Abstract
The rapid development of marine recirculating aquaculture systems (RASs) worldwide offers an efficient and sustainable approach to aquaculture. However, the slow start-up of the nitrification process under low-temperature conditions remains a significant challenge. This study evaluated multiple start-up strategies for moving bed biofilm reactors (MBBRs) operating at 13–15 °C. Among them, the salinity-gradient (SG) strategy exhibited the best performance, reducing the start-up time by 38 days compared to the control, with microbial richness (Chao1 index) reaching 396 and diversity (Shannon index) of 4.89. Inoculation with mature biofilm (MBI) also showed excellent results, shortening the start-up period by 26 days and achieving a stable total ammonia nitrogen (TAN) effluent concentration below 0.5 mg/L within 132 days. MBI exhibited the highest microbial richness (Chao1 index = 808) and diversity (Shannon index = 5.55), significantly higher than those of the control (Chao1 index = 279, Shannon index = 3.90) and other treatments. The hydraulic retention time-gradient (HRT) strategy contributed to performance improvement as well, with a 24-day reduction in start-up time and a Chao1 index of 663 and a Shannon index is 4.69. In contrast, nitrifying bacteria addition (NBA) and carrier adhesion layer modification (CALM) had limited effects on start-up efficiency or microbial diversity, with Chao1 indices of only 255 and 228, and Shannon indices were both 3.24, respectively. Overall, the results indicate that salinity acclimation, mature biofilm inoculation, and extended HRT are effective approaches for promoting microbial community adaptation and enhancing MBBR start-up under low-temperature marine conditions.
1. Introduction
China is the world’s largest aquaculture producer and the only country where aquaculture output exceeds that of capture fisheries. However, traditional aquaculture practices—such as pond culture and marine cage farming—often face challenges including low water use efficiency, rudimentary environmental protection technologies, uncontrollable water quality pollution, and inability to ensure the health and quality of aquatic products [1,2]. Moreover, the discharge of large volumes of aquaculture wastewater contributes to ecological pollution of water bodies, thereby restricting the sustainable development of the aquaculture industry [3]. Recirculating aquaculture systems (RASs), as a sustainable solution for marine aquaculture, have been widely promoted due to their efficient water reuse and pollutant control capabilities [4]. However, as the core nitrification unit within RAS, moving bed biofilm reactors (MBBRs) face significant challenges during start-up under low-temperature conditions (≤15 °C) [5,6]. Low temperatures significantly inhibit microbial activity, resulting in prolonged biofilm development periods and reduced nitrogen removal efficiency [7]. This limitation restricts the practical application of RAS in high-latitude regions or during winter, thereby creating an urgent need to develop optimized MBBR start-up strategies suitable for low-temperature conditions [5].
Nitrification in MBBRs depends on the synergistic activity of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB); however, their metabolic rates decrease exponentially as temperature declines [8,9]. Previous studies have shown that at 10 °C, the growth rate of ammonia-oxidizing bacteria (AOB) decreases by over 80% compared to that at 25 °C, significantly hindering biofilm formation and nitrogen conversion [10]. In addition, seawater aquaculture wastewater typically exhibits a high salinity of approximately 30% and elevated nitrogen loads [11]. The relatively low salt tolerance of microorganisms and hydraulic disturbances further exacerbate the challenges associated with MBBR start-up under low-temperature conditions [5].
Although previous studies have made some progress in biofilter start-up, most have focused on ambient-temperature conditions, and research on MBBR start-up strategies and their microbial mechanisms under low-temperature (≤15 °C) marine conditions remains limited. In particular, issues such as long start-up periods, severe nitrite (NO2−) accumulation, and suppressed functional microbial activity under low-temperature conditions have not been effectively addressed [12,13]. For example, mature biofilm inoculation has demonstrated promising results in freshwater systems; however, its adaptability under low-temperature and high-salinity conditions remains to be verified [14,15]. Similarly, salinity gradient acclimation and hydraulic retention time (HRT) regulation are expected to alleviate microbial stress; however, systematic evaluations of these strategies in seawater RAS remain scarce [16].
This study systematically evaluated two categories of start-up strategies for moving bed biofilm reactors (MBBRs) under low-temperature seawater conditions: microbial inoculation methods—including the addition of NBA, MBI, and CALM—and operational parameter adjustments, specifically SG implementation and HRT. This study integrates dynamic monitoring of nitrogen species concentrations with high-throughput 16S rRNA gene sequencing to identify the optimal start-up strategy that effectively shortens the nitrification lag phase. It further aims to elucidate the succession patterns of microbial communities under different start-up conditions and to reveal the adaptive mechanisms of microbial nitrification function in low-temperature, high-salinity environments. Although previous studies have explored MBBR start-up strategies in freshwater or ambient-temperature environments, systematic research on low-temperature (13–15 °C) marine recirculating aquaculture systems (marine RAS) remains significantly lacking. First, the adaptability and mechanisms of microbial inoculation strategies (e.g., mature biofilm inoculation) under low-temperature, high-salinity conditions are still unclear. Second, the effects of operational parameter adjustments, such as salinity gradient and hydraulic retention time, on the colonization and functional establishment of nitrifying bacteria at low temperatures have not been quantitatively analyzed. Third, the association mechanisms between microbial community structure and nitrification performance under different start-up strategies need further elucidation. Therefore, this study aims to systematically evaluate these strategies to fill the research gap in MBBR start-up optimization in low-temperature marine RAS and provide theoretical guidance for practical applications.
2. Materials and Methods
2.1. MBBR System and Experimental Design
The MBBR system employed in this study consisted of a reactor, biofilm carriers, a low-temperature recirculating water tank, an aeration system, a peristaltic pump, and a wastewater storage unit, as illustrated in Figure 1. The experimental MBBR reactor was fabricated from transparent acrylic, measuring 400 mm in height and 200 mm in diameter, with a total volume of approximately 10 L and an effective working volume of 8.8 L. High-density polyethylene (HDPE) K5 carriers were added into the reactor at a volumetric filling ratio of 30% (v/v), serving as attachment surfaces for microbial biofilm development [17]. The experimental water temperature was controlled at 13~15 °C. To systematically assess the effectiveness of different MBBR start-up strategies under low-temperature seawater conditions, six experimental reactors were established. Among them, Reactor NBA was designated as the uninoculated control, operated without any microbial addition or operational adjustment. Reactors NBA, MBI, and CALM were designed to evaluate three microbial inoculation strategies, namely the addition of nitrifying bacteria, inoculation with mature biofilm, and modification of the carrier adhesion layer, respectively. In contrast, SG and HRT represented operational parameter adjustment strategies, intended to alleviate the inhibitory effects of low temperature by gradually increasing the influent salinity from 10% to 30% and by extending the hydraulic retention time (HRT) from 12 h to 36 h, respectively. All reactors were continuously fed with synthetic mariculture wastewater, which was prepared using artificial seawater at a salinity of 30%. Ammonium chloride (NH4Cl) was employed as the primary nitrogen source, while sodium bicarbonate (NaHCO3) functioned both as an inorganic carbon source and as a pH buffer.
