Betulin-Hippuric Acid Conjugates: Chemistry, Antiproliferative Activity and Mechanism of Action
Abstract
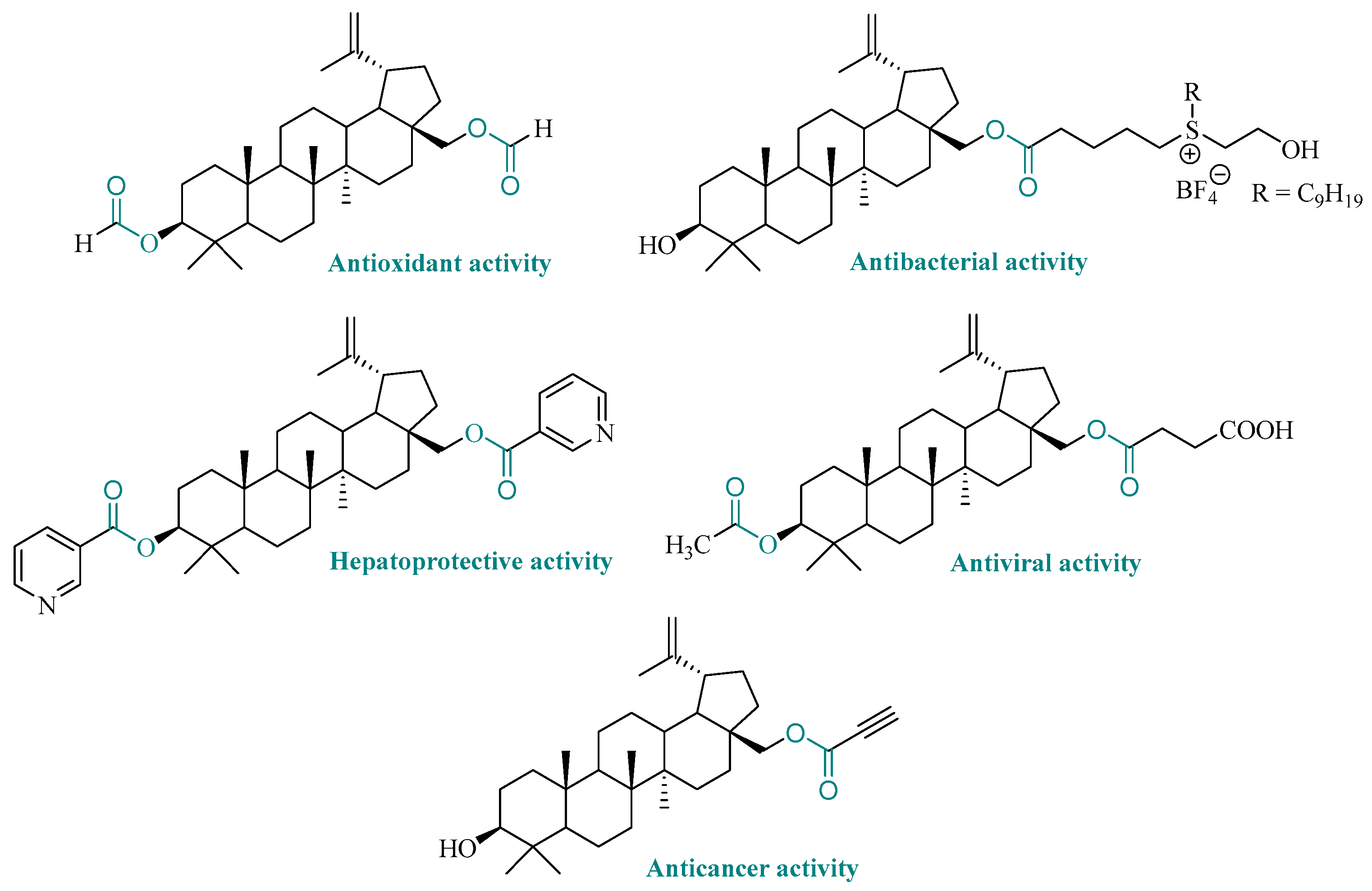
1. Introduction
2. Materials and Methods
2.1. General Techniques
2.2. Synthesis of Betulin–Hippuric Acid Conjugates 4–6 (General Procedure)
2.3. Synthesis of Compound 7
2.4. Lipophilicity Study
2.5. In Silico Study
2.6. Biological Evaluation
2.6.1. Biological Materials and Assays
2.6.2. Determination of Antiproliferative Activity
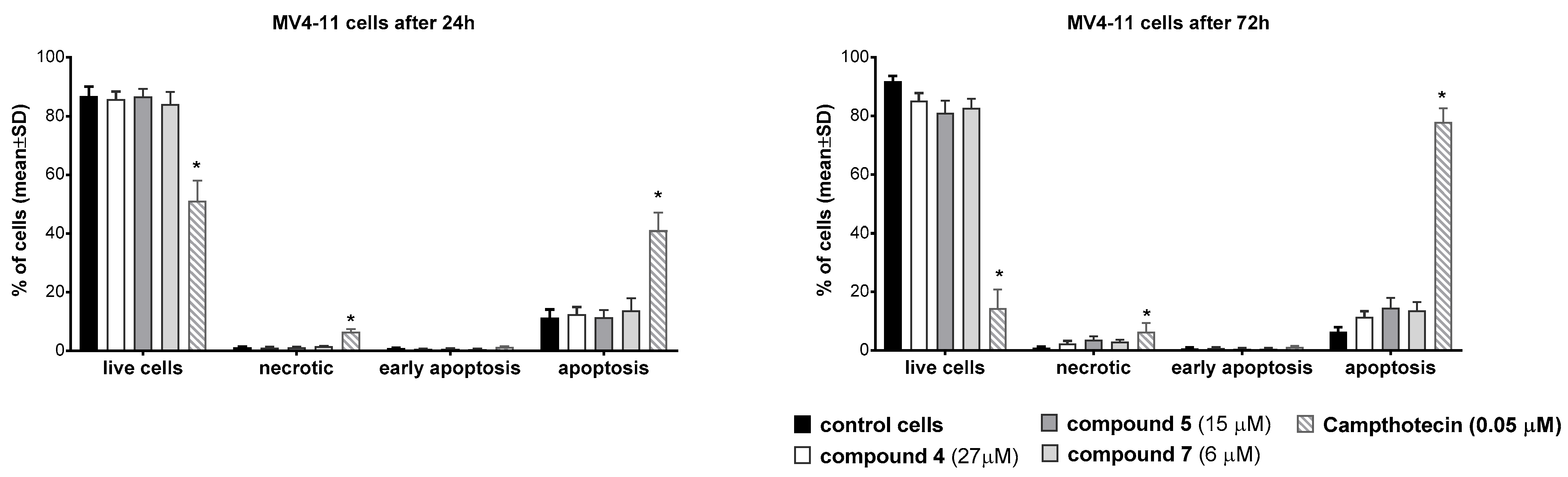
2.6.3. Apoptosis Studies Using Annexin V
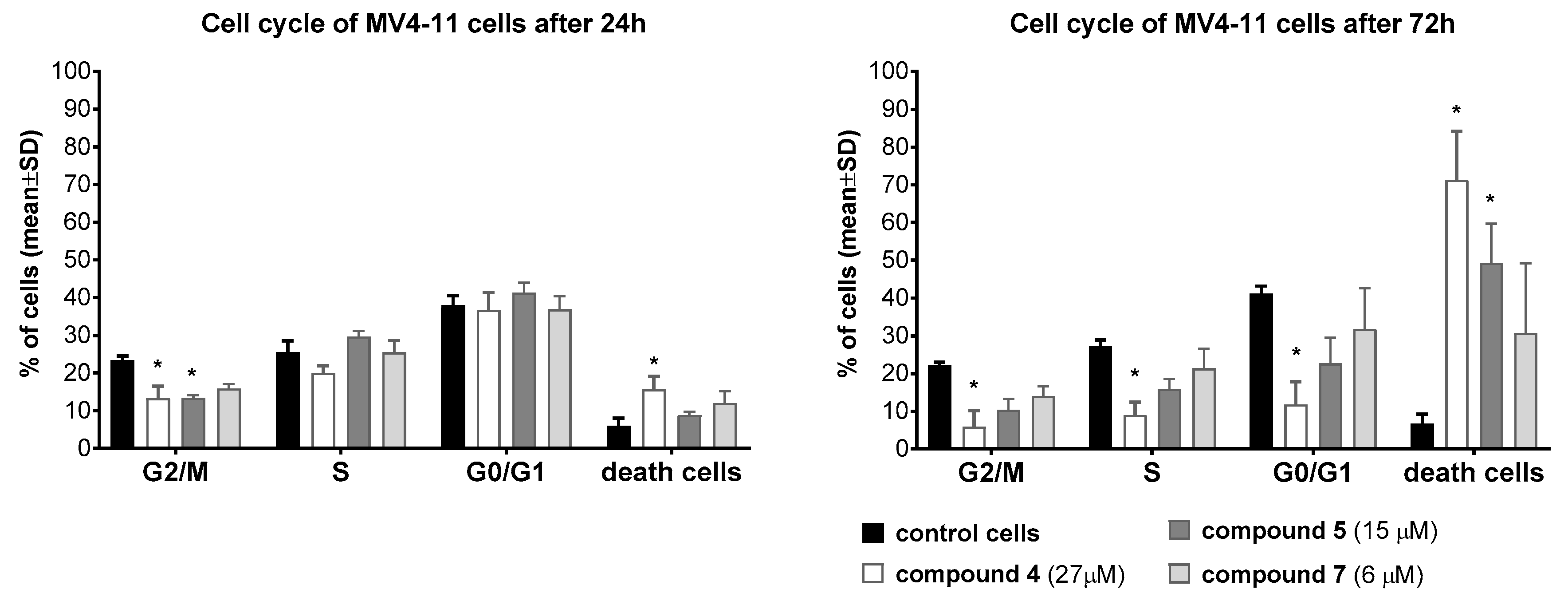
2.6.4. Cell Cycle Study
2.6.5. Statistical Analysis
3. Results and Discussion
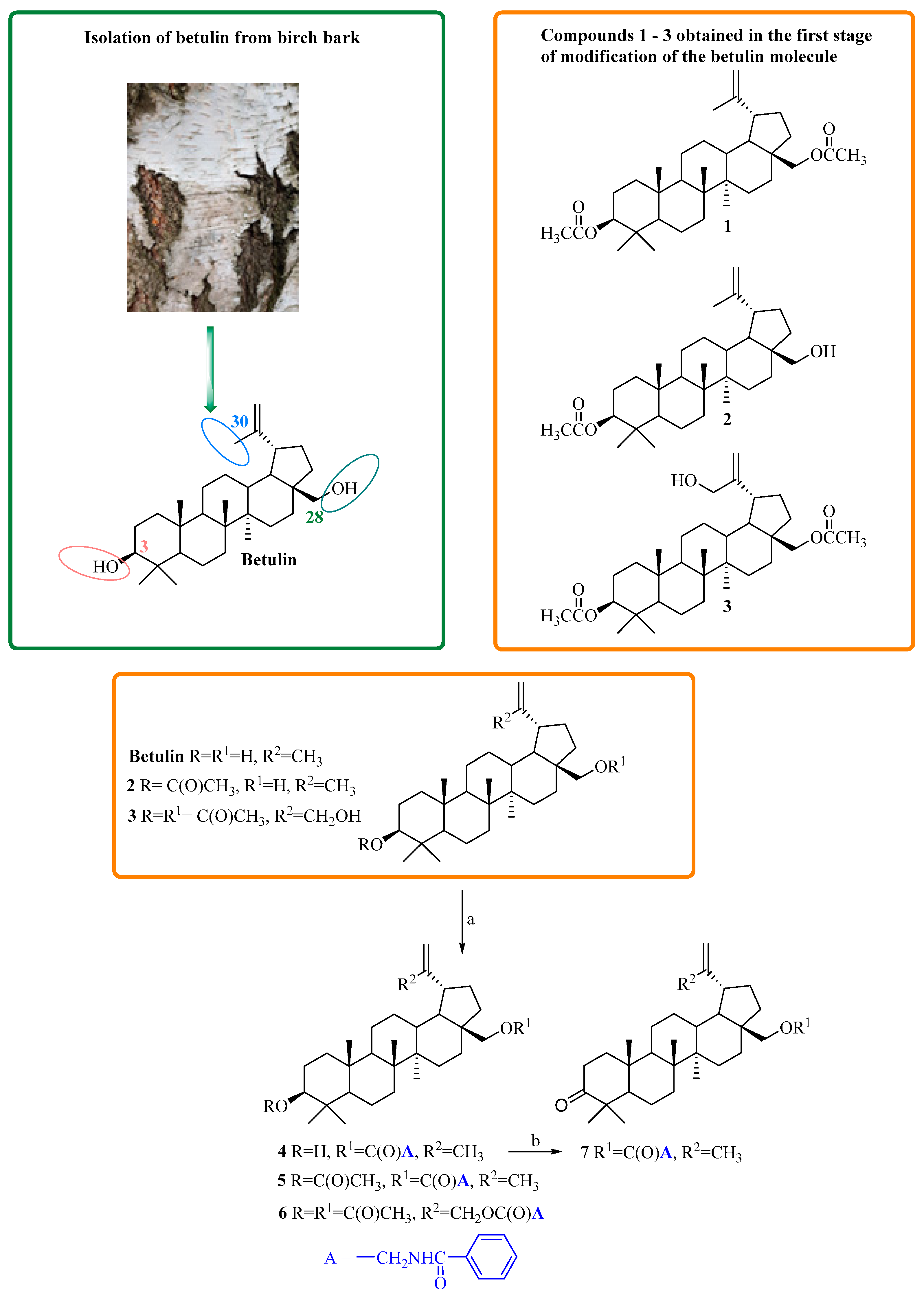
3.1. Chemisty
3.2. Experimental and Theoretical Studies of Lipophilicity
3.3. In Silico Prediction of Betulin Derivative Activity
3.4. Antiproliferative Activity
3.5. Mechanistic Studies
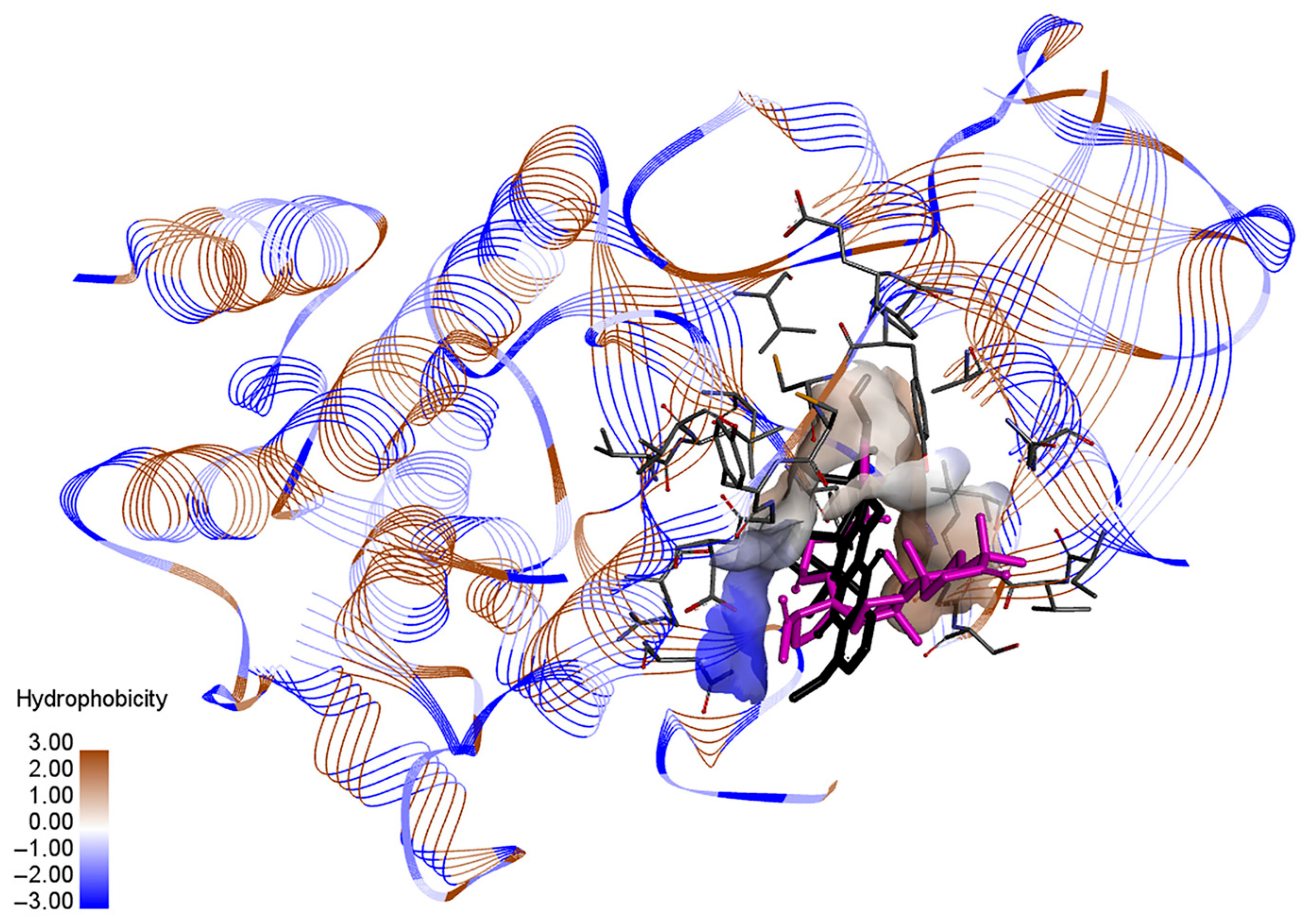
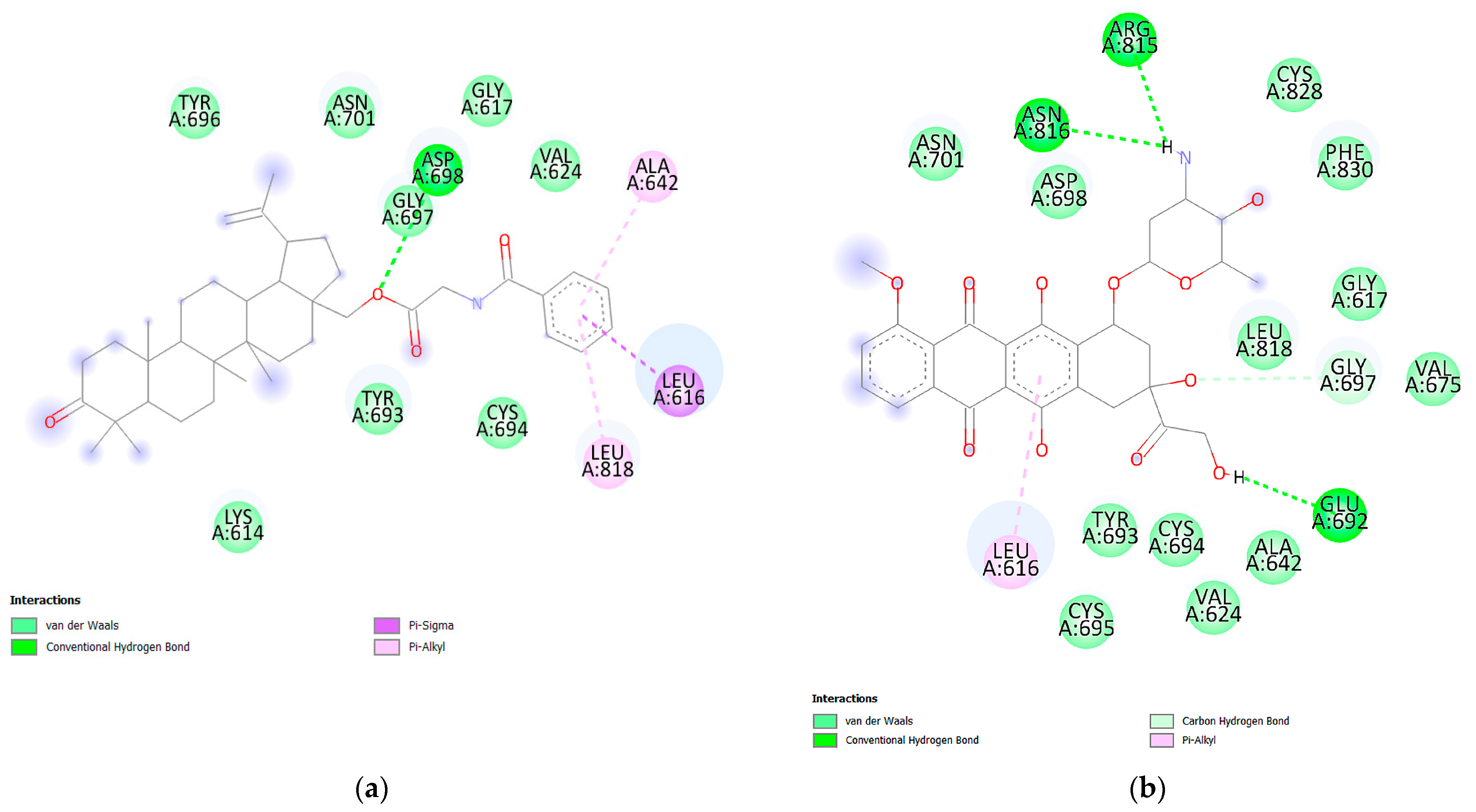
3.6. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| mp | Melting points |
| NMR | Nuclear Magnetic Resonance |
| HRMS | High-Resolution Mass Spectrometry |
| TLC | Thin-layer chromatography |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| PCC | Pyridinium chlorochromate |
| RP-TLC | Reversed-phase thin-layer chromatography |
References
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. Comp. Pharmacol. 2022, 9, 408–422. [Google Scholar] [CrossRef]
- Sarkar, P.; Alheety, M.A.; Srivastava, V. Molecular Docking and ADMET Study of Spice-Derived Potential Phytochemicals against Human DNA Topoisomerase III Alpha. Macromol. Symp. 2023, 407, 2200108. [Google Scholar] [CrossRef]
- Yuriev, E.; Holien, J.; Ramsland, P.A. Improvements, trends and new ideas in molecular docking 2012–2013 in review. J. Mol. Recognit. 2015, 28, 581–604. [Google Scholar] [CrossRef]
