Investigating the Effect of Different Bovine Colostrum Concentrations Added to Ground Rabbit Patties on the Survival of Listeria monocytogenes and Meat Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Preparation of Rabbit Meat Patties
2.3. Microbiological Analysis
2.4. Physicochemical Parameters
2.4.1. Color Evaluation
2.4.2. Thiobarbituric Acid Reactive Substance Assay (TBARS) and Sulfhydryl Group Analysis
2.4.3. Proximate Composition, Fatty Acid Analysis, and Determination of Peroxidability Index
2.5. Materials
2.6. Statistical Analysis
2.6.1. Data Analysis
2.6.2. Growth Curve Modeling
3. Results
3.1. Chemical Composition
3.2. pH, aw, and Total Viable Count Results
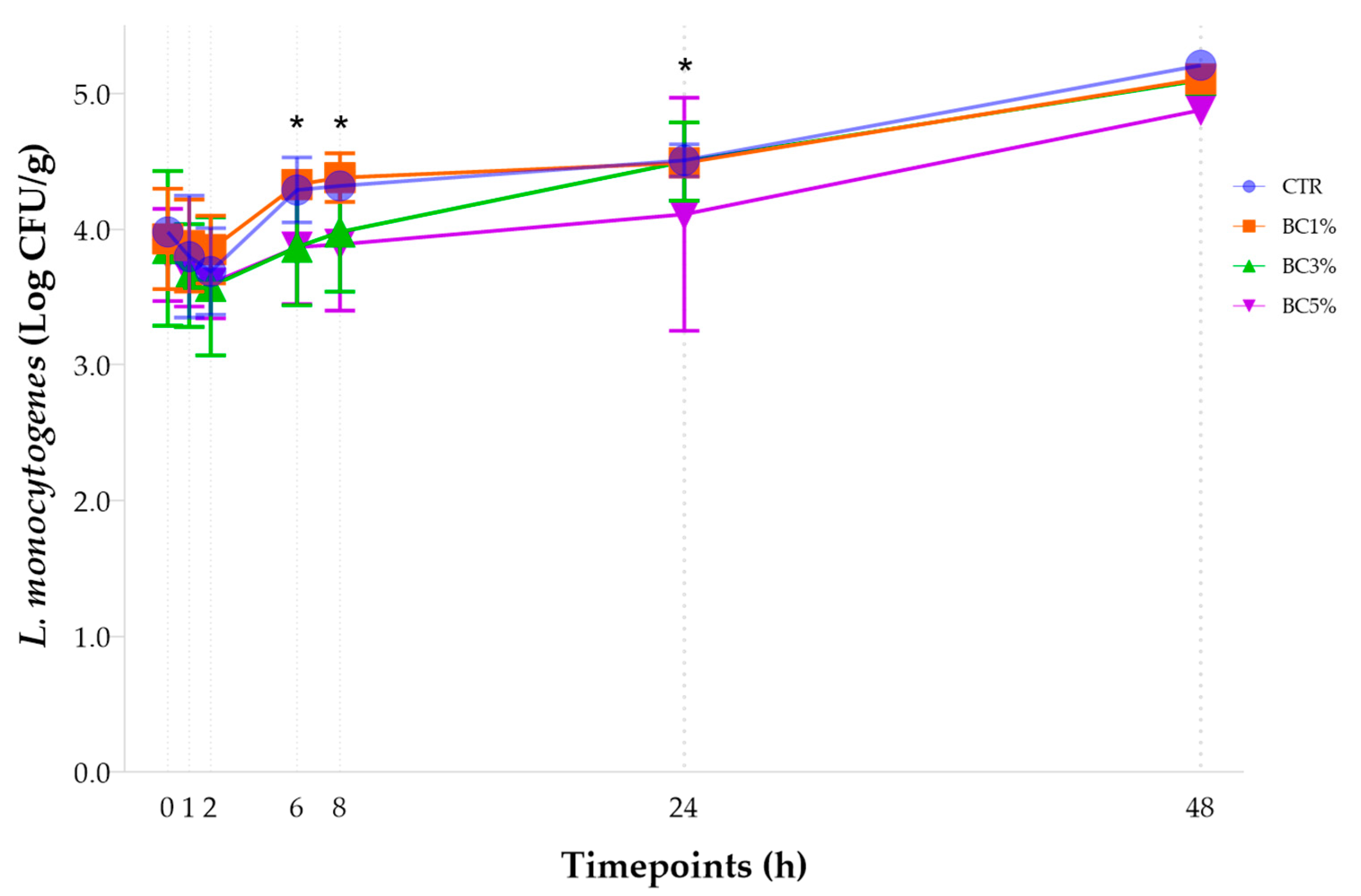
3.3. Growth Dynamics of Listeria monocytogenes: Effect of Bovine Colostrum Concentration
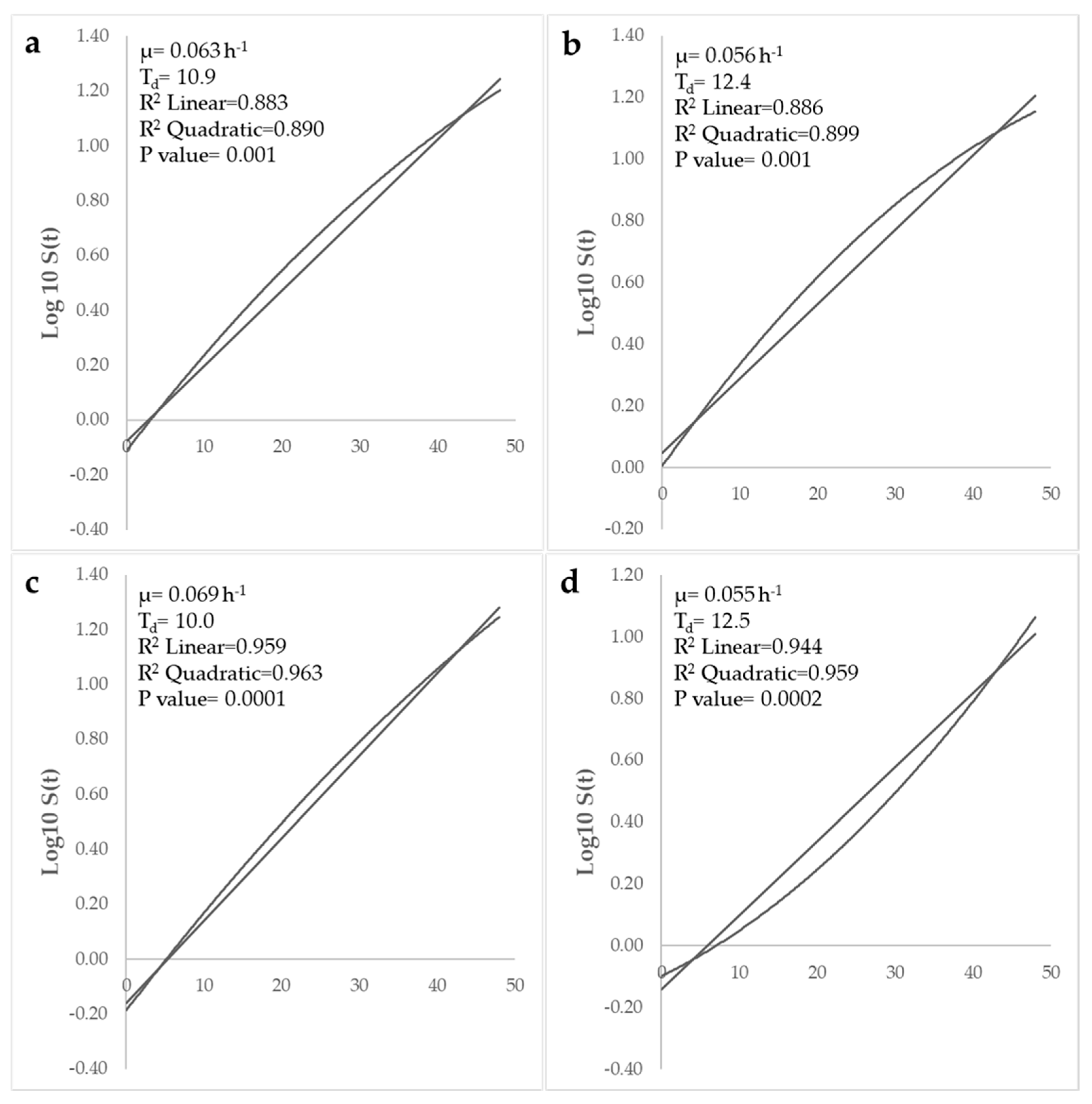
3.4. Kinetic Modeling of Listeria monocytogenes Growth: Effect of Bovine Colostrum Concentration
3.5. Colorimetric Parameters
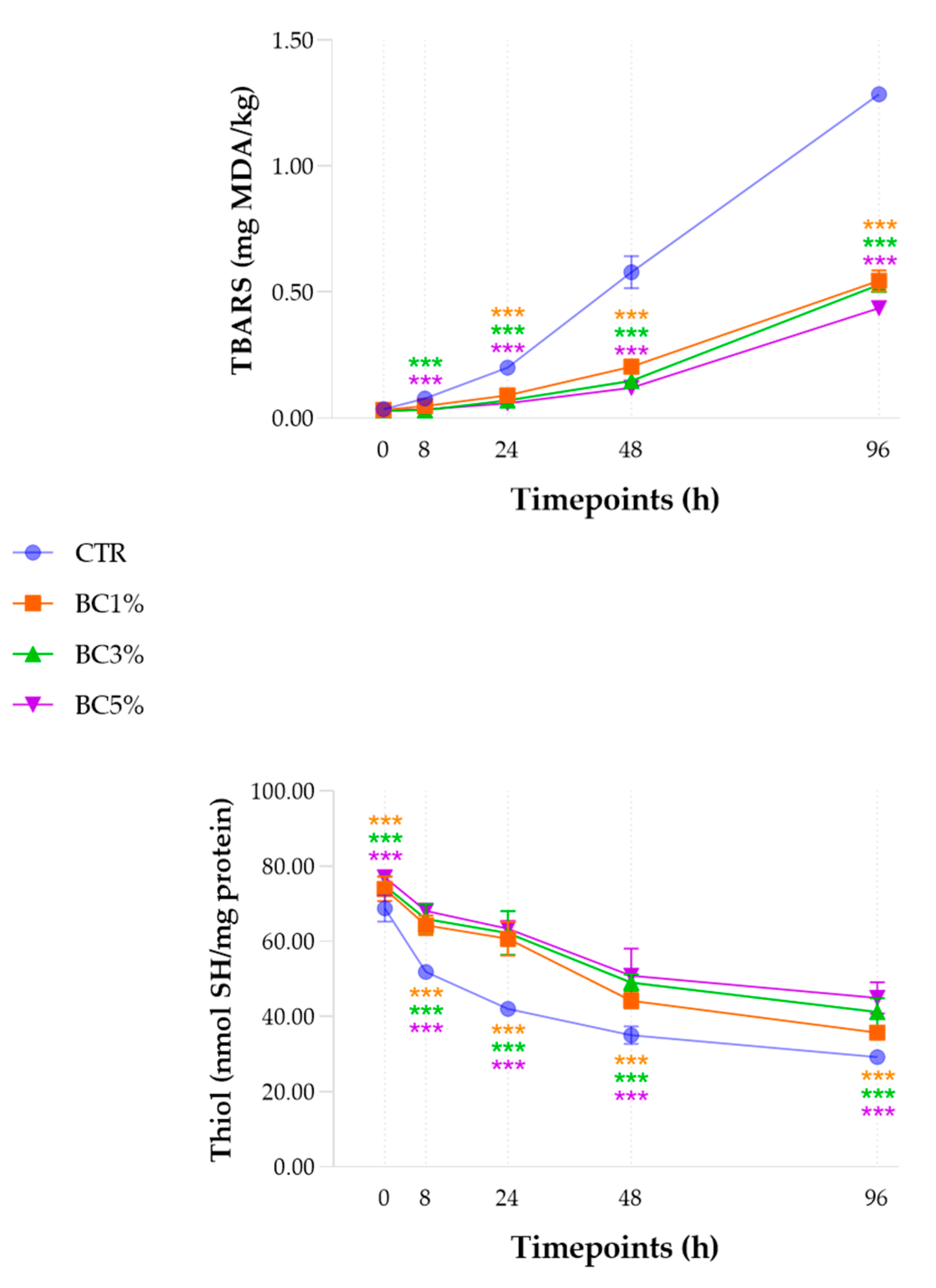
3.6. Oxidative Stability: TBARS and Thiol Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European union one health 2023 zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria Monocytogenes–How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Belias, A.; Bolten, S.; Wiedmann, M. Challenges and Opportunities for Risk- and Systems-Based Control of Listeria Monocytogenes Transmission through Food. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70071. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.F.; Hu, F.; Thakur, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of Antibacterial Effects and Fumigant Toxicity of Essential Oils Extracted from Different Plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential Oils: Antimicrobial Activities, Extraction Methods, and Their Modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Ali, M.A.; Kamal, M.M.; Rahman, M.H.; Siddiqui, M.N.; Haque, M.A.; Saha, K.K.; Rahman, M.A. Functional Dairy Products as a Source of Bioactive Peptides and Probiotics: Current Trends and Future Prospectives. J. Food Sci. Technol. 2022, 59, 1263–1279. [Google Scholar] [CrossRef]
- Ceniti, C.; Costanzo, N.; Morittu, V.M.; Tilocca, B.; Roncada, P.; Britti, D. Review: Colostrum as an Emerging Food: Nutraceutical Properties and Food Supplement. Food Rev. Int. 2023, 39, 4636–4664. [Google Scholar] [CrossRef]
- Navarré, A.; Nazareth, T.; Luz, C.; Meca, G.; Escrivá, L. Characterization of Lactic Acid Bacteria Isolated from Human Breast Milk and Their Bioactive Metabolites with Potential Application as a Probiotic Food Supplement. Food Funct. 2024, 15, 8087–8103. [Google Scholar] [CrossRef]
