Microplastics in Dairy Products: Occurrence, Characterization, Contamination Sources, Detection Methods, and Future Challenges
Abstract
1. Introduction
2. Methodology
3. Results and Discussions
3.1. Subsection Occurrence and Characterization of MPs in Dairy Products
3.2. Evaluation of Studies in Terms of MPs Properties and the Possibility of MP Contamination
3.2.1. Raw Milk
3.2.2. Milk
3.2.3. Yoghurt
3.2.4. Buttermilk
3.2.5. Butter and Sour Cream
3.2.6. Ayran (Traditional Fermented Dairy Product)
3.2.7. Milk Powder
3.3. Product-Based Comparative Assessment of MP Properties
3.4. Comparative Assessment of Countries Based on Product MP Properties
3.5. Sampling and Analytical Techniques for MP Determining and Defining in Dairy Products
- (1)
- Biological matrix elimination: Organic components in the food sample—such as proteins, lipids, and carbohydrates—are removed through enzymatic (e.g., protease, lipase) or chemical digestion methods using agents like hydrogen peroxide (H2O2) or potassium hydroxide (KOH).
- (2)
- MP isolation: MP particles are separated from the food matrix using techniques such as centrifugation, density separation with hypertonic solutions, or filtration.
- (3)
- Polymer identification: The chemical composition of isolated particles is confirmed using analytical techniques such as FT-IR, Raman spectroscopy and pyrolysis–gas chromatography/mass spectrometry (Py-GC/MS).
3.5.1. Digestion PROCESSES
3.5.2. Density Separation
3.5.3. Filtration Techniques
3.5.4. Extraction Techniques
3.5.5. Detection and Identification Methods of MPs
Microscopy Techniques
Thermal Techniques
Spectroscopy Techniques
3.6. Other Studies on MP Determination and Method Development for Milk and Dairy Products
4. Regulatory of MPs: Current Policies and Future Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR-FT-IR | Attenuated total reflectance FT-IR |
| CN | Cellulose nitrate |
| CPE | Chlorinated polyethylene |
| CR | Polychloroprene |
| DSC | Differential scanning calorimetry |
| EP | Ethylene-propylene |
| EPC | Ethylene-propylene copolymer |
| EDS | Energy-dispersive X-ray spectroscopy |
| PEVA | Poly(ethylene-vinyl acetate) |
| FESEM | Field emission SEM |
| FPA-FT-IR | Focal plane array FT-IR |
| FT-IR | Fourier-transform infrared spectroscopy |
| HDPE | High-density polyethylene |
| HNBR | Hydrogenated nitrile butadiene rubber |
| IR | Infrared |
| LDPE | Low-density polyethylene |
| MCF | Microplastic contamination factor |
| MPs | Microplastics |
| NMR | Nuclear magnetic resonance |
| NP | Neoprene |
| PA | Polyamide |
| PAM | Polyacrylamide |
| PARA | Polyaramid |
| PC | Polycarbonate |
| PE | Polyethylene |
| PEA | Polyethyl acrylate |
| PET | Polyethylene terephthalate |
| PHI | Polymer hazard index |
| PLI | Pollution load index |
| PMMA | Polymethyl methacrylate |
| PP | Polypropylene |
| PS | Polystyrene |
| PES | Polyethersulfone |
| PSU | Polysulfone |
| PTFE | Polytetrafluoroethylene |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| Py-GC/MS | Pyrolysis–gas chromatography/mass spectrometry |
| RMSECV | Root mean square error of cross-validation |
| SEM | Scanning electron microscopy |
| SPME | Solid-phase microextraction |
| TD-GC-MS | Thermal desorption gas chromatography–mass spectrometry |
| TED-GC/MS | Thermal extraction and desorption gas chromatography/mass spectrometry |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| TGA-FT-IR | Thermogravimetric analysis coupled with FT-IR |
| UV | Ultraviolet |
| µ-FT-IR | Micro-Fourier transform infrared spectroscopy |
References
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C.M. What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-Analysis and Systematic Review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackul’ak, T.; Žemlička, L.; Bírošová, L. Microplastics in the Food Chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Tian, W.; Song, P.; Zhang, H.; Duan, X.; Wei, Y.; Wang, H.; Wang, S. Microplastic Materials in the Environment: Problem and Strategical Solutions. Prog. Mater. Sci. 2023, 132, 101035. [Google Scholar] [CrossRef]