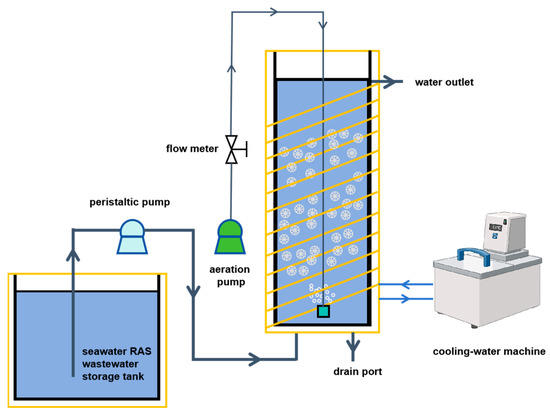
Figure 1.
The MBBR startup system for low-temperature mariculture wastewater.
2.2. Chemical Parameters and Analytical Methods
Water samples collected during the reactor experiments were immediately filtered through 0.45 μm membranes and analyzed promptly [18], or preserved appropriately by refrigeration at 4 °C prior to analysis. The monitored water quality parameters in this study included total ammonia nitrogen (TAN), nitrite nitrogen (NO2−-N), nitrate nitrogen (NO3−-N), dissolved oxygen (DO), pH, temperature, and salinity. TAN, NO2−-N, and NO3−-N were measured every two days, while pH and DO were measured weekly. The analytical methods were performed in accordance with relevant national standards and improved detection protocols, as detailed in Table 1.

Table 1.
Test items and methods.
The degradation rate of TAN (η) was calculated using the following Equation (1):
where η = degradation rate of the pollutant (%); C1 = concentration of the parameter in the influent of the biofilter (mg/L); C2 = concentration of the parameter in the effluent of the biofilter (mg/L).
2.3. Microbial High-Throughput Sequencing and Analytical Methods
Ten carriers were randomly and evenly collected from each MBBR reactor [22]. After sampling, they were placed into individually labeled sealed bags corresponding to control, NBA, MBI, SG, HRT, CALM, and immediately stored at –80 °C for subsequent analysis. At the time of sampling, the water quality criteria were a stable TAN degradation rate and a TAN concentration below 0.5 mg/L. The biofilm on the carrier media was subjected to total microbial genomic DNA extraction, PCR amplification, sequencing, and OTU clustering analysis, which were all outsourced to Mingke Biotechnology Co., Ltd. Each biofilm sample collected from the carriers was processed for genomic DNA extraction from the filtered bacterial suspension. Following DNA extraction, the quality and integrity of the genomic DNA were assessed by 1% agarose gel electrophoresis [23]. The purity of the extracted genomic DNA and the PCR products was evaluated according to the A260/A280 ratio of 1.8–2.0, following the standard guidelines for nucleic acid purity assessment (Sambrook & Russell, 2001). Subsequently, the V3–V4 regions of the bacterial 16S rRNA gene were amplified by PCR using the primer pair 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′). All samples were processed under standardized experimental conditions, with three replicates per sample. The PCR products from the same sample were pooled and subjected to electrophoresis on a 2% agarose gel. PCR fragments were then excised and purified using the AxyPrep DNA Gel Extraction Kit (AXYGEN), followed by elution with Tris-HCl buffer. The purified products were subsequently verified again by 2% agarose gel electrophoresis. Based on the preliminary quantification results from electrophoresis, the PCR products were quantified using the QuantiFluor™-ST Blue Fluorescence Quantification System (Promega). Subsequently, the PCR products were pooled in proportions corresponding to the sequencing depth requirements for each sample. The Illumina paired-end (PE) library was constructed and subsequently sequenced. After performing quality control and filtering of the DNA sequences, OTU clustering and taxonomic classification analyses were conducted. OTU clustering and taxonomic assignment were performed using the QIIME2 pipeline (version 2023.9, https://qiime2.org/) (Bolyen et al., 2019). Statistical analyses of microbial community diversity and structure were conducted in R software (version 4.2.2, R Core Team, Vienna, Austria). Based on the OTU clustering results, multiple diversity indices were calculated, and community structure was statistically analyzed at various taxonomic levels according to the taxonomic information [24]. Specifically, alpha diversity indices (Shannon, Simpson, Chao1) and beta diversity metrics were calculated using the QIIME2 pipeline (https://qiime2.org/) [25].
Given the exploratory nature of this study focusing on the comparison of startup trends and microbial community composition under different strategies, the data were primarily analyzed using descriptive statistics. Results are presented as means (of triplicate measurements where applicable) or direct observational values (e.g., Chao1, Shannon indices). No formal inferential statistical tests (e.g., ANOVA, t-test) were performed to assess the significance of differences between groups.
3. Results and Discussion
3.1. Effect of Inoculation Strategy on Reactor Start-Up in Cold Seawater
In the study on optimizing microbial inoculation strategies, nitrifying bacterial agents were added sequentially, followed by the attachment layer of the carrier and mature biofilm inoculation. The start-up performance under low-temperature conditions in mariculture wastewater was analyzed [26].
3.1.1. Effect of Nitrifying Bacteria Addition on MBBR Start-Up Performance
During the first 30 days of operation, the addition of NBA did not result in a significant reduction in the effluent TAN concentration, which is likely attributable to the microbial adaptation phase. The sudden drop in TAN concentration in the effluent on day 14 was due to the nitrifying bacterial agent added on day 13 not being uniformly mixed in the NBA reactor. Most of the bacterial powder remained in the shallow upper water zone of the MBBR, where it degraded pollutants, resulting in a lower TAN concentration measured at the sampling point near the reactor’s upper outlet. After 30 days of operation, with adaptation to the low water temperature of 13–15 °C, the microorganisms in the reactor began to regain activity and grow. The influent TAN was effectively utilized and substantially degraded, accompanied by the accumulation of NO2−-N and NO3−-N concentrations. At this stage, it can be considered that the microbial nitrification function was gradually established [27].