- Nissen, A.; Bendixen, E.; Ingvartsen, K.L.; Røntved, C.M. In-Depth Analysis of Low Abundant Proteins in Bovine Colostrum Using Different Fractionation Techniques. Proteomics 2012, 12, 2866–2878. [Google Scholar] [CrossRef]
- dos Santos, L.R.; Alía, A.; Martin, I.; Gottardo, F.M.; Rodrigues, L.B.; Borges, K.A.; Furian, T.Q.; Córdoba, J.J. Antimicrobial Activity of Essential Oils and Natural Plant Extracts against Listeria Monocytogenes in a Dry-Cured Ham-Based Model. J. Sci. Food Agric. 2022, 102, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Serdaroğlu, M. Protein Oxidation in Meat Products: Exploring the Role of Natural Antioxidants in Preservation and Quality Enhancement. Meat Technol. 2023, 64, 427–431. [Google Scholar] [CrossRef]
- Pakkanen, R.; Aalto, J. Growth Factors and Antimicrobial Factors of Bovine Colostrum. Int. Dairy J. 1997, 7, 285–297. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Silva, F.G.; Silva, S.R.; Pereira, A.M.F.; Cerqueira, J.L.; Conceição, C. A Comprehensive Review of Bovine Colostrum Components and Selected Aspects Regarding Their Impact on Neonatal Calf Physiology. Animals 2024, 14, 1130. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Agradi, S.; Curone, G.; Vigo, D.; Pastorelli, G.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; Serra, V.; et al. Effect of Bovine Colostrum Dietary Supplementation on Rabbit Meat Quality. Foods 2022, 11, 3433. [Google Scholar] [CrossRef]
- Castrica, M.; Menchetti, L.; Agradi, S.; Curone, G.; Vigo, D.; Pastorelli, G.; Pallaoro, M.; Di Giancamillo, A.; Modina, S.C.; Riva, F.; et al. Meat Quality and Sensory Traits in Rabbits Fed with Two Different Percentages of Bovine Colostrum. Meat Sci. 2024, 213, 109512. [Google Scholar] [CrossRef]
- Menchetti, L.; Taticchi, A.; Esposto, S.; Servili, M.; Ranucci, D.; Branciari, R.; Miraglia, D. The Influence of Phenolic Extract from Olive Vegetation Water and Storage Temperature on the Survival of Salmonella Enteritidis Inoculated on Mayonnaise. LWT 2020, 129, 109648. [Google Scholar] [CrossRef]
- IS0 11290-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enu-meration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 11290-2:2017/DAmd 1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enu-meration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. Amendment 1: Inclusion of storage of the samples before analysis and changes in the control strain for performance testing of culture media and reagents; International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 4833-1:2013/Amd 1:2022; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Micro-organisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization (ISO): Geneva, Switzerland, 2022.
