Abstract
Prenatal exposure to air pollution is a major public health concern due to its potential to impair fetal brain development. This study examined whether maternal inflammatory and oxidative stress biomarkers mediate the association between trimester-specific air pollutant exposure during pregnancy and infant neurodevelopment at one year. We analyzed 87 mother–infant pairs from the OBESO perinatal cohort in Mexico City. Trimester-specific exposure to CO, PM10, PM2.5, SO2, and O3 was estimated using residential geolocation. Biomarkers were measured in the first and third trimesters by protocol, and intra-pregnancy change was calculated as Δ(3T–1T) for cytokines (IL-1β, IL-6, TNFα) and oxidative stress markers (malondialdehyde (MDA), protein carbonyls (PC), and total antioxidant capacity (TAC). Infant neurodevelopment at 12 months was assessed using Bayley-III. Exploratory mediation analyses were conducted, adjusting for gestational age at birth, pre-eclampsia, gestational diabetes, fetal growth restriction, marital status, mode of delivery, and infant sex; bootstrapping was applied to obtain robust estimates. Third-trimester CO exposure was associated with poorer receptive language (coef = 0.754, p = 0.02). PM2.5 exposure showed direct effects on expressive language in the first (coef = 0.01, p = 0.04) and third trimesters (coef = 0.007, p = 0.015) in models including IL-1β. Third-trimester O3 and SO2 exposures were linked to lower expressive scores in models including TNFα (coef = 0.007, p = 0.02), MDA (coef = 0.008, p = 0.04), and PC (coef = 0.007, 95% p = 0.04). Meanwhile PM10 exposure was associated with socio-emotional outcomes in models with IL-6 and TAC (coef = 0.003, p = 0.04). These findings indicate that maternal inflammation and oxidative stress biomarkers did not mediate the associations between prenatal air pollution exposure and infant neurodevelopment, and this study cannot elucidate their specific biological role in neurodevelopment.
1. Introduction
According to the World Health Organization, more than 90% of the global population is exposed to air pollution levels exceeding recommended limits, contributing to an estimated seven million premature deaths yearly [1]. Beyond its profound health impact, air pollution imposes a substantial economic burden [2], particularly in low- and middle-income countries [3]. Densely populated urban areas with high levels of industrial activity are among the most affected [2].
The primary health consequences of air pollution include ischemic heart disease, chronic obstructive pulmonary disease, stroke, lung cancer, and respiratory infections [3]. Vulnerable populations, such as children, pregnant women, older adults, and individuals with pre-existing conditions, are disproportionately affected [4]. A growing body of evidence suggests that prenatal exposure to ambient air pollution may disrupt fetal development, with adverse effects that can persist into childhood and beyond [5].
Airborne pollutants are inhaled and processed in the lungs, where they activate immune cells, such as alveolar macrophages and neutrophils, triggering systemic low-grade inflammation by releasing cytokines such as TNF-α, IL-6, and IL-1β [6,7]. Many pollutants, particularly ultrafine particles, can cross the alveolar–capillary barrier and enter maternal circulation, where they may reach the placenta and stimulate local immune responses with further cytokine production [6,7,8]. While these molecules play essential roles in pregnancy, their excessive levels have been linked to complications, such as pre-eclampsia and gestational diabetes [9], and may disrupt fetal brain development by impairing synaptic plasticity and neuronal maturation in cortical and hippocampal regions, which are critical for cognition, emotion, and language [8,10,11,12].
In parallel, exposure to PM10, PM2.5, NO2, and O3 has been associated with oxidative stress, characterized by increased lipid peroxidation (malondialdehyde-MDA), protein damage (protein carbonyls-PC), and reduced total antioxidant capacity (TAC) [13]. Given that the fetal antioxidant system is intrinsically limited due to high metabolic demands and immature detoxification mechanisms, such an imbalance can cause oxidative damage to vulnerable neural structures in the developing brain [14,15,16,17].
Building on this evidence, it has been hypothesized that maternal systemic inflammation and oxidative stress may mediate the relationship between prenatal exposure to air pollutants and offspring neurodevelopment [18]. However, no studies have formally assessed these biological pathways using mediation analysis. [18,19]. Mediation models enable the decomposition of total effects into direct and indirect components, offering insight into potential mechanisms and improving causal inference in observational studies [20,21]. In studies examining the impact of air pollution during pregnancy and its association with neurodevelopment, preterm birth has been explored as a mediator of academic performance [22], while biological mediators such as mitochondrial function [22] and gut microbiome composition [23] have been investigated in individuals diagnosed with autism spectrum disorder. These findings highlight the importance of mediation analyses in advancing research in this field.
This study evaluated whether maternal systemic inflammation and oxidative stress mediate the association between trimester-specific prenatal exposure to air pollutants and neurodevelopmental outcomes at 12 months of age. Specifically, we examined infant performance in receptive language, expressive language, and socio-emotional domains. To our knowledge, no prior study has jointly evaluated inflammatory cytokines and oxidative stress markers as biological pathways linking prenatal air pollution exposure with infant neurodevelopment. By integrating trimester-specific pollutant exposures with dynamic maternal molecular responses, this study addresses a critical gap in the literature and provides a novel perspective on early-life environmental vulnerability.
2. Materials and Method
2.1. Study Population
This study included mother–child pairs from the OBESO perinatal cohort, an ongoing prospective birth cohort based at the Instituto Nacional de Perinatología (INPer) in Mexico City. The cohort investigates the effects of maternal obesity and environmental exposures on child development. The study was approved by the Internal Review Board of the Instituto Nacional de Perinatología (3300-11402-01-575-17; 14 January 2024), and all participants provided written informed consent before enrollment.
Pregnant women receiving prenatal care at INPer were recruited between 2017 and 2019 at the first trimester (11.0–13.6 weeks of gestation, confirmed by ultrasound) and were followed prospectively with visits scheduled in the second (18–22.6 weeks of gestation) and third trimesters (28–34.6 weeks of gestation).
Eligibility criteria included the following: singleton pregnancy, maternal age ≥ 18 years, no pre-existing conditions (e.g., diabetes mellitus, cardiovascular, infectious, autoimmune, renal, or hepatic disease), absence of major congenital malformations, and residence within the Valley of Mexico (Mexico City and the State of Mexico). The present analysis included 87 mother–infant pairs with complete data on prenatal air pollution exposure, maternal inflammatory and stress biomarkers, and infant neurodevelopment at 12 months (Figure 1).
