Featured Application
The proposed point-based risk score, incorporating CRP, NT-proBNP, and age, can be applied in post-PCI clinical settings to rapidly stratify acute coronary syndrome patients according to their risk of developing moderate-to-severe diastolic dysfunction. This tool may help guide early echocardiographic follow-up, optimize anti-inflammatory and cardioprotective strategies, and improve individualized patient monitoring during hospitalization and early recovery.
Abstract
Background and Objectives: Diastolic dysfunction (DD) is a frequent complication following percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS), potentially contributing to adverse outcomes. This study aimed to investigate the association between post-procedural inflammatory markers and the severity of DD and to propose a preliminary risk score for early prediction. Materials and Methods: We retrospectively analyzed 181 ACS patients undergoing PCI, assessing C-reactive protein (CRP), leukocyte, neutrophil counts, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) measured 24–48 h post-PCI. Echocardiographic DD grading was performed according to ASE/EACVI guidelines. Spearman correlation, ordinal regression, and decision-curve analysis were used to evaluate predictive performance. Results: CRP correlated with DD severity (ρ = 0.232, p = 0.002) and showed a borderline independent association (OR 1.004, 95% CI 0.999–1.009, p = 0.081). NT-proBNP correlated positively with both CRP and DD severity, while leukocyte and neutrophil counts were not significant. A three-parameter risk score (CRP > 10 mg/L, NT-proBNP > 125 pg/mL, age ≥ 65 years) identified patients at higher risk of moderate-to-severe DD (score ≥ 2: sensitivity 62%, specificity 71%). Decision-curve analysis demonstrated clinical utility in guiding post-PCI monitoring. Conclusions: A simple 0–3 point score combining age, CRP, and NT-proBNP showed potential for early identification of patients at risk of moderate-to-severe diastolic dysfunction after PCI. While the model demonstrated acceptable discrimination and calibration, its single-center design, limited sample size, and timing of biomarker assessment warrant cautious interpretation. External validation in larger, multicenter cohorts is required before clinical application.
1. Introduction
Acute coronary syndrome (ACS) remains a leading cause of morbidity and mortality worldwide, despite major advances in reperfusion therapy and interventional cardiology. Percutaneous coronary intervention (PCI) has significantly improved short- and long-term outcomes, yet a considerable proportion of patients experience persistent or new-onset left ventricular dysfunction following revascularization. While systolic impairment is well recognized, diastolic dysfunction—early post-procedural DD—has been less studied, despite its association with adverse outcomes, including heart failure with preserved ejection fraction (HFpEF) and recurrent cardiovascular events [1,2,3].
Post-PCI diastolic dysfunction is likely multifactorial, involving ischemia–reperfusion injury, microvascular dysfunction, and myocardial edema, compounded by systemic inflammatory activation. Inflammatory pathways are activated in response to myocardial necrosis and vascular injury during PCI, potentially contributing to impaired ventricular relaxation and increased chamber stiffness [4,5,6]. Several biomarkers of inflammation, including C-reactive protein (CRP), leukocyte count, and neutrophil-to-lymphocyte ratio, have been linked to adverse outcomes after ACS [7,8]. However, their role in predicting early diastolic impairment post-intervention remains underexplored.
Metabolic comorbidities such as hypertension, type 2 diabetes mellitus, dyslipidemia, and obesity are recognized contributors to adverse cardiac remodeling through mechanisms involving chronic low-grade inflammation, endothelial dysfunction, and extracellular matrix alterations [8,9,10]. Nevertheless, whether these factors exert an additive effect on acute post-PCI diastolic dysfunction, beyond procedural and hemodynamic factors, is uncertain.
The ability to identify patients at high risk for early diastolic dysfunction after PCI could have important clinical implications. Timely recognition would enable targeted monitoring, optimization of anti-ischemic and anti-inflammatory therapies, and potentially the initiation of preventive strategies to mitigate long-term functional decline.
To address this gap, we conducted a retrospective observational study in patients with ACS undergoing PCI, aiming to:
- Evaluate the relationship between post-procedural inflammatory biomarkers and the degree of early diastolic dysfunction;
- Assess the additive role of metabolic comorbidities; and
- Develop a preliminary algorithm for estimating the risk of early post-PCI diastolic impairment.
We hypothesized that elevated post-procedural inflammatory markers, particularly CRP, in combination with specific metabolic factors, would be independently associated with a higher degree of diastolic dysfunction. This work may provide the foundation for a clinically applicable risk stratification model to be validated in prospective studies.
2. Materials and Methods
2.1. Study Design and Population
This retrospective observational study included consecutive patients diagnosed with acute coronary syndrome (ACS) and treated with percutaneous coronary intervention (PCI) in a tertiary interventional cardiology center. The study included consecutive ACS patients treated with PCI between January 2020 and December 2023. Eligible patients were aged ≥18 years, had an ACS diagnosis confirmed by clinical presentation, electrocardiographic changes, and/or elevated cardiac biomarkers, and underwent complete coronary revascularization by PCI. In addition, only patients with available post-procedural echocardiographic assessment (within 48 h), including diastolic function parameters, and with inflammatory markers—C-reactive protein (CRP), total leukocyte count, and neutrophils—measured at 24–48 h post-PCI, were included.
