XANES Absorption Spectra of Penta-Graphene and Penta-SiC2 with Different Terminations: A Computational Study
Abstract
1. Introduction
2. Computational Methods
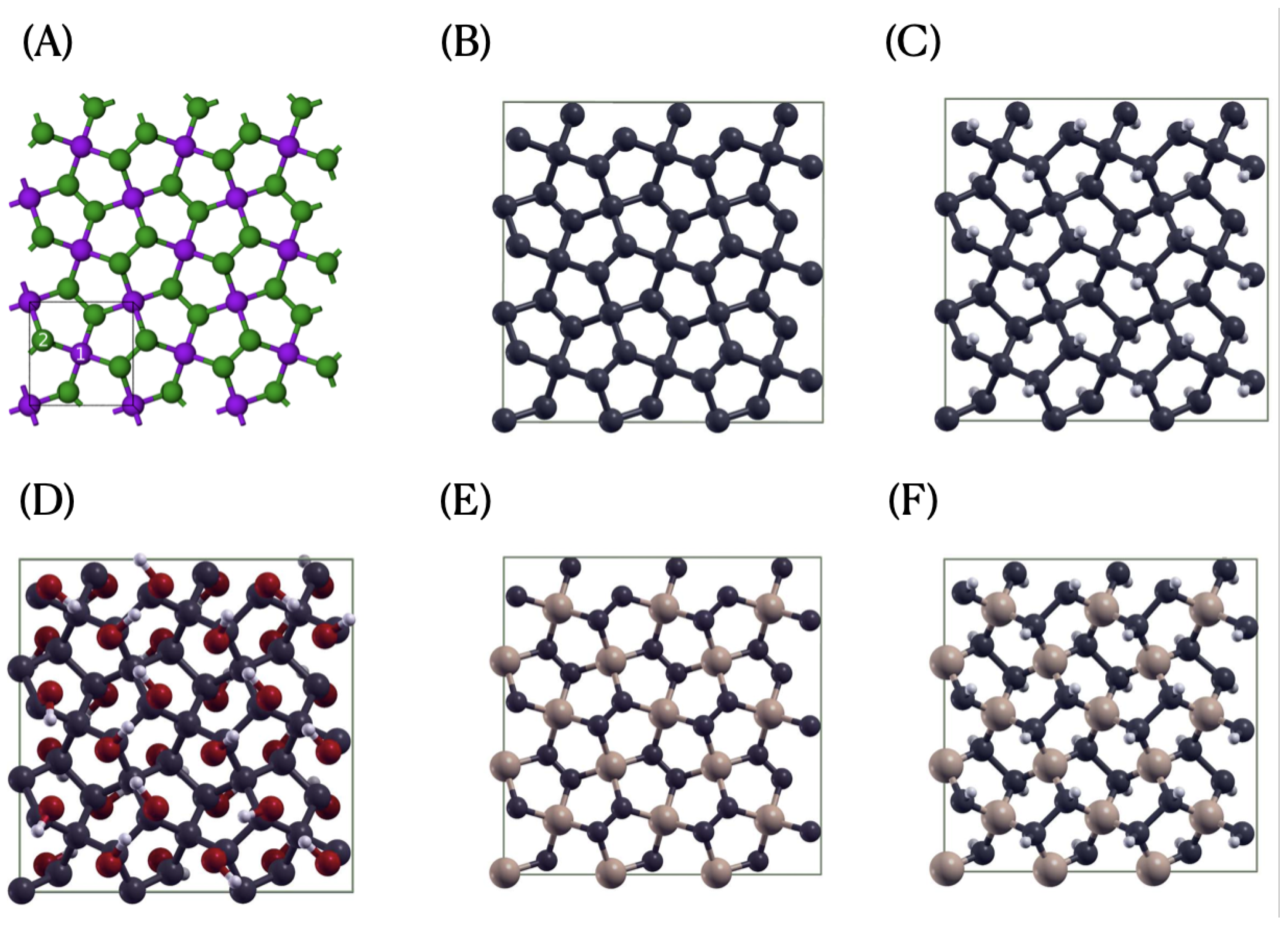
2.1. Penta-Graphene and Penta-SiC2 Models
2.2. Electronic Band Structure, Density of States, and Absorption Spectra Calculations
3. Results and Discussion
3.1. Structural and Electronic Properties
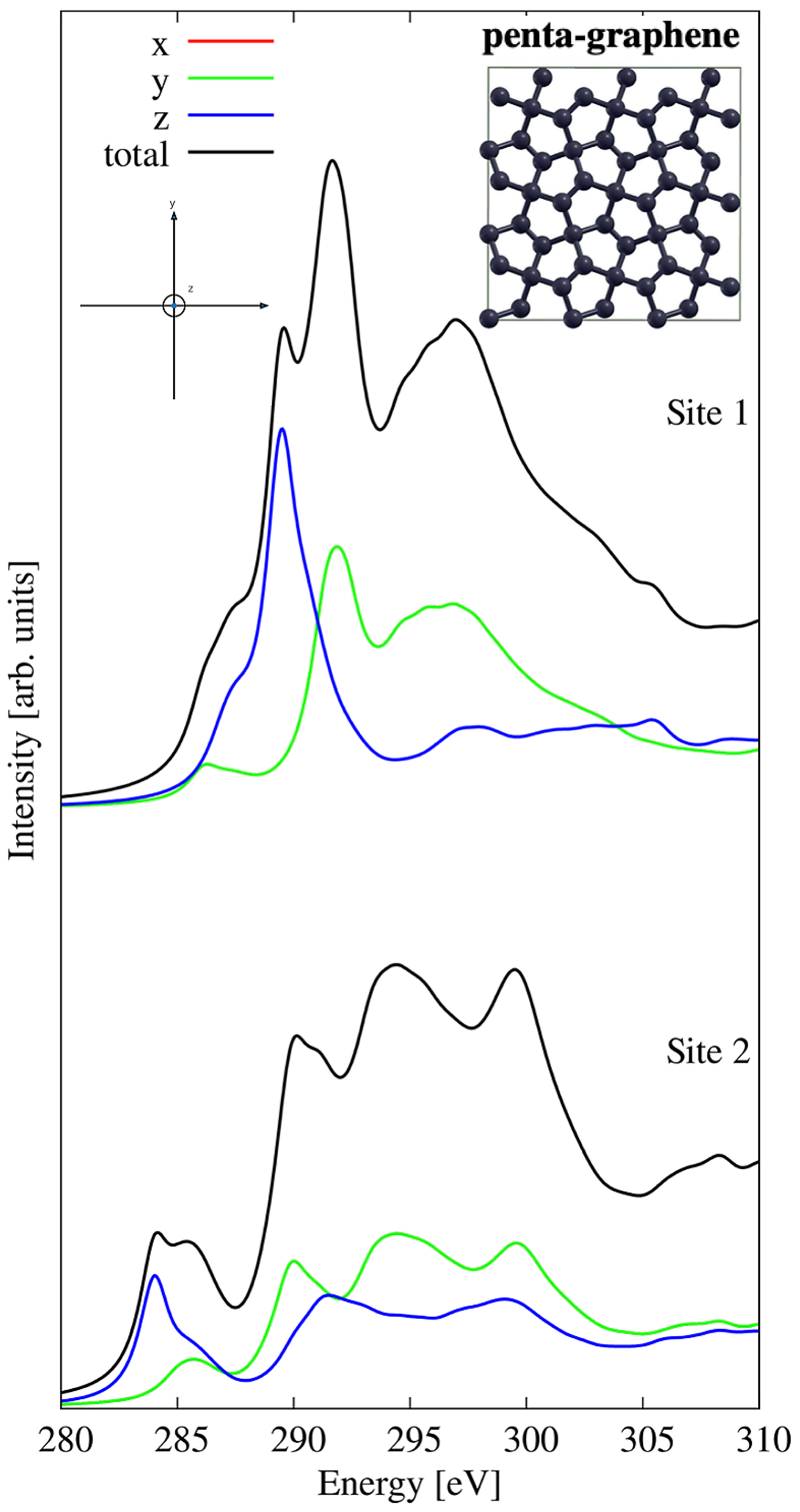
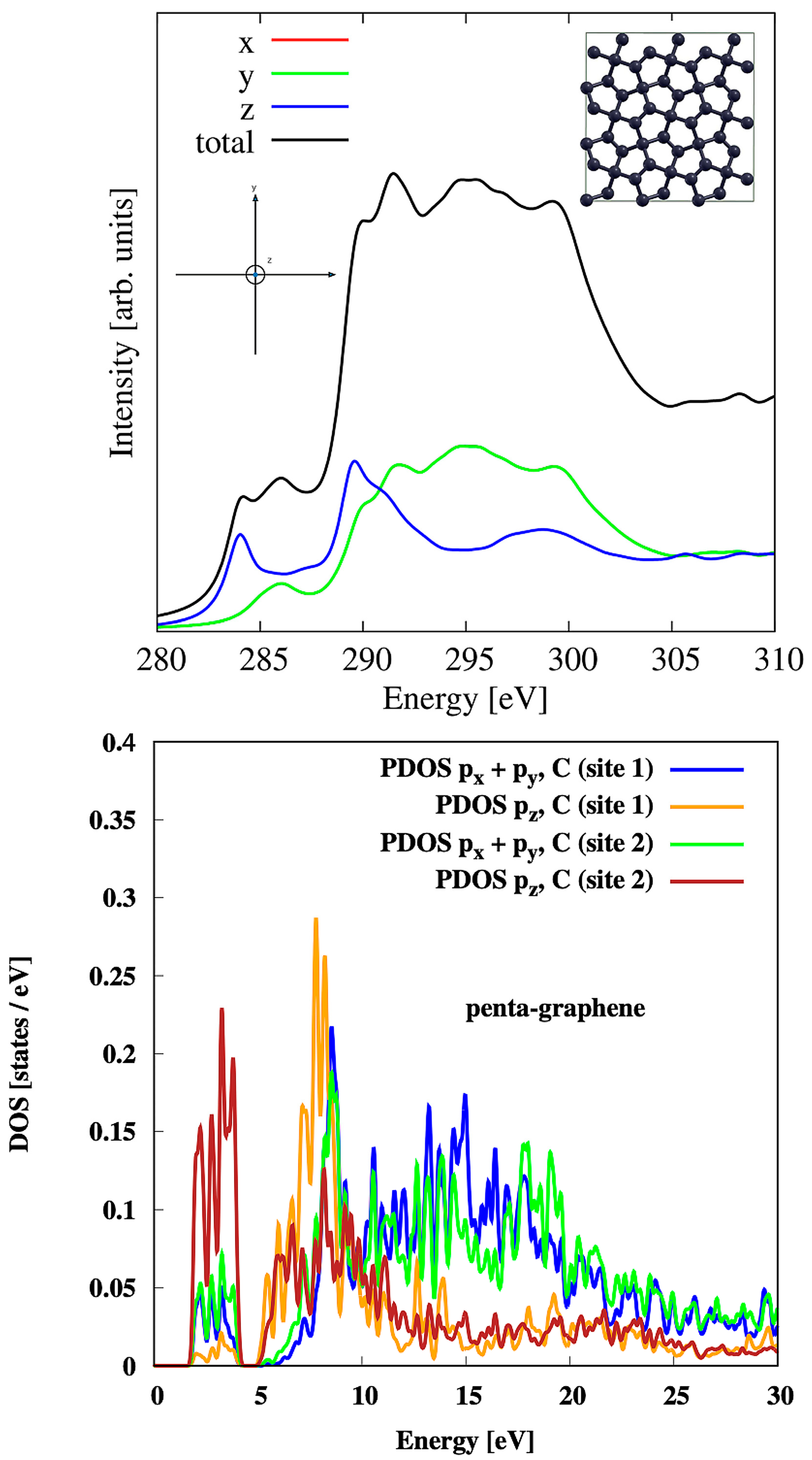
3.2. Penta-Graphene
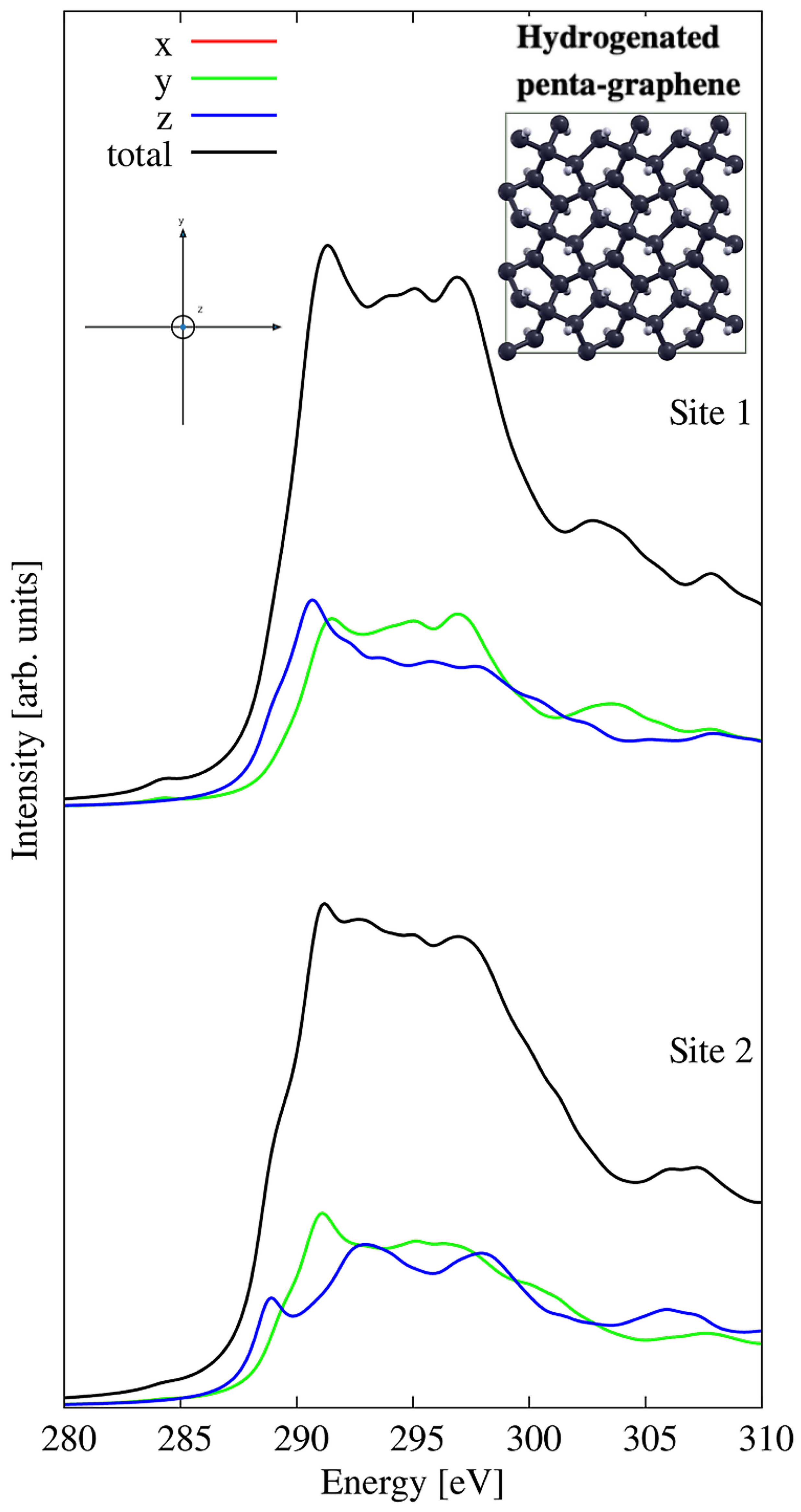
3.3. Hydrogenated Penta-Graphene
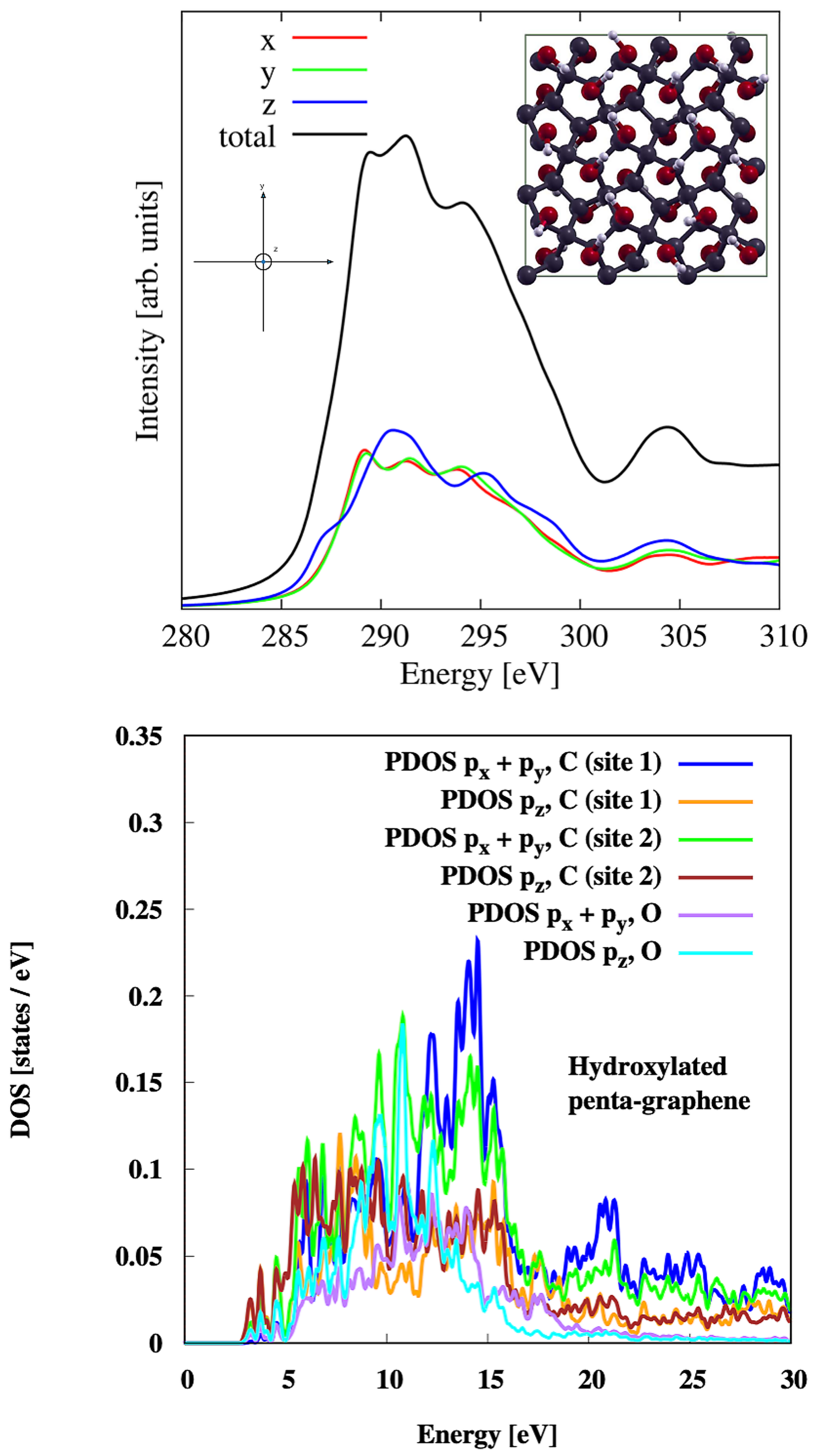
3.4. Hydroxylated Penta-Graphene
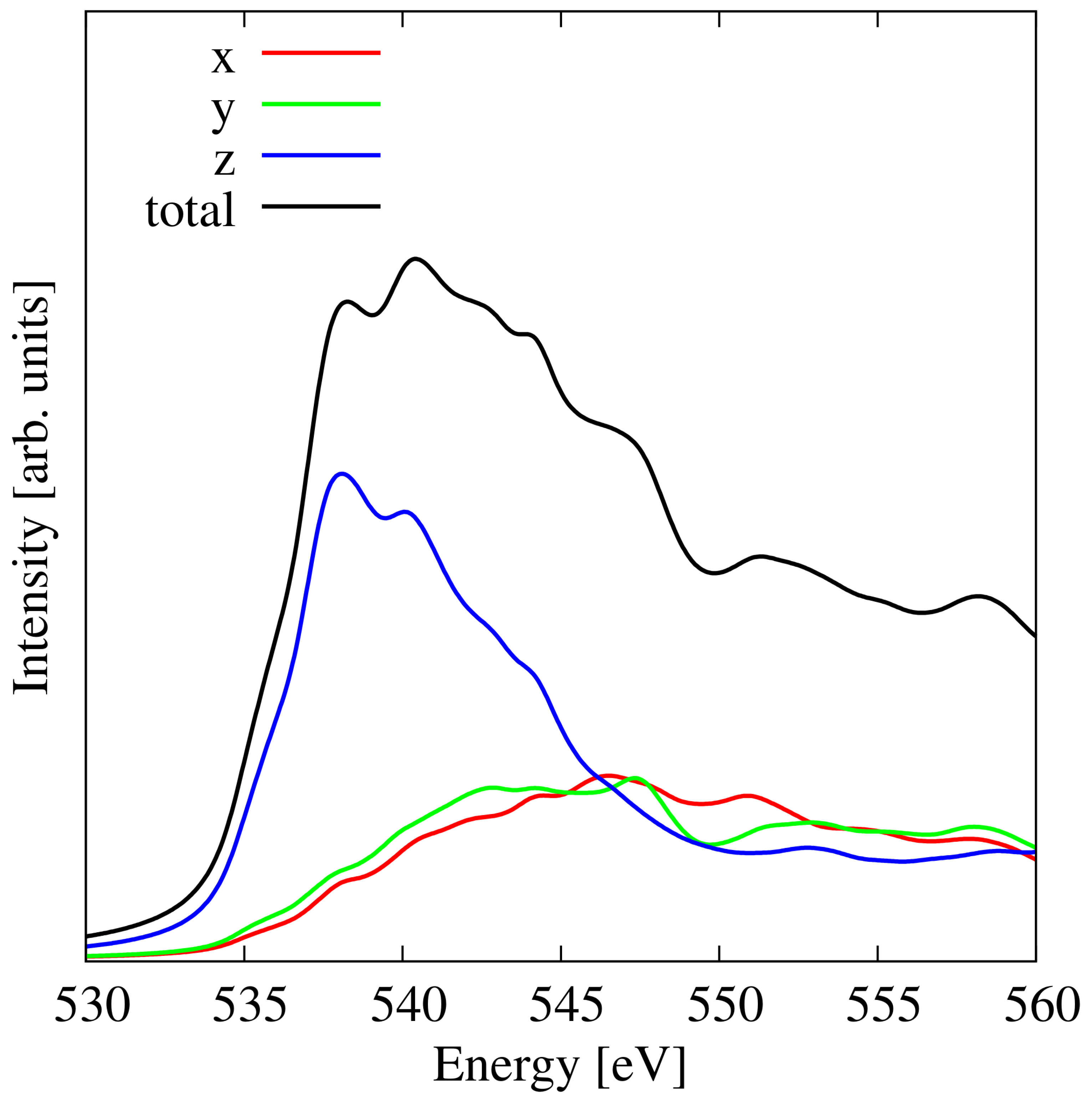
3.5. Silicon Substitution in Pristine Penta-Graphene
3.6. Silicon Substitution in Hydrogenated Penta-Graphene
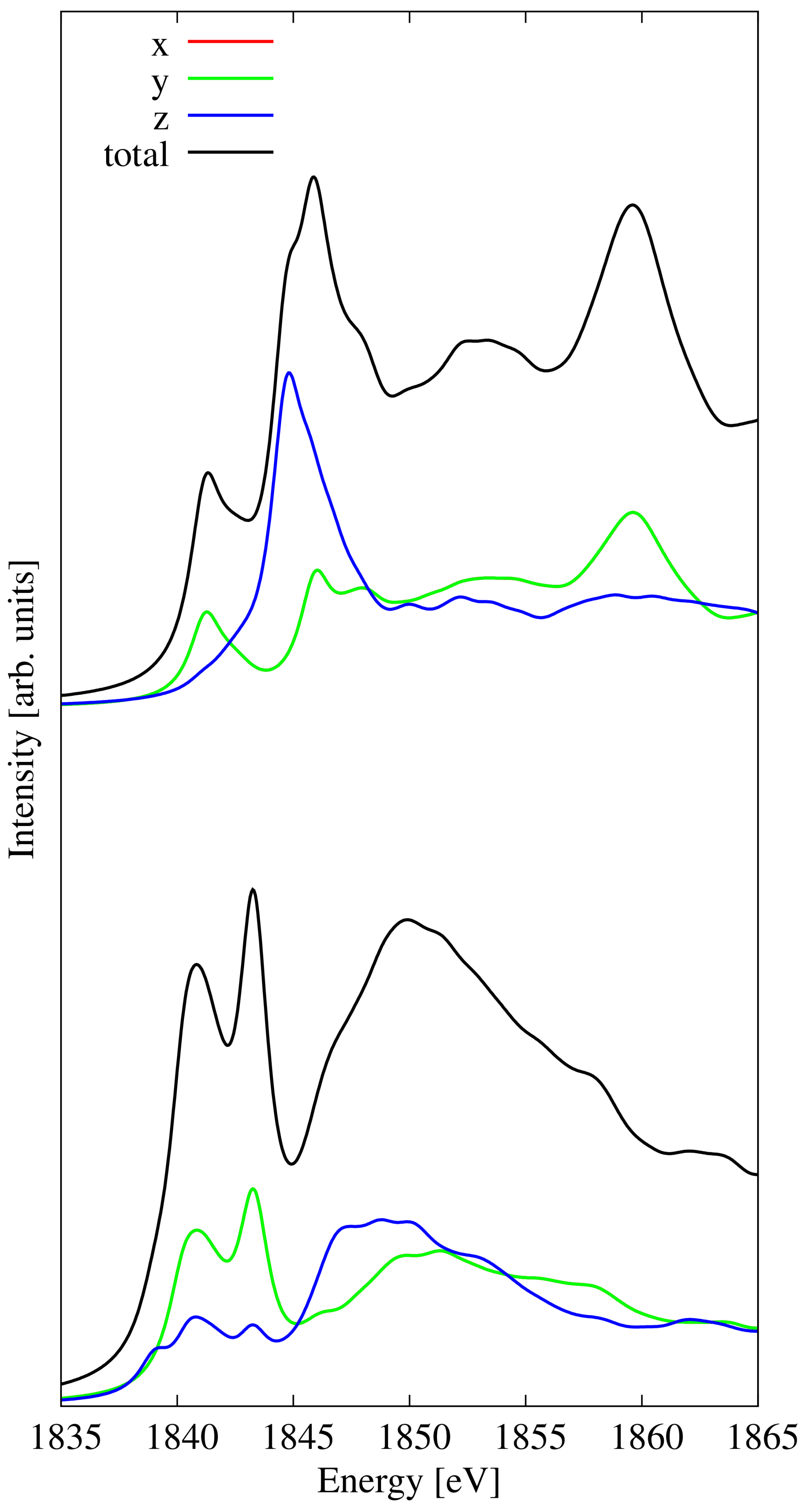
3.7. Penta-SiC2
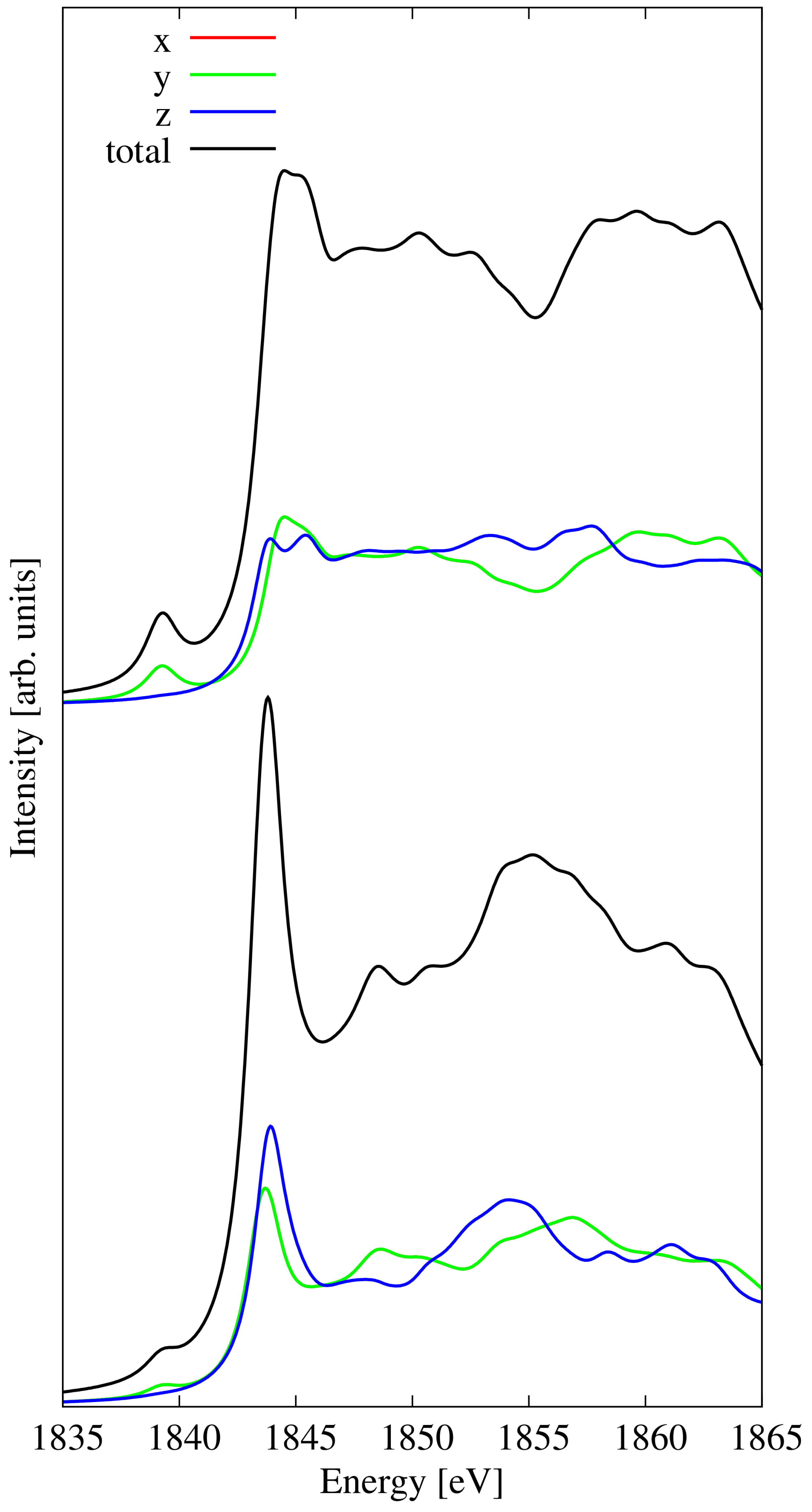
3.8. Hydrogenated Penta-SiC2
3.9. Influence of Functionalisation on C K-Edge
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Pedrielli, A.; Taioli, S.; Garberoglio, G.; Pugno, N.M. Designing graphene based nanofoams with nonlinear auxetic and anisotropic mechanical properties under tension or compression. Carbon 2017, 111, 796–806. [Google Scholar] [CrossRef]
- Morresi, T.; Pedrielli, A.; Beccara, S.a.; Gabbrielli, R.; Pugno, N.M.; Taioli, S. Structural, electronic and mechanical properties of all-sp2 carbon allotropes with density lower than graphene. Carbon 2020, 159, 512–526. [Google Scholar] [CrossRef]
- Pedrielli, A.; Taioli, S.; Garberoglio, G.; Pugno, N.M. Mechanical and thermal properties of graphene random nanofoams via Molecular Dynamics simulations. Carbon 2018, 132, 766–775. [Google Scholar] [CrossRef]
- Taioli, S.; Gabbrielli, R.; Simonucci, S.; Pugno, N.M.; Iorio, A. Lobachevsky crystallography made real through carbon pseudospheres. J. Phys. Condens. Matter 2016, 28, 13LT01. [Google Scholar] [CrossRef] [PubMed]