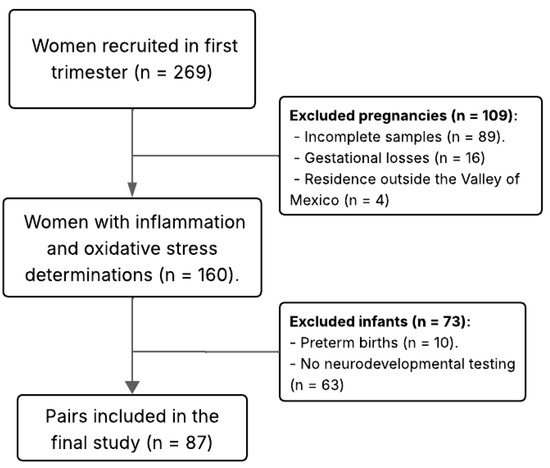
Figure 1.
Flow chart of mother–infant dyads of the OBESO perinatal cohort included in the study.
Maternal baseline sociodemographic and clinical characteristics—including age, socioeconomic status, intelligence quotient (IQ), pregestational body mass index BMI (kg/m2), and the presence of hypothyroidism—were assessed, and the occurrence of pre-eclampsia during pregnancy was recorded.
2.2. Air Pollution Exposure Assessment
Trimester-specific exposure to five air pollutants—carbon monoxide (CO), ozone (O3), sulfur dioxide (SO2), particulate matter ≤ 2.5 (PM2.5), and particulate matter ≤ 10 μm (PM10)—was estimated using daily data (January 2017 to December 2019) from the Mexico City’s official air quality monitoring network, the Red Automática de Monitoreo Atmosférico (RAMA) (http://www.aire.cdmx.gob.mx/default.php?opc=%27aKBhnmI=%27&opcion=Zg==, accessed on 20 June 2024), which includes 44 monitoring stations in the Valley of Mexico (21 in Mexico City and 13 in the State of Mexico). This approach has been implemented before for estimating ambient pollutant exposure in perinatal cohorts [13,24], as well as in non-pregnancy-related studies [25]. To estimate individual exposure to ambient air pollutants during pregnancy, the nearest air quality monitoring station was identified for each participant based on her residential location. Residential addresses were geocoded using Universal Transverse Mercator (UTM) coordinates. Geographic distances between each residence and the available monitoring stations were calculated using Geographic Information Systems (GIS).
To identify the most appropriate monitor representing each participant’s exposure, a weighted minimum distance approach was applied. This method considered not only the linear distance between residence and monitors but also the completeness of monitoring data during the gestational period. The weighted distance Dp for each monitor was calculated as follows:
where D represents the Euclidean distance (km) between residence and monitoring station, and W is a weighting factor based on the proportion of valid daily pollutant data available for the monitor during the relevant exposure window.
2.3. Maternal Inflammatory and Oxidative Stress Biomarkers
Fasting maternal blood samples were collected in red-top Vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ, USA) during the first and third trimesters of pregnancy and processed for serum separation within one hour of collection. Serum samples were stored at −70 °C until analysis.
Inflammatory cytokines (IL-6, TNF-α, IL-1β, and IL-8) were quantified using Duo-Set sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA), following the manufacturer’s protocols. Detection limits were 10, 0.3, 0.5, and 0.4 pg/mL, respectively. Oxidative stress was evaluated by measuring serum malondialdehyde (MDA) using the 1-methyl-2-phenylindole method [22], protein carbonyls (PC) by the dinitrophenylhydrazine method [23], and total antioxidant capacity (TAC) using CUPRAC method [24]. All assays were run in duplicate, and intra-assay coefficients of variation were <10%.
Changes in biomarker concentrations were calculated as the arithmetic difference between third- and first-trimester levels to reduce potential confounding by pre-pregnancy exposures.
2.4. Neurodevelopment Assessment
Infant neurodevelopment was assessed at 12 months of age using the Bayley Scales of Infant Development, Third Edition (Bayley-III), administered by trained psychologists blinded to exposure data and independent from the environmental assessment team. For this study, we focused on three domains: receptive language, expressive language, and socio-emotional development. Raw scores were converted to age-standardized scaled scores (mean = 10, SD = 3) according to Bayley-III guidelines. Impaired neurodevelopment was defined as a scaled score of 7 or less (1 SD below the mean) in one or more of the assessed neurodevelopmental domains.
2.5. Statistical Analysis
Descriptive statistics were used to characterize the study population. Bivariate analyses were performed to assess associations between sociodemographic and clinical variables, pollutant exposures, and biomarker levels. Normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Group differences were analyzed using t-tests for normally distributed continuous variables, Mann–Whitney U tests for non-normally distributed variables, and chi-square tests for categorical variables. All statistical analyses were conducted using R version 4.4.1 in RStudio (version 2024.09.1 + 394, “Cranberry Hibiscus”).
2.6. Mediation Analysis
Exploratory mediation analyses were conducted to test whether changes in maternal inflammatory and oxidative stress biomarkers mediated the association between trimester-specific pollutant exposures and infant neurodevelopmental outcomes, as illustrated in Figure 2. Separate models were constructed for each pair of pollutants (trimester-specific mean) and mediators (arithmetic difference Δ3T–1T). As a consequence, in all cases, references to the second trimester correspond exclusively to pollutant exposures, which were estimated separately for each trimester (T1, T2, and T3); inflammatory and oxidative stress biomarkers were consistently modeled as the difference between third- and first-trimester levels (Δ3T–1T). These analyses followed a classical mediation framework and were not intended to infer causal or effect modification pathways.
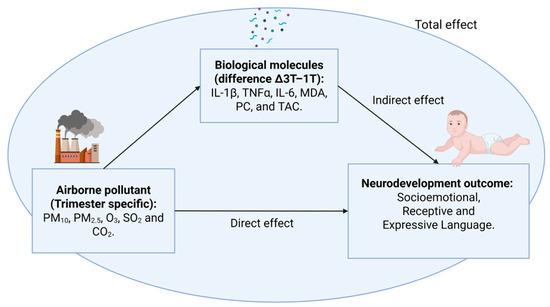
Figure 2.
