Mechanistic Insights into 5-Fluorouracil Adsorption on Clinoptilolite Surfaces: Optimizing DFT Parameters for Natural Zeolites, Part II
Abstract
1. Introduction and State of the Literature
2. Model Development and Computational Workflow
2.1. Construction of the Surface Models
- General guidelines for surface models construction:
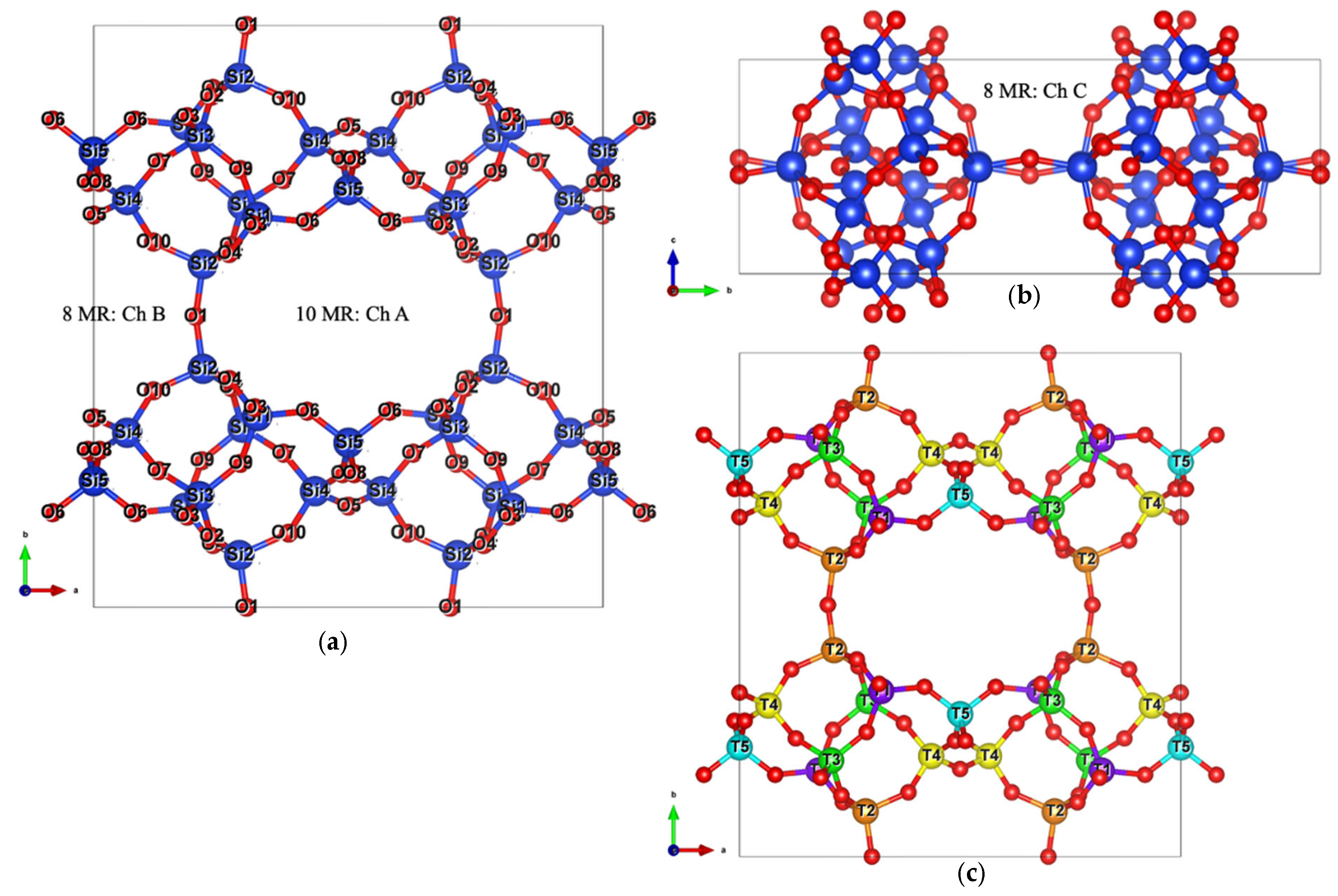
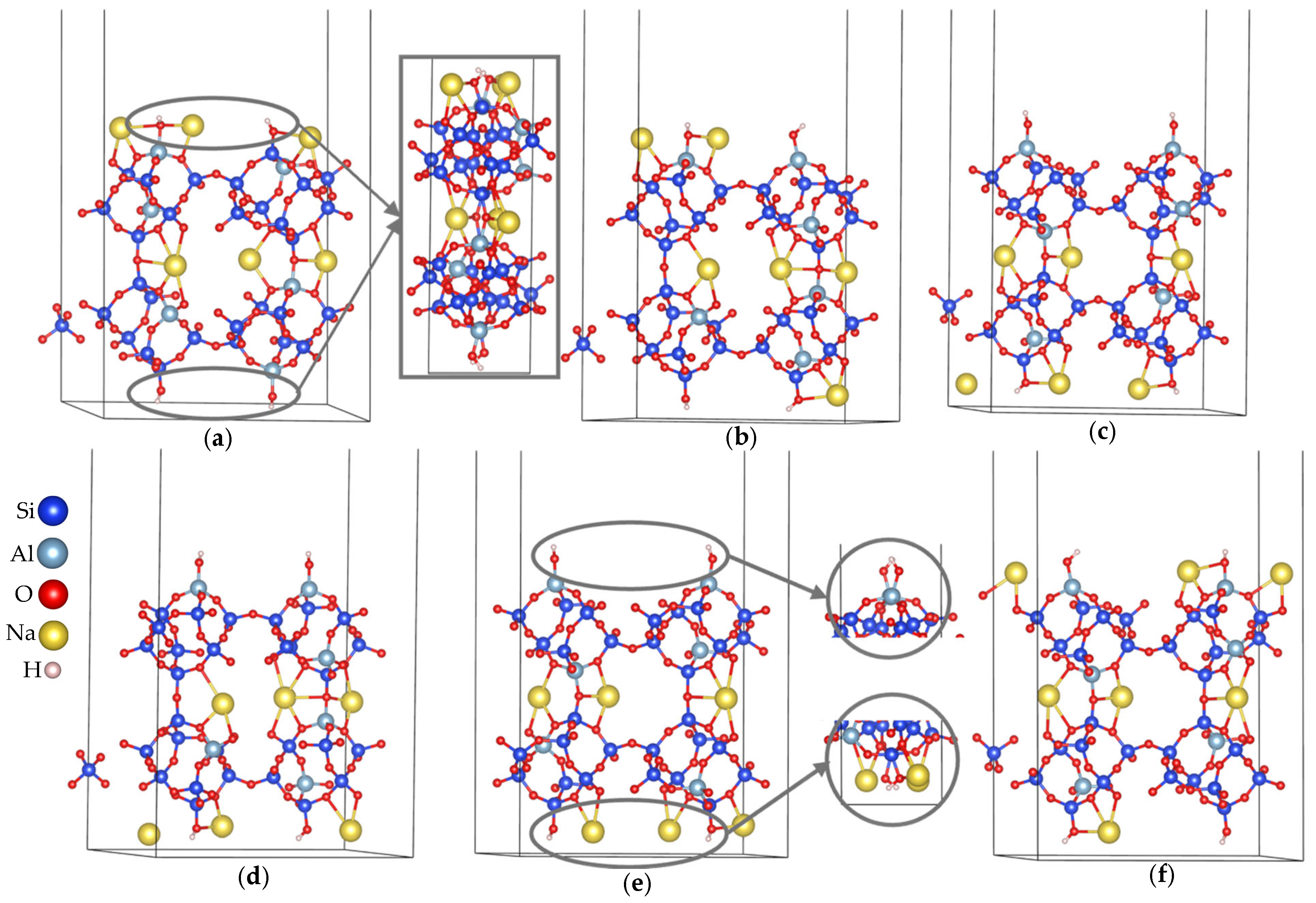
- Build a supercell by repeating the fully optimized bulk unit cell along all three spatial directions to ensure adequate size for surface termination and convergence testing: in Figure 3a, the fully optimized bulk unit cell is shown inside the grey box.
- Select the crystallographic plane of interest—ideally one that represents a natural cleavage surface or has been previously studied in the literature (either experimentally or theoretically). This aids in validating the structural and energetic features of the generated surface model (Figure 3b).
- Orient the crystal structure such that the chosen Miller plane is aligned horizontally or vertically; this eases the imagination and visualization of the cleaving process and the trimming of atoms (Figure 3c).
- Define a parallel plane to the selected crystallographic plane to control slab thickness. This plane should be placed to preserve the periodicity and symmetry of the bulk structure (Figure 3c).
- Add two bounding planes perpendicular to the initial pair, as well as two additional planes along the third spatial dimension. These define the three-dimensional boundaries of the slab (Figure 3d).
- Trim atoms outside the defined volume and apply the appropriate transformation matrix—typically a covariant transformation or a linear combination of the original lattice vectors—to generate the correct supercell orientation (Figure 3d).