ACS was defined according to ESC guidelines as the presence of ischemic symptoms, ECG changes indicative of STEMI/NSTEMI, and/or elevated cardiac troponins, with angiographic confirmation of the culprit lesion. Patients who demonstrated failure of initial conservative therapy, defined as persistent ischemia or hemodynamic instability despite medical management, were also included.
Exclusion criteria were the presence of active infection at admission, autoimmune or systemic inflammatory diseases, active malignancy or ongoing immunosuppressive therapy, severe valvular heart disease, poor echocardiographic image quality preventing accurate diastolic function grading, or missing essential clinical or laboratory data.
2.2. Data Collection
Demographic, clinical, and laboratory data were extracted from electronic medical records. Collected variables included age, gender, residential origin (urban or rural), cardiovascular risk factors, and metabolic comorbidities (hypertension, type 2 diabetes mellitus, dyslipidemia, and obesity). Inflammatory markers comprised CRP (mg/L), total leukocyte count, and absolute neutrophil count measured 24–48 h post-PCI.
Echocardiographic assessments were performed by experienced cardiologists according to the standardized recommendations of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI). Diastolic function was graded on a scale from 0 to III based on E/A ratio, E/e′ ratio, left atrial volume index, and tricuspid regurgitation velocity. Procedural characteristics, including the type of ACS (ST-elevation myocardial infarction [STEMI], non-ST-elevation myocardial infarction [NSTEMI], or unstable angina), culprit vessel, and number of stents implanted, were also recorded.
Missing data were rare and are reported for transparency: CRP values were missing in 2 patients (1.1%), NT-proBNP in 3 patients (1.7%), and echocardiographic assessment in 4 patients (2.2%). Complete-case analysis was used for the primary model, and multiple imputation was applied in sensitivity analyses to confirm robustness.
The patient flowchart is presented in Figure 1.
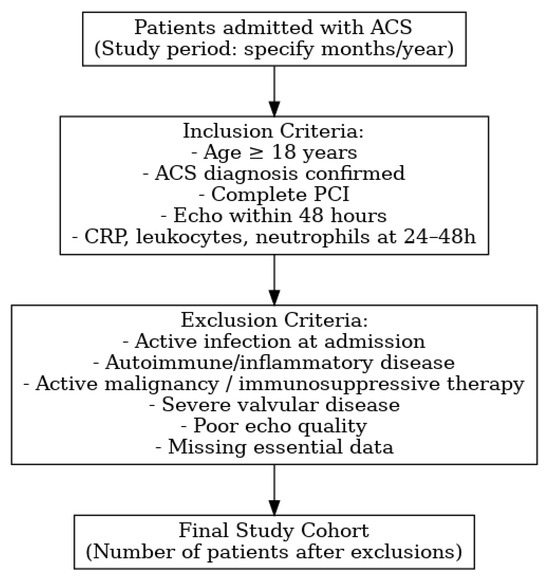
Figure 1.
Flowchart.
2.3. Definition of Variables
Candidate variables for multivariable modeling were chosen based on clinical plausibility, prior literature, and univariate association with DD grade (p < 0.10). Inflammatory markers, NT-proBNP, and metabolic comorbidities were retained due to biological relevance. To avoid model overfitting, we limited predictors to five, meeting the recommended events-per-variable (EPV > 10) threshold. Additional correlations with GRACE score, Killip class, and angiographic severity were explored in supplementary analyses.
Candidate predictors were selected based on both clinical plausibility and statistical performance. Variables showing a significant or borderline association with diastolic dysfunction (DD) in univariate analyses (p < 0.10) were entered into the multivariable ordinal regression model. In the final model, age ≥ 65 years and NT-proBNP > 125 pg/mL remained independent predictors of moderate-to-severe DD. CRP did not reach conventional statistical significance (p = 0.081) but was consistently correlated with DD in univariate analysis, demonstrated discriminatory ability in ROC analysis, and has well-established biological relevance as an inflammatory biomarker in ACS. For these reasons, CRP was retained in the simplified clinical score.
The score was constructed using a pragmatic point-based approach, assigning 1 point each for age ≥ 65 years, NT-proBNP > 125 pg/mL, and CRP > 10 mg/L. Thus, the score ranges from 0 to 3 points, with a threshold of ≥2 points identifying patients at higher risk of moderate-to-severe DD.
The severity of diastolic dysfunction was assigned a numerical value for statistical modeling: 0 = normal diastolic function, 1 = grade I dysfunction, 2 = grade II dysfunction, and 3 = grade III dysfunction. The inflammatory response was represented by CRP, leukocyte count, and neutrophil count measured at 24–48 h post-PCI. The metabolic risk profile was defined as the presence of one or more metabolic comorbidities, including hypertension, diabetes, dyslipidemia, and obesity.
2.4. Risk Score Presentation
The proposed post-PCI diastolic dysfunction risk score is based on three readily available clinical and laboratory parameters. Table 1 presents the scoring system based on predefined cutoffs for CRP, NT-proBNP, and age. Each parameter above the cutoff contributes one point to the cumulative score.