Analytical framework. Schematic representation of the mediation analysis conducted in this study. Input variables include trimester-specific exposure to airborne pollutants, while outcome variables correspond to infant neurodevelopmental domains (receptive language, expressive language, and socio-emotional development). Mediators are maternal inflammatory and oxidative stress biomarkers. The diagram illustrates the decomposition of total effects into direct and indirect effects through the proposed biological pathways.
All models were adjusted for relevant clinical and sociodemographic covariates, including gestational age at birth, pre-eclampsia, gestational diabetes, fetal growth restriction, maternal educational level, socioeconomic status, marital status, mode of delivery, and infant sex. Mediation analyses were performed in R using the mediation package (version 4.5.0), with additional support from the lmtest (version 0.9-40) and sandwich (version 3.1-1) packages to obtain heteroskedasticity-consistent standard errors. To ensure robust estimation of the mediation effects, nonparametric bootstrapping with 100 simulations was conducted for each model, allowing for the construction of 95% confidence intervals for both the indirect (mediated) and direct pathways.
3. Results
This study included 87 mother–infant pairs from the OBESO perinatal cohort at INPer in Mexico City who met the established inclusion criteria and had complete data on neurodevelopmental assessment at 12 months of age. Based on their performance in the Bayley-III scales, infants were classified as having either normal or altered neurodevelopment across three domains: receptive language (Normal = 51, Altered = 36), expressive language (Normal = 47, Altered = 40), and socio-emotional development (Normal = 66, Altered = 21). These classifications were used to define comparison groups for subsequent analyses. The mean maternal age was 29.9 years, and the average pregestational BMI (pBMI) was 27.46 kg/m2. No significant differences between groups were found in demographic or clinical characteristics (Table 1).

Table 1.
Maternal sociodemographic and clinical characteristics by neurodevelopmental domain at 12 months.
Mean trimester-specific pollutant exposures were compared across neurodevelopmental outcome groups (Table 2). Infants with altered receptive language had significantly higher second-trimester exposure to PM10 (31.79 ± 10.96 µg/m3 vs. 27.61 ± 10.98 µg/m3, p = 0.04) and third-trimester exposure to CO (0.43 ± 0.11 ppb vs. 0.38 ± 0.11 ppb, p = 0.05). In addition, infants with altered expressive language also had greater second-trimester exposure to PM2.5 (15.32 ± 7.05 µg/m3 vs. 12.12 ± 8.28 µg/m3, p = 0.05). Infants with impaired socio-emotional skills had significantly higher exposure to PM10 in the third trimester (30.41 ± 9.74 µg/m3 vs. 25.2 ± 9.39 µg/m3, p = 0.01).

Table 2.
Trimester-specific average pollutant exposure by neurodevelopmental domain at 12 months.
Inflammatory and oxidative stress biomarkers were measured in maternal serum during the first and third trimesters of pregnancy (Table 3). For each biomarker, the arithmetic difference between third- and first-trimester concentrations was calculated to reduce the influence of pre-pregnancy baseline levels. Bivariate analyses showed no significant differences in these biomarkers’ changes between infants with normal and altered scores across any neurodevelopmental domains.

Table 3.
Maternal inflammatory and oxidative stress biomarkers during pregnancy by neurodevelopmental outcome domain at 12 months.
Mediation models were used to assess whether inflammatory and oxidative stress markers mediated associations between prenatal pollutant exposure and neurodevelopmental outcomes (Figure 3, Figure 4 and Figure 5). The results presented correspond to models in which a significant mediation effect was observed, while all evaluated models are available in the Supplementary materials (Supplementary Tables S1–S3).
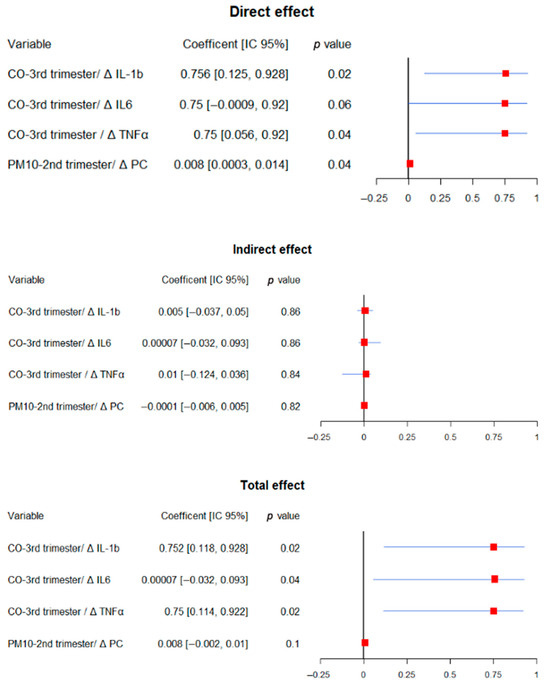
Figure 3.
Summary of significance mediation analysis for receptive language. The figure displays direct effects in the upper panel, indirect effects in the middle panel, and total effects in the lower panel. Within each panel, the left columns correspond to the different mediation models and present the coefficients with their 95% confidence intervals (lower and upper limits) and p-values. Each line in the forest plot represents a predictor variable and its association with receptive language outcomes. Red squares indicate point estimates, while horizontal blue lines depict the 95% confidence intervals. Intervals not crossing the vertical reference line at β = 0 indicate statistically significant associations. All models were adjusted for gestational age at birth, pre-eclampsia, gestational diabetes, fetal growth restriction, maternal education, socioeconomic status, marital status, mode of delivery, and infant sex.
