Substitution of Fossil Layers with Biobased Ones in Sustainable Cellulosic Packaging for Dairy Products
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The Production of the Materials
2.2. Characterization of the Materials
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in Sustainable Food Packaging: From Eco-Friendly Materials to Innovative Technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Zhong, G.-J.; Olah, A.; Li, Z.-M.; Baer, E.; Zhu, L. Promising Strategies and New Opportunities for High Barrier Polymer Packaging Films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Trinh, B.M.; Chang, B.P.; Mekonnen, T.H. The Barrier Properties of Sustainable Multiphase and Multicomponent Packaging Materials: A Review. Prog. Mater. Sci. 2023, 133, 101071. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent Advances in Cellulose-Based Hydrophobic Food Packaging. Emergent Mater. 2022, 5, 703–718. [Google Scholar] [CrossRef]
- Antony, T.; Cherian, R.M.; Varghese, R.T.; Kargarzadeh, H.; Ponnamma, D.; Chirayil, C.J.; Thomas, S. Sustainable Green Packaging Based on Nanocellulose Composites-Present and Future. Cellulose 2023, 30, 10559–10593. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Yekta, R.; Abedi-Firoozjah, R.; Azimi Salim, S.; Khezerlou, A.; Abdolmaleki, K. Application of Cellulose and Cellulose Derivatives in Smart/Intelligent Bio-Based Food Packaging. Cellulose 2023, 30, 9925–9953. [Google Scholar] [CrossRef]
- Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A. Cellulose Structure and Property Changes Indicated via Wetting-Drying Cycles. Polym. Degrad. Stab. 2019, 167, 33–43. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research Progress of Nanocellulose-Based Food Packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Huang, K.; Maltais, A.; Wang, Y. Enhancing Water Resistance of Regenerated Cellulose Films with Organosilanes and Cellulose Nanocrystals for Food Packaging. Carbohydr. Polym. Technol. Appl. 2023, 6, 100391. [Google Scholar] [CrossRef]