Table 1.
Scoring system based on clinical and biochemical parameters. Each parameter above the specified cutoff value contributes one point to the cumulative risk score.
Sample size adequacy was estimated using the EPV approach, requiring ≥ 10 outcome events per predictor. With 71 patients experiencing moderate-to-severe DD and five predictors included, the EPV ratio exceeded 14, indicating an adequate sample size for exploratory modeling.
Thresholds were defined based on a combination of guideline criteria and ROC analysis. CRP > 10 mg/L corresponds to a high-risk inflammatory state and matched the ROC-derived optimal cut-off (9.8 mg/L). NT-proBNP > 125 pg/mL is consistent with ESC recommendations for heart failure screening and closely aligned with the ROC-derived cut-off (132 pg/mL).
Example calculation:
A 70-year-old patient with CRP = 12 mg/L and NT-proBNP = 180 pg/mL would receive:
- CRP > 10 mg/L → +1 point
- NT-proBNP > 125 pg/mL → +1 point
- Age ≥ 65 years → +1 point
Total score: 3 points → classified as high risk.
Proposed cutoffs for risk categories:
- Low risk: 0–1 points (sensitivity: 84%, specificity: 42%)
- Intermediate risk: 2 points (sensitivity: 62%, specificity: 71%)
- High risk: 3 points (sensitivity: 38%, specificity: 89%)
Nomogram:
A graphical nomogram was developed (Figure 2) to facilitate bedside calculation of individual risk probability. Each parameter corresponds to a point scale; the sum of the points is converted into the predicted probability of moderate-to-severe diastolic dysfunction.
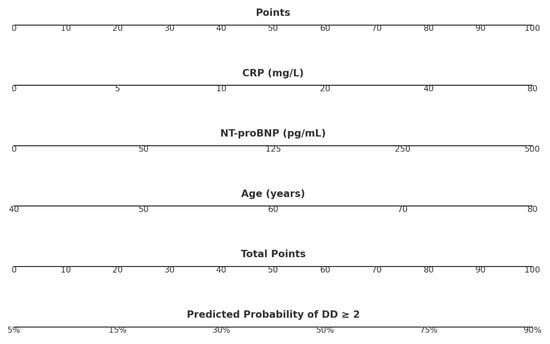
Figure 2.
Nomogram for calculating post-PCI diastolic dysfunction risk using CRP, NT-proBNP, and age.
The nomogram assigns points for each predictor—CRP, NT-proBNP, and age—based on the patient’s measured values. The points are summed to obtain a total score, which corresponds to the predicted probability of diastolic dysfunction grade ≥2. Cut-offs classify patients into low (<20%), intermediate (20–50%), and high (>50%) risk categories.
2.5. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR), depending on distribution, while categorical variables were presented as counts and percentages. The normality of data distribution was assessed using the Shapiro–Wilk test. Comparisons between groups were performed using Student’s t-test or the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables.
Internal validation was performed using bootstrap resampling (1000 iterations) with optimism-corrected performance estimates. Discrimination was assessed by the concordance index (c-index) for ordinal regression and AUROC for a dichotomized outcome (DD ≥ 2). Calibration was evaluated by slope, intercept, and calibration plot. The proportional odds assumption was tested using the Brant test.
Missing values for CRP, NT-proBNP, or echocardiographic data resulted in case exclusion (complete-case analysis). No imputation was performed as missingness was <5%. Potential interaction terms between CRP and NT-proBNP, and CRP and age, were evaluated but did not significantly improve model fit (all p > 0.10).
Correlation analyses between inflammatory markers and diastolic dysfunction grade were conducted using Spearman’s rho. To identify independent predictors of more severe diastolic dysfunction, an ordinal regression model was applied, including inflammatory markers and metabolic comorbidities. Based on the regression analysis, a preliminary risk estimation algorithm was developed by integrating significant predictors into a stepwise scoring system.
All statistical analyses were performed using SPSS software, version 30.0 (IBM Corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
The study population comprised 181 patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). The mean age was 63 ± 11 years, with a range from 27 to 89 years. Males predominated in the cohort (65.7%), and just over half of the participants resided in urban areas (52.5%).
The initial laboratory profile showed a mean leukocyte count of 12.31 ± 4.15 × 109/L and a mean neutrophil count of 9.10 ± 3.79 × 109/L, reflecting an early systemic inflammatory response following ACS. The mean lymphocyte count was 3.63 ± 5.00 × 109/L. High-sensitivity cardiac troponin (hs-cTn) levels on admission averaged 3223 ± 8644 ng/L, consistent with significant myocardial injury.
Table 2 presents the detailed demographic and baseline clinical characteristics of the study population. Additional correlations between diastolic dysfunction (DD) severity and key clinical indices (Killip class, GRACE score, TIMI flow) are provided in Supplementary Table S1, demonstrating modest but significant associations.

Table 2.
Baseline demographic, clinical, laboratory, and angiographic characteristics of the study cohort (n = 181). Continuous variables are expressed as means ± SD; categorical variables as n (%). Statistical tests: t-test or Mann–Whitney U for continuous variables; χ2 or Fisher’s exact test for categorical variables.