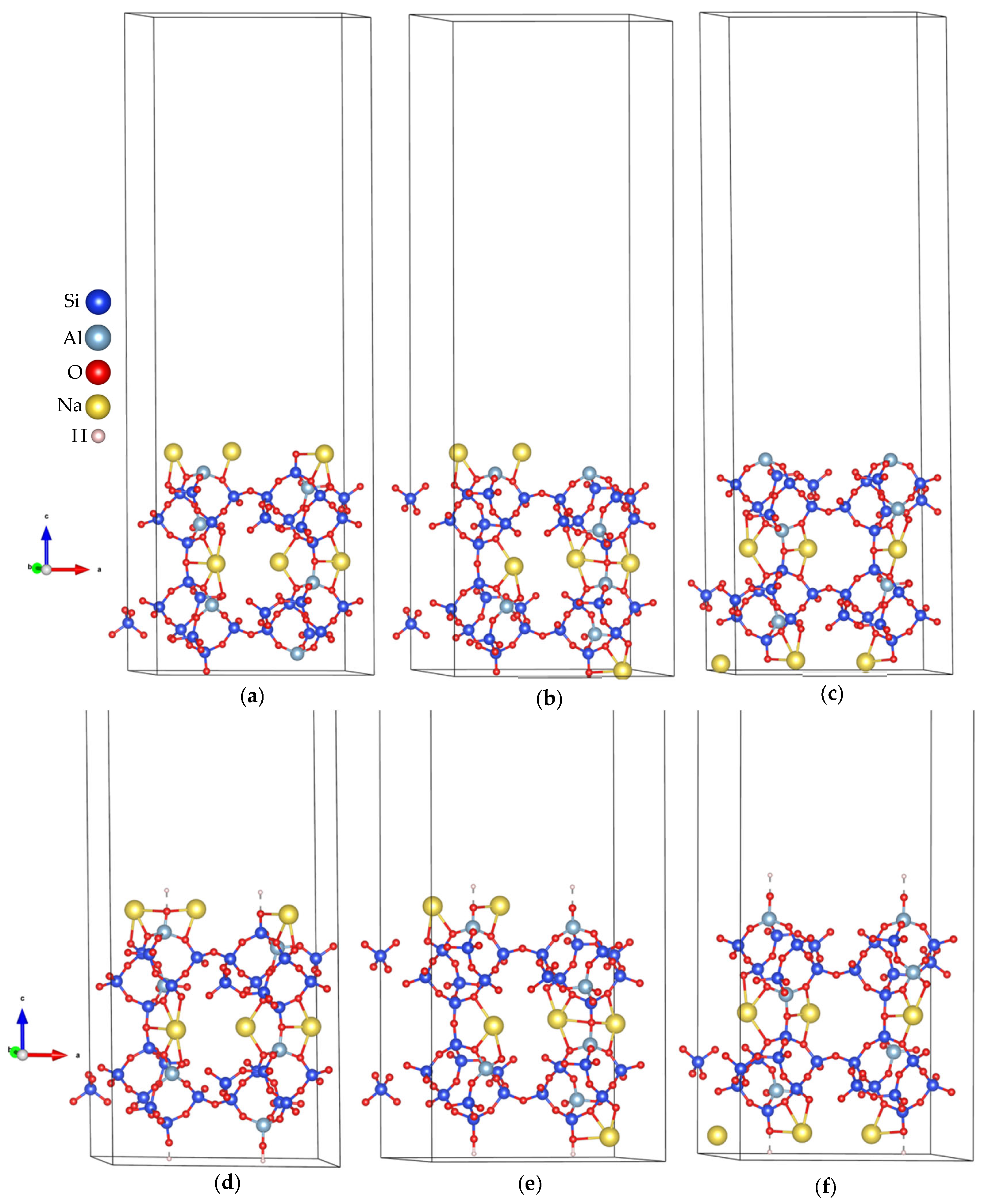
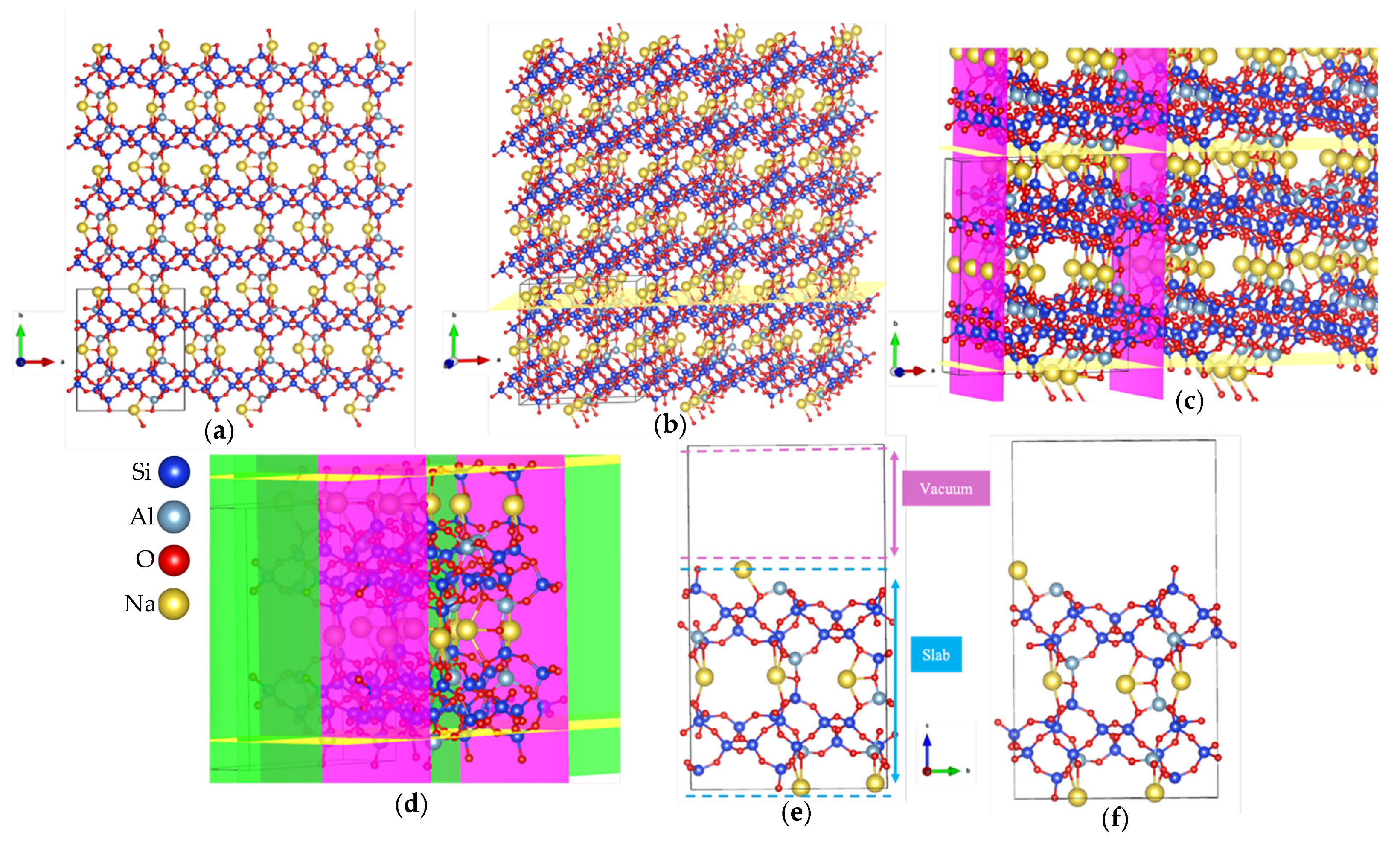
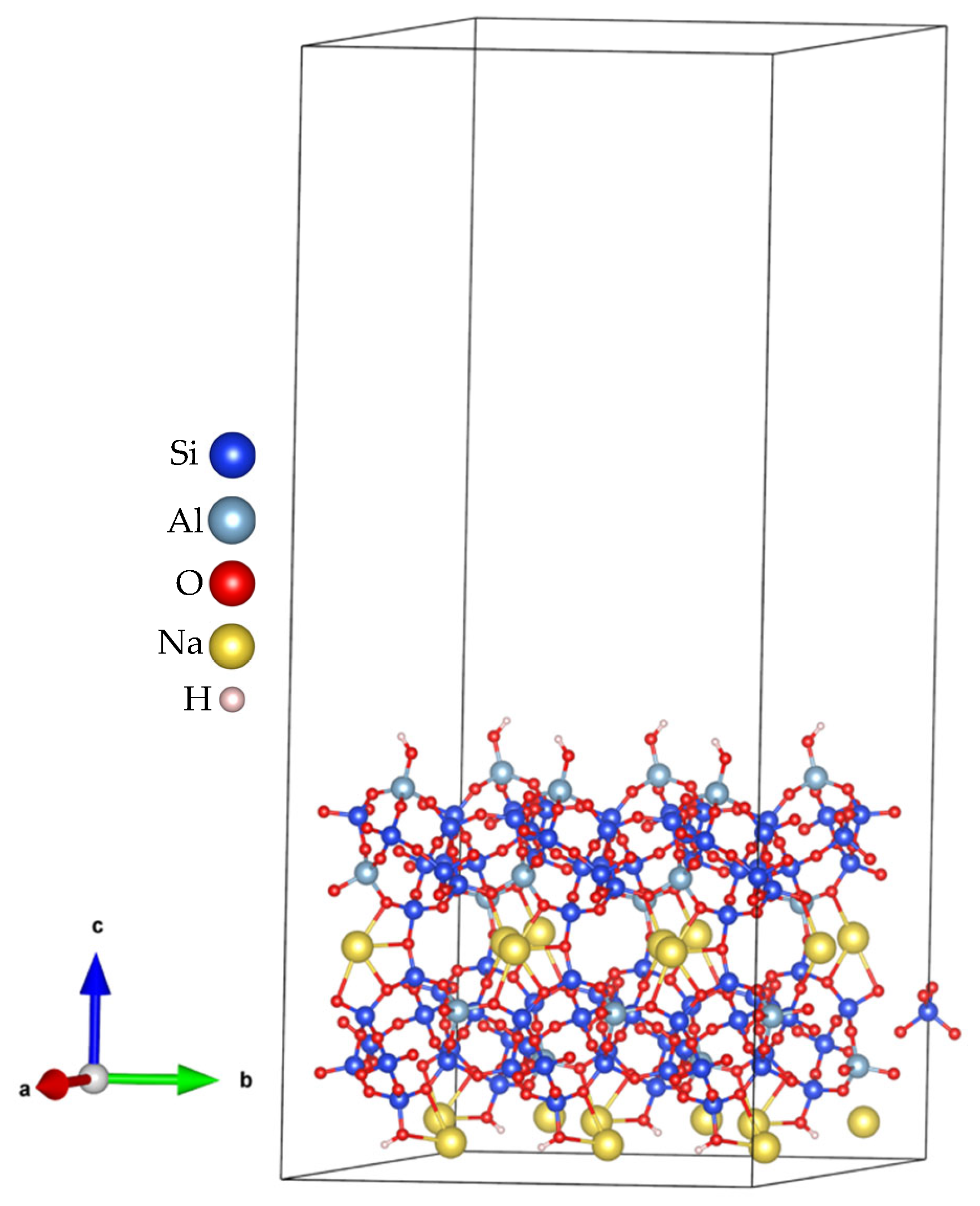
- Export the resulting model in Vienna Ab initio Simulation Package (VASP) format with Cartesian coordinates, which facilitates the addition of vacuum spacing (usually along the z-axis) for subsequent DFT calculations. Some resulting models are shown in Figure 3e,f for the (010) plane.