- Fang, Y.; Li, J.; Li, Q.; Zeng, C.; Jiang, Y.; Kong, W.; Zhu, M. Fully Biobased Fluorine-Free Water- and Oil-Resistant Cellulose-Chitosan Pulp Molding with Enhanced Durability and Closed-Loop Recyclability. Ind. Crops Prod. 2025, 230, 121032. [Google Scholar] [CrossRef]
- Sethi, J.; Wågberg, L.; Larsson, P.A. Water-Resistant Hybrid Cellulose Nanofibril Films Prepared by Charge Reversal on Gibbsite Nanoclays. Carbohydr. Polym. 2022, 295, 119867. [Google Scholar] [CrossRef]
- Sharma, K.; Choudhary, P.; Majeed, A.; Guleria, S.; Kumar, M.; Rana, A.K.; Rajauria, G. Cellulose Based Membranes, Hydrogels and Aerogels for Water Treatment Application. Ind. Crops Prod. 2025, 225, 120474. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Hart, P.; Thakur, V.K. Cellulose-Alginate Hydrogels and Their Nanocomposites for Water Remediation and Biomedical Applications. Environ. Res. 2024, 243, 117889. [Google Scholar] [CrossRef]
- Sharma, D.; Harte, F.M.; Ziegler, G.R. Fabrication and Physicomechanical Performance of Casein-Hydroxypropyl Methylcellulose Nanofibers. J. Colloid Interface Sci. 2025, 693, 137601. [Google Scholar] [CrossRef]
- Li, C.-Y.; You, J.-L.; Liu, I.-T.; Istiqomah, A.; Liao, Y.-C. Mercerized Bacterial Cellulose/Chitosan/Waterborne Polyurethane Composites for Sustainable and Effective Food Preservation Packaging. Chem. Eng. J. 2025, 512, 162332. [Google Scholar] [CrossRef]
- Singh, A.K.; Itkor, P.; Lee, Y.S. State-of-the-Art Insights and Potential Applications of Cellulose-Based Hydrogels in Food Packaging: Advances towards Sustainable Trends. Gels 2023, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mann, B.; Sharma, R.; Verma, A.; Panjagari, N.R.; Gandhi, K. Identification of Polymer Additives from Multilayer Milk Packaging Materials by Liquid-Solid Extraction Coupled with GC-MS. Food Packag. Shelf Life 2022, 34, 100975. [Google Scholar] [CrossRef]
- Pinto, M.S.; Pires, A.C.S.; Sant’Ana, H.M.P.; Soares, N.F.F.; Carvalho, A.F. Influence of Multilayer Packaging and Microfiltration Process on Milk Shelf Life. Food Packag. Shelf Life 2014, 1, 151–159. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Ščetar, M. Multilayer Packaging Materials; Wiley: Hoboken, NJ, USA, 2021; ISBN 9781119825081. [Google Scholar]