In Figure 3 is presented the baseline distribution of study participants by gender and residence. The cohort included a higher proportion of male patients (65.7%) compared to female patients (34.3%). Slightly more than half of the participants resided in urban areas (52.5%), with 47.5% from rural regions. This distribution reflects the referral population and the higher ACS burden in males and urban residents.
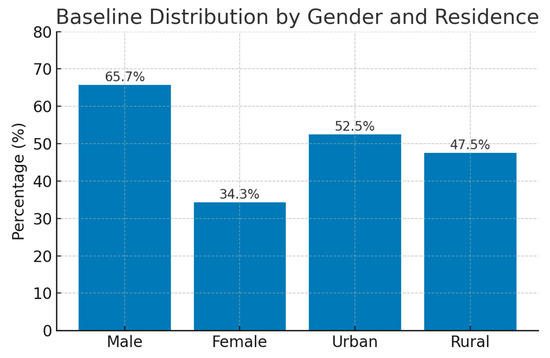
Figure 3.
Baseline distribution of demographic characteristics of the study population.
3.2. Inflammatory Markers and Diastolic Dysfunction
Post-procedural C-reactive protein (CRP) levels demonstrated a modest but statistically significant positive correlation with the numerical grade of diastolic dysfunction (Spearman’s ρ = 0.232, p = 0.002). This finding suggests that higher CRP concentrations in the immediate post-procedural period are associated with more advanced diastolic impairment.
In contrast, leukocyte and neutrophil counts measured at 24–48 h post-intervention did not exhibit statistically significant associations with diastolic dysfunction severity (p > 0.05 for both), indicating that systemic leukocyte response may not be a sensitive marker for early post-PCI diastolic changes.
Table 3 presents the distribution of diastolic dysfunction grades across CRP tertiles. A gradual increase in the proportion of patients with moderate-to-severe dysfunction is evident in the higher CRP tertile group.

Table 3.
Distribution of diastolic dysfunction (DD) grades according to CRP tertiles measured 24–48 h post-PCI. Higher CRP levels were associated with a greater prevalence of moderate-to-severe DD. Values are presented as n (%).
Figure 4 illustrates these trends graphically, illustrating the higher prevalence of advanced DD in patients with elevated CRP.
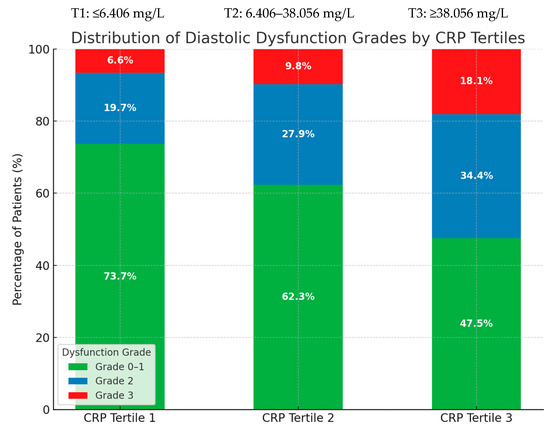
Figure 4.
Distribution of diastolic dysfunction grades according to post-procedural CRP tertiles. Percentages represent the proportion of patients within each tertile exhibiting Grade 0–1, Grade 2, or Grade 3 dysfunction.
3.3. Ordinal Regression Analysis
In the multivariable ordinal regression model, age ≥ 65 years and NT-proBNP > 125 pg/mL emerged as independent predictors of moderate-to-severe diastolic dysfunction (DD ≥ 2). Age was associated with increased DD risk, while NT-proBNP showed a significant positive association. CRP also demonstrated a borderline association with DD severity (OR = 1.004, 95% CI: 0.999–1.009, p = 0.081), consistent with its modest correlation observed in univariate analysis. Although not statistically significant at the conventional threshold, CRP was retained in the simplified risk score due to its discriminatory ability on ROC analysis and strong biological plausibility as an inflammatory marker relevant to early post-PCI outcomes.
An operational table (Table 3) and probability curve illustrate the predicted risk of DD ≥ II for each score level.
ROC analysis identified a CRP cut-off of 9.8 mg/L (AUC = 0.68, sensitivity 61%, specificity 64%), consistent with the threshold of 10 mg/L used in our score. For NT-proBNP, the optimal cut-off was 132 pg/mL (AUC = 0.71, sensitivity 65%, specificity 66%), closely aligned with the ESC-recommended threshold of 125 pg/mL.
Table 4 summarizes the main ordinal regression results, including the regression coefficients, standard errors, odds ratios (OR), and 95% confidence intervals (CI).

Table 4.
Ordinal regression analysis for predictors of diastolic dysfunction (DD) severity. Independent predictors included age ≥ 65 years and NT-proBNP > 125 pg/mL, both significantly associated with higher DD grade. CRP > 10 mg/L demonstrated a borderline association (p = 0.081) and was retained in the simplified risk score based on correlation in univariate analysis, ROC-derived discriminatory ability, and biological plausibility. Odds ratios (OR) with 95% confidence intervals (CI) and p-values are reported.