Combined with Figure 2a, the effluent TAN concentration in the NBA reactor supplemented with nitrifying bacterial agent dropped below 0.5 mg/L and stabilized after 112 days of operation, which is 6 days earlier than the blank control. In terms of TAN degradation performance, the addition of the nitrifying bacterial agent was slightly superior to that of the blank control. On day 134, the accumulation of NO2−-N in the NBA reactor supplemented with nitrifying bacterial agent reached a peak concentration of 2.88 mg/L. This milestone indicates that before day 134, ammonia oxidation was the dominant reaction in the reactor, whereas after day 134, the nitrification process shifted to nitrite oxidation. Figure 2b shows that the NO2−-N concentration in the blank control declined from its peak at day 122 to below 0.5 mg/L by day 158, while in the NBA reactor supplemented with nitrifying bacterial agent, the NO2−-N concentration started to decrease from day 134 and only fell below 0.5 mg/L by day 160. Meanwhile, the NO3−-N concentration also indicates that in the NBA reactor supplemented with nitrifying bacterial agent (Figure 2c), NO2−-N was not rapidly converted to NO3−-N. Significant and rapid accumulation of NO3−-N only occurred after the NO2−-N reached its peak concentration. After 120 days of operation, the concentration trends of TAN, NO2−-N, and NO3−-N in the NBA reactor supplemented with nitrifying bacterial agent closely resembled those of the blank control. This phenomenon may be attributed, on one hand, to the gradual loss of the nitrifying bacterial population in the NBA reactor over time, and on the other hand, to the intrinsic microbial community composition of the reactor following the bacterial agent addition [28].
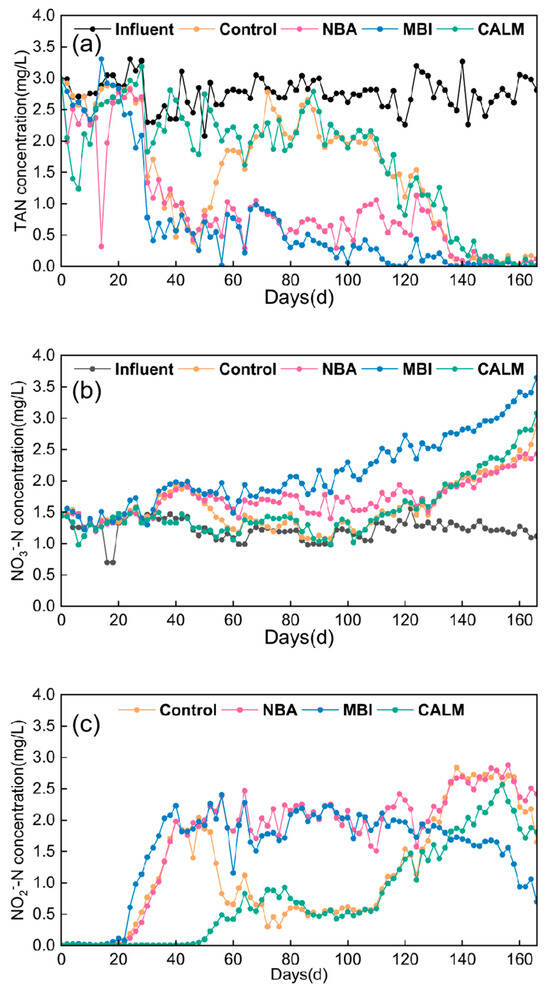
Figure 2.
Variations in pollutant concentrations under different microbial inoculation approaches. (a) TAN; (b) NO3−-N; (c) NO2−-N concentration.
In summary, the nitrifying bacterial agent treatment completed the transition from ammonia oxidation to nitrification 12 days later than the blank control. Using stable effluent TAN concentration and NO2−-N concentration below 0.5 mg/L as criteria, the blank control reached this stage at day 158, whereas the NBA-supplemented reactor did so at day 162. In this experiment, the addition of nitrifying bacterial agent did not accelerate the low-temperature nitrification startup of the MBBR reactor, and no enhancement in nitrification performance was observed compared to the blank control.
3.1.2. Effect of Mature Biofilm Inoculation on MBBR Start-Up Performance
By day 24 of operation, as the microorganisms adapted to the low water temperature of 13–15 °C, their activity began to recover and grow actively. TAN in the reactor was utilized and oxidatively degraded by the microbes, leading to the accumulation of NO2−-N and NO3−-N concentrations in the effluent, indicating that nitrification had started. Figure 2a shows that after 22 days of operation, the effluent TAN concentration in the reactor inoculated with mature biofilm was significantly lower than that of the blank control. After 30 days, the TAN degradation performance of MBI was clearly superior to that of the control. Considering an effluent TAN concentration below 0.5 mg/L as the criterion, the reactor inoculated with mature biofilm required 70 days to reach this level, whereas the blank control required 118 days. Compared to control, the inoculation with mature biofilm shortened the time to achieve this TAN concentration by 48 days, demonstrating that MBI significantly enhanced TAN degradation in the effluent [29]. The reactor inoculated with mature biofilm reached a peak NO2−-N accumulation of 2.28 mg/L at day 72, after which NO2−-N gradually degraded (Figure 2b). The rapid accumulation of NO3−-N observed after day 72 further indicates that NO2−-N was oxidized and converted into NO3−-N (Figure 2c). Marking day 72 as the peak of NO2−-N accumulation, it can be inferred that the process before day 72 was dominated by ammonia oxidation, while after day 72, the system transitioned into the nitrification stage. Meanwhile, the blank control reached its NO2−-N accumulation peak of 2.84 mg/L only at day 122, indicating that the reactor inoculated with mature biofilm completed the ammonia oxidation stage 50 days earlier. Using an effluent NO2−-N concentration below 0.5 mg/L as the criterion for the nitrification stage, MBI achieved this threshold at day 132, whereas the blank control reached it at day 158. Compared to control, inoculation with mature biofilm shortened the time to reach this standard by 26 days.
Using stable effluent TAN concentration and NO2−-N levels below 0.5 mg/L as criteria, the reactor inoculated with mature biofilm required 132 days to reach this standard, which is 26 days shorter than the blank control. This indicates that the inoculation of mature biofilm effectively promoted the maturation and stabilization of nitrification function, thereby enabling successful nitrification startup of the MBBR under low-temperature conditions treating marine aquaculture wastewater [30] (Figure 2).
3.1.3. Effect of Carrier-Attached Biofilm on MBBR Start-Up Performance
In the influent conditions of the MBBR reactor with the biofilm-coated carriers CALM, all parameters were identical to those of the blank control except for the carriers used. The CALM reactor employed K5 carriers with an external biofilm coating, whereas the other reactors utilized conventional K5 carriers without the coating. Combined with Figure 2a, the effluent TAN concentration in the MBBR reactor with biofilm-coated carriers CALM decreased slowly in a stepwise manner, exhibiting two major phases of significant TAN degradation. The first occurred around day 30, coinciding with microbial adaptation and recovery of activity under the low water temperature of 13–15 °C. The second phase took place around day 90, attributed to microbial growth and proliferation reaching a certain stage in the low-temperature seawater environment. The effluent TAN concentration in the CALM reactor dropped below 0.5 mg/L by day 112, which is 6 days earlier than the blank control that reached this level at day 118, indicating a relatively weak promotion of TAN degradation. Due to the slow TAN degradation in the MBBR reactor with biofilm-coated carriers CALM, the NO2−-N concentration reached its maximum accumulation peak of 2.57 mg/L only at day 130, indicating the completion of the ammonia oxidation stage. After day 130, the main reaction in the reactor shifted to the nitrification stage. Using an effluent NO2−-N concentration of 0.5 mg/L as the benchmark, the CALM reactor completed the NO2−-N degradation relatively quickly, reaching this concentration level by day 146—12 days earlier than the blank control—demonstrating superior nitrification performance (Figure 2c). Additionally, Figure 2 shows that the overall concentration trends of TAN, NO2−-N, and NO3−-N in the CALM reactor during the startup period were similar to those of the blank control, but with better nitrification performance compared to the control.