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioproc Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Standard Methods for The Analysis of Oils, Fats and De-Rivatives. In Method 2.411, 7th ed.; International Union of Pure and Applied Chemistry: Zürich, Switzerland; Blackwell Scientific Publications: London, UK, 1987. [Google Scholar]
- Failla, S.; Buttazzoni, L.; Zilio, D.M.; Contò, M.; Renzi, G.; Castellini, C.; Amato, M.G. An Index to Measure the Activity Attitude of Broilers in Extensive System. Poult. Sci. 2021, 100, 101279. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Sagai, M. Species differences in lipid peroxide levels in lung tissue and investigation of their determining factors. Lipids 1986, 21, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Senecal, A.; Chinachoti, P.; Faustman, C. Effect of Water Activity on Lipid Oxidation and Protein Solubility in Freeze-Dried Beef during Storage. J. Food Sci. 2002, 67, 2512–2516. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid Oxidation in Food Science and Nutritional Health: A Comprehensive Review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Claus, J.R.; Hunt’, M.C.; Kastner, C.L.; Hall, W. Effects of Substituting Added Water for Fat on the Textural, Sensory, and Processing Characteristics of Bologna. J. Muscle Foods 1990, 1, 1–21. [Google Scholar] [CrossRef]
- Miklos, R.; Mora-Gallego, H.; Larsen, F.H.; Serra, X.; Cheong, L.Z.; Xu, X.; Arnau, J.; Lametsch, R. Influence of Lipid Type on Water and Fat Mobility in Fermented Sausages Studied by Low-Field NMR. Meat Sci. 2014, 96, 617–622. [Google Scholar] [CrossRef]
- Hao, F.; Lu, L.; Ge, C. Effect of Fat Content on Water Sorption Properties of Biscuits Studied by Nuclear Magnetic Resonance. J. Food Nutr. Res. 2014, 2, 814–818. [Google Scholar] [CrossRef][Green Version]
- Eker, F.; Akda¸sçi, A.; Duman, H.; Mert, Y.; Yalçınta¸s, Y.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef]
- Ramani, A.; Taherabbas, S.; Manik, S. Bovine Colostrum as a Promising Nutraceutical: A Systematic Review. Sustain. Food Technol. 2024, 2, 531–547. [Google Scholar] [CrossRef]
- Mehra, R.; Garhwal, R.; Sangwan, K.; Guiné, R.P.F.; Lemos, E.T.; Buttar, H.S.; Visen, P.K.S.; Kumar, N.; Bhardwaj, A.; Kumar, H. Insights into the Research Trends on Bovine Colostrum: Beneficial Health Perspectives with Special Reference to Manufacturing of Functional Foods and Feed Supplements. Nutrients 2022, 14, 659. [Google Scholar] [CrossRef]
- Iram, D.; Sansi, M.S.; Meena, S.; Puniya, A.K.; Vij, S. In Vitro Antimicrobial and Synergistic Effect of Fermented Indian Zebu (Sahiwal) Cow Colostrum Whey Derived Peptides with Lactobacillus Rhamnosus against Pathogenic Bacteria. J. Food Sci. Technol. 2023, 60, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 2073/2005; Microbiological criteria for foodstuffs (Text with EEA relevance). European Commission: Brussels, Belgium, 2005.
- Commission Regulation (EU) No 2024/2895; Amending Regula-tion (EC) No 2073/2005 as regards Listeria monocytogens (Text with EEA relevance). European Commission: Brussels, Belgium, 2024.