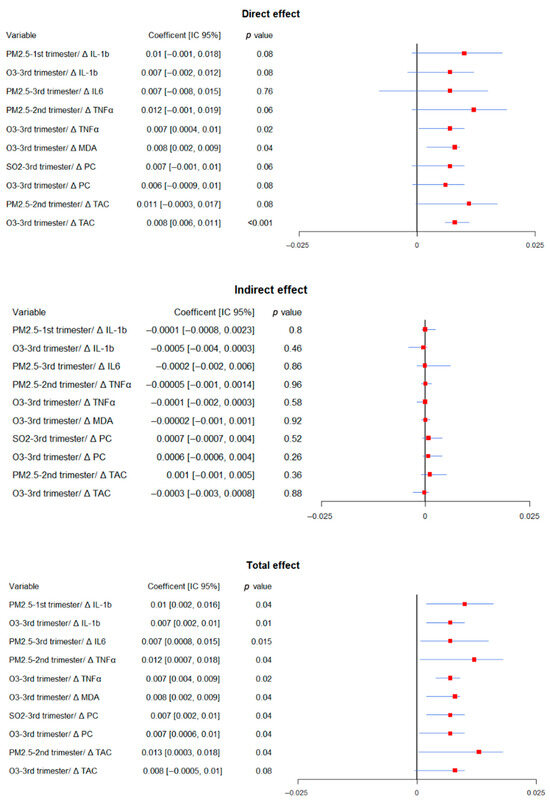
Figure 4.
Summary of significant mediation analysis for expressive language. The figure displays direct effects in the upper panel, indirect effects in the middle panel, and total effects in the lower panel. Within each panel, the left columns correspond to the different mediation models and present the coefficients with their 95% confidence intervals (lower and upper limits) and p-values. Each line in the forest plot represents a predictor variable and its association with expressive language outcomes. Red squares indicate point estimates, while horizontal blue lines depict the 95% confidence intervals. Intervals not crossing the vertical reference line at β = 0 indicate statistically significant associations. All models were adjusted for gestational age at birth, pre-eclampsia, gestational diabetes, fetal growth restriction, maternal education, socioeconomic status, marital status, mode of delivery, and infant sex.
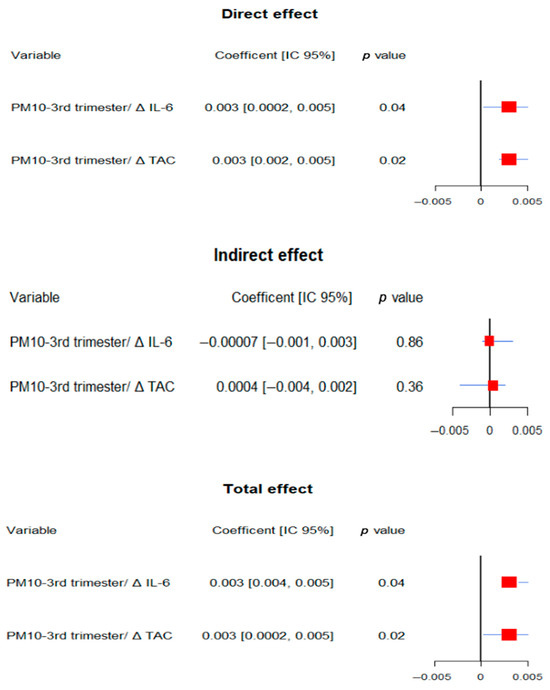
Figure 5.
Summary of significant mediation analysis for socioemotional outcomes. The figure displays direct effects in the upper panel, indirect effects in the middle panel, and total effects in the lower panel. Within each panel, the left columns correspond to the different mediation models and present the coefficients with their 95% confidence intervals (lower and upper limits) and p-values. Each line in the forest plot represents a predictor variable and its association with socioemotional outcomes. Red squares indicate point estimates, while horizontal blue lines depict the 95% confidence intervals. Intervals not crossing the vertical reference line at β = 0 indicate statistically significant associations. All models were adjusted for gestational age at birth, pre-eclampsia, gestational diabetes, fetal growth restriction, maternal education, socioeconomic status, marital status, mode of delivery, and infant sex.
For receptive language, no biomarker significantly mediated the associations. However, third-trimester CO exposure showed a direct effect when we evaluated Δ IL-1β (coef = 0.754, 95% CI: 0.12 to 0.93 p = 0.02) and Δ TNFα (coef = 0.75, 95% CI: 0.056 to 0.92, p = 0.04). It was similar in total effects Δ IL-1β (coef = 0.75, 95% CI: 0.12 to 0.93 p = 0.02), Δ TNFα (coef =0.75, 95% CI: 0.11 to 0.92, p = 0.02), and Δ IL-6 (coef = 0.0001, 95% CI: 0.032 to 0.093, p = 0.02). Interestingly, second-trimester PM10 exposure became significant in the mediation model (direct effect: coef = 0.008, 95% CI: 0.0003 to 0.014, p = 0.04), despite being non-significant in the direct model, indicating a suppression effect.
In expressive language models, PM2.5 exposure in the first (coef = 0.01, 95% CI: 0.002 to 0.016, p = 0.04) and third trimesters (coef = 0.007, 95% CI: 0.008 to 0.01, p = 0.015) showed significant direct associations when IL-1β was included as a mediator, although IL-1β itself was not statistically significant. PM2.5 exposure in the second trimester showed significant total effects in models including TNFα (coef = 0.012, 95% CI: 0.0007 to 0.018, p = 0.04) and TAC (coef = 0.013, 95% CI: 0.0003 to 0.018, p = 0.04), although no significant indirect effects were detected.
PM2.5 in the third trimester had a significant total effect (coef = 0.007, 95% CI: 0.008 to 0.015, p = 0.015) in models including IL-6 as a mediator, but neither direct nor indirect effects were significant. Third-trimester exposure to O3 was associated with poorer expressive language scores in models including TNFα (total and direct effects: coef = 0.007, 95% CI: 0.0004 to 0.009, p = 0.02) and MDA (coef = 0.008, 95% CI: 0.002 to 0.009, p = 0.04). TAC also showed a significant direct effect (coef = 0.008, 95% CI: 0.006 to 0.011, p = 0.0001), without mediation. SO2 exposure was associated with significant total effects when PC were included as mediators (coef = 0.007, 95% CI: 0.002 to 0.001, p = 0.04), although no significant indirect effect emerged.
For socio-emotional development, third-trimester PM10 exposure was significantly associated with adverse outcomes in models including IL-6 (total and direct effects: coef = 0.003, 95% CI: 0.004 to 0.005, p = 0.04) and TAC (total effect: coef = 0.003, 95% CI: 0.0002 to 0.005, p = 0.04; direct effect: p = 0.02), although no significant mediation was observed.