Overall, compared to the blank control, the biofilm-coated carriers CALM demonstrated a promotion effect by shortening the TAN degradation period by 6 days and accelerating the NO2−-N degradation and conversion by 12 days. Thus, the biofilm-coated carriers CALM reduced the time required for nitrification startup under low-temperature conditions by 12 days.
Comparative analysis of nitrification performance under different microbial inoculation methods indicates that the reactor inoculated with MBI exhibits a clear advantage in promoting biofilm formation and establishing complete nitrification functionality during low-temperature startup of the seawater aquaculture wastewater MBBR. MBI achieved the fastest reduction in TAN concentration as well as the most rapid accumulation and subsequent degradation of NO2−-N. Following MBI, the reactor CALM demonstrated intermediate performance, while the reactor supplemented with NBA showed the poorest results. The suboptimal performance of the NBA is likely attributable to the small inoculation dosage used in this study and the susceptibility of the bacterial agent to washout.
3.2. Effect of Operational Parameters on Reactor Start-Up in Cold Marine Wastewater
3.2.1. Effect of Salinity Gradient on MBBR Start-Up Performance
In the SG reactor with the salinity gradient, on day 80, when the salinity was adjusted from 20‰ to 30‰, a full water replacement was not performed; instead, 30‰ influent was continuously pumped in to gradually replace the reactor water and increase the concentration. As shown clearly in Figure 3, the concentrations of TAN, NO2−-N, and NO3−-N remained essentially unchanged at this time, indicating that the microbial community in the SG reactor was relatively stable. Excluding the full water replacement event on day 30, the SG reactor had already achieved effective TAN degradation by day 18, and the subsequent TAN degradation performance remained largely stable thereafter. Figure 3 clearly shows that the effluent concentrations of TAN, NO2−-N, and NO3−-N in the salinity gradient reactor SG remained relatively stable after day 36. The introduction of 30‰ influent at day 80 did not significantly affect the concentrations of these three nitrogen species in the effluent. The NO3−-N accumulation concentration remained nearly constant at approximately 2.0 mg/L, with notable changes only occurring after day 88. After day 88, a rapid increase in NO3−-N concentration was observed (Figure 3b), while the NO2−-N concentration in the reactor rapidly decreased from 3.0 mg/L to below 1.5 mg/L. By day 120, the effluent NO2−-N concentration had dropped below 0.5 mg/L (Figure 3b), indicating substantial conversion of NO2−-N to accumulated NO3−-N, which stabilized at approximately 3.75 mg/L. The salinity gradient reactor SG not only achieved rapid TAN degradation to minimal effluent concentrations and NO2−-N accumulation, but also exhibited a significant advantage over the blank control at day 120, as the NO2−-N concentration in SG was reduced to 0.5 mg/L.
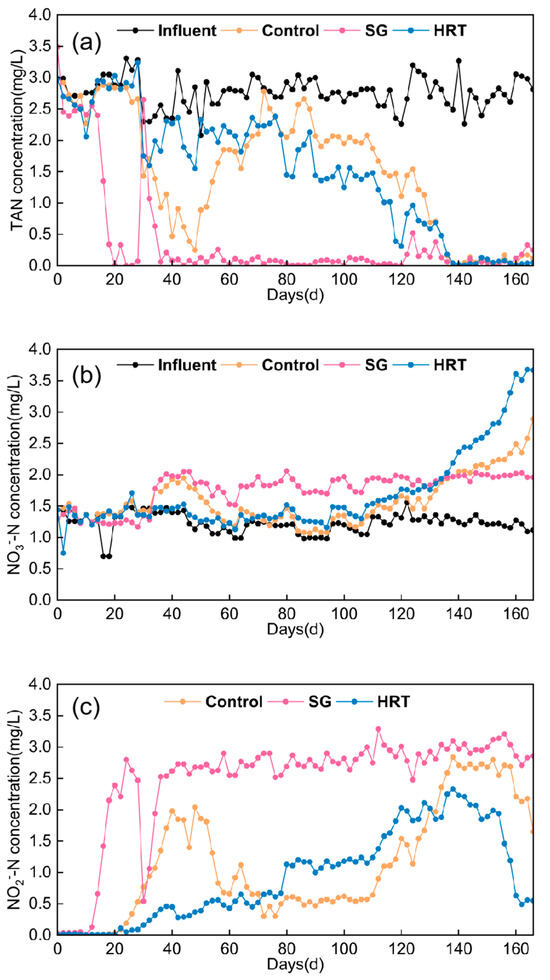
Figure 3.
Variations in pollutant concentrations under parameter regulation approaches. (a) TAN; (b) NO3−-N; (c) NO2−-N concentration.
The results from the salinity gradient reactor SG indicate that gradually increasing the influent salinity from low levels to normal seawater salinity enables the MBBR biofilter reactor to enter the ammonia oxidation stage more rapidly, followed by the nitrification stage, where NO2−-N is oxidized and accumulated as NO3−-N. This approach can significantly enhance the nitrification startup of MBBR systems under low-temperature conditions.
3.2.2. Effect of Hydraulic Retention Time on MBBR Start-Up Performance
Figure 3a shows around day 28 of the startup operation, as microorganisms adapted to the environmental conditions, the effluent TAN concentrations in both the blank control and HRT reactors exhibited similar declining trends. It was evident that the initial hydraulic retention time of 12 h in the HRT reactor did not promote nitrification under these conditions. After day 30, the hydraulic retention time in the HRT reactor was adjusted to 24 h, matching that of the blank control. However, possibly due to insufficient microbial growth during the prior shorter HRT period, the TAN degradation performance of HRT was even inferior to that of, accompanied by low accumulation of NO2−-N and NO3−-N (Figure 3b,c). Following the extension of the HRT reactor’s HRT to 36 h after day 80, significant changes in effluent concentrations were observed: TAN was substantially degraded, NO2−-N concentration rapidly accumulated, and nitrifying bacteria gradually oxidized NO2−-N to NO3−-N, resulting in its accumulation. Based on Figure 3c, the NO2−-N peak concentration observed at day 110 in the HRT reactor under extended hydraulic retention time (HRT) can be considered a turning point. Prior to this, the reactor was primarily in the ammonia oxidation stage, after which microbial activity shifted to the nitrification stage. The HRT reactor reached the NO2−-N accumulation peak 12 days earlier than the blank control, which peaked at day 122, and subsequently began to decline. Using an effluent NO2−-N concentration below 0.5 mg/L as the benchmark, the HRT reactor achieved this level by day 134, whereas the blank control reached it at day 158. Thus, the HRT reactor established mature nitrification performance 24 days faster than the control, primarily due to the significant promotion of microbial nitrification activity when the HRT was extended to 36 h, thereby enabling successful nitrification startup.