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.-M.; Dong, T.; Anderson, A.; et al. Sustainable Bioplastics Derived from Renewable Natural Resources for Food Packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Kochersperger, S.; Schabel, S. Recyclability of Paper-Based Composites for Packaging Applications–The Role of Evaluation Methods. Chem. Ing. Tech. 2024, 96, 891–901. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef]
- Ciawi, Y.; Tonyes, S.G.; Utami Dwipayanti, N.M. Multilayer Packaging Recycling: Challenges, Current Practices, and Future Prospects. Acad. Environ. Sci. Sustain. 2025, 2, 1–12. [Google Scholar] [CrossRef]
- Anwar, M.A.; Suprihatin; Sasongko, N.A.; Najib, M.; Pranoto, B. Challenges and Prospects of Multilayer Plastic Waste Management in Several Countries: A Systematic Literature Review. Case Stud. Chem. Environ. Eng. 2024, 10, 100911. [Google Scholar] [CrossRef]
- Verma, S.K.; Prasad, A.; Sonika; Katiyar, V. State of Art Review on Sustainable Biodegradable Polymers with a Market Overview for Sustainability Packaging. Mater. Today Sustain. 2024, 26, 100776. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Rabnawaz, M. Sustainable Packaging Systems Using Renewable Materials; American Chemical Society: Washington, DC, USA, 2025; pp. 2024–2026. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Gigante, V.; Aliotta, L.; Lazzeri, A. Recyclability Perspectives of the Most Diffused Biobased and Biodegradable Plastic Materials. Macromol 2024, 4, 401–419. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- de Perre, D.; Serbruyns, L.; Coltelli, M.-B.; Gigante, V.; Aliotta, L.; Lazzeri, A.; Geerinck, R.; Verstichel, S. Tuning Biodegradation of Poly (Lactic Acid) (PLA) at Mild Temperature by Blending with Poly (Butylene Succinate-Co-Adipate) (PBSA) or Polycaprolactone (PCL). Materials 2024, 17, 5436. [Google Scholar] [CrossRef]
