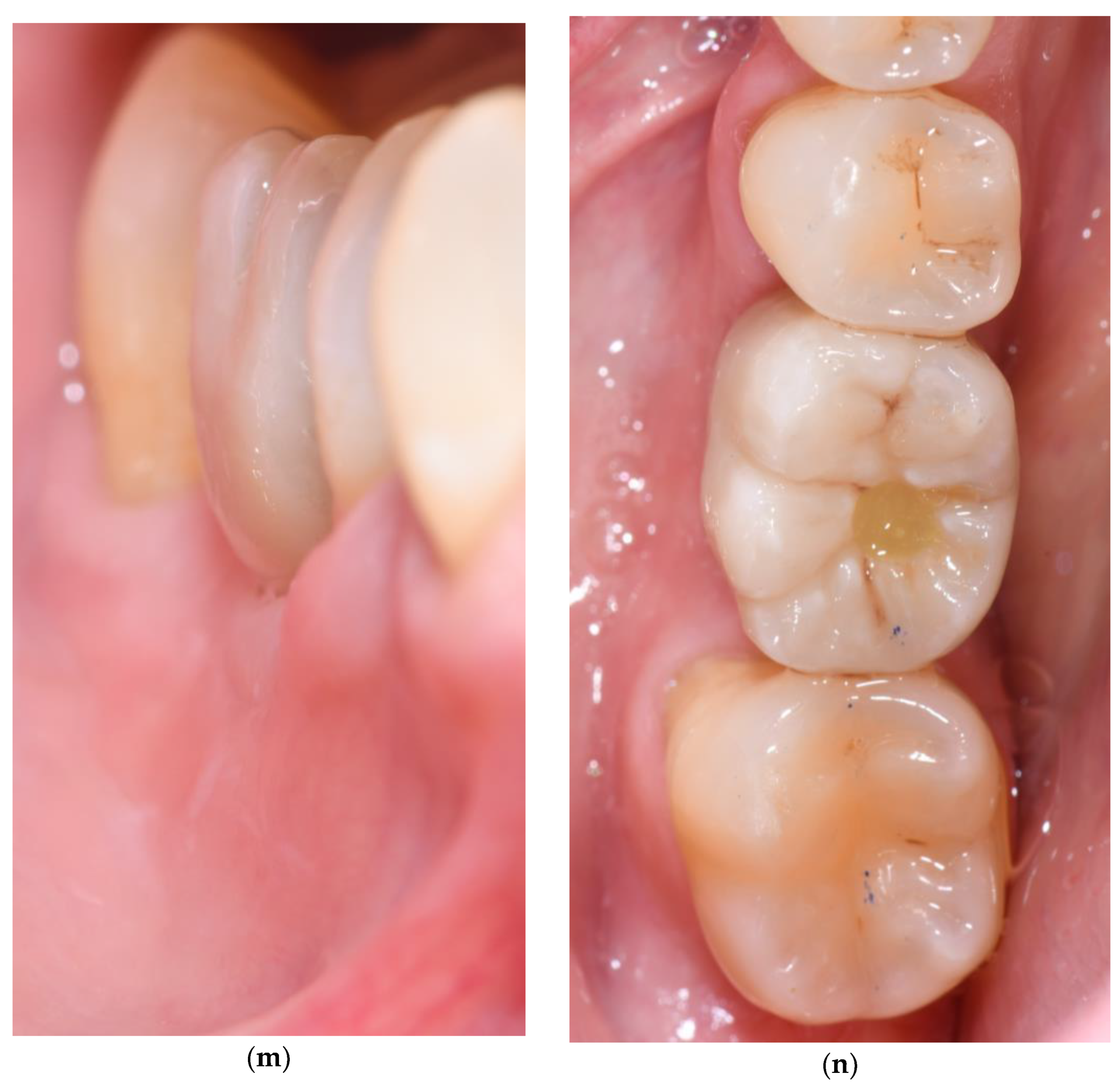
3.3. Soft Tissue Augmentation After Implant Installation
4. Discussion and Clinical Recommendations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Glossary and Abbreviations
| CTG | Connective Tissue Graft |
| CTGS | Connective Tissue Graft Substitute |
| KT | Keratinized Tissue |
| pADM | Porcine Acellular Dermal Matrices |
| hADM | Human Acellular Dermal Matrices |
| CM | Collagen Matrix |
| VCMX | Volume-stable Collagen Matrix |
References
- Reddy, K.S.; Biswas, S.; Sarangi, S.; Chaurasia, A.; Reddy, M.P.; Jose, A.T.; Kashwani, R. Impact of Smoking on Dental Implant: A Review. Bioinformation 2024, 20, 1750–1753. [Google Scholar] [CrossRef]
- Heydari, M.; Ataei, A.; Riahi, S.M. Long-Term Effect of Keratinized Tissue Width on Peri-Implant Health Status Indices: An Updated Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2021, 36, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Longoni, S.; Tinto, M.; Pacifico, C.; Sartori, M.; Andreano, A. Effect of Peri-Implant Keratinized Tissue Width on Tissue Health and Stability: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2019, 34, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Mahardawi, B.; Jiaranuchart, S.; Damrongsirirat, N.; Arunjaroensuk, S.; Mattheos, N.; Somboonsavatdee, A.; Pimkhaokham, A. The Lack of Keratinized Mucosa as a Risk Factor for Peri-Implantitis: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 3778. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Schwarz, F.; Sader, R. Influence of Width of Keratinized Tissue on the Prevalence of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2022, 33 (Suppl. 23), 8–31. [Google Scholar] [CrossRef]
- Ravidà, A.; Arena, C.; Tattan, M.; Caponio, V.C.A.; Saleh, M.H.A.; Wang, H.L.; Troiano, G. The Role of Keratinized Mucosa Width as a Risk Factor for Peri-Implant Disease: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 287–300. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H.L. Peri-Implant Soft Tissue Phenotype Modification and Its Impact on Peri-Implant Health: A Systematic Review and Network Meta-Analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef]
- Barootchi, S.; Mancini, L.; Heck, T.; Zucchelli, G.; Stefanini, M.; Kazarian, E.; Rasperini, G.; Wang, H.L.; Tavelli, L. Reliability Assessment of the Classification for Facial Peri-Implant Soft Tissue Dehiscence/Deficiencies (PSTDs): A Multi-Center Inter-Rater Agreement Study of Different Skill-Level Practitioners. J. Periodontol. 2022, 93, 1173–1182. [Google Scholar] [CrossRef]
- Jung, R.E.; Holderegger, C.; Sailer, I.; Khraisat, A.; Suter, A.; Hämmerle, C.H. The Effect of All-Ceramic and Porcelain-Fused-to-Metal Restorations on Marginal Peri-Implant Soft Tissue Color: A Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2008, 28, 357–365. [Google Scholar]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Discoloration of the Peri-Implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef]
- Oh, S.L. Peri-Implantitis Associated with a Pre-Existing Pathology. J. Oral Implantol. 2017, 43, 232–236. [Google Scholar] [CrossRef]