- Takibayeva, A.T.; Zhumabayeva, G.K.; Bakibaev, A.A.; Demets, O.V.; Lyapunova, M.V.; Mamaeva, E.A.; Yerkassov, R.S.; Kassenov, R.Z.; Ibrayev, M.K. Methods of Analysis and Identification of Betulin and Its Derivatives. Molecules 2023, 28, 5946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y. Exploring Ester Prodrugs: A Comprehensive Review of Approaches, Applications, and Methods. Pharmacol. Pharm. 2024, 15, 269–284. [Google Scholar] [CrossRef]
- Larsen, E.M.; Johnson, R.J. Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Dev. Res. 2019, 80, 33–47. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2023, 10, e23416. [Google Scholar] [CrossRef] [PubMed]
- Arrous, S.; Boudebouz, I.; Parunov, I.; Plotnikov, E.; Voronova, O. New Synthetic Method and Antioxidant Activity of Betulin Diformate and Allobetulin Formate. Chem. Nat. Compd. 2019, 55, 1094–1097. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, R.; Ma, Z.; Zuo, W.; Zhu, M. S-Alkylated sulfonium betulin derivatives: Synthesis, antibacterial activities, and wound healing applications. Bioorganic Chem. 2025, 154, 108056. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Nawrot, D.A.; Alakurtti, S.; Ghemtio, L.; Yli-Kauhaluoma, J.; Tammela, P. Screening and Characterisation of Antimicrobial Properties of Semisynthetic Betulin Derivatives. PLoS ONE 2014, 9, e102696. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, Y.; Wang, R.; Zhu, M. Synthesis of polymerizable betulin maleic diester derivative for dental restorative resins with antibacterial activity. Dent. Mater. 2024, 40, 941–950. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Smirnova, I.E.; Medvedeva, N.I.; Lopatina, T.V.; Chudov, I.V.; Sharipov, A.R.; Ziganshin, A.S.; Thao, T.T.P. Hepatoprotective Activity of Betulin and Dipterocarpol Derivatives. Russ. J. Bioorganic Chem. 2019, 45, 558–565. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Smirnova, I.E.; Baltina, L.A.; Boreko, E.I.; Savinova, O.V.; Pokrovskii, A.G. Antiviral Activity of Acyl Derivatives of Betulin and Betulinic and Dihydroquinopimaric Acids. Russ. J. Bioorganic Chem. 2018, 44, 740–744. [Google Scholar] [CrossRef]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Wietrzyk, J.; Sadowska, J.; Boryczka, S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Shakhtshneider, T.P.; Mikhailenko, M.A.; Malyar, Y.N.; Kichkailo, A.S.; Drebushchak, V.A.; Kuznetsov, B.N. Preparation and Antitumor Activity of Betulin Dipropionate and its Composites. Biointerface Res. Appl. Chem. 2022, 12, 6873–6894. [Google Scholar] [CrossRef]