Although most studies indicate that at ambient or higher temperatures, shorter hydraulic retention times (HRT) [31] can enhance material circulation within reactors, allowing microorganisms to have increased contact with pollutants, thereby promoting biofilm development, attachment, and nitrification, microbial activity is generally inhibited under low water temperatures of 13–15 °C. Under such conditions, faster influent flow rates may not effectively improve nitrification system establishment; instead, longer HRTs are more conducive to the utilization, growth, and proliferation of nitrifying bacteria. Overall, after adjusting the hydraulic retention time (HRT) to 36 h, the HRT reactor outperformed the blank control in various aspects. Moreover, by day 134 of operation, HRT accelerated the biofilm formation startup of the MBBR treating low-temperature seawater aquaculture wastewater, demonstrating superior nitrification performance.
Overall, both strategies contribute to the nitrification startup of MBBR treating seawater wastewater under low-temperature conditions, with the salinity gradient reactor SG showing the most pronounced promotion effect, followed by the hydraulic retention time reactor HRT.
3.3. Analysis of Microbial Community Structure and Diversity Under Different MBBR Start-Up Methods
3.3.1. Analysis of Microbial Community Abundance and Diversity
The Chao 1 index, which reflects community richness, was calculated at a 97% similarity threshold [32,33]. The Chao indices for the six samples were 279, 255, 808, 396, 663, and 228, respectively, indicating a richness order of MBI > HRT > SG > control > NBA > CALM. The lowest microbial richness was observed in reactor CALM, potentially due to the detachment of the external adhesion layer from the carrier surface under hydraulic shear over time. This detachment likely led to a loss of biofilm-forming microorganisms, resulting in the lowest detectable richness among all samples.
At a 97% similarity threshold, OTU clustering of all sequences resulted in a total of 2573 OTUs across the dataset [34]. The number of OTUs detected in the control samples through CALM was 269, 240, 798, 391, 654, and 221, respectively. A Venn diagram was constructed to visualize the shared and unique OTUs among the six samples [35]. As shown in Figure 4a, a total of 66 OTUs were shared across all samples, while the number of unique OTUs for control to CALM were 56, 38, 405, 98, 212, and 26, respectively. Clearly, the mature biofilm inoculation sample MBI exhibited the highest OTU richness, followed by the extended HRT sample. In contrast, the carrier adhesion layer sample CALM had the lowest OTU count, reflecting lower microbial diversity.
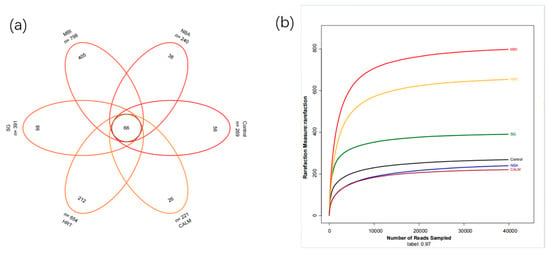
Figure 4.
(a) Venn diagram showing the distribution of operational taxonomic units (OTUs) among six start-up strategies of the moving bed biofilm reactor (MBBR); (b) rarefaction curve can be used to compare species richness among samples with different sequencing depths, and also to assess whether the sequencing depth of the samples is sufficient and reasonable.
To evaluate sequencing depth and species richness, rarefaction curves were generated based on the number of sequences sampled versus the number of observed OTUs. The rarefaction curves of all six samples approached saturation at 97% sequence similarity, indicating that the sequencing depth was sufficient (Figure 4b). Additional sequencing would yield only a small number of new OTUs [34], suggesting that the majority of microbial diversity in the samples had been captured. These results confirm that the sequencing effort was adequate and representative, and reliably reflects the microbial community composition of biofilms developed under various MBBR start-up strategies in low-temperature mariculture wastewater conditions [36].
The Shannon and Simpson indices are both widely used metrics for evaluating microbial diversity within a community. A higher Shannon index indicates greater community diversity, while a higher Simpson index suggests lower diversity. The Shannon indices for the six biofilm samples were 3.90, 3.24, 5.55, 4.89, 4.69, and 3.24 for control through CALM, respectively. These results reveal a consistent trend with microbial richness, with the diversity ranking as follows: MBI > SG > HRT > control > CALM > NBA.
In addition, the rank–abundance curve (Figure 5) provides further insight into microbial diversity by simultaneously illustrating species richness and evenness. On the horizontal axis, the width of the curve reflects species richness—the broader the curve, the higher the richness. The smoothness of the curve reflects species evenness—a flatter curve indicates a more uniform species distribution. MBI exhibited both the highest microbial richness and the most even distribution of species. HRT and SG also showed relatively high diversity and uniformity, whereas NBA and CALM displayed the narrowest and steepest curves, suggesting limited richness and poor evenness.
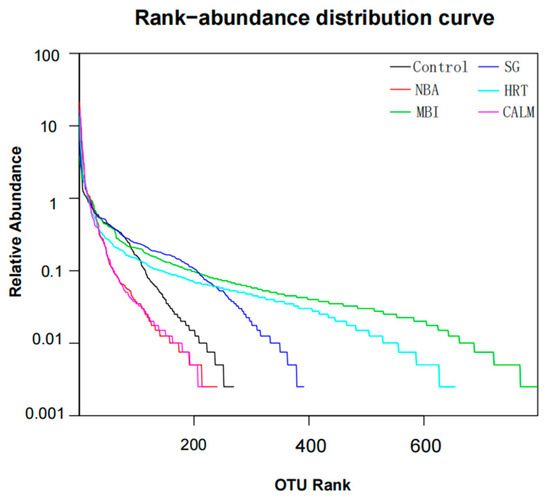
Figure 5.
Rank–abundance curves can be used to explain two aspects of diversity, namely species richness and species evenness.
3.3.2. Analysis of Dominant Microbial Community Distribution
The phylum- and genus-level biomarkers in the overall biofilm community were screened for a detailed analysis because of their clearer functional annotation [22].
Dominant Microbes Distribution at Phylum Level
The distribution and composition of microbial communities at the phylum level across the six carrier biofilm samples are shown in Figure 6. A total of 10 phyla were identified in the six samples (with those having relative abundances below 1% grouped as “others”). Previous studies [36] have shown that many heterotrophic nitrifying and denitrifying bacteria involved in nitrogen cycling in aquatic environments belong to the phylum Proteobacteria, which also adheres well to surfaces and thus facilitates biofilm formation. In aquaculture systems, Proteobacteria are recognized as the dominant phylum [37], and in conjunction with Bacteroidetes and Actinobacteria, they contribute significantly to the degradation of nitrogenous organic pollutants in water bodies. Firmicutes, mostly Gram-positive bacteria, are primarily involved in nitrification and denitrification processes [38]. In this study, the relative abundance of Firmicutes ranged from 1.82% to 19.68% across all samples, with the highest abundance observed in the salinity-gradient reactor SG (19.68%), followed by the extended-HRT reactor, while the lowest abundance was detected in the carrier adhesion layer reactor CALM.