- Morresi, T.; Binosi, D.; Simonucci, S.; Piergallini, R.; Roche, S.; Pugno, N.M.; Simone, T. Exploring event horizons and Hawking radiation through deformed graphene membranes. 2D Mater. 2020, 7, 041006. [Google Scholar] [CrossRef]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling Experimental Evidence for Graphenelike Two-Dimensional Silicon. Phys. Rev. Lett. 2012, 108, 155501. [Google Scholar] [CrossRef]
- Meyer, J.C.; Chuvilin, A.; Algara-Siller, G.; Biskupek, J.; Kaiser, U. Selective Sputtering and Atomic Resolution Imaging of Atomically Thin Boron Nitride Membranes. Nano Lett. 2009, 9, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q.; Meng, R.; Jiang, J.; Liang, Q.; Tan, C.; Sun, X. The electronic and optical properties of novel germanene and antimonene heterostructures. J. Mater. Chem. C 2016, 4, 5434–5441. [Google Scholar] [CrossRef]
- Matthes, L.; Pulci, O.; Bechstedt, F. Massive Dirac quasiparticles in the optical absorbance of graphene, silicene, germanene, and tinene. J. Phys. Condens. Matter 2013, 25, 395305. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Q. Pentagon-based 2D materials: Classification, properties and applications. Phys. Rep. 2022, 964, 1–42. [Google Scholar] [CrossRef]
- Shah, N.A.; Janjua, R.A.; Jin, Y.; Evans, J.; Shen, J.Q.; He, S. Theoretical development in the realm of pentagonal 2D-materials. J. Appl. Phys. 2024, 136, 130701. [Google Scholar] [CrossRef]
- Bykov, M.; Bykova, E.; Ponomareva, A.V.; Tasnádi, F.; Chariton, S.; Prakapenka, V.B.; Glazyrin, K.; Smith, J.S.; Mahmood, M.F.; Abrikosov, I.A.; et al. Realization of an Ideal Cairo Tessellation in Nickel Diazenide NiN2: High-Pressure Route to Pentagonal 2D Materials. ACS Nano 2021, 15, 13539–13546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Wang, Q.; Chen, X.; Kawazoe, Y.; Jena, P. Penta-graphene: A new carbon allotrope. Proc. Natl. Acad. Sci. USA 2015, 112, 2372–2377. [Google Scholar] [CrossRef]