- Kozicka, D.; Krześniak, M.; Grymel, M.; Adamek, J.; Łasut-Szyszka, B.; Cichoń, T.; Kuźnik, A. Ultrasound-assisted synthesis of new bisphosphonate–betulin conjugates and preliminary evaluation of their cytotoxic activity. RSC Adv. 2025, 15, 4086–4094. [Google Scholar] [CrossRef]
- Rzepka, Z.; Bębenek, E.; Chrobak, E.; Wrześniok, D. Synthesis and Anticancer Activity of Indole-Functionalized Derivatives of Betulin. Pharmaceutics 2022, 14, 2372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.-M. Recent Updates on Anticancer Activity of Betulin and Betulinic Acid Hybrids (A Review). Russ. J. Gen. Chem. 2023, 93, 610–627. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, Z.; Chen, Y.; Ma, Z.; Zuo, W.; Zhu, M. Synthesis and Cytotoxicity Evaluation of Betulin Cycloolefin Derivatives by Ruthenium-Catalyzed Ring-Closing Metathesis. J. Nat. Prod. 2023, 86, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Snyder, J.K. Preparation of a 24-nor-1,4-dien-3-one triterpene derivative from betulin: A new route to 24-nortriterpene analogues. J. Org. Chem. 2002, 67, 2864–2873. [Google Scholar] [CrossRef]
- Chrobak, E.; Marciniec, K.; Dąbrowska, A.; Pęcak, P.; Bębenek, E.; Kadela-Tomanek, M.; Bak, A.; Jastrzębska, M.; Boryczka, S. New phosphorus analogs of bevirimat: Synthesis, evaluation of anti-HIV-1 activity and molecular docking study. Int. J. Mol. Sci. 2019, 20, 5209. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Chung, B.Y.; Bentley, M.D.; Alford, A.R. Colorado potato beetle antifeedants by simple modification of the birchbark triterpene betulin. J. Agric. Food Chem. 1995, 43, 2513–2516. [Google Scholar] [CrossRef]