4. Discussion
This study conducted exploratory mediation analyses to evaluate whether changes in maternal oxidative stress and inflammatory biomarker concentrations between the first and third trimesters of pregnancy could explain the association between prenatal exposure to air pollutants and neurodevelopmental outcomes at one year of age. Several airborne pollutants—specifically CO, PM2.5, PM10, O3, and sulfur dioxide SO2—were independently associated with alterations in infant neurodevelopment, particularly in receptive and expressive language domains. Despite these consistent direct associations, no statistically significant indirect effects were detected through the evaluated biomarkers, suggesting that maternal systemic inflammation and oxidative stress do not mediate the observed relationships.
In the receptive language domain, we observed that third-trimester exposure to CO was significantly associated with poorer outcomes, independent of maternal levels of IL-1β, IL-6, or TNFα. The significant bivariate association suggests a direct relationship potentially involving biological mechanisms not captured by the measured mediators. Mechanistically, CO has been proposed to induce fetal neuroinflammation and early maternal immune activation [25]. In murine models, prenatal CO exposure has been linked to impairments in spatial memory and social behavior [26], as well as disrupted neuronal migration in the fetal cerebral cortex [27]. Although evidence on CO’s effect on maternal inflammation is limited, studies have shown that CO exposure increases the expression of SOD-1, SOD-2, HO-1, iNOS, and nitrotyrosine [28], contributing to systemic hypoxia due to its 240-fold greater binding affinity for hemoglobin compared to oxygen, with consequent cardiovascular and neurological effects [29,30] Complementing our findings, a cohort study in rural Guatemala found that higher third-trimester CO exposure—measured in households using wood-burning cookstoves—was associated with poorer cognitive outcomes in children aged 6 to 7 years, particularly in memory, visuospatial abilities, and fine motor coordination [31]. While the affected domains differ from those assessed in our study, these results may reflect a shared developmental vulnerability to prenatal CO exposure. Early deficits in receptive language, such as those we observed at 12 months, could signal broader neurodevelopmental disturbances that manifest later in childhood across multiple domains.
Second-trimester PM10 exposure was also linked to receptive language impairment, particularly in models that included PC, suggesting that higher PM10 levels and insufficient oxidative stress defenses, may adversely affect early brain development. Third-trimester PM10 exposure was linked to socio-emotional impairments, aligning with previous studies reporting PM10—related alterations in brain volume and [32] white matter microstructure during childhood and adolescence [32,33].
In the expressive language domain, PM2.5 exposure during all trimesters was consistently associated with adverse outcomes, although only second-trimester exposure reached statistical significance in bivariate analyses. In mediation models, total effects were significant in several cases, but no clear mediating or direct pathways emerged. These results support prior evidence that PM2.5 is one of the most neurotoxic airborne pollutants, implicated in deficits in cognition, learning, memory, executive function, adaptive behavior, and attention [34,35,36]. In addition, several studies have identified the second trimester as a sensitive window during which exposure to PM2.5 is associated with adverse neurodevelopmental outcomes. Exposure during mid-pregnancy (approximately 20–24 weeks of gestation) has been linked to lower language scores at age two [37], and exposure around 17–18 weeks of gestation has been associated with reduced cognitive performance (MDI scores). This period coincides with critical stages of synaptogenesis and neuronal migration in brain regions involved in language development [38] These associations are likely mediated by their impact on brain development, including thickness and volume of the thalamus [39], size of the hippocampus and corpus callosum [40], impaired white matter development [40,41], and disruptions in synaptic pruning, myelination, and interconnectivity [39,42].
The total effect of third-trimester SO2 exposure was observed in the model evaluating PC as a mediator. However, studies assessing SO2 exposure remain inconclusive regarding its association with neurodevelopmental outcomes. The findings most comparable to ours are from a study conducted in China by Yu et al., which reported associations with lower motor and language scores [43]. In contrast, three cohort studies in children with autism spectrum disorder did not identify significant associations with neurodevelopmental outcomes [44,45,46]. These results suggest that SO2 exposure may exert subtle effects on neurodevelopment without leading to significant alterations.
O3 exposure during the third trimester was associated with poorer expressive language scores, particularly in models that included TAC as a covariate. This suggests that both increased O3 exposure and a decrease in antioxidant defenses are strongly associated with adverse outcomes. While the role of O3 in neurodevelopment has received less attention, [41] prenatal exposure has been linked to intellectual disability and social integration difficulties [36,47]. Recent evidence suggests it may disrupt functional connectivity in brain regions involved in emotion and social cognition, such as the amygdala and hippocampus [42].
Despite the lack of statistically significant mediation by inflammatory or oxidative stress biomarkers, our findings suggest a biological interaction between prenatal pollutant exposure and maternal physiological responses that may influence fetal neurodevelopment. This is consistent with experimental data showing that pollutants activate inflammatory pathways (e.g., IL-1β, IL-6, TNF-α, CRP), oxidative stress responses (e.g., MDA, 8-OHdG, hypoxanthine), and innate immune signaling such as the NLRP3 inflammasome [36,48]. While the selected biomarkers may not have captured the full extent of these responses, their inclusion modified effect estimates in some models, indicating a possible modulatory role [48,49].
We observed no significant differences in maternal biomarker concentrations between infants with normal vs. altered neurodevelopment, nor did these biomarkers mediate the associations in formal models. This may reflect the involvement of alternative biological pathways such as epigenetic regulation, hormonal signaling, or placental function.
A major strength of this study is the simultaneous integration and evaluation of maternal inflammatory and oxidative stress biomarkers, providing a broader and more biologically comprehensive perspective on maternal physiological responses to environmental exposures during pregnancy. By collecting biological data at two key gestational time points (first and third trimesters), we were able to assess dynamic changes in these molecular pathways across pregnancy, rather than relying on single time-point measurements. Additionally, using a well-characterized perinatal cohort with prospective follow-up and standardized neurodevelopmental assessments at 12 months strengthens the internal validity of our findings. The trimester-specific estimation of pollutant exposures further enhances temporal resolution in exposure assessment. Finally, the application of exploratory mediation models enabled the investigation of potential biological pathways linking environmental exposures to early neurodevelopment, moving beyond traditional exposure–outcome associations and offering a mechanistic framework to guide future research and targeted prevention strategies.