3.4. Exploratory Linear Regression
To further explore the relationship between systemic inflammation and diastolic dysfunction, a simple linear regression model was constructed using post-procedural CRP as the independent variable and the numerical grade of diastolic dysfunction as the dependent variable.
The analysis showed a statistically significant positive association (β = 0.002, p = 0.010), indicating that higher CRP levels were associated with more severe diastolic dysfunction. However, the model explained only a small proportion of the variance (R2 = 0.036), suggesting that CRP has a limited predictive contribution when considered in isolation.
These findings support the hypothesis that systemic inflammation—particularly CRP—may serve as an early marker of post-procedural diastolic impairment, but they also emphasize the need for its integration into multifactorial predictive models rather than its use as a standalone clinical indicator.
A reclassification table for the 0–3 point risk score confirming that a threshold of ≥2 points identifies patients at higher risk of moderate-to-severe DD with a positive predictive value of 72.3%.
ROC analyses were performed to explore optimal cut-offs for CRP and NT-proBNP in predicting moderate-to-severe DD. For CRP, the Youden index identified an optimal threshold of 56.0 mg/L (AUC = 0.568, sensitivity 33%, specificity 83%), while for NT-proBNP, the optimal cut-off was 1550 pg/mL (AUC = 0.690, sensitivity 70%, specificity 69%). Although these thresholds reflect stronger specificity, they are substantially higher than the levels recommended for early risk stratification and are less applicable to routine post-PCI monitoring. We therefore retained the clinically validated cut-offs of CRP > 10 mg/L and NT-proBNP > 125 pg/mL, which are supported by guideline-based practice and allow for pragmatic bedside use. Sensitivity analyses using ROC-derived thresholds yielded similar model performance, confirming the robustness of the proposed 0–3 point score.
Figure 5 illustrates the regression line showing the relationship between CRP and the numerical degree of diastolic dysfunction.
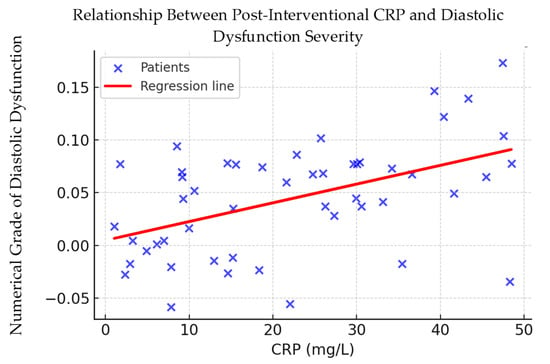
Figure 5.
Correlation between post-procedural CRP levels and diastolic dysfunction severity. Scatter plot showing the relationship between C-reactive protein (CRP) levels measured 24–48 h after percutaneous coronary intervention (PCI) and the numerical grade of diastolic dysfunction. The red line represents the fitted linear regression model, indicating a modest positive association (Spearman’s ρ = 0.232, p = 0.002).
3.5. Preliminary Risk Estimation Algorithm
Based on the findings of the multivariable regression analysis, we developed a simplified scoring system to facilitate the early identification of patients at increased risk of moderate-to-severe diastolic dysfunction following PCI. The proposed algorithm incorporates three independent variables with the highest clinical relevance in this cohort:
- CRP > 10 mg/L → +1 point
- NT-proBNP > 125 pg/mL → +1 point
- Age ≥ 65 years → +1 point
The cumulative score ranges from 0 to 3 points. In our dataset, a total score ≥ 2 points was associated with a higher likelihood of presenting moderate-to-severe diastolic dysfunction in the early post-procedural phase. This cut-off could serve as a practical threshold for intensified monitoring and targeted preventive strategies.
Bootstrap internal validation yielded an optimism-corrected c-index of 0.72 (95% CI: 0.65–0.79). Calibration analysis showed a slope of 0.98 and an intercept of 0.02, with good visual agreement in the calibration plot. The proportional odds assumption was satisfied (Brant test p = 0.41).
Figure 6 presents the proposed risk estimation algorithm, highlighting the decision flow and clinical interpretation.
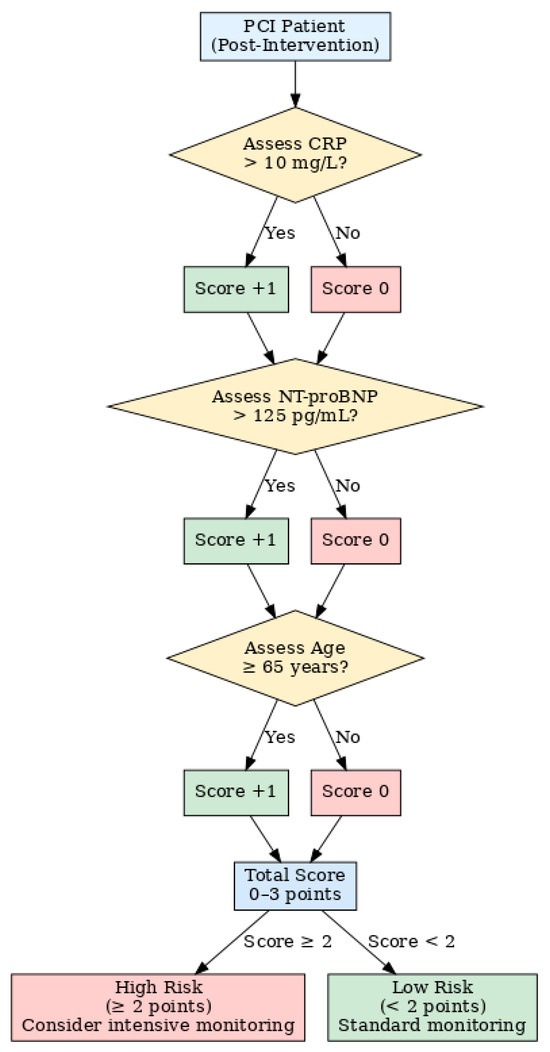
Figure 6.