- Chrobak, E.; Świtalska, M.; Wietrzyk, J.; Bębenek, E. Synthesis, Structure, and In Vitro Biological Evaluation of Semi-Synthetic Derivatives of Betulin. Appl. Sci. 2024, 14, 9970. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, characterization, and in vitro cancer cell growth inhibition evaluation of novel phosphatidylcholines with anisic and veratric acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13, 1105. [Google Scholar] [CrossRef]
- Boparai, A.; Niazi, J.; Bajwa, N.; Singh, P.A. Betulin a pentacyclic triterpenoid: An hour to rethink the compound. Open Access J. Trans. Med. Res. 2017, 1, 53–59. [Google Scholar] [CrossRef]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A solvent-reagent selection guide for Steglich-type esterification of carboxylic acids. Green. Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Rageh, A.H.; Atia, N.N.; Abdel-Rahman, H.A. Lipophilicity estimation of statins as a decisive physicochemical parameter for their hepato-selectivity using reversed-phase thin layer chromatography. J. Pharm. Biomed. Anal. 2017, 142, 7–14. [Google Scholar] [CrossRef]
- SwissADME SwissDrugDesign. Available online: http://www.swissadme.ch/ (accessed on 28 May 2025).
- Molinspiration Chemoinformatics, Slovak Republic. Available online: http://www.molinspiration.com/services/logp.html (accessed on 27 May 2025).
- ChemDraw: ChemDraw Ultra, version 12; PerkinElmer Informatics; CambridgeSoft: Cambridge, MA, USA, 2007.
- Yalçin-Özkat, G. Computational studies with flavonoids and terpenoids as BRPF1 inhibitors: In silico biological activity prediction, molecular docking, molecular dynamics simulations, MM/PBSA calculations. SAR QSAR Environ. Res. 2022, 33, 533–550. [Google Scholar] [CrossRef]
- Islam, S.; Hosen, M.A.; Ahmad, S.; Qamar, M.T.U.; Dey, S.; Hasan, I.; Fujii, Y.; Ozeki, Y.; Kawsar, S.M.A. Synthesis, antimicrobial, anticancer activities, PASS prediction, molecular docking, molecular dynamics and pharmacokinetic studies of designed methyl α-D-glucopyranoside esters. J. Mol. Struct. 2022, 1260, 132761. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Hushmandi, K.; Mirzaei, S.; Bokaie, S.; Bigham, A.; Makvandi, P.; Rabiee, N.; Thakur, V.K.; Kumar, A.P.; Sharifi, E.; et al. Chitosan-based nanoscale systems for doxorubicin delivery: Exploring biomedical application in cancer therapy. Bioeng. Transl. Med. 2023, 8, e10325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ding, X.F.; Shen, J.Y.; Zhang, X.P.; Ding, X.W.; Xu, B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J. Zhejiang Univ. Sci. B 2017, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Arifian, H.; Maharani, R.; Megantara, S.; Gazzali, A.M.; Muchtaridi, M. Amino-Acid-Conjugated Natural Compounds: Aims, Designs and Results. Molecules 2022, 27, 7631. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Drąg, M.; Poręba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef]
- Drąg-Zalesińka, M.; Wysocka, T.; Borska, S.; Drąg, M.; Poręba, M.; Choromańska, A.; Kulbacka, J.; Saczko, J. The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment—In vitro studies. Biomed. Pharmacother. 2015, 72, 91–97. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Watz, C.G.; Bora, L.; Dehelean, C.A.; Diaconeasa, Z.; Dinu, S.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Danciu, C. Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation. Plants 2024, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xu, X.; Liu, J.; Jia, Q.; Ke, C.; Zhang, H.; Xu, C.; Ou, E.; Tan, W.; Zhao, Y. Mitochondria-Targeted Triphenylphosphonium Conjugated C-3 Modified Betulin: Synthesis, Antitumor Properties and Mechanism of Action. ChemMedChem 2022, 17, e202100659. [Google Scholar] [CrossRef]
- Hata, K.; Hori, K.; Ogasawara, H.; Takahashi, S. Anti-leukemia activities of Lup-28-al-20(29)-en-3-one, a lupane triterpene. Toxicol. Lett. 2003, 143, 1–7. [Google Scholar] [CrossRef]
- Cao, T.; Jiang, N.; Liao, H.; Shuai, X.; Su, J.; Zheng, Q. The FLT3-ITD mutation and the expression of its downstream signaling intermediates STAT5 and Pim-1 are positively correlated with CXCR4 expression in patients with acute myeloid leukemia. Sci. Rep. 2019, 9, 12209. [Google Scholar] [CrossRef]