- Mann, S.; Curone, G.; Chandler, T.L.; Moroni, P.; Cha, J.; Bhawal, R.; Zhang, S. Heat Treatment of Bovine Colostrum: I. Effects on Bacterial and Somatic Cell Counts, Immunoglobulin, Insulin, and IGF-I Concentrations, as Well as the Colostrum Proteome. J. Dairy. Sci. 2020, 103, 9368–9383. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.R.; Qureshi, A.I.; Hussain, S.A.; Fayaz, H.; Sofi, A.H. Design and Development of Meat-Based Functional Foods. In Hand Book of Processed Functional Meat Products; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 129–156. ISBN 9783031698682. [Google Scholar]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The Hurdle Approach-A Holistic Concept for Controlling Food Safety Risks Associated with Pathogenic Bacterial Contamination of Leafy Green Vegetables. A Review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef] [PubMed]

| Group | Batch | Timepoints (h) | Parameters | |||
|---|---|---|---|---|---|---|
| L. monocytogenes | pH, aw, and Total Viable Count | Proximate Composition, FA 1 Profile and PI 2 | Color, TBARS, and Sulfhydryl | |||
| CTR | B1 B2 B3 | 0 | ✔ 3 | ✔ 4 | ✔ 4 | ✔ 4 |
| 1 | ✔ | |||||
| 2 | ✔ | |||||
| 6 | ✔ | |||||
| 8 | ✔ | ✔ 4 | ||||
| 24 | ✔ | ✔ 4 | ||||
| 48 | ✔ | ✔ 4 | ✔ 4 | |||
| 96 | ✔ 4 | |||||
| BC1% | B1 B2 B3 | 0 | ✔ 3 | ✔ 4 | ✔ 4 | ✔ 4 |
| 1 | ✔ | |||||
| 2 | ✔ | |||||
| 6 | ✔ | |||||
| 8 | ✔ | ✔ 4 | ||||
| 24 | ✔ | ✔ 4 | ||||
| 48 | ✔ | ✔ 4 | ✔ 4 | |||
| 96 | ✔ 4 | |||||
| BC3% | B1 B2 B3 | 0 | ✔ 3 | ✔ 4 | ✔ 4 | ✔ 4 |
| 1 | ✔ | |||||
| 2 | ✔ | |||||
| 6 | ✔ | |||||
| 8 | ✔ | ✔ 4 | ||||
| 24 | ✔ | ✔ 4 | ||||
| 48 | ✔ | ✔ 4 | ✔ 4 | |||
| 96 | ✔ 4 | |||||
| BC5% | B1 B2 B3 | 0 | ✔ 3 | ✔ 4 | ✔ 4 | ✔ 4 |
| 1 | ✔ | |||||
| 2 | ✔ | |||||
| 6 | ✔ | |||||
| 8 | ✔ | ✔ 4 | ||||
| 24 | ✔ | ✔ 4 | ||||
| 48 | ✔ | ✔ 4 | ✔ 4 | |||
| 96 | ✔ 4 | |||||
| Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | pH | aw | Total Viable Count (Log CFU/g) | Δ (Log CFU/g) | |||||
| 0 | 48 | 0 | 48 | 0 | 48 | ||||
| CTR | 5.68 ± 0.009 c | 5.36 ± 0.010 c | *** | 0.973 ± 0.020 ns | 0.943 ± 0.024 ns | 3.81 ± 0.021 b | 4.70 ± 0.013 b | *** | +0.89 |
| BC1% | 6.44 ± 0.030 b | 5.94 ± 0.010 b | *** | 0.956 ± 0.015 ns | 0.970 ± 0.017 ns | 3.60 ± 0.012 c | 4.08 ± 0.022 c | *** | +0.48 |
| BC3% | 6.49 ± 0.022 ab | 6.03 ± 0.019 a | *** | 0.964 ± 0.007 ns | 0.948 ± 0.024 ns | 3.95 ± 0.015 a | 4.85 ± 0.014 a | *** | +0.90 |
| BC5% | 6.53 ± 0.021 a | 6.01 ± 0.027 a | *** | 0.962 ± 0.013 ns | 0.964 ± 0.011 ns | 3.57 ± 0.009 c | 3.92 ± 0.022 d | *** | +0.35 |
| <0.001 | <0.001 | ns | ns | <0.001 | <0.001 | ||||
| Groups | Timepoints (h) | p Value for Time Effect | Δ (Log CFU/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 6 | 8 | 24 | 48 | |||
| CTR | 3.98 ± 0.07 Ca | 3.80 ± 0.45 Ca | 3.69 ± 0.32 Ca | 4.29 ± 0.24 BCa | 4.32 ± 0.08 BCa | 4.58 ± 0.12 Ba | 5.21 ± 0.01 Aa | <0.05 | +1.23 |