2.2. Computational Details
2.2.1. Workflow for Surface Models Optimizations
2.2.2. Force Fields and Molecular Dynamics Workflow for Adsorption Simulations
- Hybrid Simulated Annealing and Parallel Tempering Approach
2.2.3. From Force Fields to DFT Optimization and Adsorption Energy Evaluation
3. Results and Discussion
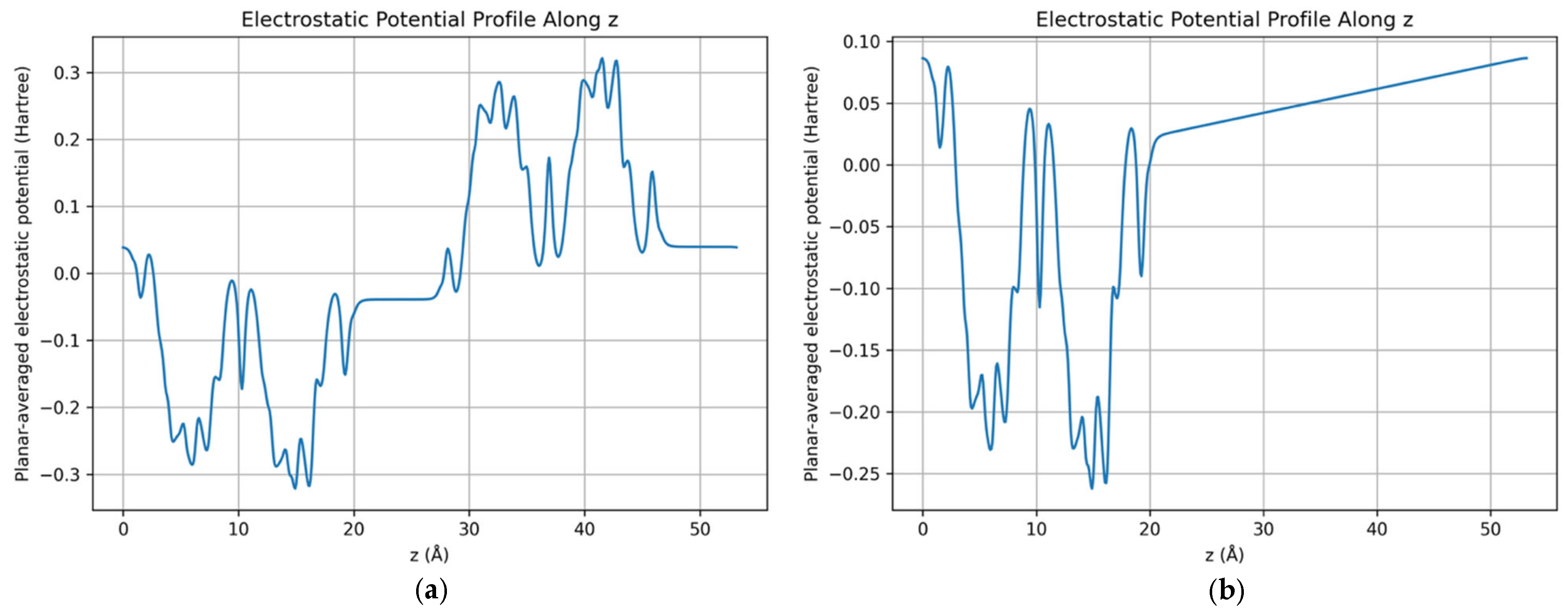
3.1. Optimizations of Surface Models
| &DFT &V_HARTREE_CUBE STRIDE 1 1 1 &END V_HARTREE_CUBE &END PRINT &END DFT |
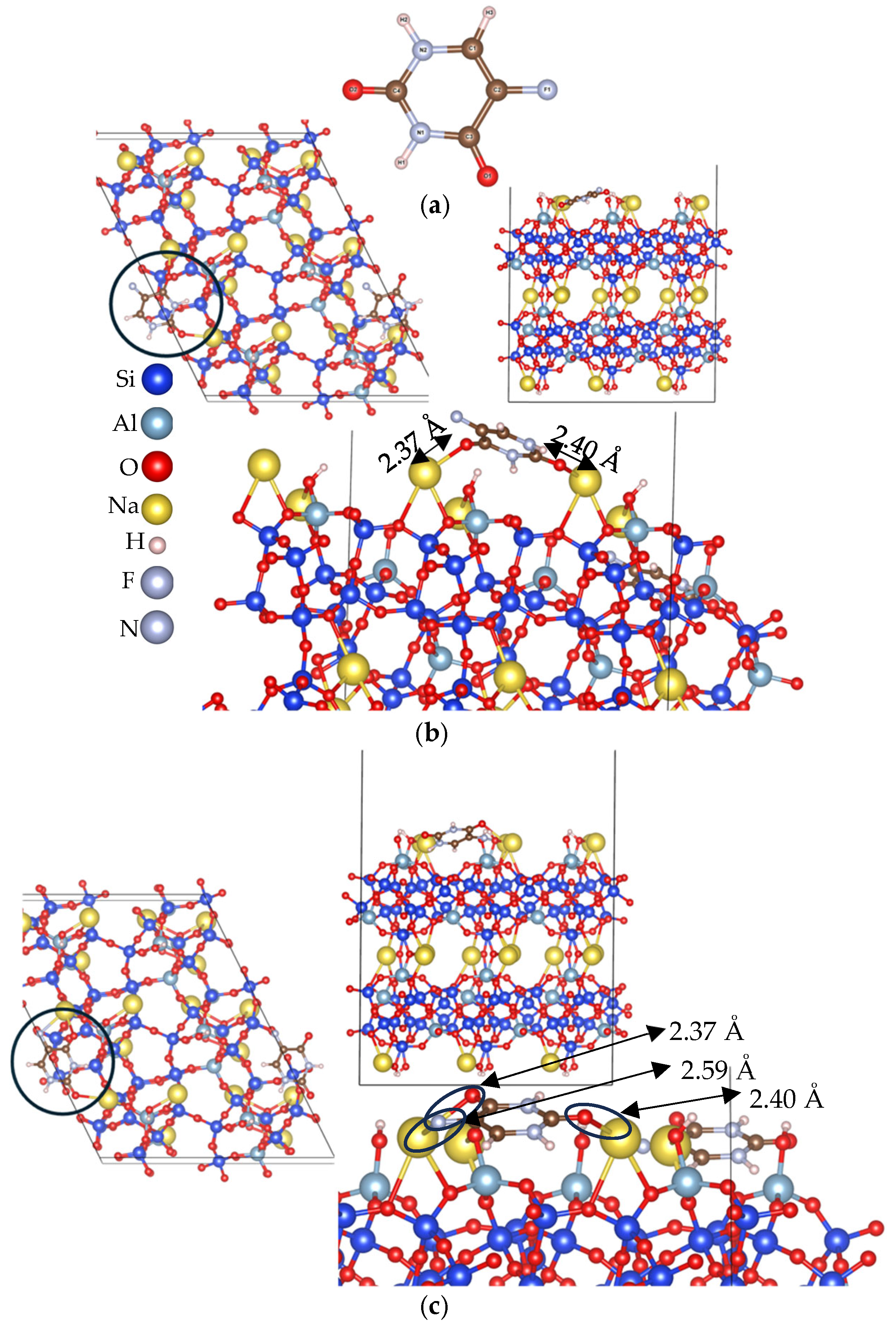
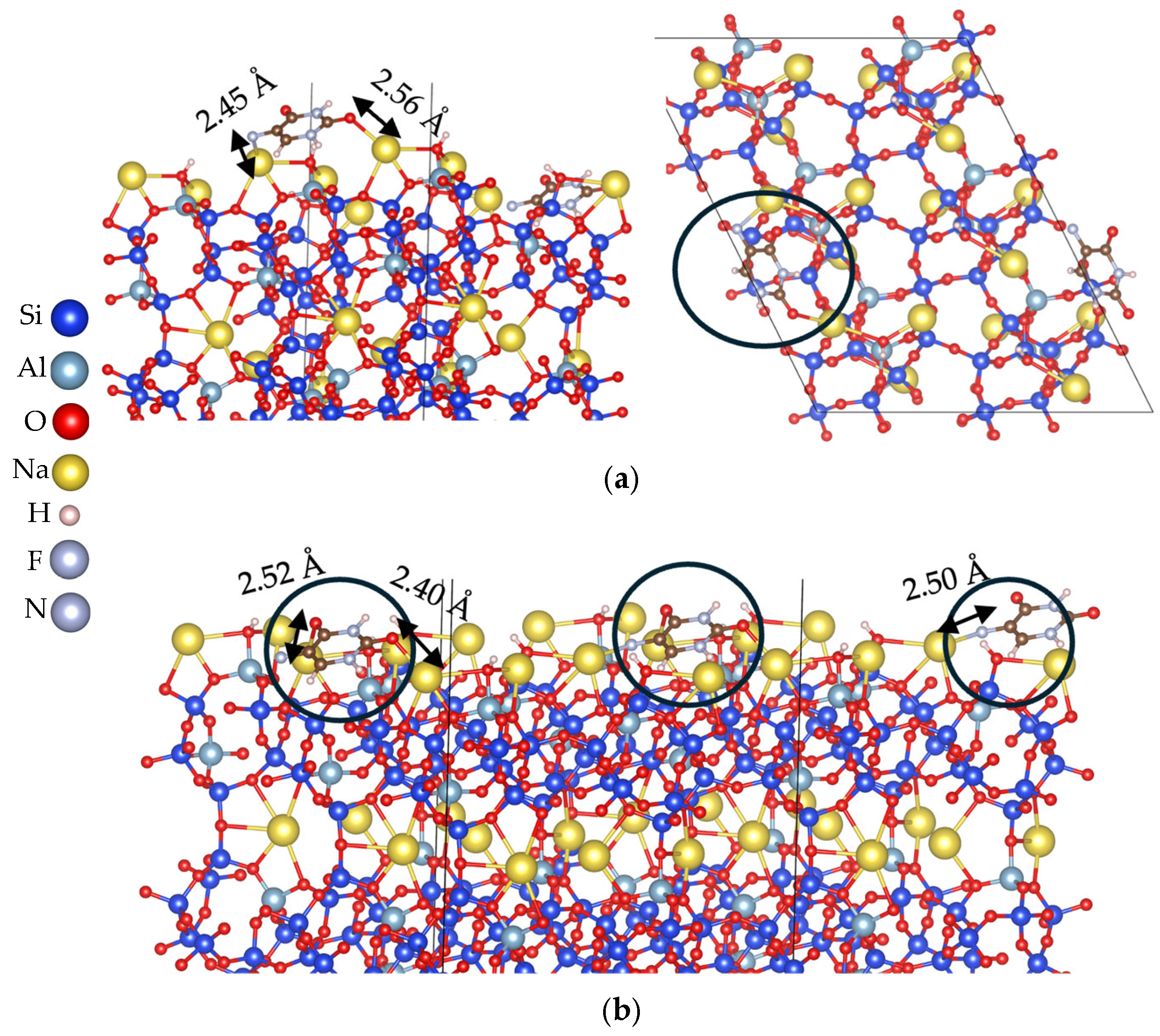
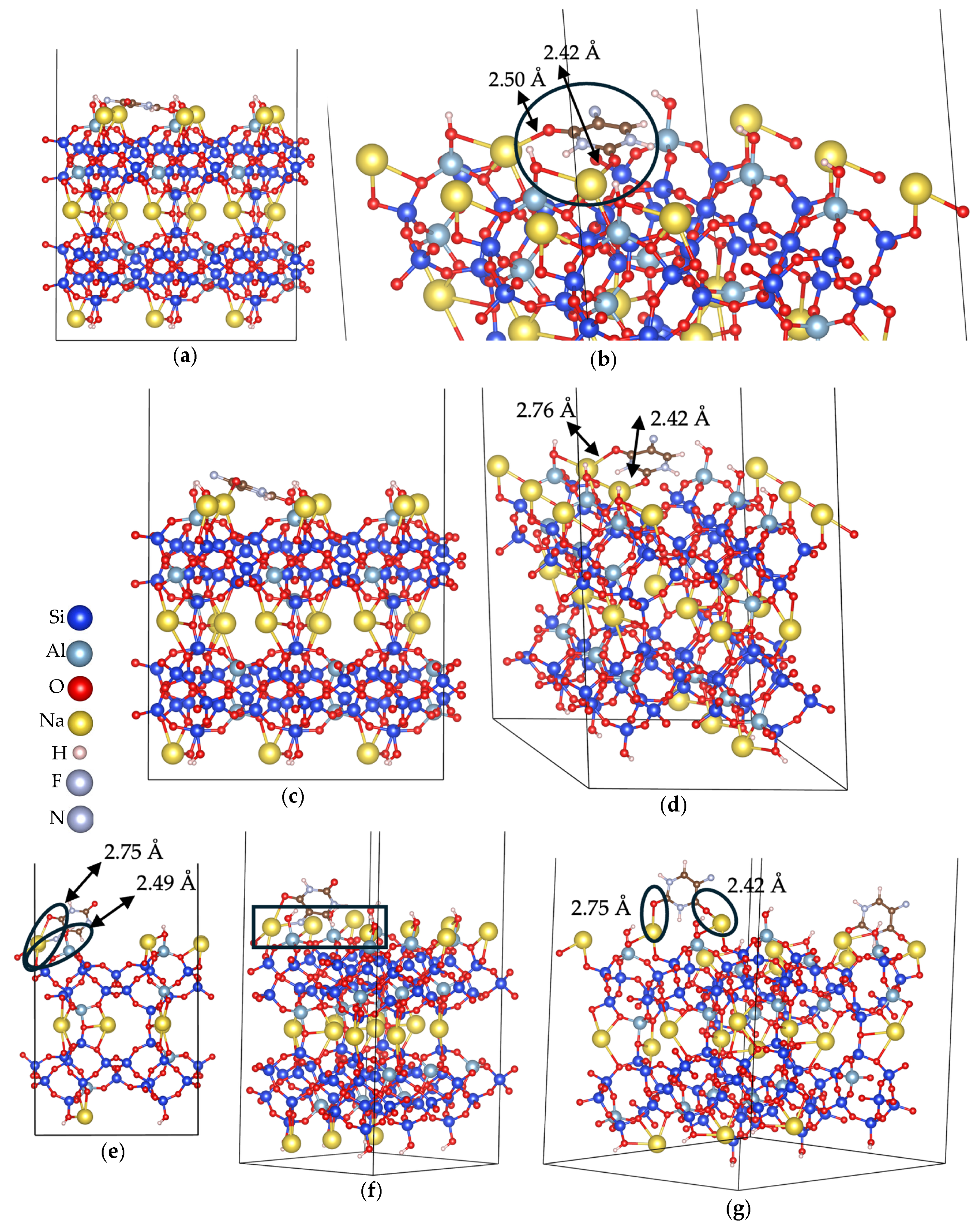
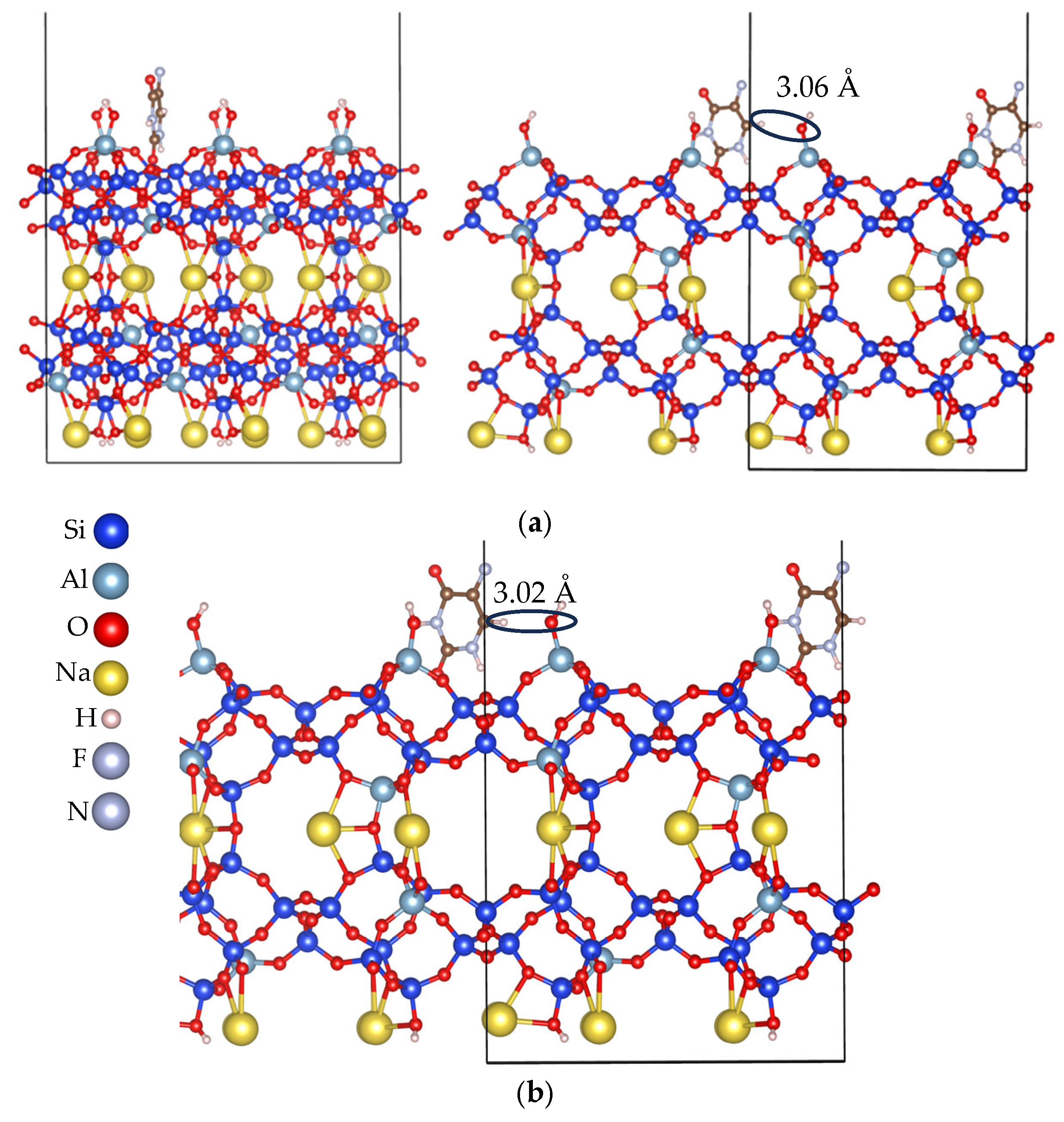
3.2. Adsorption Energy Evaluation of 5-Fluorouracil on Clinoptilolite Surfaces
3.2.1. Cation-Rich Surfaces
3.2.2. Cation-Free Surfaces
3.3. Comparison with Other Experimental and Computational Works
3.4. Implications of Our Trends for Experimental Design
3.5. Charge Transfer/Protonation State of 5-FU at the Surface
3.6. Entropic Effects
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PubChem Fluorouracil. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3385 (accessed on 7 July 2025).
- Sayed, M.A.; El-Zeiny, H.M.; Khim, J.S.; Ajarem, J.S.; Allam, A.A.; Abukhadra, M.R. Insight into the Loading Properties of Na+ Green-Functionalized Clinoptilolite as a Potential Carrier for the 5-Fluorouracil Drug, Its Release Kinetics, and Cytotoxicity. ACS Omega 2022, 7, 6991–7001. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Faisal Jabar, M.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef]
- Long, S.; Wang, J.; Weng, F.; Pei, Z.; Zhou, S.; Sun, G.; Xiang, D. ECM1 Regulates the Resistance of Colorectal Cancer to 5-FU Treatment by Modulating Apoptotic Cell Death and Epithelial-Mesenchymal Transition Induction. Front. Pharmacol. 2022, 13, 1005915. [Google Scholar] [CrossRef]
- Noordhuis, P.; Holwerda, U.; der Wilt, C.L.V.; Groeningen, C.J.V.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil Incorporation into RNA and DNA in Relation to Thymidylate Synthase Inhibition of Human Colorectal Cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef]