- Du, K.; He, Y.; Fu, J.; Xue, G.; Zhang, Z.; Li, X.; Zhi, Y. Design, Synthesis, and Biological Evaluation of Potent FLT3 Inhibitor for Acute Myeloid Leukemia (AML) Treatment. Drug Dev. Res. 2025, 86, e70119. [Google Scholar] [CrossRef] [PubMed]
- Larrosa-Garcia, M.; Baer, M.R. FLT3 Inhibitors in Acute Myeloid Leukemia: Current Status and Future Directions. Mol. Cancer Ther. 2017, 16, 991–1001. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 10 June 2025).
- Kawase, T.; Nakazawa, T.; Eguchi, T.; Tsuzuki, H.; Ueno, Y.; Amano, Y.; Suzuki, T.; Mori, M.; Yoshida, T. Effect of Fms-like tyrosine kinase 3 (FLT3) ligand (FL) on antitumor activity of gilteritinib, a FLT3 inhibitor, in mice xenografted with FL-overexpressing cells. Oncotarget 2019, 10, 6111–6123. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A. 03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dessault Systemes BIOVIA. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/ (accessed on 20 July 2025).
- Egbuna, C.; Patrick-Iwuanyanwu, K.C.; Onyeike, E.N.; Khan, J.; Alshehri, B. FMS-like tyrosine kinase-3 (FLT3) inhibitors with better binding affinity and ADMET properties than sorafenib and gilteritinib against acute myeloid leukemia: In silico studies. J. Biomol. Struct. Dyn. 2021, 40, 12248–12259. [Google Scholar] [CrossRef]
| Compound | RM0 | r | b | logPTLC |
|---|---|---|---|---|
| betulin | 5.07 | 0.993 | −0.0573 | 6.46 |
| 2 | 6.24 | 0.995 | −0.0685 | 7.82 |
| 3 | 5.75 | 0.997 | −0.0652 | 7.25 |
| 4 | 5.33 | 0.988 | −0.0623 | 6.76 |
| 5 | 6.63 | 0.989 | −0.0753 | 8.27 |
| 6 | 6.04 | 0.994 | −0.0699 | 7.58 |
| 7 | 5.41 | 0.996 | −0.0627 | 6.85 |
| Compound | iLogP a | MLOGP a | WLOGP a | miLogP b | CLogP c | XLOGP3 a |
|---|---|---|---|---|---|---|
| betulin | 4.47 | 6.00 | 7.00 | 7.16 | 8.53 | 8.28 |
| 2 | 4.74 | 6.20 | 7.57 | 7.87 | 9.47 | 8.86 |
| 3 | 4.91 | 5.60 | 7.11 | 7.33 | 8.63 | 8.18 |
| 4 | 5.03 | 6.09 | 7.98 | 8.39 | 10.15 | 9.86 |
| 5 | 5.47 | 6.35 | 8.55 | 8.78 | 11.10 | 10.44 |
| 6 | 6.12 | 5.83 | 8.09 | 8.50 | 10.26 | 9.76 |
| 7 | 4.86 | 6.01 | 8.19 | 8.26 | 9.75 | 9.54 |
| Activity | Pa (Pi) | ||||
|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | Doxorubicin | |