However, several limitations should be acknowledged. Our biomarker panel was restricted to a subset of markers of systemic inflammation and oxidative stress, which may not fully capture the complexity of immune and redox pathways involved in prenatal environmental exposures. Additional biomarkers—such as CRP, glutathione, or 8-OHdG—could provide a more comprehensive picture of the mechanisms at play. Biomarker concentrations were only available for the first and third trimesters, and changes were estimated as a simple difference between these time points; this approach may overlook non-linear or fluctuating patterns across gestation, particularly during the second trimester. Pollutant exposure was derived from trimester-specific arithmetic means from fixed-site monitoring stations, which may not reflect individual-level variation related to mobility, indoor exposure, and microenvironmental conditions, potentially leading to exposure misclassification. However, recent studies have adopted similar methodologies for estimating ambient pollutant exposure in perinatal cohorts [13,24], as well as in non-pregnancy-related studies [25]. Although we adjusted for key demographic and clinical covariates, residual confounding cannot be ruled out. The relatively small sample size (n = 87) limited statistical power to detect indirect effects in mediation models and increased the likelihood of Type II error. Finally, although multiple associations were tested, no formal correction for multiple comparisons was applied, given the exploratory nature of the study. These considerations underscore the need for cautious interpretation and highlight the importance of replication in larger, longitudinal studies.
These findings highlight the importance of considering gestational timing and pollutant-specific effects when evaluating neurodevelopmental risk. It is important to note that the mediation models applied in this study are exploratory and do not imply causal relationships; instead, they aim to identify potential biological pathways and generate hypotheses for future research. Fetal brain vulnerability to air pollutants involves complex biological processes, potentially including disruptions in maternal oxidative stress responses. Future research should incorporate broader panel biomarkers to better understand the pathways through which prenatal air pollution exposure contributes to adverse neurodevelopmental outcomes. In addition to oxidative stress and inflammation, future studies should explore alternative or complementary mechanisms—such as epigenetic regulation, endocrine disruption, and placental response pathways—to provide a more comprehensive understanding of fetal vulnerability. A deeper understanding of these mechanisms could inform targeted public health interventions and help design clinical strategies to identify at-risk pregnancies and implement early developmental surveillance or preventive care.
5. Conclusions
Although exploratory mediation models did not yield statistically significant indirect effects, our findings indicate pollutant- and trimester-specific associations with early neurodevelopment, particularly in receptive, expressive, and socio-emotional domains. Including maternal inflammatory and oxidative stress biomarkers in the models modified the effect estimates. However, they could not be linked to the causal pathway between prenatal pollutant exposure and neurodevelopmental outcomes. Given the modest sample size and reliance on biomarker changes between the first and third trimesters, the study may have been underpowered to detect subtle mediation effects, and results should be interpreted with caution.
Our findings highlight the importance of considering gestational timing, pollutant-specific mechanisms, and maternal physiological responses in future research, and underscore the need for larger, longitudinal studies with more comprehensive biomarker panels to clarify the biological pathways through which prenatal air pollution exposure may affect child neurodevelopment.
Importantly, this study is novel in integrating trimester-specific exposures with maternal inflammatory and oxidative stress biomarkers, and in jointly evaluating their role as potential mediators or modulators of the neurodevelopmental effects of prenatal air pollution. While exploratory, this approach provides new insights into maternal molecular responses during pregnancy and their potential relevance for fetal programming, underscoring the value of replication in larger cohorts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15179753/s1, Table S1: Mediation models for receptive language; Table S2: Mediation models for expressive Language; Table S3: Mediation models for socioemotional language.
Author Contributions
J.A.M.-O. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: G.E.-G. and J.A.M.-O. Acquisition, analysis, or interpretation of data: J.A.M.-O., G.E.-G., A.E.-N., A.M.-E., J.M.S.-P., A.C.-E., S.M.-M. and M.T.-C. Drafting of the manuscript: J.A.M.-O. Critical revision of the manuscript for important intellectual content: G.E.-G., I.C.-A., O.P.-P. and A.C.-E. Statistical analysis: J.A.M.-O. and E.R.-M. Administrative, technical, or material support: G.E.-G. and B.V.S.-R. Study supervision: G.E.-G. and S.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institute of Perinatology (3300-11402-01-575-17) and FOSISS-CONACyT (2015-3-261661) to G.E.-G.
Institutional Review Board Statement
This study involving human participants was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the National Institute of Perinatology Review Board (3300-11402-01-575-17 and 14 January 2024).
Informed Consent Statement
The participants provided written informed consent to participate in this study.
Data Availability Statement
Due to the inclusion of sensitive clinical and geographic information, public sharing of the dataset is restricted to protect participant confidentiality. The data are not deposited in a public repository but may be made available upon request; J.A.M.-O. is responsible for the integrity, storage, and controlled access to the dataset.