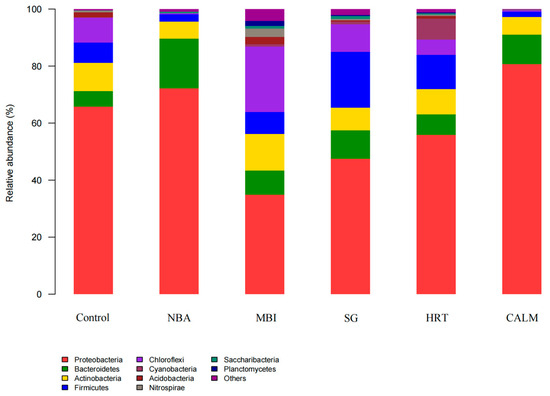
Figure 6.
Relative abundance of bacterial communities at the phylum-level under different MBBR start-up strategies. Sequencing was performed using 16S rRNA gene high-throughput sequencing, and relative abundance is presented as percentages. The figure highlights shifts in dominant genera across treatments, providing insight into microbial adaptation under varying start-up conditions.
Based on the combined analysis of the sample clustering tree and bar chart (as shown in Figure 7), the phylum-level similarity among groups reveals that, taking control as the uninoculated control, the microbial community composition of the extended HRT reactor was the most similar to that of the control. This was followed by the salinity gradient reactor SG, the nitrifying bacteria addition reactor NBA, and the carrier adhesion layer reactor CALM. In contrast, the mature biofilm inoculation reactor MBI showed the greatest dissimilarity from the control.
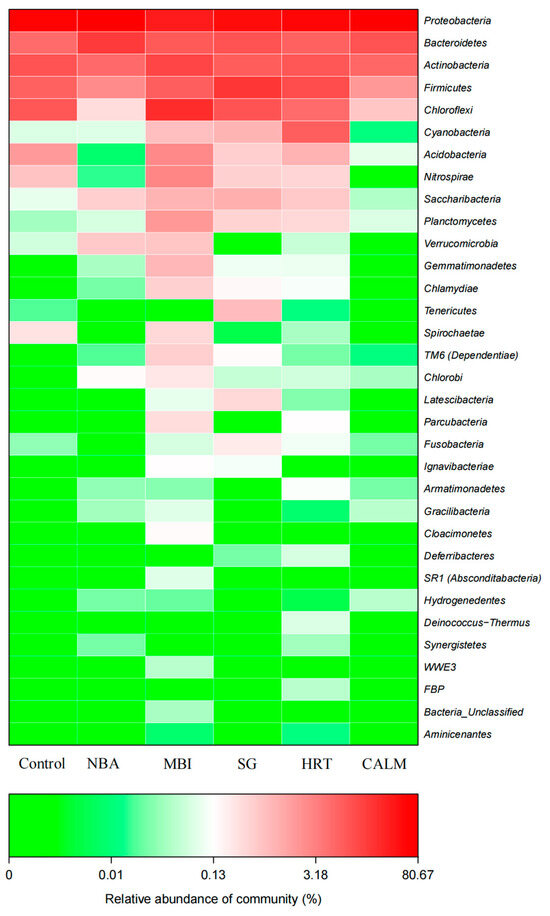
Figure 7.
Heatmap of microbial communities at the phylum-level across six MBBR start-up strategies. Clustering was performed based on Bray–Curtis distances of relative abundance profiles. The color scale represents the relative abundance (%) of bacterial phyla, illustrating similarities and differences in community composition under different start-up strategies.
Dominant Microbial Distribution at Genus Level
To more precisely identify the dominant genera within the microbial communities, taxonomic classification was conducted at the genus level. For clarity and consistency, all genera with relative abundances below 1% were grouped under “Others.” The genus-level composition and relative abundance profiles of the microbial communities from the six biofilm samples are illustrated in Figure 8.
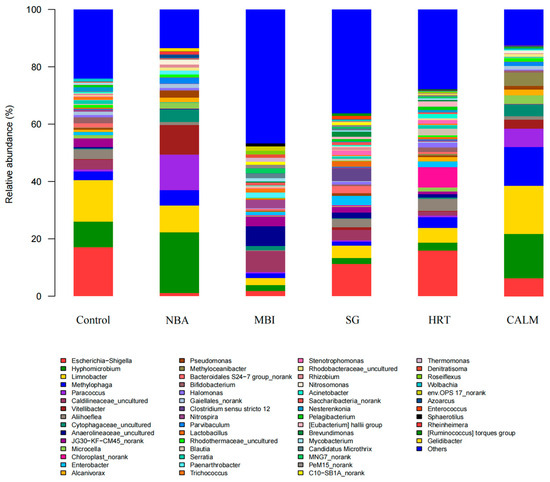
Figure 8.
Hierarchical clustering of microbial communities at the genus-level across six MBBR start-up strategies. Data were obtained from 16S rRNA gene sequencing; color intensity represents relative abundance, illustrating differences in microbial community composition under different start-up strategies.
Escherichia-Shigella, Limnobacter, Hyphomicrobium, and Methylophaga were identified as the core dominant genera shared across all six biofilm samples. Comparative analysis of genus-level relative abundances reveals that microbial diversity and richness were markedly higher in reactors MBI, SG, and HRT than in control, NBA, and CALM, as visually depicted in the heatmap and bar chart. Notably, in the NBA, Paracoccus (12.40%), Vitellibacter (10.22%), and Hyphomicrobium (21.13%) exhibited the highest relative abundances among all samples, likely reflecting the core composition of the commercial nitrifying bacterial inoculum itself. In the MBI reactor, two uncultured genera, Caldilineaceae_uncultured (7.26%) and Anaerolineaceae_uncultured (6.86%), were the most abundant. These taxa are members of the phylum Chloroflexi and the order Anaerolineales, known for their filamentous morphology and anaerobic metabolism, which may facilitate early-stage biofilm development and substrate degradation under low-temperature conditions. For SG, Clostridium sensu stricto 12—a Firmicutes-affiliated spore-forming genus with strong hydrolytic and acidogenic capabilities—was detected exclusively in this group at a relative abundance of 4.41%. This genus plays a key role in the breakdown of polysaccharides, proteins, and complex organic compounds through hydrolytic enzyme activity, suggesting a unique contribution to organic matter degradation under salinity stress [39]. Limnobacter, a heterotrophic bacterium known to exhibit syntrophic interactions with ammonia-oxidizing bacteria (AOB), was most abundant in the CALM reactor (16.68%) and least abundant in MBI (2.44%). Its high relative abundance in CALM may contribute to enhanced nitrification performance by providing a supportive microenvironment for AOB, reducing external perturbations [40].