- Oh, S.L.; Chung, M.K. Creeping Attachment Following Free Gingival Grafts around Dental Implants Exhibiting Mucosal Recession with a Lack of Keratinised Mucosa: A Case Series. Int. J. Oral Implantol. 2020, 13, 401–409. [Google Scholar]
- Roccuzzo, M.; Grasso, G.; Dalmasso, P. Keratinized Mucosa around Implants in Partially Edentulous Posterior Mandible: 10-Year Results of a Prospective Comparative Study. Clin. Oral Implants Res. 2016, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Pagliaro, U.; Nieri, M. Soft Tissue Management at Implant Sites. J. Clin. Periodontol. 2008, 35, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gasser, T.J.W.; Hämmerle, C.H.F.; Strauss, F.J.; Jung, R.E. Soft Tissue Augmentation with a Volume-stable Collagen Matrix or an Autogenous Connective Tissue Graft at Implant Sites: Five-year Results of a Randomized Controlled Trial Post Implant Loading. J. Periodontol. 2023, 94, 230–243. [Google Scholar] [CrossRef]
- Montero, E.; Molina, A.; Matesanz, P.; Monje, A.; Sanz-Sánchez, I.; Herrera, D. Efficacy of Soft Tissue Substitutes, in Comparison with Autogenous Grafts, in Surgical Procedures Aiming to Increase the Peri-implant Keratinized Mucosa: A Systematic Review. Clin. Oral Implants Res. 2022, 33, 32–46. [Google Scholar] [CrossRef]
- Rotundo, R.; Pancrazi, G.L.; Grassi, A.; Ceresoli, L.; Di Domenico, G.L.; Bonafede, V. Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review. Materials 2024, 17, 1221. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, Á.; Vallecillo, C.; Lynch, C.D.; Osorio, M.T.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers 2020, 12, 1850. [Google Scholar] [CrossRef]
- Fischer, K.R.; Testori, T.; Wachtel, H.; Mühlemann, S.; Happe, A.; Del Fabbro, M. Soft Tissue Augmentation Applying a Collagenated Porcine Dermal Matrix during Second Stage Surgery: A Prospective Multicenter Case Series. Clin. Implant. Dent. Relat. Res. 2019, 21, 923–930. [Google Scholar] [CrossRef]
- Wolff, J.; Farré-Guasch, E.; Sándor, G.K.; Gibbs, S.; Jager, D.J.; Forouzanfar, T. Soft Tissue Augmentation Techniques and Materials Used in the Oral Cavity: An Overview. Implant. Dent. 2016, 25, 427–434. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Extracellular Matrix-Based Scaffolding Technologies for Periodontal and Peri-Implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackermann, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In Vitro and in Vivo Characterization of Porcine Acellular Dermal Matrix for Gingival Augmentation Procedures. J. Periodontal Res. 2014, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.-J.; Zöller, J.E. Biodegradation Pattern and Tissue Integration of Native and Cross-Linked Porcine Collagen Soft Tissue Augmentation Matrices—An Experimental Study in the Rat. Head Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized Controlled Clinical Study Evaluating Effectiveness and Safety of a Volume-stable Collagen Matrix Compared to Autogenous Connective Tissue Grafts for Soft Tissue Augmentation at Implant Sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Yu, S.H.; Tseng, S.C.; Wang, H.L. Classification of Soft Tissue Grafting Materials Based on Biologic Principles. Int. J. Periodontics Restor. Dent. 2018, 38, 849–854. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Schlegel, K.A.; Gammel, L.; Moest, T. Gingiva Thickening with a Porcine Collagen Matrix in a Preclinical Dog Model: Histological Outcomes. J. Clin. Periodontol. 2019, 46, 1273–1281. [Google Scholar] [CrossRef]