- Einollahzadeh, H.; Dariani, R.; Fazeli, S. Computing the band structure and energy gap of penta-graphene by using DFT and G0W0 approximations. Solid State Commun. 2016, 229, 1–4. [Google Scholar] [CrossRef]
- Taioli, S.; Umari, P.; De Souza, M.M. Electronic properties of extended graphene nanomaterials from GW calculations. Phys. Status Solidi B 2009, 246, 2572–2576. [Google Scholar] [CrossRef]
- Umari, P.; Petrenko, O.; Taioli, S.; De Souza, M.M. Communication: Electronic band gaps of semiconducting zig-zag carbon nanotubes from many-body perturbation theory calculations. J. Chem. Phys. 2012, 136, 181101. [Google Scholar] [CrossRef]
- Le, M.Q. Mechanical properties of penta-graphene, hydrogenated penta-graphene, and penta-CN2 sheets. Comput. Mater. Sci. 2017, 136, 181–190. [Google Scholar] [CrossRef]
- Rajbanshi, B.; Sarkar, S.; Mandal, B.; Sarkar, P. Energetic and electronic structure of penta-graphene nanoribbons. Carbon 2016, 100, 118–125. [Google Scholar] [CrossRef]
- Yuan, P.F.; Zhang, Z.H.; Fan, Z.Q.; Qiu, M. Electronic structure and magnetic properties of penta-graphene nanoribbons. Phys. Chem. Chem. Phys. 2017, 19, 9528–9536. [Google Scholar] [CrossRef]
- Minaie, B.; Ketabi, S.A.; Sousa, J.M.D. First-principles calculations of the electronic and optical properties of penta-graphene monolayer: Study of many-body effects. arXiv 2024, arXiv:2401.05429. [Google Scholar]
- Xu, W.; Zhang, G.; Li, B. Thermal conductivity of penta-graphene from molecular dynamics study. J. Chem. Phys. 2015, 143, 154703. [Google Scholar] [CrossRef]
- Wang, F.Q.; Yu, J.; Wang, Q.; Kawazoe, Y.; Jena, P. Lattice thermal conductivity of penta-graphene. Carbon 2016, 105, 424–429. [Google Scholar] [CrossRef]
- Wang, F.Q.; Liu, J.; Li, X.; Wang, Q.; Kawazoe, Y. Weak interlayer dependence of lattice thermal conductivity on stacking thickness of penta-graphene. Appl. Phys. Lett. 2017, 111, 192102. [Google Scholar] [CrossRef]