Limnobacter, a heterotrophic bacterium that has a syntrophic relationship with AOB, may indirectly enhance nitrification efficiency in the CALM reactor due to its high abundance (16.68%), by providing a protective microenvironment for AOB and alleviating the inhibitory effects of low-temperature and high-salinity stress on the ammonia oxidation process. In addition, the potential synergy between filamentous taxa, such as Caldilineaceae_uncultured, and nitrite-oxidizing bacteria may enhance biofilm stability and promote nitrite conversion under high-salinity conditions.
Ammonia-oxidizing bacteria (AOB) can be categorized into five genera based on their cell morphology and cytoplasmic membrane structure, among which Nitrosomonas and Nitrosospira are the most commonly observed [41]. Notably, Nitrosomonas is recognized as the primary contributor to ammonia oxidation [42]. In this study, Nitrosomonas was detected at relatively higher abundances in the NBA (1.62%) and CALM (1.05%), while its abundance remained below 0.2% in all other reactors (Figure 9). The genus Nitrospira, a representative nitrite-oxidizing bacterium (NOB), was exclusively detected in the MBI with a relative abundance of 2.86%, the highest among all samples. This enrichment may explain the superior nitrification performance observed in MBI.
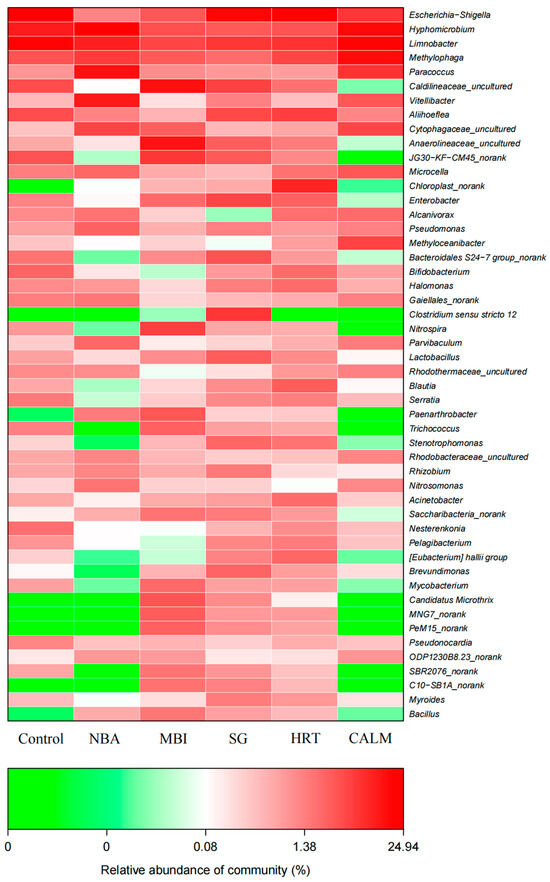
Figure 9.
Heatmap of microbial communities at the genus-level across six MBBR start-up strategies. The color scale represents the relative abundance (%) of bacterial phyla, illustrating similarities and differences in community composition under different start-up strategies.
4. Conclusions
This study investigated the biofilm formation and nitrification optimization during the startup of a moving bed biofilm reactor treating wastewater from a seawater recirculating aquaculture system under low-temperature conditions (13–15 degree). Overall, MBI could significantly shorten the startup period while enhancing nitrification efficiency. Both SG and HRT also effectively promoted startup, reducing startup time by 38 and 24 days compared to the control, respectively. Microbial community analysis revealed that although dominant microbial taxa varied among strategies, all effectively supported nitrogen pollutant degradation. Parallel trends were observed between nitrification performance and microbial community development: systems achieving faster start-up (MBI, SG, HRT) exhibited higher richness (Chao1 index) and diversity (Shannon index), along with enrichment of nitrifying taxa. Based on these findings, it is recommended to prioritize biofilm inoculation under low-temperature conditions to ensure rapid and stable nitrification. In the absence of mature biofilm inoculum, gradual salinity adjustment or HRT extension can be employed to facilitate microbial acclimation and nitrification startup.
Author Contributions
Conceptualization, J.Y. and D.L.; methodology, J.Y., S.L., J.D., Q.L., Y.L., G.L. and J.G.; formal analysis, J.Y. and S.L.; investigation, J.Y. and S.L.; discussion, S.L., J.D., K.Y., Q.L., Y.L., G.L., J.G. and D.L.; resources, D.L.; data curation, J.D.; writing—original draft preparation, J.Y. and S.L.; writing—review and editing, J.Y., S.L, J.D., Q.L., Y.L., G.L. and J.G.; supervision, K.Y., G.L. and D.L.; project administration, K.Y.; funding acquisition, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the Sannong Jiufang Research Project at Zhejiang Province (No. 2025SNJF013) and the National Key R&D Program of China (No. 2024YFD2400100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, P.; Ji, J.; Zhang, Y. Aquaculture extension system in China: Development, challenges, and prospects. Aquac. Rep. 2020, 17, 100339. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S. Current status of industrialized aquaculture in China: A review. Environ. Sci. Pollut. Res. 2023, 30, 32278–32287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Liu, H.; Zhang, Y.; Zhou, Q.; Wen, X.; Guo, W.; Zhang, Z. A systematic review on aquaculture wastewater: Pollutants, impacts, and treatment technology. Environ. Res. 2024, 262, 119793. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Young, B.; Delatolla, R.; Kennedy, K.; Laflamme, E.; Stintzi, A. Low temperature MBBR nitrification: Microbiome analysis. Water Res. 2017, 111, 224–233. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, W.; Kong, Y.; Cai, Y.; Huang, Z.; Wu, T.; Zhang, M.; Nie, H.; Wang, Y. Effects of low temperature on the microbial community of MBBR filler biofilm. Water Sci. Technol. 2024, 90, 3166–3179. [Google Scholar] [CrossRef]
- Nengzi, L.; Li, H.; Ke, D.; Wu, X.; Meng, L.; Fang, Y.; Hu, Q. Influence of Temperature on the Removal Efficiency of Organic Matter and Ammonia from Micro-Polluted Source Water. Water 2023, 15, 2695. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Lai, C.-Y.; Zhao, H.-P. Salinity acclimation of nitrifying microorganisms: Nitrification performance, microbial community, osmotic adaptation strategies. J. Hazard. Mater. Adv. 2024, 15, 100448. [Google Scholar] [CrossRef]
- Juliet, J.; Du, Z.; Sebastian, B. Ammonia-Oxidizing Bacteria Maintain Abundance but Lower amoA-Gene Expression during Cold Temperature Nitrification Failure in a Full-Scale Municipal Wastewater Treatment Plant. Microbiol. Spectr. 2023, 11, e2522–e2571. [Google Scholar]
- Bassin, J.P.; Kleerebezem, R.; Rosado, A.S.; van Loosdrecht, M.M.; Dezotti, M. Effect of Different Operational Conditions on Biofilm Development, Nitrification, and Nitrifying Microbial Population in Moving-Bed Biofilm Reactors. Environ. Sci. Technol. 2012, 46, 1546–1555. [Google Scholar] [CrossRef]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L.; Liu, D. Fast start-up strategies of MBBR for mariculture wastewater treatment. J. Environ. Manag. 2019, 248, 109267. [Google Scholar] [CrossRef]