Acknowledgments
We extend our sincere gratitude to the entire operational and field staff of the OBESO cohort. Their dedication, effort, and meticulous work in participant follow-up and data collection were essential to the success of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yi, C.; Wang, Q.; Qu, Y.; Niu, J.; Oliver, B.G.; Chen, H. In-Utero Exposure to Air Pollution and Early-Life Neural Development and Cognition. Ecotoxicol. Environ. Saf. 2022, 238, 113589. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, R.; Kan, H. Air Pollution, Disease Burden, and Health Economic Loss in China. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2017; Volume 1017, pp. 233–242. [Google Scholar]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-Year Trends of the Global Burden of Disease Attributable to Ambient Air Pollution: An Analysis of Data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Atuyambe, L.M.; Arku, R.E.; Naidoo, N.; Kapwata, T.; Asante, K.P.; Cissé, G.; Simane, B.; Wright, C.Y.; Berhane, K. The Health Impacts of Air Pollution in the Context of Changing Climate in Africa: A Narrative Review with Recommendations for Action. Ann. Glob. Health 2024, 90, 76. [Google Scholar] [CrossRef]
- Gheissari, R.; Liao, J.; Garcia, E.; Pavlovic, N.; Gilliland, F.D.; Xiang, A.H.; Chen, Z. Health Outcomes in Children Associated with Prenatal and Early-Life Exposures to Air Pollution: A Narrative Review. Toxics 2022, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Johnson, N.M.; Hoffmann, A.R.; Behlen, J.C.; Lau, C.; Pendleton, D.; Harvey, N.; Shore, R.; Li, Y.; Chen, J.; Tian, Y.; et al. Air Pollution and Children’s Health—A Review of Adverse Effects Associated with Prenatal Exposure from Fine to Ultrafine Particulate Matter. Environ. Health Prev. Med. 2021, 26, 72. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal Immune Activation and Neuroinflammation in Human Neurodevelopmental Disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Kwon, H.K.; Choi, G.B.; Huh, J.R. Maternal Inflammation and Its Ramifications on Fetal Neurodevelopment. Trends Immunol. 2022, 43, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Juan-Reyes, S.S.; Gómez-Oliván, L.M.; Juan-Reyes, N.S.; Islas-Flores, H.; Dublán-García, O.; Orozco-Hernández, J.M.; Pérez-Álvarez, I.; Mejía-García, A. Women with Preeclampsia Exposed to Air Pollution during Pregnancy: Relationship between Oxidative Stress and Neonatal Disease—Pilot Study. Sci. Total Environ. 2023, 871, 161858. [Google Scholar] [CrossRef] [PubMed]
- Dowell, J.; Elser, B.A.; Schroeder, R.E.; Stevens, H.E. Cellular Stress Mechanisms of Prenatal Maternal Stress: Heat Shock Factors and Oxidative Stress. Neurosci. Lett. 2019, 709, 134368. [Google Scholar] [CrossRef]
- Lavigne, E.; Yasseen, A.S.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Brook, J.R.; Crouse, D.L.; Burnett, R.T.; Chen, H.; et al. Ambient Air Pollution and Adverse Birth Outcomes: Differences by Maternal Comorbidities. Environ. Res. 2016, 148, 457–466. [Google Scholar] [CrossRef]
- Christensen, G.M.; Marcus, M.; Vanker, A.; Eick, S.M.; Malcolm-Smith, S.; Suglia, S.F.; Chang, H.H.; Zar, H.J.; Stein, D.J.; Hüls, A. Joint Effects of Indoor Air Pollution and Maternal Psychosocial Factors during Pregnancy on Trajectories of Early Childhood Psychopathology. Am. J. Epidemiol. 2024, 193, 1352–1361. [Google Scholar] [CrossRef]
- Carter, S.A.; Rahman, M.M.; Lin, J.C.; Chow, T.; Yu, X.; Martinez, M.P.; Levitt, P.; Chen, Z.; Chen, J.C.; Eckel, S.P.; et al. Maternal Exposure to Aircraft Emitted Ultrafine Particles during Pregnancy and Likelihood of ASD in Children. Environ. Int. 2023, 178, 108061. [Google Scholar] [CrossRef]
- Annavarapu, R.N.; Kathi, S. Cognitive Disorders in Children Associated with Urban Vehicular Emissions. Environ. Pollut. 2016, 208, 74–78. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of Air Pollution on the Nervous System and Its Possible Role in Neurodevelopmental and Neurodegenerative Disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Ananth, C.V.; Brandt, J.S. A Principled Approach to Mediation Analysis in Perinatal Epidemiology. Am. J. Obstet. Gynecol. 2022, 226, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Pelham, W.E.; Smyth, H.; MacKinnon, D.P. Confounding in Statistical Mediation Analysis: What It Is and How to Address It. J. Couns. Psychol. 2017, 64, 659–671. [Google Scholar] [CrossRef]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudière, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademi˙r, S.E.; Altun, M. Total Antioxidant Capacity Assay of Human Serum Using Copper(II)-Neocuproine as Chromogenic Oxidant: The CUPRAC Method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Ghahari, N.; Yousefian, F.; Najafi, E. Prenatal Exposure to Ambient Air Pollution and Autism Spectrum Disorders: Results from a Family-Based Case-Control Study. JCPP Adv. 2023, 3, e12129. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A.; Supplee, W.W.; Koffler, K.; Cheng, Y.; Quezado, Z.M.N.; Levy, R.J. Carbon Monoxide Incompletely Prevents Isoflurane-Induced Defects in Murine Neurodevelopment. Neurotoxicol. Teratol. 2017, 61, 92–103. [Google Scholar] [CrossRef]
- Trentini, J.F.; O’Neill, J.T.; Poluch, S.; Juliano, S.L. Prenatal Carbon Monoxide Impairs Migration of Interneurons into the Cerebral Cortex. Neurotoxicology 2016, 53, 31–44. [Google Scholar] [CrossRef]