- Behzad, S.; Chegel, R. The evolution of penta-graphene thermoelectrics: External fields as a key enabler for High-Performance devices. Mater. Sci. Eng. B 2024, 300, 117082. [Google Scholar] [CrossRef]
- Stauber, T.; Beltrán, J.I.; Schliemann, J. Tight-binding approach to penta-graphene. Sci. Rep. 2016, 6, 22672. [Google Scholar] [CrossRef]
- Haberer, D.; Vyalikh, D.V.; Taioli, S.; Dora, B.; Farjam, M.; Fink, J.; Marchenko, D.; Pichler, T.; Ziegler, K.; Simonucci, S.; et al. Tunable Band Gap in Hydrogenated Quasi-Free-Standing Graphene. Nano Lett. 2010, 10, 3360–3366. [Google Scholar] [CrossRef]
- Haberer, D.; Petaccia, L.; Farjam, M.; Taioli, S.; Jafari, S.A.; Nefedov, A.; Zhang, W.; Calliari, L.; Scarduelli, G.; Dora, B.; et al. Direct observation of a dispersionless impurity band in hydrogenated graphene. Phys. Rev. B 2011, 83, 165433. [Google Scholar] [CrossRef]
- Berdiyorov, G.R.; Dixit, G.; Madjet, M.E. Band gap engineering in penta-graphene by substitutional doping: First-principles calculations. J. Phys. Condens. Matter 2016, 28, 475001. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wang, F.Q.; Guo, Y.; Liu, J.; Wang, Q. Tuning the electronic and mechanical properties of penta-graphene via hydrogenation and fluorination. Phys. Chem. Chem. Phys. 2016, 18, 14191–14197. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Wang, X.F. First-Principles Study of CO, C2H2, and C2H4 Adsorption on Penta-Graphene for Transformer Oil Gas Sensing Applications. C 2025, 11, 49. [Google Scholar] [CrossRef]
- Santos, R.M.d.; Sousa, L.E.d.; Galvão, D.S.; Ribeiro, L.A. Tuning Penta-Graphene Electronic Properties Through Engineered Line Defects. Sci. Rep. 2020, 10, 8014. [Google Scholar] [CrossRef]
- Santos, R.M.d.; da Cunha, W.F.; de Sousa Junior, R.T.; Giozza, W.F.; Ribeiro Junior, L.A. Tuning magnetic properties of penta-graphene bilayers through doping with boron, nitrogen, and oxygen. Sci. Rep. 2020, 10, 16748. [Google Scholar] [CrossRef]