- Hao, J.; Stavljenić Milašin, I.; Batu Eken, Z.; Mravak-Stipetic, M.; Pavelić, K.; Ozer, F. Effects of Zeolite as a Drug Delivery System on Cancer Therapy: A Systematic Review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef]
- Spanakis, M.; Bouropoulos, N.; Theodoropoulos, D.; Sygellou, L.; Ewart, S.; Moschovi, A.M.; Siokou, A.; Niopas, I.; Kachrimanis, K.; Nikolakis, V.; et al. Controlled Release of 5-Fluorouracil from Microporous Zeolites. Nanomedicine 2014, 10, 197–205. [Google Scholar] [CrossRef]
- Fischer, M. Adsorption of 5-Fluorouracil, an Anticancer Drug, in Faujasite-Type Zeolites: Understanding Storage and Release with Density Functional Theory Calculations. CrystEngComm 2024, 26, 3795–3807. [Google Scholar] [CrossRef]
- Vilaça, N.; Amorim, R.; Machado, A.F.; Parpot, P.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Fonseca, A.M.; Neves, I.C.; Baltazar, F. Potentiation of 5-Fluorouracil Encapsulated in Zeolites as Drug Delivery Systems for in Vitro Models of Colorectal Carcinoma. Colloids Surf. B Biointerfaces 2013, 112, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Datt, A.; Burns, E.A.; Dhuna, N.A.; Larsen, S.C. Loading and Release of 5-Fluorouracil from HY Zeolites with Varying SiO2/Al2O3 Ratios. Microporous Mesoporous Mater. 2013, 167, 182–187. [Google Scholar] [CrossRef]
- Abd-Elsatar, A.G.; Farag, M.M.; Youssef, H.F.; Salih, S.A.; Mounier, M.M.; El-Meliegy, E. Different Zeolite Systems for Colon Cancer Therapy: Monitoring of Ion Release, Cytotoxicity and Drug Release Behavior. Prog Biomater 2019, 8, 101–113. [Google Scholar] [CrossRef]
- Klimeš, J.; Tew, D.P. Efficient and Accurate Description of Adsorption in Zeolites. J. Chem. Phys. 2019, 151, 234108. [Google Scholar] [CrossRef]
- Simonetti, S.; Compañy, A.D.; Pronsato, E.; Juan, A.; Brizuela, G.; Lam, A. Density Functional Theory Based-Study of 5-Fluorouracil Adsorption on β-Cristobalite (1 1 1) Hydroxylated Surface: The Importance of H-Bonding Interactions. Appl. Surf. Sci. 2015, 359, 474–479. [Google Scholar] [CrossRef]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface Energies of Elemental Crystals. Sci Data 2016, 3, 160080. [Google Scholar] [CrossRef]