| BC1% | 3.93 ± 0.37 Ca | 3.88 ± 0.34 Ca | 3.85 ± 0.25 Ca | 4.33 ± 0.10 BCa | 4.38 ± 0.18 BCa | 4.49 ± 0.03 Bab | 5.11 ± 0.08 Aa | <0.01 | +1.18 |
| BC3% | 3.86 ± 0.57 BCa | 3.66 ± 0.38 Ca | 3.58 ± 0.51 Ca | 3.87 ± 0.43 BCb | 3.98 ± 0.44 BCab | 4.16 ± 0.29 Bab | 5.10 ± 0.04 Aa | <0.001 | +1.24 |
| BC5% | 3.81 ± 0.34 BCa | 3.66 ± 0.23 Ca | 3.60 ± 0.26 Ca | 3.87 ± 0.42 BCb | 3.90 ± 0.49 BCb | 4.11 ± 0.86 Bb | 4.88 ± 0.09 Aa | <0.05 | +1.07 |
| p Value for Group Effect | >0.05 | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 | >0.05 | ||
| Parameters | Timepoints (h) | p Value | RMSE | ||||
|---|---|---|---|---|---|---|---|
| 0 | 8 | 24 | 48 | 96 | |||
| L* | 49.49 b | 51.58 a | 50.29 ab | 48.72 b | 48.64 b | <0.001 | 1.47 |
| a* | −1.62 b | −1.42 b | −1.43 b | −1.46 b | −1.02 a | 0.003 | 0.23 |
| b* | 6.92 b | 7.25 b | 7.84 ab | 7.79 ab | 8.15 a | <0.001 | 0.69 |
| C* | 7.15 b | 7.43 b | 8.00 a | 7.94 ab | 8.24 a | 0.002 | 0.67 |
| H° | 76.11 c | 78.10 bc | 78.92 b | 79.21 b | 82.75 a | <0.001 | 2.28 |
| TBARS (mg MDA/kg) | 0.03 d | 0.05 d | 0.11 c | 0.26 b | 0.70 a | <0.001 | 0.02 |
| Sulfhydryl (nmol SH/mg protein) | 73.65 a | 62.57 b | 57.08 c | 44.80 d | 37.77 e | <0.001 | 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrica, M.; Rinaldi, S.; Contò, M.; Curone, G.; Quattrone, A.; Balzaretti, C.M.; Brecchia, G.; Giaccone, V.; Failla, S. Investigating the Effect of Different Bovine Colostrum Concentrations Added to Ground Rabbit Patties on the Survival of Listeria monocytogenes and Meat Quality. Appl. Sci. 2025, 15, 10019. https://doi.org/10.3390/app151810019
Castrica M, Rinaldi S, Contò M, Curone G, Quattrone A, Balzaretti CM, Brecchia G, Giaccone V, Failla S. Investigating the Effect of Different Bovine Colostrum Concentrations Added to Ground Rabbit Patties on the Survival of Listeria monocytogenes and Meat Quality. Applied Sciences. 2025; 15(18):10019. https://doi.org/10.3390/app151810019
Chicago/Turabian StyleCastrica, Marta, Simona Rinaldi, Michela Contò, Giulio Curone, Alda Quattrone, Claudia M. Balzaretti, Gabriele Brecchia, Valerio Giaccone, and Sebastiana Failla. 2025. "Investigating the Effect of Different Bovine Colostrum Concentrations Added to Ground Rabbit Patties on the Survival of Listeria monocytogenes and Meat Quality" Applied Sciences 15, no. 18: 10019. https://doi.org/10.3390/app151810019
APA StyleCastrica, M., Rinaldi, S., Contò, M., Curone, G., Quattrone, A., Balzaretti, C. M., Brecchia, G., Giaccone, V., & Failla, S. (2025). Investigating the Effect of Different Bovine Colostrum Concentrations Added to Ground Rabbit Patties on the Survival of Listeria monocytogenes and Meat Quality. Applied Sciences, 15(18), 10019. https://doi.org/10.3390/app151810019