- Morales-Ferreiro, J.; Silva-Oelker, G.; Kumar, C.; Zambra, C.; Liu, Z.; Diaz-Droguett, D.; Celentano, D. Tuning the Electronic Bandgap of Penta-Graphene from Insulator to Metal Through Functionalization: A First-Principles Calculation. Nanomaterials 2024, 14, 1751. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.T.; Thao, P.T.B.; Thuan, L.V.P.; Chuong, D.H. First-principles study of electronic and optical properties of defective sawtooth penta-graphene nanoribbons. Comput. Mater. Sci. 2022, 203, 111065. [Google Scholar] [CrossRef]
- Einollahzadeh, H.; Fazeli, S.M.; Dariani, R.S. Studying the electronic and phononic structure of penta-graphane. Sci. Technol. Adv. Mater. 2016, 17, 610–617. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, Y.; Chen, C.P.; Yu, H.X.; Zhao, L.S.; Wang, X.C. Tuning the electronic and magnetic properties of penta-graphene using a hydrogen atom: A theoretical study. RSC Adv. 2017, 7, 40200–40207. [Google Scholar] [CrossRef]
- Ebrahimi, S. Effect of hydrogen coverage on the buckling of penta-graphene by molecular dynamics simulation. Mol. Simul. 2016, 42, 1485–1489. [Google Scholar] [CrossRef]
- Nazir, M.A.; Hassan, A.; Shen, Y.; Wang, Q. Research progress on penta-graphene and its related materials: Properties and applications. Nano Today 2022, 44, 101501. [Google Scholar] [CrossRef]
- Ewels, C.P.; Rocquefelte, X.; Kroto, H.W.; Rayson, M.J.; Briddon, P.R.; Heggie, M.I. Predicting experimentally stable allotropes: Instability of penta-graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 15609–15612. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, J.I.; Sławińska, J.; Le Lay, G.; Marele, A.C.; Gómez-Rodríguez, J.M.; Dávila, M.E. Unveiling the pentagonal nature of perfectly aligned single-and double-strand Si nano-ribbons on Ag(110). Nat. Commun. 2016, 7, 13076. [Google Scholar] [CrossRef]