| Antineoplastic | 0.871 (0.002) | 0.901 (0.005) | 0.900 (0.005) | 0.881 (0.005) | 0.960 (0.004) |
| Antineoplastic (lung cancer) | 0.738 (0.005) | 0.785 (0.004) | 0.759 (0.005) | 0.709 (0.005) | 0.311 (0.038) |
| Antineoplastic (melanoma) | 0.736 (0.004) | 0.759 (0.004) | 0.747 (0.004) | 0.786 (0.003) | 0.215 (0.049) |
| Antineoplastic (colorectal cancer) | 0.693 (0.005) | 0.783 (0.005) | 0.796 (0.005) | 0.730 (0.005) | - |
| Antineoplastic (colon cancer) | 0.687 (0.005) | 0.780 (0.005) | 0.794 (0.005) | 0.727 (0.005) | - |
| Antineoplastic (ovarian cancer) | 0.613 (0.005) | 0.666 (0.004) | 0.584 (0.005) | 0.602 (0.005) | - |
| Antineoplastic (breast cancer) | 0.604 (0.010) | 0.666 (0.007) | 0.569 (0.013) | 0.614 (0.009) | 0.372 (0.037) |
| Antineoplastic (cervical cancer) | 0.601 (0.004) | 0.659 (0.004) | 0.719 (0.004) | 0.609 (0.004) | 0.245 (0.028) |
| Antineoplastic (thyroid cancer) | 0.579 (0.001) | 0.621 (0.001) | 0.570 (0.001) | 0.558 (0.001) | - |
| Antineoplastic (endocrine cancer) | 0.555 (0.002) | 0.591 (0.002) | 0.546 (0.002) | 0.537 (0.002) | - |
| Prostate cancer treatment | 0.432 (0.017) | 0.475 (0.013) | 0.517 (0.008) | 0.448 (0.015) | - |
| Antileukemic | 0.414 (0.023) | 0.456 (0.018) | 0.349 (0.034) | 0.490 (0.015) | 0.478 (0.016) |
| Antineoplastic (pancreatic cancer) | 0.355 (0.030) | 0.351 (0.031) | 0.276 (0.079) | 0.398 (0.017) | 0.224 (0.143) |
| Antineoplastic (lymphocytic leukemia) | 0.289 (0.019) | 0.300 (0.017) | 0.392 (0.009) | 0.319 (0.015) | - |
| Antineoplastic (liver cancer) | 0.250 (0.042) | - | - | 0.187 (0.102) | 0.257 (0.038) |
| Antineoplastic (squamous cell carcinoma) | 0.177 (0.027) | 0.199 (0.023) | 0.141 (0.039) | 0.207 (0.021) | 0.200 (0.022) |
| Antineoplastic (glioblastoma multiforme) | - | 0.200 (0.063) | 0.185 (0.083) | 0.201 (0.061) | - |
| Antineoplastic (renal cancer) | - | 0.150 (0.113) | 0.147 (0.119) | - | 0.231 (0.026) |
| Antineoplastic (uterine cancer) | - | - | - | - | 0.620 (0.003) |
| Antineoplastic (bladder cancer) | - | - | - | - | 0.483 (0.003) |