- Sun, W.; Ceder, G. Efficient Creation and Convergence of Surface Slabs. Surf. Sci. 2013, 617, 53–59. [Google Scholar] [CrossRef]
- Saeed, L.; Fischer, M. Computational Insights into Aluminum and Cation Placement in Clinoptilolite: Optimizing DFT Parameters for Natural Zeolites, Part I. Appl. Sci. 2025, 15, 3955. [Google Scholar] [CrossRef]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.A.; Ceder, G. Python Materials Genomics (Pymatgen): A Robust, Open-Source Python Library for Materials Analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J Appl Cryst 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- VESTA. Available online: https://jp-minerals.org/vesta/en/ (accessed on 23 June 2025).
- Sun, W.; Ceder, G. A Topological Screening Heuristic for Low-Energy, High-Index Surfaces. Surf. Sci. 2018, 669, 50–56. [Google Scholar] [CrossRef]
- Armbruster, T. Clinoptilotite-Heulandite: Applications and Basic Research. Stud. Surf. Sci. Catal. 2001, 135, 13–27. [Google Scholar] [CrossRef]
- Witman, M.; Ling, S.; Boyd, P.; Barthel, S.; Haranczyk, M.; Slater, B.; Smit, B. Cutting Materials in Half: A Graph Theory Approach for Generating Crystal Surfaces and Its Prediction of 2D Zeolites. ACS Cent. Sci. 2018, 4, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lunevich, L.; Sanciolo, P.; Smallridge, A.; Gray, S.R. Silica Scale Formation and Effect of Sodium and Aluminium Ions-29Si NMR Study. Environ. Sci. Water Res. Technol. 2016, 2, 174–185. [Google Scholar] [CrossRef]
- El-Manharawy, S.; Hafez, A. Dehydration Model for RO-Membrane Fouling: A Preliminary Approach. Desalination 2003, 153, 95–107. [Google Scholar] [CrossRef]
- El-Manharawy, S.; Hafez, A. Technical Management of RO System. Desalination 2000, 131, 173–188. [Google Scholar] [CrossRef]
- Tasci, E.S. How to Prepare an Input File for Surface Calculations. 2013. Available online: https://www.emresururi.com/physics/wp-content/uploads/2013/09/ETasci_2013_SurfacePreparationHowTo.pdf (accessed on 29 April 2024).