- Berdiyorov, G.R.; Madjet, M.E.A. First-principles study of electronic transport and optical properties of penta-graphene, penta-SiC2 and penta-CN2. RSC Adv. 2016, 6, 50867–50873. [Google Scholar] [CrossRef]
- Xu, Y.; Ning, Z.; Zhang, H.; Ni, G.; Shao, H.; Peng, B.; Zhang, X.; He, X.; Zhu, Y.; Zhu, H. Anisotropic ultrahigh hole mobility in two-dimensional penta-SiC2 by strain-engineering: Electronic structure and chemical bonding analysis. RSC Adv. 2017, 7, 45705–45713. [Google Scholar] [CrossRef]
- Lopez-Bezanilla, A.; Littlewood, P. σ-π-Band Inversion in a Novel Two-Dimensional Material. J. Phys. Chem. C 2015, 119, 150724095829005. [Google Scholar] [CrossRef]
- Chabi, S.; Kadel, K. Two-dimensional silicon carbide: Emerging direct band gap semiconductor. Nanomaterials 2020, 10, 2226. [Google Scholar] [CrossRef]
- Jiang, H. Band gaps from the Tran-Blaha modified Becke-Johnson approach: A systematic investigation. J. Chem. Phys. 2013, 138, 134115. [Google Scholar] [CrossRef] [PubMed]
- Nakada, K.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys. Rev. B 1996, 54, 17954–17961. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Miao, W.; Zhao, L.; Wang, J. Graphene nanoribbons: Current status and challenges as quasi-one-dimensional nanomaterials. Rev. Phys. 2023, 10, 100082. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Wang, J. Electronic structures of Penta-SiC2 and g-SiC3 nanoribbons: A first-principles study. Materials 2023, 16, 4041. [Google Scholar] [CrossRef]
- Taioli, S.; Simonucci, S.; Calliari, L.; Dapor, M. Electron spectroscopies and inelastic processes in nanoclusters and solids: Theory and experiment. Phys. Rep. 2010, 493, 237–319. [Google Scholar] [CrossRef]
- Taioli, S.; Dapor, M. Advancements in secondary and backscattered electron energy spectra and yields analysis: From theory to applications. Surf. Sci. Rep. 2025, 80, 100646. [Google Scholar] [CrossRef]