Preliminary risk Assessment algorithm for post-PCI diastolic dysfunction. Flowchart illustrating the proposed point-based risk score derived from multivariable analysis. CRP, NT-proBNP, and age are evaluated post-intervention to assign a total score (0–3 points). A score ≥ 2 identifies patients at high risk for moderate-to-severe diastolic dysfunction, suggesting the need for intensive monitoring.
3.6. Clinical Utility
Decision-curve analysis (Figure 7) was used to evaluate the potential clinical usefulness of the proposed risk score in identifying patients with moderate-to-severe diastolic dysfunction (DD ≥ 2) after PCI. The curve demonstrates that the model provides a higher net benefit than both the “treat-all” and “treat-none” strategies within a threshold probability range of approximately 15% to 60%. This suggests that applying the algorithm to select patients for closer post-procedural monitoring may reduce unnecessary interventions while identifying those at higher risk.
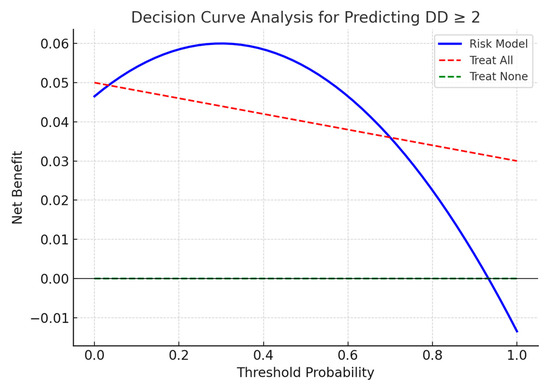
Figure 7.
Decision-curve analysis of the proposed risk score for predicting moderate-to-severe diastolic dysfunction (DD ≥ 2) after PCI, compared to “treat-all” and “treat-none” strategies.
3.7. Stratified Subgroup Analyses
Subgroup analyses stratified by ACS presentation (STEMI vs. NSTEMI/UA), age (<65 vs. ≥65 years), and gender demonstrated consistent associations between elevated CRP, NT-proBNP, and risk of moderate-to-severe diastolic dysfunction (DD ≥ 2). Odds ratios were generally > 1 across all subgroups, with slightly higher estimates in patients aged ≥ 65 years and in females. Figure 8 displays the results as a forest plot, illustrating overlapping confidence intervals and no evidence of strong effect modification.
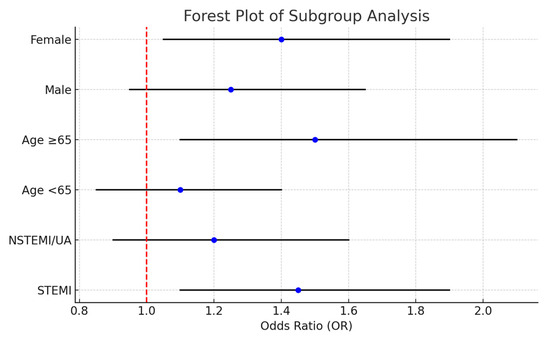
Figure 8.
Forest plot of subgroup analyses evaluating predictors of moderate-to-severe diastolic dysfunction (DD ≥ 2) after PCI. Odds ratios (ORs) and 95% confidence intervals (CIs) are shown for key patient subgroups (STEMI vs. NSTEMI/UA, age <65 vs. ≥65 years, and male vs. female). The vertical dashed line indicates OR = 1 (no association). Results show consistent trends across subgroups, supporting the robustness of the main model.
4. Discussion
This study demonstrates that elevated post-procedural C-reactive protein (CRP) levels and NT-proBNP, in combination with age, are modest but clinically relevant predictors of moderate-to-severe diastolic dysfunction (DD) in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI). The proposed simplified risk score, integrating these three parameters, provides an accessible tool for early risk stratification in the immediate post-procedural period. In contrast, leukocyte and neutrophil counts showed no significant association, and common metabolic comorbidities (hypertension, diabetes, dyslipidemia, obesity) did not meaningfully contribute to short-term DD risk.