- Tasker, P.W. The Stability of Ionic Crystal Surfaces. J. Phys. C Solid State Phys. 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Finnis, M.W. The Theory of Metal—Ceramic Interfaces. J. Phys. Condens. Matter 1996, 8, 5811–5836. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package—Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and Accurate Density Functional Calculations Using a Mixed Gaussian and Plane Waves Approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Lippert, G.; Hutter, J.; Parrinello, M. The Gaussian and Augmented-Plane-Wave Density Functional Method for Ab Initio Molecular Dynamics Simulations. Theor. Chem. Acc. Theory Comput. Model. (Theor. Chim. Acta) 1999, 103, 124–140. [Google Scholar] [CrossRef]
- Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic Separable Dual-Space Gaussian Pseudopotentials from H to Rn. Phys. Rev. B 1998, 58, 3641–3662. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty Years of Density Functional Theory in Computational Chemistry: An Overview and Extensive Assessment of 200 Density Functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- BROYDEN, C.G. The Convergence of a Class of Double-Rank Minimization Algorithms 1. General Considerations. IMA J. Appl. Math. 1970, 6, 76–90. [Google Scholar] [CrossRef]
- Fletcher, R.; Powell, M.J.D. A Rapidly Convergent Descent Method for Minimization. Comput. J. 1963, 6, 163–168. [Google Scholar] [CrossRef]
- Goldfarb, D. A Family of Variable-Metric Methods Derived by Variational Means. Math. Comput. 1970, 24, 23–26. [Google Scholar] [CrossRef]
- Shanno, D.F. Conditioning of Quasi-Newton Methods for Function Minimization. Math. Comput. 1970, 24, 647–656. [Google Scholar] [CrossRef]
- Bilal, M.; Alay-e-Abbas, S.M.; Sluydts, M.; Batool, J.; Laref, A.; Abbas, G.; Amin, N. DFT Insights into Surface Properties of Anti-Perovskite 3D Topological Crystalline Insulators: A Case Study of (001) Surfaces of Ca3SnO. Phys. Lett. A 2021, 408, 127469. [Google Scholar] [CrossRef]
- Reid, T.K.; Alpay, S.P.; Balatsky, A.V.; Nayak, S.K. First-Principles Modeling of Binary Layered Topological Insulators: Structural Optimization and Exchange-Correlation Functionals. Phys. Rev. B 2020, 101, 085140. [Google Scholar] [CrossRef]
- Schultz, P.A. First-Principles Calculations of Metal Surfaces. I. Slab-Consistent Bulk Reference for Convergent Surface Properties. Phys. Rev. B 2021, 103, 195426. [Google Scholar] [CrossRef]
- Martin, R.M. Electronic Structure: Basic Theory and Practical Methods, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-108-55558-6. [Google Scholar]
- Vande Vondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef]
- González, M.A. Force Fields and Molecular Dynamics Simulations. JDN 2011, 12, 169–200. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Physics Today; Elsevier: Amsterdam, The Netherlands, 1996; Volume 50. [Google Scholar]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; In’ T Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A Flexible Simulation Tool for Particle-Based Materials Modeling at the Atomic, Meso, and Continuum Scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: https://www.lammps.org/#gsc.tab=0 (accessed on 5 July 2025).
- LAMMPS Tutorials. Available online: https://lammpstutorials.github.io/ (accessed on 5 July 2025).
- Brauer, J.; Fischer, M. Computational Screening of Hydrophobic Zeolites for the Removal of Emerging Organic Contaminants from Water. ChemPhysChem 2024, 25, e202400347. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Lin, T.-J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Force Field for Computation of Conformational Energies, Structures, and Vibrational Frequencies of Aromatic Polyesters. J. Comput. Chem. 1994, 15, 752–768. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Sun, H.; Ren, P.; Fried, J.R. The COMPASS Force Field: Parameterization and Validation for Phosphazenes. Comput. Theor. Polym. Sci. 1998, 8, 229–246. [Google Scholar] [CrossRef]
- Sekulla, M.; Kohns, M.; Richter, M. Adsorption of CO2 on Gold Surfaces: Adsorbate Density Assumption Investigated Using Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2023, 62, 19884–19892. [Google Scholar] [CrossRef]
- Lammps/Tools/Msi2lmp/README at Develop Lammps/Lammps. Available online: https://github.com/lammps/lammps/blob/develop/tools/msi2lmp/README (accessed on 5 July 2025).
- BIOVIA, Dassault Systèmes. DS BIOVIA Materials Studio 2024; Dassault Systèmes: San Diego, CA, USA, 2024. [Google Scholar]
- Patiño, C.M.; Alzate-Vargas, L.; Li, C.; Haley, B.P.; Strachan, A. LAMMPS Data-File Generator. 2021. Available online: https://nanohub.org/resources/struc2lammpsdf (accessed on 7 May 2025).
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by Simulated Annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Romeo, F.; Sangiovanni-Vincentelli, A. Convergence and Finite-Time Behavior of Simulated Annealing. In Proceedings of the 1985 24th IEEE Conference on Decision and Control, Fort Lauderdale, FL, USA, 11–13 December 1985; pp. 761–767. [Google Scholar]
- Geyer, C.J. Markov Chain Monte Carlo Maximum Likelihood. In Computing Science and Statistics, Proceedings of the 23rd Symposium on the Interface, Seattle, WA, USA, 21–24 April 1991; Interface Foundation of North America: Atlanta, Georgia.
- Sugita, Y.; Okamoto, Y. Replica-Exchange Molecular Dynamics Method for Protein Folding. Chem. Phys. Lett. 1999, 314, 141–151. [Google Scholar] [CrossRef]
- Rathore, N.; Chopra, M.; De Pablo, J.J. Optimal Allocation of Replicas in Parallel Tempering Simulations. J. Chem. Phys. 2005, 122, 024111. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.; Schwartzentruber, T. Using Simulated Annealing with Parallel Tempering to Create Low-Defect Silica Surfaces. Available online: https://www.lammps.org/workshops/Aug11/Norman/poster.pdf (accessed on 25 March 2025).
- Kannan, S.; Zacharias, M. Simulated Annealing Coupled Replica Exchange Molecular Dynamics—An Efficient Conformational Sampling Method. J. Struct. Biol. 2009, 166, 288–294. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. An Efficient Orbital Transformation Method for Electronic Structure Calculations. J. Chem. Phys. 2003, 118, 4365–4369. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple-PBE. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, H. Computational Insight into the Bioapplication of 2D Materials: A Review. Nano Today 2023, 53, 102007. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic Factors Determining the Reactivity of Metal Surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Competitive Paths for Methanol Decomposition on Pt(111). J. Am. Chem. Soc. 2004, 126, 3910–3919. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kirvassilis, D.; Xu, L.; Mavrikakis, M. Atomic and Molecular Adsorption on Ni(111). Surf. Sci. 2019, 679, 240–253. [Google Scholar] [CrossRef]
- Xu, L.; Kirvassilis, D.; Bai, Y.; Mavrikakis, M. Atomic and Molecular Adsorption on Fe(110). Surf. Sci. 2018, 667, 54–65. [Google Scholar] [CrossRef]
- Liu, H.; Liu, F.; Zong, H.; Ding, X.; Sun, J. Initial Oxidation Behavior of α-U and γ-U Surfaces. J. Nucl. Mater. 2024, 592, 154964. [Google Scholar] [CrossRef]
- Galimberti, D.R.; Sauer, J. Chemically Accurate Vibrational Free Energies of Adsorption from Density Functional Theory Molecular Dynamics: Alkanes in Zeolites. J. Chem. Theory Comput. 2021, 17, 5849–5862. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Abdelrahman, O.A. A Universal Descriptor for the Entropy of Adsorbed Molecules in Confined Spaces. ACS Cent. Sci. 2018, 4, 1235–1243. [Google Scholar] [CrossRef]
- Patriksson, A.; Van Der Spoel, D. A Temperature Predictor for Parallel Tempering Simulations. Phys. Chem. Chem. Phys. 2008, 10, 2073. [Google Scholar] [CrossRef]
- Replica Exchange. Available online: https://epcced.github.io/archer2-advanced-use-of-lammps/05-replica-exchange/index.html (accessed on 15 January 2025).