- Yano, J.; Yachandra, V.K. X-ray absorption spectroscopy. Photosynth. Res. 2009, 102, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Rossi, F.; Attolini, G.; Salviati, G.; Iannotta, S.; Aversa, L.; Verucchi, R.; Nardi, M.; Fukata, N.; Dierre, B.; et al. Enhancement of the core near-band-edge emission induced by an amorphous shell in coaxial one-dimensional nanostructure: The case of SiC/SiO2core/shell self-organized nanowires. Nanotechnology 2010, 21, 345702. [Google Scholar] [CrossRef]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Gougoussis, C.; Calandra, M.; Seitsonen, A.P.; Mauri, F. First-principles calculations of X-ray absorption in a scheme based on ultrasoft pseudopotentials: From α-quartz to high-Tc compounds. Phys. Rev. B 2009, 80, 075102. [Google Scholar] [CrossRef]
- Available online: http://www.quantum-espresso.org (accessed on 1 March 2025).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Minaie, B.; Ketabi, S.K.; De Sousa, J.M. Theoretical calculations of the optoelectronic properties of a penta-graphene monolayer: Study of many-body effects. J. Comput. Electron. 2024, 23, 1102–1110. [Google Scholar] [CrossRef]
- Pacilé, D.; Papagno, M.; Rodríguez, A.F.; Grioni, M.; Papagno, L.; Girit, U.O.; Meyer, J.C.; Begtrup, G.E.; Zettl, A. Near-Edge X-Ray Absorption Fine-Structure Investigation of Graphene. Phys. Rev. Lett. 2008, 101, 066806. [Google Scholar] [CrossRef]
- Papagno, M.; Fraile Rodríguez, A.; Girit, u.; Meyer, J.; Zettl, A.; Pacilé, D. Polarization-dependent C K near-edge X-ray absorption fine-structure of graphene. Chem. Phys. Lett. 2009, 475, 269–271. [Google Scholar] [CrossRef]
- Christiansen, D.; Selig, M.; Biegert, J.; Knorr, A. Theory of x-ray absorption spectroscopy: A microscopic Bloch equation approach for two-dimensional solid states. Phys. Rev. Res. 2023, 5, 023002. [Google Scholar] [CrossRef]