- Gigante, V.; Coltelli, M.-B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat Die Extruded Biocompatible Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate) (PBS) Based Films. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened Poly (Lactic Acid)—PLA Formulations by Binary Blends with Poly(Butylene Succinate-Co-Adipate)—PBSA and Their Shape Memory Behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Dal Pont, B.; Miketa, F.; Coltelli, M.-B.; Lazzeri, A. Tearing Fracture of Poly(Lactic Acid) (PLA)/ Poly(Butylene Succinate-Co-Adipate) (PBSA) Cast Extruded Films: Effect of the PBSA Content. Eng. Fract. Mech. 2023, 289, 109450. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Aliotta, L.; Fasano, G.; Miketa, F.; Brkić, F.; Alonso, R.; Romei, M.; Cinelli, P.; Canesi, I.; Gigante, V.; et al. Recyclability Studies on Poly(Lactic Acid)/Poly(Butylene Succinate-Co-Adipate) (PLA/PBSA) Biobased and Biodegradable Films. Macromol. Mater. Eng. 2023, 308, 2300136. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Talha, M.; Maan, A.A.; Khan, M.K.I.; Tanveer, M.; Arif, S.; Butt, M.S.; Nazir, A. Extending Bio-Based and Biodegradable Thermoplastics in Food Packaging: A Focus on Multiphase Systems. Food Front. 2025, 6, 1129–1172. [Google Scholar] [CrossRef]
- Itabana, B.E.; Pal, A.K.; Mohanty, A.K.; Misra, M. Biodegradable Blown Film Composite from Poly (Butylene Adipate-Co-Terephthalate) and Talc: Effect of Uniaxial Stretching on Mechanical and Barrier Properties. Food Packag. Shelf Life 2023, 39, 101147. [Google Scholar] [CrossRef]
- Lin, G.; Xu, J.; Wu, M.; Sun, Q.; Zhu, S.; Wang, B.; Zhang, W. Cellulose Nanofibril/Talc Composite Films with Excellent Barrier Properties by Alternate Hierarchical Method. ACS Appl. Polym. Mater. 2023, 5, 9180–9191. [Google Scholar] [CrossRef]
- Calambas, H.L.; Fonseca, A.; Adames, D.; Aguirre-Loredo, Y.; Caicedo, C. Physical-Mechanical Behavior and Water-Barrier Properties of Biopolymers-Clay Nanocomposites. Molecules 2021, 26, 6734. [Google Scholar] [CrossRef] [PubMed]
- Röhrl, M.; Timmins, R.L.; Rosenfeldt, S.; Schuchardt, D.D.; Uhlig, F.; Nürmberger, S.; Breu, J. Stretchable Clay Nanocomposite Barrier Film for Flexible Packaging. ACS Appl. Mater. Interfaces 2023, 15, 22524–22531. [Google Scholar] [CrossRef]