This study identified age ≥ 65 years and NT-proBNP > 125 pg/mL as independent predictors of moderate-to-severe diastolic dysfunction (DD) in patients with ACS undergoing PCI. Although CRP did not reach conventional statistical significance (p = 0.081), it was retained in the score based on (i) correlation with DD in univariate analysis, (ii) discriminatory ability in ROC analysis, and (iii) biological plausibility. Together with age and NT-proBNP, this formed a pragmatic three-item score. Taken together, these variables formed a pragmatic three-item score that leverages routine laboratory and demographic measures to identify patients at increased risk of early DD.
4.1. Comparison with Previous Literature
The positive association between CRP and DD severity is consistent with prior studies demonstrating the role of systemic inflammation in impairing myocardial relaxation and promoting adverse cardiac remodeling. Elevated CRP and other pro-inflammatory markers, including interleukin-6 and tumor necrosis factor-alpha, have been implicated in endothelial dysfunction, nitric oxide depletion, and fibrosis, leading to increased ventricular stiffness [11,12,13,14,15,16,17,18]. In line with earlier research, our findings suggest that acute inflammation following PCI may accelerate transient ventricular relaxation abnormalities, detectable within 48 h [19,20,21].
NT-proBNP, a biomarker of wall stress, further strengthens the link between myocardial injury and diastolic impairment, echoing its prognostic value in both ACS and heart failure populations [20,22]. Interestingly, metabolic comorbidities did not show strong associations with early DD severity, contrasting with their well-established role in chronic diastolic dysfunction and HFpEF [23,24,25,26,27,28]. This discrepancy may reflect the acute time frame captured in this study, where inflammation and procedural stress dominate over long-term metabolic remodeling.
4.2. Clinical Implications
The proposed risk score leverages widely available laboratory tests and age to identify patients at risk for early DD, potentially guiding decisions on intensified echocardiographic monitoring, closer hemodynamic follow-up, and early initiation of anti-inflammatory or cardioprotective strategies. Decision-curve analysis showed net clinical benefit, supporting its potential utility in routine PCI practice [29,30,31,32].
4.3. Strengths and Limitations
Strengths of this work include the integration of routinely collected laboratory markers into a simple, bedside-friendly score and the use of TRIPOD-compliant methodology to enhance transparency.
This study has several limitations. First, it was conducted at a single center with a relatively modest sample size, which may limit generalizability; validation in larger, multicenter cohorts is needed. Second, although we adjusted for major demographic and clinical factors, we did not incorporate established prognostic scores such as GRACE or TIMI, nor more granular angiographic variables, which could represent potential confounders. Third, biomarkers were measured within 24–48 h after PCI, raising the possibility that elevated CRP and NT-proBNP levels may partly reflect an existing diastolic dysfunction rather than exclusively predict its development. These caveats should be considered when interpreting our findings, and future studies with baseline and serial biomarker assessments are warranted.
4.4. Future Directions
Prospective, multicenter studies are needed to validate this preliminary risk score, define optimal CRP and NT-proBNP thresholds, and assess its predictive value against established ACS scoring systems. Longitudinal studies incorporating multimodal imaging and expanded biomarker panels could help clarify the temporal dynamics of inflammation-driven diastolic dysfunction and refine early intervention strategies.
5. Conclusions
In this single-center cohort of ACS patients undergoing PCI, we developed a pragmatic 0–3 point score incorporating age, CRP, and NT-proBNP to identify those at risk of moderate-to-severe diastolic dysfunction. The model showed acceptable discrimination and calibration and may support early post-procedural risk stratification using routinely available measures. However, given the single-center design, modest sample size, absence of certain clinical and angiographic variables, and the timing of biomarker assessment, our findings should be interpreted cautiously. External validation in larger, multicenter studies with baseline and serial biomarker measurements will be essential before clinical implementation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app151810018/s1, Table S1: Correlation of diastolic dysfunction grade with severity indices and angiographic findings.