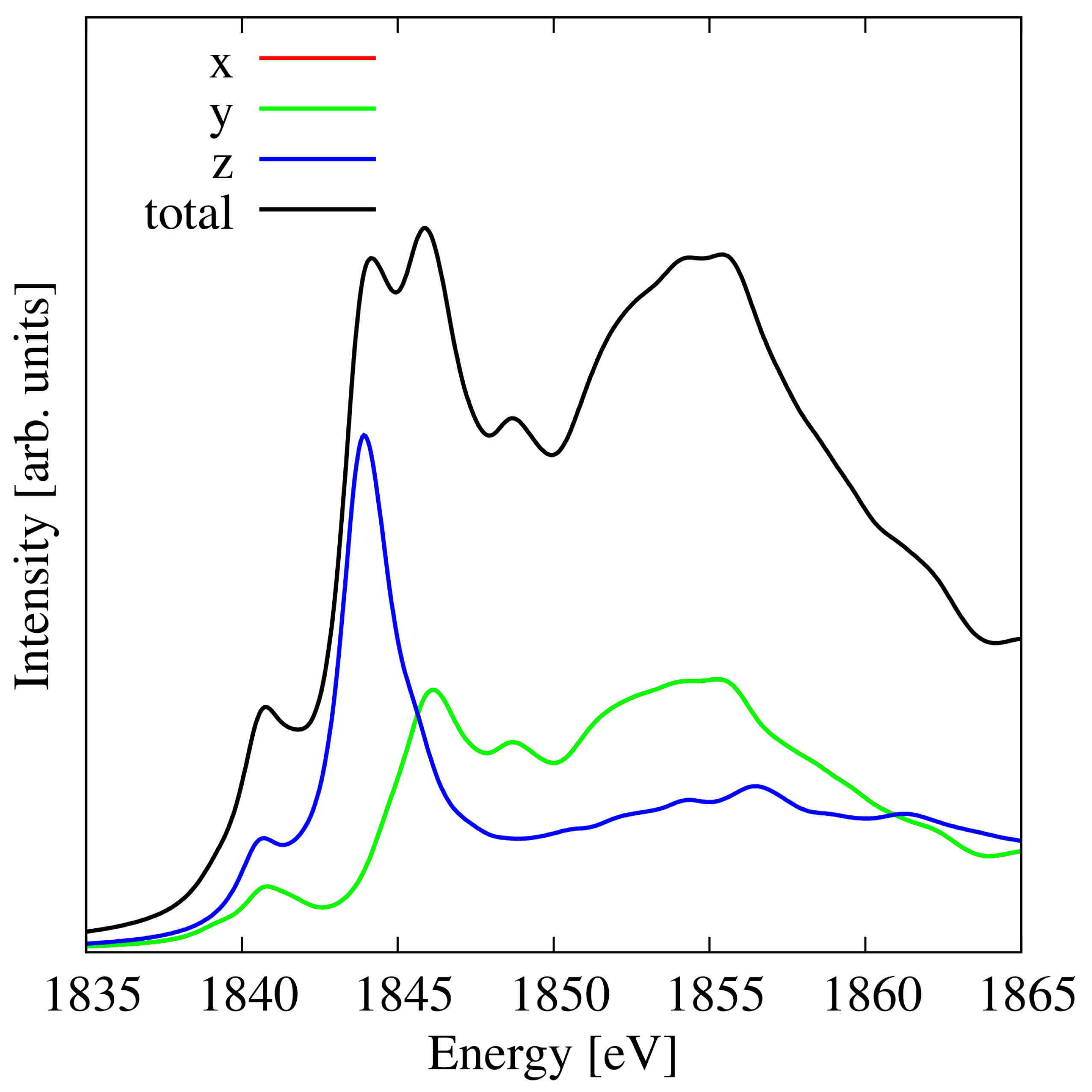
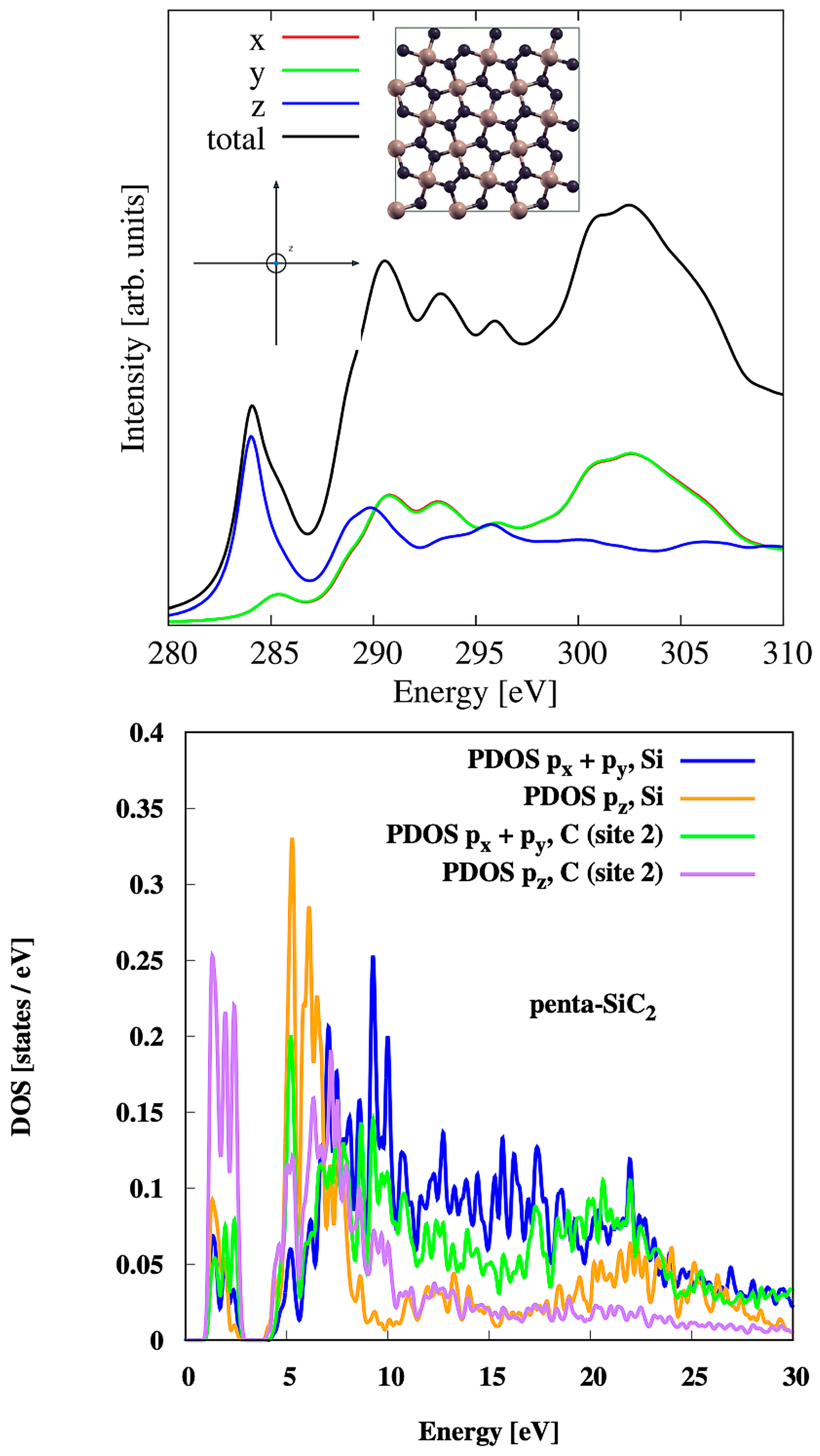

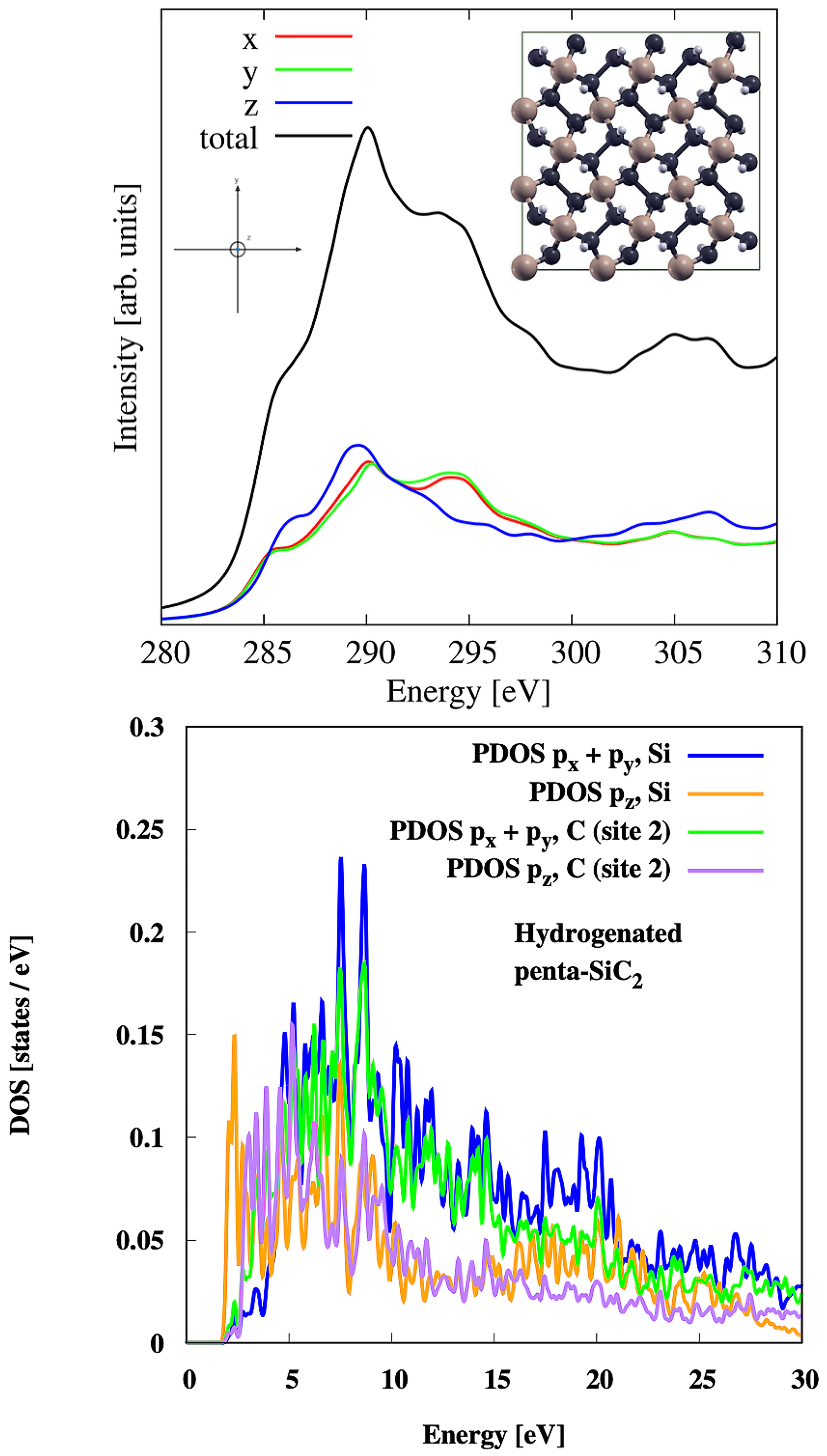
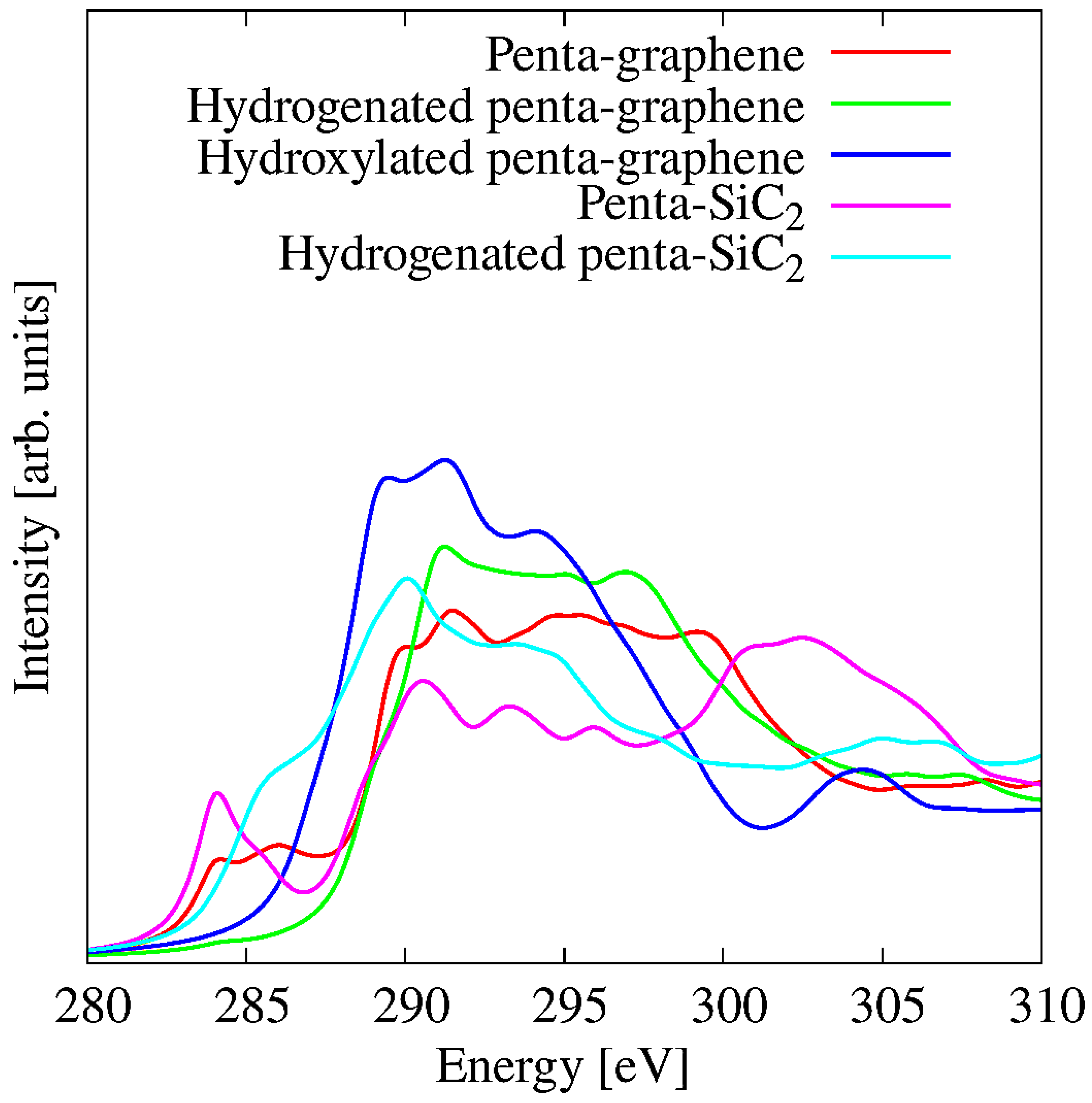
| Material | Pristine | Hydrogenated | Hydroxylated |
|---|---|---|---|
| Penta-graphene | yes | yes | yes |
| Penta-graphene + Si sub. | yes | yes | — |
| Penta-SiC2 | yes | yes | — |
| Structure (Cell Parameter a in Å) | Bond Distances (Å) |
|---|---|
| Penta-graphene () | C (site 2)–C (site 2): 1.338 C (site 1)–C (site 2): 1.549 |
| Hydrogenated penta-graphene () | C (site 2)–H: 1.101 C (site 1)–C (site 2): 1.553 C (site 2)–C (site 2): 1.553 |
| Hydroxylated penta-graphene () | C (site 2)–O: 1.432 C (site 1)–C (site 2): 1.576 C (site 2)–C (site 2): 1.576 O–H: 1.030 |
| Penta-SiC2 () | C (site 2)–Si: 1.900 C (site 2)–C (site 2): 1.363 |
| Hydrogenated penta-SiC2 () | C (site 2)–Si: 1.906 C (site 2)–C (site 2): 1.580 C (site 2)–H: 1.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrielli, A.; Morresi, T.; Taioli, S. XANES Absorption Spectra of Penta-Graphene and Penta-SiC2 with Different Terminations: A Computational Study. Appl. Sci. 2025, 15, 9812. https://doi.org/10.3390/app15179812
Pedrielli A, Morresi T, Taioli S. XANES Absorption Spectra of Penta-Graphene and Penta-SiC2 with Different Terminations: A Computational Study. Applied Sciences. 2025; 15(17):9812. https://doi.org/10.3390/app15179812
Chicago/Turabian StylePedrielli, Andrea, Tommaso Morresi, and Simone Taioli. 2025. "XANES Absorption Spectra of Penta-Graphene and Penta-SiC2 with Different Terminations: A Computational Study" Applied Sciences 15, no. 17: 9812. https://doi.org/10.3390/app15179812
APA StylePedrielli, A., Morresi, T., & Taioli, S. (2025). XANES Absorption Spectra of Penta-Graphene and Penta-SiC2 with Different Terminations: A Computational Study. Applied Sciences, 15(17), 9812. https://doi.org/10.3390/app15179812