- Sadiku, E.R.; Babul Reddy, A.; Gnanasekarana, D.; Oboirien, B.; Aderibigbe, B.A.; Varaprasad, K.; Goddeti, S.M.R. Chapter 12-Nanostructured Polymer Blends for Gas/Vapor Barrier and Dielectric Applications. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; Micro and Nano Technologies; William Andrew Publishing: Boston, MA, USA, 2016; pp. 239–259. ISBN 978-0-323-39408-6. [Google Scholar]
- Tan, B.; Thomas, N.L. Tortuosity Model to Predict the Combined Effects of Crystallinity and Nano-Sized Clay Mineral on the Water Vapour Barrier Properties of Polylactic Acid. Appl. Clay Sci. 2017, 141, 46–54. [Google Scholar] [CrossRef]
- Meng, Q.; Heuzey, M.-C.; Carreau, P.J. Control of Thermal Degradation of Polylactide/Clay Nanocomposites during Melt Processing by Chain Extension Reaction. Polym. Degrad. Stab. 2012, 97, 2010–2020. [Google Scholar] [CrossRef]
- Scatto, M.; Salmini, E.; Castiello, S.; Coltelli, M.-B.; Conzatti, L.; Stagnaro, P.; Andreotti, L.; Bronco, S. Plasticized and Nanofilled Poly(Lactic Acid)-Based Cast Films: Effect of Plasticizer and Organoclay on Processability and Final Properties. J. Appl. Polym. Sci. 2013, 127, 4947–4956. [Google Scholar] [CrossRef]
- Ann Bazar, J.; Rahimi, M.; Fathinia, S.; Jafari, M.; Chipakwe, V.; Chehreh Chelgani, S. Talc Flotation—An Overview. Minerals 2021, 11, 662. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Coiai, S.; Bronco, S.; Passaglia, E. Nanocomposites Based on Phyllosilicates: From Petrochemicals to Renewable Thermoplastic Matrices. In Advanced Nanomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 403–458. ISBN 9783527628940. [Google Scholar]
- Peng, H.; Li, P.; Yang, Q. Investigation of the Catalytic Pyrolysis Of Polyester/Viscose Fibers with Monometallic-Supported Montmorillonite. Fibers Polym. 2025, 26, 1163–1173. [Google Scholar] [CrossRef]

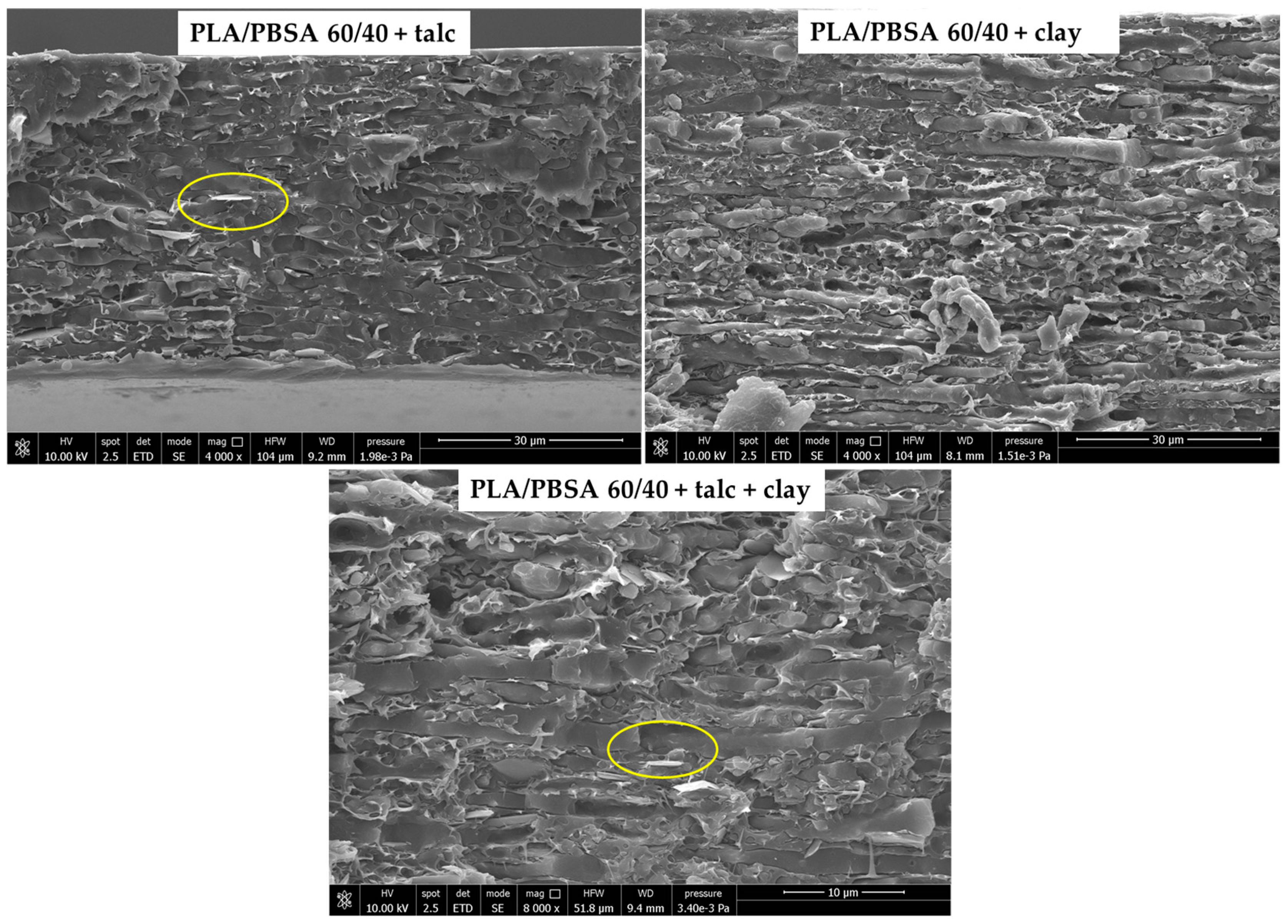


| Samples | PBSA (% by Weight) | PLA (% by Weight) | Talc (% by Weight) | Clay (% by Weight) |
|---|---|---|---|---|
| PLA/PBSA 60/40 | 40 | 60 | - | - |
| PLA/PBSA 60/40 + clay | 38.1 | 57.1 | - | 4.8 |
| PLA/PBSA 60/40 + talc | 38.1 | 57.1 | 4.8 | - |
| PLA/PBSA 60/40 + talc + clay | 36.4 | 54.5 | 4.6 | 4.6 |