- Eshamuddin, M.; Zuccaro, G.; Nourrit, G.; Albasi, C. The influence of process operating conditions on the microbial community structure in the moving bed biofilm reactor at phylum and class level: A review. J. Environ. Chem. Eng. 2024, 12, 113266. [Google Scholar] [CrossRef]
- Gichana, Z.M.; Liti, D.; Waidbacher, H.; Zollitsch, W.; Drexler, S.; Waikibia, J. Waste management in recirculating aquaculture system through bacteria dissimilation and plant assimilation. Aquac. Int. 2018, 26, 1541–1572. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O. Salinity Acclimation Strategies in Nitrifying Bioreactors. Front. Mar. Sci. 2022, 9, 867592. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, W.; Song, Y.; Xia, S.; Hermanowicz, S.W. The characteristics of extracellular polymeric substances and soluble microbial products in moving bed biofilm reactor-membrane bioreactor. Bioresour. Technol. 2013, 148, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, S. An experimental study on nitrification biofilm performances using a series reactor system. Aquac. Eng. 1999, 20, 245–259. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e73. [Google Scholar] [CrossRef]
- Norwitz, G.; Keliher, P.N. Spectrophotometric determination of nitrite with composite reagents containing sulphanilamide, sulphanilic acid or 4-nitroaniline as the diazotisable aromatic amine and N-(1-naphthyl)ethylenediamine as the coupling agent. Analyst 1984, 109, 1281–1286. [Google Scholar] [CrossRef]
- Sánchez Rojas, F.; Cano Pavón, J.M. Spectrophotometry|Biochemical Applications; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 366–372. [Google Scholar]
- Qiu, H.; Zhao, W.; Zhao, Z.; Bai, M.; Bi, X.; Zhou, X.; Wang, Y.; Su, S.; Qin, Y.; Wang, C. Nitrogen removal activity and functional microbial community structure in IFAS, activated sludge, and MBBR systems under different salinity conditions. J. Water Process. Eng. 2025, 76, 108285. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Zhang, Z.; Luo, W.; Wu, H.; Zhang, L.; Zhang, X.; Zhao, T. Performance and microbial ecology of a novel moving bed biofilm reactor process inoculated with heterotrophic nitrification-aerobic denitrification bacteria for high ammonia nitrogen wastewater treatment. Bioresour. Technol. 2020, 315, 123813. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Song, Z.-Y.; Hu, P.; Jing, S.-Y.; Li, W.-P. Analysis of Microbial Community Characteristics and Function Prediction of MBBR with Magnetic Biocarriers at Low Temperature. Huan Jing Ke Xue = Huanjing Kexue 2023, 44, 889–899. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2 (vol 37, pg 852, 2019). Nat. Biotechnol. 2019, 37, 1091. [Google Scholar]
- Tadda, M.A.; Li, C.; Gouda, M.; Abomohra, A.E.-F.; Shitu, A.; Ahsan, A.; Zhu, S.; Liu, D. Enhancement of nitrite/ammonia removal from saline recirculating aquaculture wastewater system using moving bed bioreactor. J. Environ. Chem. Eng. 2021, 9, 105947. [Google Scholar] [CrossRef]
- Hoang, V.; Delatolla, R.; Laflamme, E.; Gadbois, A. An investigation of moving bed biofilm reactor nitrification during long-term exposure to cold temperatures. Water Environ. Res. 2014, 86, 36–42. [Google Scholar] [CrossRef]
- Knapp, C.W.; Graham, D.W. Nitrite-oxidizing bacteria guild ecology associated with nitrification failure in a continuous-flow reactor. FEMS Microbiol. Ecol. 2007, 62, 195–201. [Google Scholar] [CrossRef]
- Hou, Z.; Dong, W.; Wang, H.; Zhao, Z.; Li, Y.; Liu, H.; Shi, K.; Liang, Q.; Peng, Y. Rapid start-up of mainstream partial denitrification /anammox and enhanced nitrogen removal through inoculation of precultured biofilm for treating low-strength municipal sewage. Bioresour. Technol. 2024, 411, 131320. [Google Scholar] [CrossRef]
- Zhu, S.; Shen, J.; Ruan, Y.; Guo, X.; Ye, Z.; Deng, Y.; Shi, M. The effects of different seeding ratios on nitrification performance and biofilm formation in marine recirculating aquaculture system biofilter. Environ. Sci. Pollut. Res. 2016, 23, 14540–14548. [Google Scholar] [CrossRef]
- Aslam, Z.; Alam, P.; Islam, R.; Khan, A.H.; Samaraweera, H.; Hussain, A.; Zargar, T.I. Recent developments in moving bed biofilm reactor (MBBR) for the treatment of phenolic wastewater—A review. J. Taiwan Inst. Chem. Eng. 2025, 166, 105517. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Q.; Zeng, H.; Hu, X. Lake microbiome composition determines community adaptability to warming perturbations. Ecol. Process. 2024, 13, 33. [Google Scholar] [CrossRef]
- Song, Y.; Wang, P.; Li, G.; Zhou, D. Relationships between functional diversity and ecosystem functioning: A review. Ecol. Front. 2014, 34, 85–91. [Google Scholar] [CrossRef]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E.; Kolokotronis, S.-O. Next Generation Sequencing to Define Prokaryotic and Fungal Diversity in the Bovine Rumen. PLoS ONE 2012, 7, e48289. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Colombo, S.; Arioli, S.; Guglielmetti, S.; Lunelli, F.; Mora, D.; Simonet, P. Virome-associated antibiotic-resistance genes in an experimental aquaculture facility. FEMS Microbiol. Ecol. 2016, 92, fiw003. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Xu, X.; Zhang, C.; Wang, D.; Yang, F. Achieving mainstream nitrogen removal through simultaneous partial nitrification, anammox and denitrification process in an integrated fixed film activated sludge reactor. Chemosphere 2018, 203, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-D.; Noguera, D.R. Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res. 2004, 38, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Kikuchi, S.; Fujitani, H.; Ishii, K.; Isshiki, R.; Sekiguchi, Y.; Tsuneda, S. Characterisation of bacteria representing a novel Nitrosomonas clade: Physiology, genomics and distribution of missing ammonia oxidizer. Environ. Microbiol. Rep. 2023, 15, 404–416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).