- Tommasato, G.; Del Fabbro, M.; Oliva, N.; Khijmatgar, S.; Grusovin, M.G.; Sculean, A.; Canullo, L. Autogenous Graft versus Collagen Matrices for Peri-Implant Soft Tissue Augmentation. A Systematic Review and Network Meta-Analysis. Clin. Oral Investig. 2024, 28, 300. [Google Scholar] [CrossRef]
- Nabers, J.M. Free Gingival Grafts. Periodontics 1966, 4, 243–245. [Google Scholar]
- Sullivan, H.C.; Atkins, J.H. Free Autogenous Gingival Grafts. I. Principles of Successful Grafting. Periodontics 1968, 6, 121–129. [Google Scholar]
- Thoma, D.S.; Gil, A.; Hämmerle, C.H.F.; Jung, R.E. Management and Prevention of Soft Tissue Complications in Implant Dentistry. Periodontol. 2000 2022, 88, 116–129. [Google Scholar] [CrossRef]
- Zucchelli, G.; Mazzotti, C. Mucogingival Esthetic Surgery Around Implants, 1st ed.; Quintessence Publishing: New Malden, UK, 2022. [Google Scholar]
- Bressan, E.; Zucchelli, G.; Tommasato, G.; Pesce, P.; Canullo, L.; Grusovin, M.G. Consensus Report by the Italian Academy of Osseointegration on the Importance of Peri-Implant Soft Tissues. Medicina 2024, 60, 1393. [Google Scholar] [CrossRef]
| Matrix Type | Source | Resorption | Fibroblasts Proliferation | Angiogenesis | Volume Stability | Keratinization | Indications |
|---|---|---|---|---|---|---|---|
| hADM/pADM [22,25] | Human/Porcine | Moderate | Moderate | Moderate | Moderate | Limited | Esthetic zones, thin biotype |
| VCMX (Crosslinked) [26,27] | Porcine | Slow | Moderate-high | Moderate | High | Moderate | Posterior sites, soft tissue bulking |
| Non-Crosslinked CM [27,29] | Porcine | Fast | Strong | High | Low-moderate | High-moderate | KT gain, minor thickness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mounssif, I.; Bentivogli, V.; Rendón, A.; Mazzotti, C.; De Rubertis, I.; Zucchelli, G.; Stefanini, M. Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes. Appl. Sci. 2025, 15, 10178. https://doi.org/10.3390/app151810178
Mounssif I, Bentivogli V, Rendón A, Mazzotti C, De Rubertis I, Zucchelli G, Stefanini M. Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes. Applied Sciences. 2025; 15(18):10178. https://doi.org/10.3390/app151810178
Chicago/Turabian StyleMounssif, Ilham, Valentina Bentivogli, Alexandra Rendón, Claudio Mazzotti, Isabella De Rubertis, Giovanni Zucchelli, and Martina Stefanini. 2025. "Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes" Applied Sciences 15, no. 18: 10178. https://doi.org/10.3390/app151810178
APA StyleMounssif, I., Bentivogli, V., Rendón, A., Mazzotti, C., De Rubertis, I., Zucchelli, G., & Stefanini, M. (2025). Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes. Applied Sciences, 15(18), 10178. https://doi.org/10.3390/app151810178