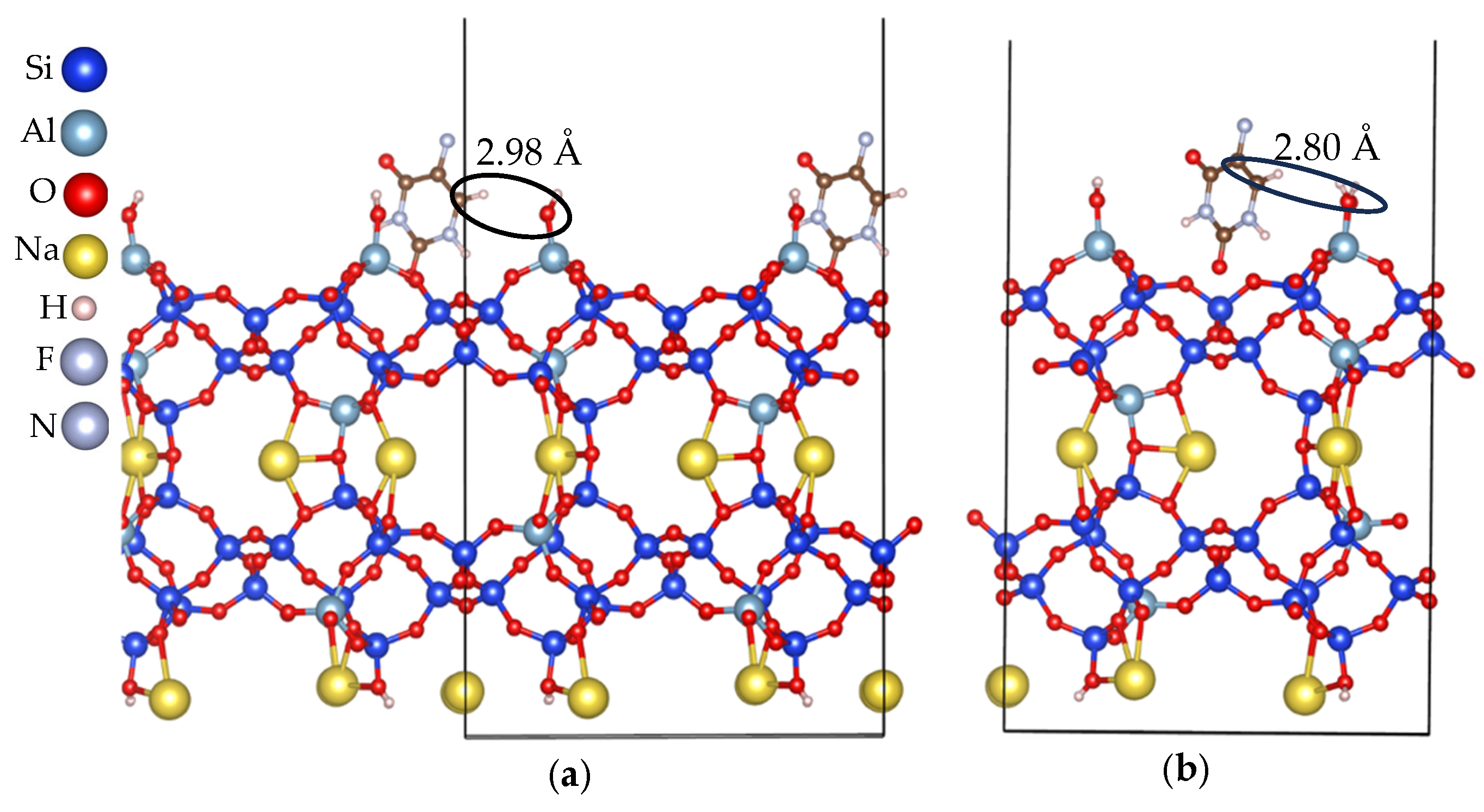
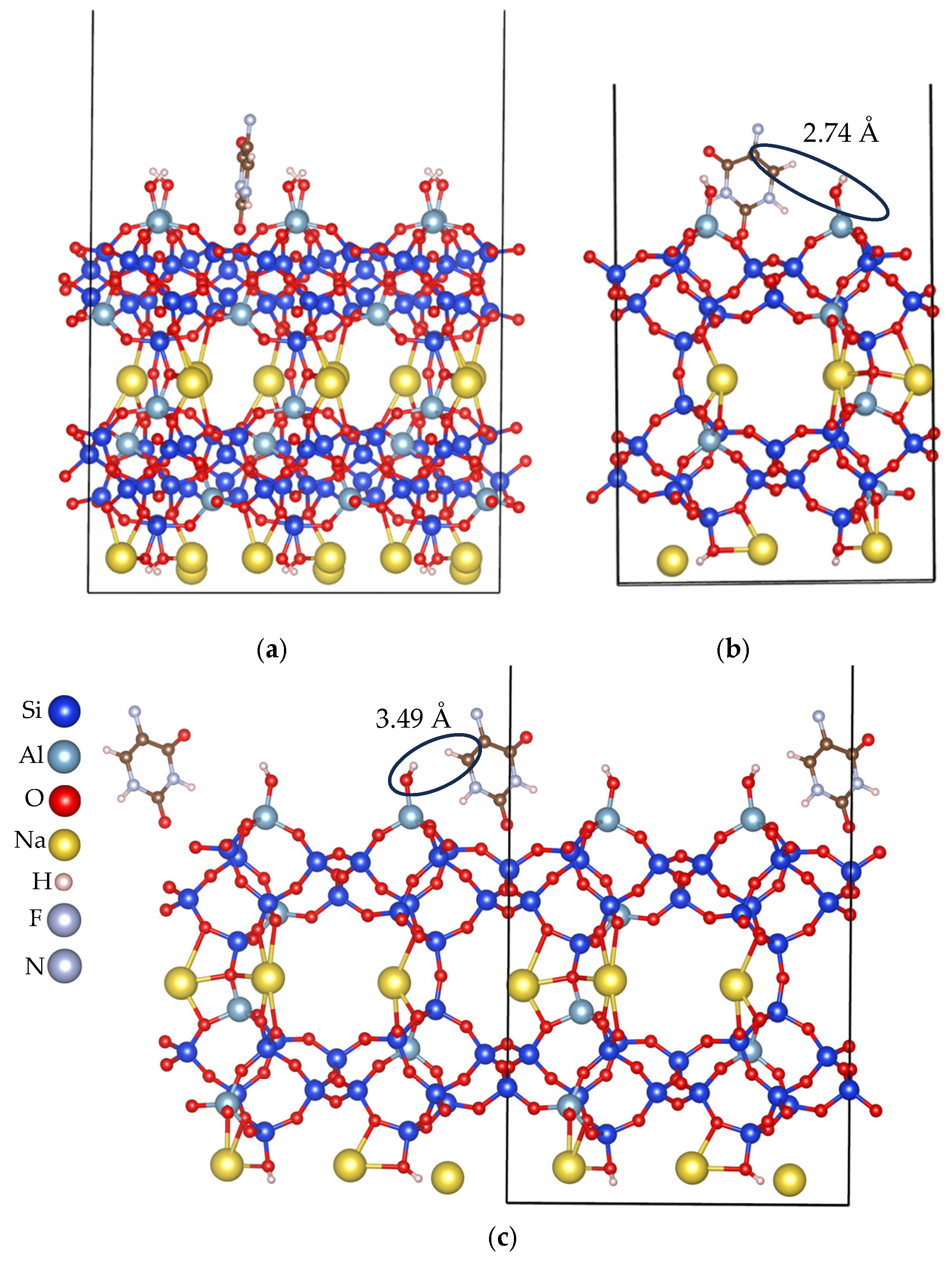
| Slab ID | Number of Na Cations at the Surface or Exposed Ring Type | Lowest Eads (kJ/mol) |
|---|---|---|
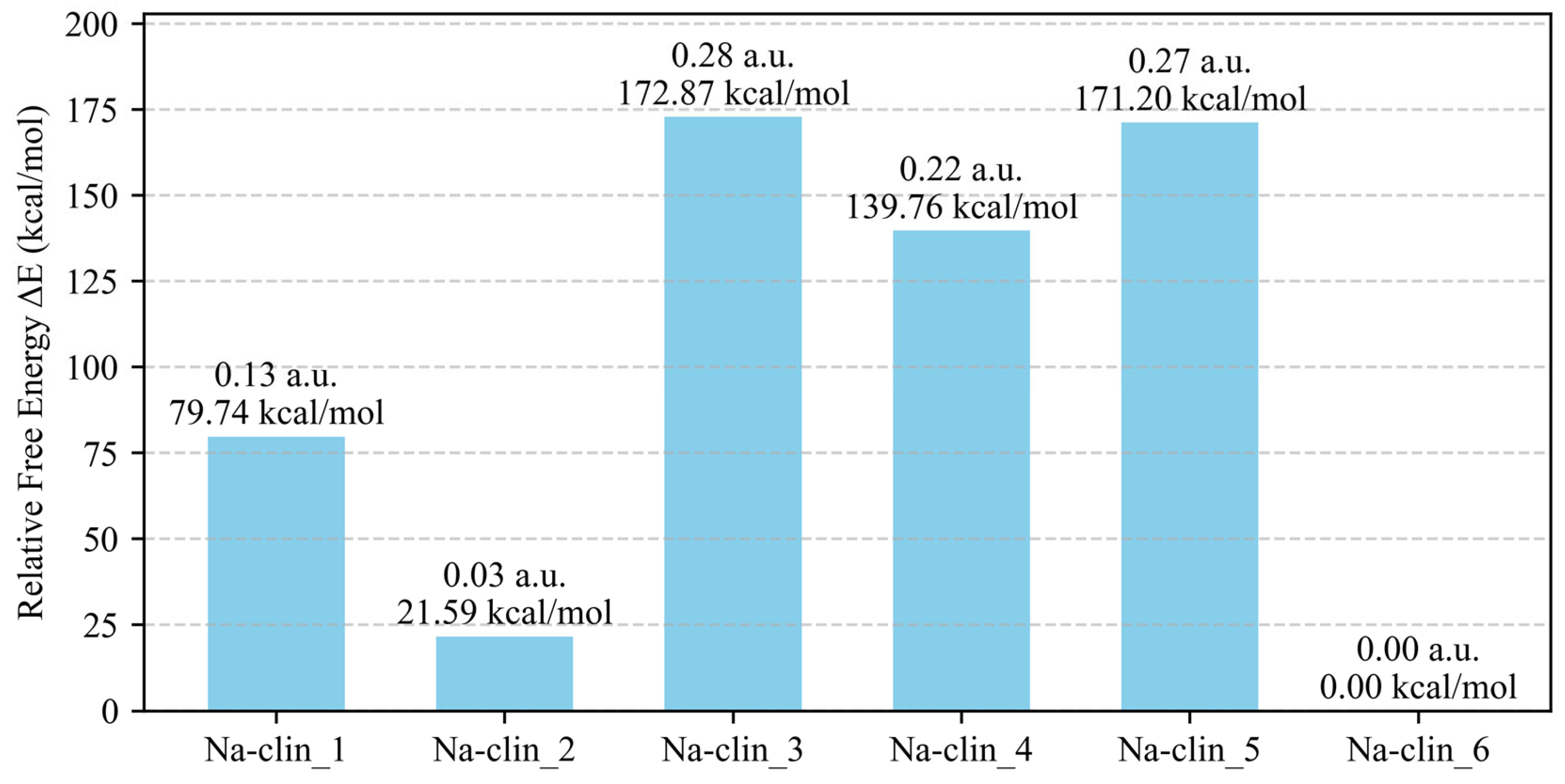
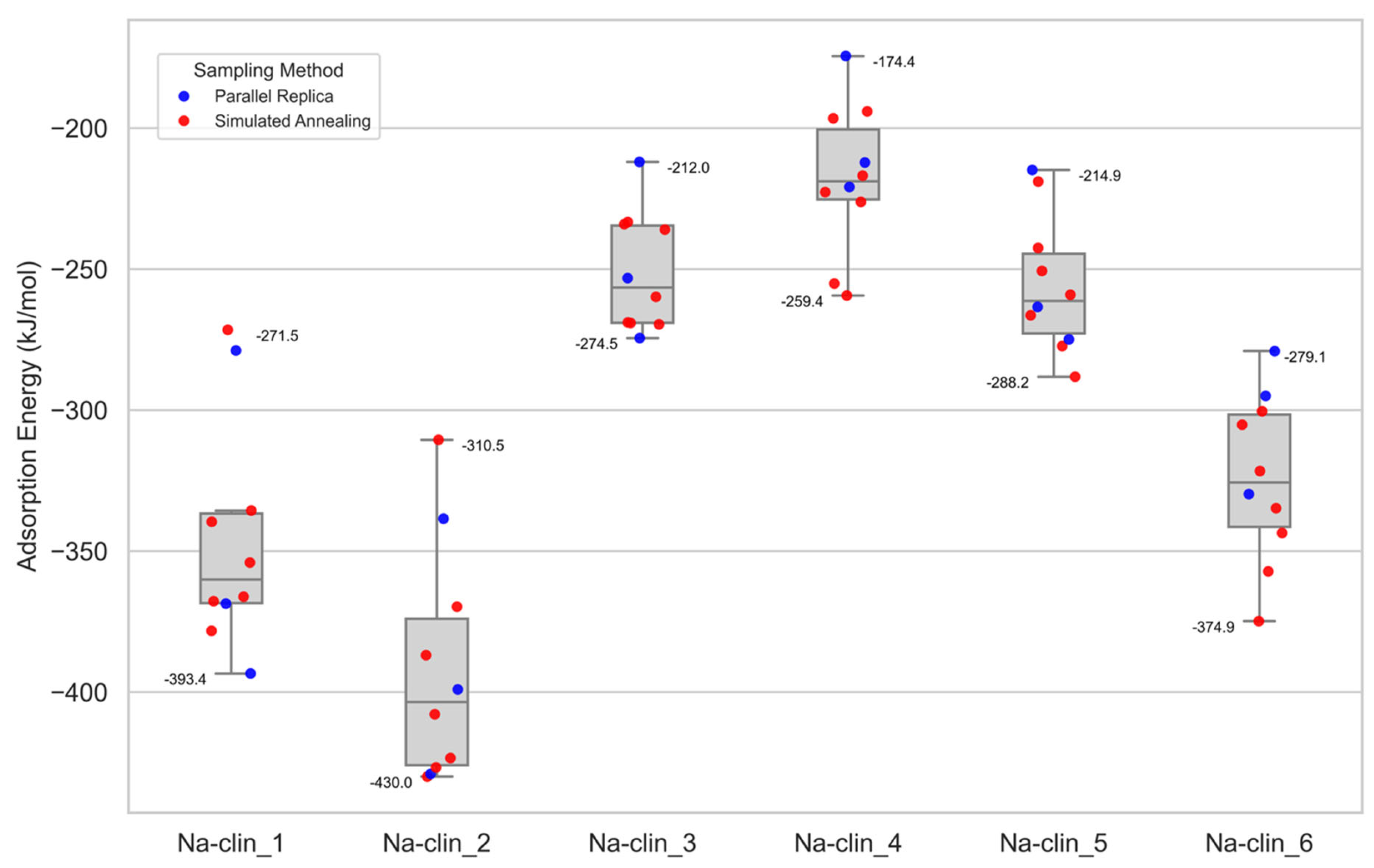
| Na-clin_1 | 3 Na cations | −393.4 |
| Na-clin_2 | 2 Na cations | −430.0 |
| Na-clin_3 | 8 MR (PR sampling) | −274.5 |
| Na-clin_4 | 8 MR (SA sampling) | −295.4 |
| Na-clin_5 | 8 MR (both sampling) | −288.2 |
| Na-clin_6 | 3 Na cations | −374.9 |
| Bonding Class | Example System | Reference | Reported Adsorption Enthalpy/Energy |
|---|---|---|---|
| Moderate molecular chemisorption (−80 to −200 kJ/mol) | CO chemisorption on Ni(111) | [73] | −1.19 eV (≈−115 kJ/mol) |
| Strong atomic/dissociative chemisorption (~−200–−400 kJ/mol) | OH adsorption on Pt(111) | [74] | ~−2.1 eV (≈−204 kJ/mol) |
| Atomic O from dissociative O2 adsorption on Ni(111) | [75] | −2.28 eV(≈−220 kJ/mol) | |
| Very strong chemisorption/initial oxidation (−400 to −700 kJ/mol) | 5-FU adsorption on Na-clinoptilolite (this work) | This study | −174.4 to −430 kJ/mol |
| Adsorption on Fe(110): lowest—NH3 (molecular) = −0.62 eV (−59.8 kJ/mol); highest—CH (molecular) =−5.24 eV(−505.6 kJ/mol) | [76] | Overall range: −59.8 to −505.6 kJ/mol (molecular fragments and atomic species) | |