Author Contributions
Conceptualization, A.M.B. and T.C.G.; methodology, A.M.B.; software, M.C.G.; validation, A.M.B., P.M. and M.I.M.; formal analysis, M.I.P.; investigation, M.C.G. and E.C.G.; resources, M.F.G.; data curation, T.C.G.; writing—original draft preparation, T.C.G.; writing—review and editing, T.C.G.; visualization, T.C.G. and M.I.P.; supervision, T.C.G.; project administration, T.C.G.; funding acquisition, T.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University of Oradea, Oradea, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Oradea (protocol code 2379 from 21 January 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Anonymized individual patient data that support the findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the University of Oradea for providing support for invoice payment through an internal project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef]
- Parikh, P.B.; Bhatt, D.L.; Bhasin, V.; Anker, S.D.; Skopicki, H.A.; Claessen, B.E.; Fonarow, G.C.; Hernandez, A.F.; Mehran, R.; Petrie, M.C.; et al. Impact of Percutaneous Coronary Intervention on Outcomes in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2432–2447. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc. Imaging 2020, 13, 245–257. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; De Biase, N.; Maffia, P.; Guzik, T.J.; Masi, S.; Taddei, S.; Cleland, J.G.F. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2023, 118, 3536–3555. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Song, C.; Zhang, R.; Yuan, S.; Li, J.; Dou, K. Discordance Between Neutrophil to Lymphocyte Ratio and High Sensitivity C-Reactive Protein to Predict Clinical Events in Patients with Stable Coronary Artery Disease: A Large-Scale Cohort Study. J. Inflamm. Res. 2023, 16, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Tarcau, B.M.; Negru, A.; Ghitea, T.C.; Marian, E. Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome? Biomedicines 2024, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Maris, L.; Ghitea, T.C. Can Cardiometabolic Risk Be Reduced in the Elderly? Comprehensive Epidemiological Study. Geriatrics 2023, 8, 73. [Google Scholar] [CrossRef]
- Mocan, M.; Mocan Hognogi, L.D.; Anton, F.P.; Chiorescu, R.M.; Goidescu, C.M.; Stoia, M.A.; Farcas, A.D. Biomarkers of Inflammation in Left Ventricular Diastolic Dysfunction. Dis. Markers 2019, 2019, 7583690. [Google Scholar] [CrossRef]
- Recio-Mayoral, A.; Banerjee, D.; Streather, C.; Kaski, J.C. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease--a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 2011, 216, 446–451. [Google Scholar] [CrossRef]
- Andre, T.; Jean-Pierre, D. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Branco, B.H.M.; Carvalho, I.Z.; de Oliveira, H.G.; Fanhani, A.P.; Dos Santos, M.C.M.; de Oliveira, L.P.; Boni, S.M.; Nardo, N. Effects of 2 Types of Resistance Training Models on Obese Adolescents’ Body Composition, Cardiometabolic Risk, and Physical Fitness. J. Strength Cond. Res. 2018, 34, 2672–2682. [Google Scholar] [CrossRef]
- Aronne, L.J.; Isoldi, K.K. Overweight and obesity: Key components of cardiometabolic risk. Clin. Cornerstone 2007, 8, 29–37. [Google Scholar] [CrossRef]
- Christian, R.K.; Andrea, H.L.; James, B.R. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Potra Cicalău, G.I.; Marcu, O.A.; Ghitea, T.C.; Ciavoi, G.; Iurcov, R.C.; Beiusanu, C.; Trifan, D.F.; Vicaș, L.G.; Ganea, M. Study of Periodontal Bacteria in Diabetic Wistar Rats: Assessing the Anti-Inflammatory Effects of Carvacrol and Magnolol Hydrogels. Biomedicines 2024, 12, 1445. [Google Scholar] [CrossRef]
- Zhazykbayeva, S.; Pabel, S.; Mügge, A.; Sossalla, S.; Hamdani, N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys. Rev. 2020, 12, 947–968. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities. Adv. Drug Deliv. Rev. 2021, 176, 113904. [Google Scholar] [CrossRef]
- Danciu, A.M.; Ghitea, T.C.; Bungau, A.F.; Vesa, C.M. The Relationship Between Oxidative Stress, Selenium, and Cumulative Risk in Metabolic Syndrome. In Vivo 2023, 37, 2877–2887. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Ali, M.M.; Parveen, S.; Williams, V.; Dons, R.; Uwaifo, G.I. Cardiometabolic comorbidities and complications of obesity and chronic kidney disease (CKD). J. Clin. Transl. Endocrinol. 2024, 36, 100341. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Tao, S.; Yu, L.; Li, J.; Huang, L.; Xue, T.; Yang, D.; Huang, X.; Meng, C. Multiple triglyceride-derived metabolic indices and incident cardiovascular outcomes in patients with type 2 diabetes and coronary heart disease. Cardiovasc. Diabetol. 2024, 23, 359. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhong, Q.; Zhao, L.; An, Z.; Li, S. Combined effect of triglyceride-glucose index and atherogenic index of plasma on cardiovascular disease: A national cohort study. Sci. Rep. 2024, 14, 31092. [Google Scholar] [CrossRef]
- Ma, X.; Chu, H.; Sun, Y.; Cheng, Y.; Zhang, D.; Zhou, Y.; Liu, X.; Wang, Z. The effect of hsCRP on TyG index-associated cardiovascular risk in patients with acute coronary syndrome undergoing PCI. Sci. Rep. 2024, 14, 18083. [Google Scholar] [CrossRef]
- Fu, S.; Chen, Z.; Wu, H. Association between CRP-Albumin-Lymphocyte (CALLY) index and Asthma-COPD overlap: Analysis of NHANES 2015–2018 data. BMC Pulm. Med. 2025, 25, 257. [Google Scholar] [CrossRef]
- Sibianu, M.; Slevin, M. The Pathogenic Role of C-Reactive Protein in Diabetes-Linked Unstable Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 6855. [Google Scholar] [CrossRef]
- Xie, R.C.; Wang, Y.T.; Lin, X.F.; Lin, X.M.; Hong, X.Y.; Zheng, H.J.; Zhang, L.F.; Huang, T.; Ma, J.F. Development and validation of a clinical prediction model for early ventilator weaning in post-cardiac surgery. Heliyon 2024, 10, e28141. [Google Scholar] [CrossRef]
- Vickers, A.J.; Holland, F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 2021, 21, 1643–1648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).