| Antineoplastic (brain cancer) | - | - | - | - | 0.317 (0.020) |
| Antineoplastic (sarcoma) | - | - | - | - | 0.311 (0.015) |
| Compound | IC50 [µM] ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| MV4-11 | A549 | MCF-7 | PC-3 | HCT116 | MiaPaca-2 | Hs249T | MCF-10A | |
| 4 | 17.6 ± 4.5 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 5 | 9.7 ± 0.6 | 98.9 ± 21.8 | >100 | >100 | 92.9 ± 27.7 | >100 | >100 | >100 |
| 6 | 31.4 ± 4.3 | >100 | >100 | >100 | 32.2 ± 2.1 | >100 | >100 | >100 |
| 7 | 4.2 ± 1.2 | >100 | >100 | 84.9 ± 31.2 | 89.5 ± 14.6 | >100 | 96.5 ± 33.4 | >100 |
| hippuric acid | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| betulin | 32.8 ± 12.9 | 14.0 ± 1.1 | 43.6 ± 15.8 | 51.5 ± 9.9 | 37.7 ± 7.5 | >100 | 58.5 ± 15.6 | 98.5 ± 0.9 |
| doxorubicin | 0.021 ± 0.008 | 0.040 ± 0.012 | 0.150 ± 0.015 | 1.27 ± 0.2 | 0.118 ± 0.02 | - | - | 0.114 ± 0.013 |
| Compound | (ΔG) [kcal/mol] |
|---|---|
| 4 | −6.9 |
| 5 | −6.3 |
| 6 | −4.8 |
| 7 | −7.3 |
| doxorubicin | −7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świtalska, M.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Bębenek, E. Betulin-Hippuric Acid Conjugates: Chemistry, Antiproliferative Activity and Mechanism of Action. Appl. Sci. 2025, 15, 9824. https://doi.org/10.3390/app15179824
Świtalska M, Chrobak E, Kadela-Tomanek M, Wietrzyk J, Bębenek E. Betulin-Hippuric Acid Conjugates: Chemistry, Antiproliferative Activity and Mechanism of Action. Applied Sciences. 2025; 15(17):9824. https://doi.org/10.3390/app15179824
Chicago/Turabian StyleŚwitalska, Marta, Elwira Chrobak, Monika Kadela-Tomanek, Joanna Wietrzyk, and Ewa Bębenek. 2025. "Betulin-Hippuric Acid Conjugates: Chemistry, Antiproliferative Activity and Mechanism of Action" Applied Sciences 15, no. 17: 9824. https://doi.org/10.3390/app15179824
APA StyleŚwitalska, M., Chrobak, E., Kadela-Tomanek, M., Wietrzyk, J., & Bębenek, E. (2025). Betulin-Hippuric Acid Conjugates: Chemistry, Antiproliferative Activity and Mechanism of Action. Applied Sciences, 15(17), 9824. https://doi.org/10.3390/app15179824