| Samples | MVR (cm3/10 min) | MFR (g/10 min) |
|---|---|---|
| PLA/PBSA 60/40 | 4.4 ± 0.1 a | 5.0 ± 0.2 |
| PLA/PBSA 60/40 + clay | 37.9 ± 1.9 b | 42.1 ± 2.1 |
| PLA/PBSA 60/40 + talc | 23.6 ± 2.1 c | 26.7 ± 2.3 |
| PLA/PBSA 60/40 + talc + clay | 33.4 ± 1.1 d | 37.1 ± 1.2 |
| Samples | Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|
| PLA/PBSA 60/40 | 19.4 ± 4.5 a | 45.8 ± 10.3 a |
| PLA/PBSA 60/40 + clay | 18.6 ± 4.0 a | 5.8 ± 0.5 b |
| PLA/PBSA 60/40 + talc | 24.3 ± 2.1 b | 7.0 ± 0.4 c |
| PLA/PBSA 60/40 + talc + clay | 16.9 ± 4.6 a | 5.4 ± 0.5 b |
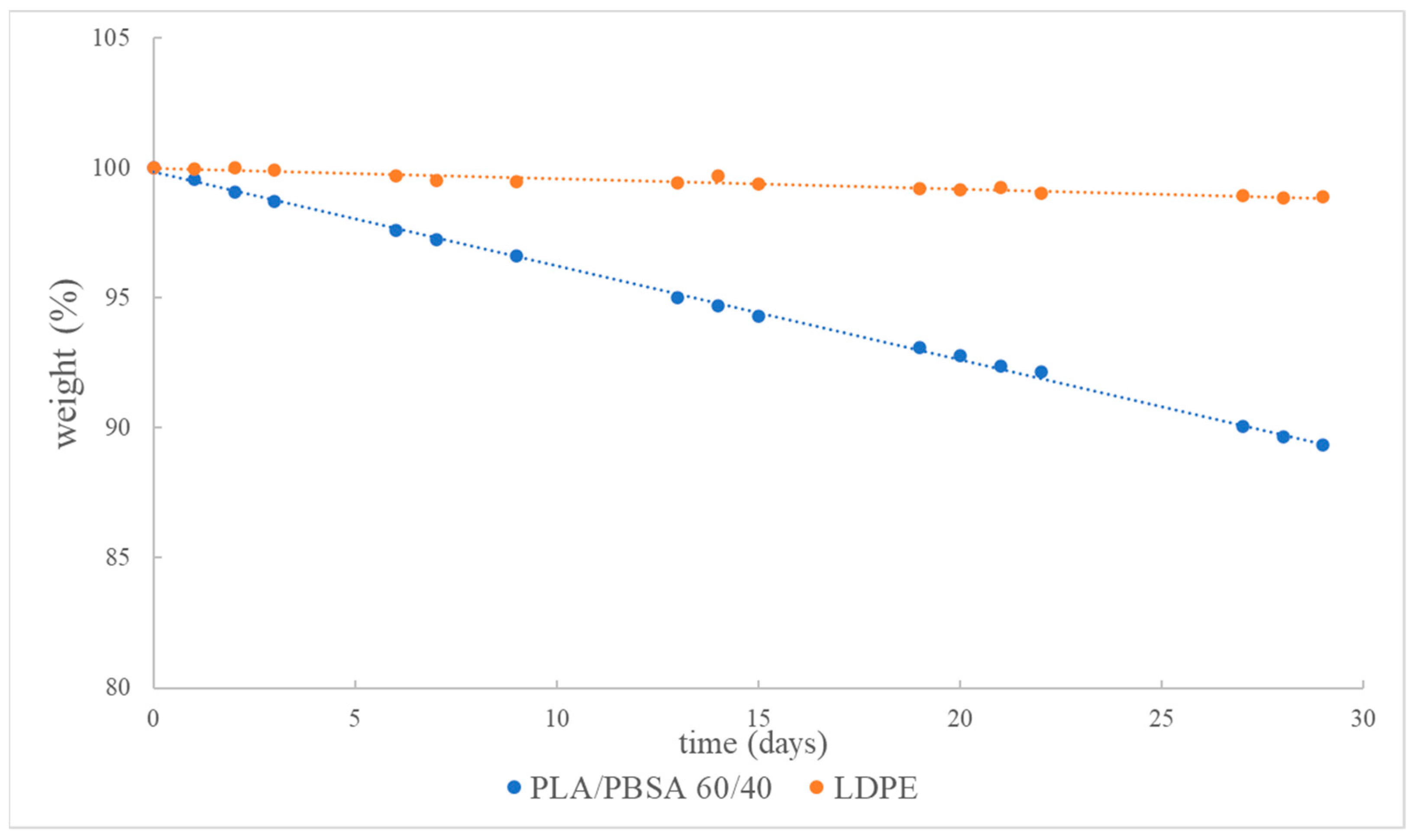
| Packaging | Mass Loss in 30 Days (%) |
|---|---|
| PLA/PBSA 60/40 | 9.7 a |
| PLA/PBSA 60/40 + clay | 16.4 c |
| PLA/PBSA 60/40 + talc | 10.9 b |
| PLA/PBSA 60/4 + talc + clay | 10.7 b |
| Packaging Material | Fitting Equation | R2 | Loss at One Week | Loss at 10 Days |
|---|---|---|---|---|
| PLA/PBSA 60/40 | Y = −0.35679 X + 99.76824 | 0.999 | −2.73% | −3.80% |
| LDPE (Commercial) | Y = −0.04171 X + 100.0376 | 0.962 | −0.25% | −0.38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltelli, M.-B.; Giangrandi, S.; Tricoli, V.; Panariello, L.; Lazzeri, A. Substitution of Fossil Layers with Biobased Ones in Sustainable Cellulosic Packaging for Dairy Products. Appl. Sci. 2025, 15, 9615. https://doi.org/10.3390/app15179615
Coltelli M-B, Giangrandi S, Tricoli V, Panariello L, Lazzeri A. Substitution of Fossil Layers with Biobased Ones in Sustainable Cellulosic Packaging for Dairy Products. Applied Sciences. 2025; 15(17):9615. https://doi.org/10.3390/app15179615
Chicago/Turabian StyleColtelli, Maria-Beatrice, Simone Giangrandi, Vincenzo Tricoli, Luca Panariello, and Andrea Lazzeri. 2025. "Substitution of Fossil Layers with Biobased Ones in Sustainable Cellulosic Packaging for Dairy Products" Applied Sciences 15, no. 17: 9615. https://doi.org/10.3390/app15179615
APA StyleColtelli, M.-B., Giangrandi, S., Tricoli, V., Panariello, L., & Lazzeri, A. (2025). Substitution of Fossil Layers with Biobased Ones in Sustainable Cellulosic Packaging for Dairy Products. Applied Sciences, 15(17), 9615. https://doi.org/10.3390/app15179615