- Lopez, I.A.; Acuna, D.; Beltran-Parrazal, L.; Lopez, I.E.; Amarnani, A.; Cortes, M.; Edmond, J. Evidence for Oxidative Stress in the Developing Cerebellum of the Rat after Chronic Mild Carbon Monoxide Exposure (0.0025% in Air). BMC Neurosci. 2009, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- André, L.; Gouzi, F.; Thireau, J.; Meyer, G.; Boissiere, J.; Delage, M.; Abdellaoui, A.; Feillet-Coudray, C.; Fouret, G.; Cristol, J.P.; et al. Carbon Monoxide Exposure Enhances Arrhythmia after Cardiac Stress: Involvement of Oxidative Stress. Basic Res. Cardiol. 2011, 106, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.J. Carbon Monoxide Pollution and Neurodevelopment: A Public Health Concern. Neurotoxicol. Teratol. 2015, 49, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dix-Cooper, L.; Eskenazi, B.; Romero, C.; Balmes, J.; Smith, K.R. Neurodevelopmental Performance among School Age Children in Rural Guatemala Is Associated with Prenatal and Postnatal Exposure to Carbon Monoxide, a Marker for Exposure to Woodsmoke. Neurotoxicology 2012, 33, 246–254. [Google Scholar] [CrossRef]
- Bos, B.; Barratt, B.; Batalle, D.; Gale-Grant, O.; Hughes, E.J.; Beevers, S.; Cordero-Grande, L.; Price, A.N.; Hutter, J.; Hajnal, J.V.; et al. Prenatal Exposure to Air Pollution Is Associated with Structural Changes in the Neonatal Brain. Environ. Int. 2023, 174, 107921. [Google Scholar] [CrossRef]
- Kusters, M.S.W.; López-Vicente, M.; Muetzel, R.L.; Binter, A.-C.; Petricola, S.; Tiemeier, H.; Guxens, M. Residential Ambient Air Pollution Exposure and the Development of White Matter Microstructure throughout Adolescence. Environ. Res. 2024, 262, 119828. [Google Scholar] [CrossRef]
- Sukumaran, K.; Botternhorn, K.L.; Schwartz, J.; Gauderman, J.; Cardenas-Iniguez, C.; McConnell, R.; Hackman, D.A.; Berhane, K.; Ahmadi, H.; Abad, S.; et al. Associations between Fine Particulate Matter Components, Their Sources, and Cognitive Outcomes in Children Ages 9-10 Years Old from the United States. Environ. Health Perspect. 2024, 132, 107009. [Google Scholar] [CrossRef] [PubMed]
- McGuinn, L.A.; Bellinger, D.C.; Colicino, E.; Coull, B.A.; Just, A.C.; Kloog, I.; Osorio-Valencia, E.; Schnaas, L.; Wright, R.J.; Téllez-Rojo, M.M.; et al. Prenatal PM2.5 Exposure and Behavioral Development in Children from Mexico City. Neurotoxicology 2020, 81, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, X.; Li, H.; Jiang, Y.; Zhu, X.; Zhang, Y.; Niu, Y.; Liu, C.; Ji, J.; Chillrud, S.N.; et al. Cardiovascular Effects of Traffic-Related Air Pollution: A Multi-Omics Analysis from a Randomized, Crossover Trial. J. Hazard. Mater. 2022, 435, 129031. [Google Scholar] [CrossRef] [PubMed]
- Morgan, Z.E.M.; Bailey, M.J.; Trifonova, D.I.; Naik, N.C.; Patterson, W.B.; Lurmann, F.W.; Chang, H.H.; Peterson, B.S.; Goran, M.I.; Alderete, T.L. Prenatal Exposure to Ambient Air Pollution Is Associated with Neurodevelopmental Outcomes at 2 Years of Age. Environ. Health 2023, 22, 11. [Google Scholar] [CrossRef]
- Perera, F.; Miao, Y.; Ross, Z.; Rauh, V.; Margolis, A.; Hoepner, L.; Riley, K.W.; Herbstman, J.; Wang, S. Prenatal Exposure to Air Pollution during the Early and Middle Stages of Pregnancy Is Associated with Adverse Neurodevelopmental Outcomes at Ages 1 to 3 Years. Environ. Health 2024, 23, 95. [Google Scholar] [CrossRef]
- Cserbik, D.; Chen, J.C.; McConnell, R.; Berhane, K.; Sowell, E.R.; Schwartz, J.; Hackman, D.A.; Kan, E.; Fan, C.C.; Herting, M.M. Fine Particulate Matter Exposure during Childhood Relates to Hemispheric-Specific Differences in Brain Structure. Environ. Int. 2020, 143, 105933. [Google Scholar] [CrossRef]
- Guxens, M.; Lubczyńska, M.J.; Pérez-Crespo, L.; Muetzel, R.L.; El Marroun, H.; Basagaña, X.; Hoek, G.; Tiemeier, H. Associations of Air Pollution on the Brain in Children: A Brain Imaging Study. Res. Rep. Health Eff. Inst. 2022, 2022, 209. [Google Scholar]
- Burnor, E.; Cserbik, D.; Cotter, D.L.; Palmer, C.E.; Ahmadi, H.; Eckel, S.P.; Berhane, K.; McConnell, R.; Chen, J.C.; Schwartz, J.; et al. Association of Outdoor Ambient Fine Particulate Matter with Intracellular White Matter Microstructural Properties among Children. JAMA Netw. Open 2021, 4, e2138300. [Google Scholar] [CrossRef]
- Cotter, D.L.; Campbell, C.E.; Sukumaran, K.; McConnell, R.; Berhane, K.; Schwartz, J.; Hackman, D.A.; Ahmadi, H.; Chen, J.C.; Herting, M.M. Effects of Ambient Fine Particulates, Nitrogen Dioxide, and Ozone on Maturation of Functional Brain Networks across Early Adolescence. Environ. Int. 2023, 177, 108001. [Google Scholar] [CrossRef]
- Yu, T.; Zhou, L.; Xu, J.; Kan, H.; Chen, R.; Chen, S.; Hua, H.; Liu, Z.; Yan, C. Effects of Prenatal Exposures to Air Sulfur Dioxide/Nitrogen Dioxide on Toddler Neurodevelopment and Effect Modification by Ambient Temperature. Ecotoxicol. Environ. Saf. 2022, 230, 113118. [Google Scholar] [CrossRef]
- Ha, Y.W.; Kim, T.H.; Kang, D.R.; Park, K.S.; Shin, D.C.; Cho, J.; Kim, C. Estimation of Attributable Risk and Direct Medical and Non-Medical Costs of Major Mental Disorders Associated with Air Pollution Exposures Among Children and Adolescents in the Republic of Korea, 2011–2019. J. Korean Med. Sci. 2024, 39, e218. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Min, W.K.; Choi, Y.J.; Jin, S.; Park, K.H.; Kim, S. The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina 2023, 59, 951. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Cardoso Melo, R.D.; de Vries, M.; Lakerveld, J.; Zijlema, W.; Hartman, C.A. Longitudinal Effects of Environmental Noise and Air Pollution Exposure on Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder during Adolescence and Early Adulthood: The TRAILS Study. Environ. Res. 2023, 227, 115704. [Google Scholar] [CrossRef] [PubMed]
- Grineski, S.E.; Renteria, R.; Bakian, A.; Collins, T.W.; VanDerslice, J.; Alexander, C.J.; Bilder, D. Prenatal Ozone Exposure and Risk of Intellectual Disability. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Q.; Hua, C.; Ci, X. Melatonin Alleviates Particulate Matter-Induced Liver Fibrosis by Inhibiting ROS-Mediated Mitophagy and Inflammation via Nrf2 Activation. Ecotoxicol. Environ. Saf. 2023, 268, 115717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gong, X.; Han, B.; Chu, M.; Gong, C.; Yang, J.; Chen, L.; Wang, J.; Bai, Z.; Zhang, Y. Ambient PM2.5 Exposures and Systemic Inflammation in Women with Early Pregnancy. Sci. Total Environ. 2022, 829, 154564. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).