| Atomic O on U surfaces (α-U and γ-U) across sites and facets | [77] | Range: −4.64 to −5.96 eV (−448 to −575 kJ/mol), atomic species. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, L.; Fischer, M. Mechanistic Insights into 5-Fluorouracil Adsorption on Clinoptilolite Surfaces: Optimizing DFT Parameters for Natural Zeolites, Part II. Appl. Sci. 2025, 15, 9535. https://doi.org/10.3390/app15179535
Saeed L, Fischer M. Mechanistic Insights into 5-Fluorouracil Adsorption on Clinoptilolite Surfaces: Optimizing DFT Parameters for Natural Zeolites, Part II. Applied Sciences. 2025; 15(17):9535. https://doi.org/10.3390/app15179535
Chicago/Turabian StyleSaeed, Lobna, and Michael Fischer. 2025. "Mechanistic Insights into 5-Fluorouracil Adsorption on Clinoptilolite Surfaces: Optimizing DFT Parameters for Natural Zeolites, Part II" Applied Sciences 15, no. 17: 9535. https://doi.org/10.3390/app15179535
APA StyleSaeed, L., & Fischer, M. (2025). Mechanistic Insights into 5-Fluorouracil Adsorption on Clinoptilolite Surfaces: Optimizing DFT Parameters for Natural Zeolites, Part II. Applied Sciences, 15(17), 9535. https://doi.org/10.3390/app15179535