- Edirisooriya, E.M.N.T.; Senanayake, P.S.; Ahasan, T.; Xu, P.; Wang, H. Comprehensive Insights into Photoreforming of Waste Plastics for Hydrogen Production. Catalysts 2025, 15, 453. [Google Scholar] [CrossRef]
- Trueba, D.; Palos, R.; Crespo, I.; Veloso, A.; Azkoiti, M.J.; Bilbao, J.; Gutiérrez, A. Production of Plastic-Derived Fuel by Cohydrocracking of Different Polyethylene Terephthalate (PET) with Vacuum Gas Oil (VGO). Energy Fuels 2025, 39, 3598–3610. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, H.; Liu, Y.; Shen, Y.; Liu, G.; Su, X. Global Meta-Analysis Reveals Differential Effects of Microplastics on Soil Ecosystem. Sci. Total Environ. 2023, 867, 161403. [Google Scholar] [CrossRef] [PubMed]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Singh, J.; Mishra, P.P. Microplastics in Polar Regions: An Early Warning to the World’s Pristine Ecosystem. Sci. Total Environ. 2021, 784, 147149. [Google Scholar] [CrossRef]
- Özçifçi, Z.; Basaran, B.; Akçay, H.T. Microplastic Contamination and Risk Assessment in Table Salts: Turkey. Food Chem. Toxicol. 2023, 175, 113698. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Adjama, I.; Dave, H.; Balarabe, B.Y.; Masiyambiri, V.; Marycleopha, M. Microplastics in Dairy Products and Human Breast Milk: Contamination Status and Greenness Analysis of Available Analytical Methods. J. Hazard. Mater. Lett. 2024, 5, 100120. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of Micron-Sized Microplastic Particles from Simulated Drinking Water via Alum Coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Khan, A.; Qadeer, A.; Wajid, A.; Ullah, Q.; Rahman, S.U.; Ullah, K.; Safi, S.Z.; Ticha, L.; Skalickova, S.; Chilala, P.; et al. Microplastics in Animal Nutrition: Occurrence, Spread, and Hazard in Animals. J. Agric. Food Res. 2024, 17, 101258. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric Microplastics: A Review on Current Status and Perspectives. Earth Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Z.; Peng, G.; Luo, L.; Liu, K.; Huang, X.; Xu, Y.; Shen, Q.; Li, D. Microplastic Abundance and Distribution in a Central Asian Desert. Sci. Total Environ. 2021, 800, 149529. [Google Scholar] [CrossRef] [PubMed]
- Bodor, A.; Feigl, G.; Kolossa, B.; Mészáros, E.; Laczi, K.; Kovács, E.; Perei, K.; Rákhely, G. Soils in Distress: The Impacts and Ecological Risks of (Micro)Plastic Pollution in the Terrestrial Environment. Ecotoxicol. Environ. Saf. 2024, 269, 115807. [Google Scholar] [CrossRef]
- Nakano, H.; Alfonso, M.B.; Jandang, S.; Imai, K.; Arakawa, H. Microplastic Pollution Indexes in the Coastal and Open Ocean Areas around Japan. Reg. Stud. Mar. Sci. 2024, 69, 103287. [Google Scholar] [CrossRef]
- Rosso, B.; Scoto, F.; Hallanger, I.G.; Larose, C.; Gallet, J.C.; Spolaor, A.; Bravo, B.; Barbante, C.; Gambaro, A.; Corami, F. Characteristics and Quantification of Small Microplastics (<100 Μm) in Seasonal Svalbard Snow on Glaciers and Lands. J. Hazard. Mater. 2024, 467, 133723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, J.; Liu, M.; Wang, R.; Zhang, B.; Meng, X.Z.; Zhang, S. Seasonal Variations of Microplastics in Surface Water and Sediment in an Inland River Drinking Water Source in Southern China. Sci. Total Environ. 2024, 908, 168241. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef]
- Nakat, Z.; Dgheim, N.; Ballout, J.; Bou-Mitri, C. Occurrence and Exposure to Microplastics in Salt for Human Consumption, Present on the Lebanese Market. Food Control 2023, 145, 109414. [Google Scholar] [CrossRef]
- Gautam, R.; Jo, J.H.; Acharya, M.; Maharjan, A.; Lee, D.E.; Pramod, P.B.; Kim, C.Y.; Kim, K.S.; Kim, H.A.; Heo, Y. Evaluation of Potential Toxicity of Polyethylene Microplastics on Human Derived Cell Lines. Sci. Total Environ. 2022, 838, 156089. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You Are What You Eat: Microplastics in the Feces of Young Men Living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, Y.; Wu, X.; Cao, X.; Zhang, P.; Liang, Z.; Zhang, J.; Zhang, Y.; Gao, P.; Fu, Y.; et al. Microplastics in Human Skeletal Tissues: Presence, Distribution and Health Implications. Environ. Int. 2025, 196, 109316. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An Emerging Role of Microplastics in the Etiology of Lung Ground Glass Nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Hu, C.J.; Garcia, M.A.; Nihart, A.; Liu, R.; Yin, L.; Adolphi, N.; Gallego, D.F.; Kang, H.; Campen, M.J.; Yu, X. Microplastic Presence in Dog and Human Testis and Its Potential Association with Sperm Count and Weights of Testis and Epididymis. Toxicol. Sci. 2024, 200, 235–240. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Lee, D.W.; Jung, J.; Park, S.A.; Lee, Y.; Kim, J.; Han, C.; Kim, H.C.; Lee, J.H.; Hong, Y.C. Microplastic Particles in Human Blood and Their Association with Coagulation Markers. Sci. Rep. 2024, 14, 30419. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Aytan, Ü.; Şentürk, Y.; Özçifçi, Z.; Akçay, H.T. Microplastic Contamination in Some Beverages Marketed in Türkiye: Characteristics, Dietary Exposure and Risk Assessment. Food Chem. Toxicol. 2024, 189, 114730. [Google Scholar] [CrossRef]
- Pham, D.T.; Kim, J.; Lee, S.H.; Kim, J.; Kim, D.; Hong, S.; Jung, J.; Kwon, J.H. Analysis of Microplastics in Various Foods and Assessment of Aggregate Human Exposure via Food Consumption in Korea. Environ. Pollut. 2023, 322, 121153. [Google Scholar] [CrossRef] [PubMed]
- Özçifçi, Z.; Dizman, S.; Basaran, B.; Aliu, H.; Akçay, H.T. Occurrence and Evaluation of Microplastics in Honeys: Dietary Intake and Risk Assessment. J. Food Compos. Anal. 2025, 145, 107831. [Google Scholar] [CrossRef]
- Jasso–Salcedo, A.B.; Díaz–Cruz, C.A.; Rivera–Vallejo, C.C.; Jiménez–Regalado, E.J.; Aguirre–Loredo, R.Y. Human Consumption of Microplastics via Food Type and Habits: Recent Review. Water Air Soil Pollut. 2024, 235, 139. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Abedi, D.; Niari, M.H.; Ramavandi, B.; De-la-Torre, G.E.; Renner, G.; Schmidt, T.C.; Dobaradaran, S. Microplastics and Phthalate Esters in Yogurt and Buttermilk Samples: Characterization and Health Risk Assessment. J. Environ. Health Sci. Eng. 2025, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Visentin, E.; Manuelian, C.L.; Niero, G.; Benetti, F.; Perini, A.; Zanella, M.; Pozza, M.; De Marchi, M. Characterization of Microplastics in Skim-Milk Powders. J. Dairy Sci. 2024, 107, 5393–5401. [Google Scholar] [CrossRef]
- Wu, R.T.; Cai, Y.F.; Chen, Y.X.; Yang, Y.W.; Xing, S.C.; Liao, X. Di Occurrence of Microplastic in Livestock and Poultry Manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- van der Veen, I.; van Mourik, L.M.; van Velzen, M.J.M.; Groenewoud, Q.R.; Leslie, H.A. Plastic Particles in Livestock Feed, Milk, Meat and Blood; Amsterdam, 2022. Available online: https://vakbladvoedingsindustrie.nl/storage/app/media/Rapporten/rapporten%202022/07-juli/VOE-2022-JUL-PLASTICSOUP.pdf (accessed on 5 July 2025).
- Meyer, G.; Puig-Lozano, R.; Fernández, A. Anthropogenic Litter in Terrestrial Flora and Fauna: Is the Situation as Bad as in the Ocean? A Field Study in Southern Germany on Five Meadows and 150 Ruminants in Comparison with Marine Debris. Environ. Pollut. 2023, 323, 121304. [Google Scholar] [CrossRef]
- Chang, X.; Li, Y.; Han, Y.; Fang, Y.; Xiang, H.; Zhao, Z.; Zhao, B.; Zhong, R. Polystyrene Exposure Induces Lamb Gastrointestinal Injury, Digestive Disorders and Inflammation, Decreasing Daily Gain, and Meat Quality. Ecotoxicol. Environ. Saf. 2024, 277, 116389. [Google Scholar] [CrossRef] [PubMed]
- Shelver, W.L.; McGarvey, A.M.; Billey, L.O. Disposition of [14C]-Polystyrene Microplastics after Oral Administration to Lactating Sheep. Food Addit. Contam.—Part A 2024, 41, 1132–1143. [Google Scholar] [CrossRef]
- Bahrani, F.; Mohammadi, A.; Dobaradaran, S.; De-la-Torre, G.E.; Arfaeinia, H.; Ramavandi, B.; Saeedi, R.; Tekle-Röttering, A. Occurrence of Microplastics in Edible Tissues of Livestock (Cow and Sheep). Environ. Sci. Pollut. Res. 2024, 31, 22145–22157. [Google Scholar] [CrossRef]
- Rbaibi Zipak, S.; Muratoglu, K.; Buyukunal, S.K. Microplastics in Raw Milk Samples from the Marmara Region in Turkey. J. Fur Verbraucherschutz Und Leb. 2024, 19, 175–186. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.; Xu, S.; He, L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, G.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Cocca, M.; Colavita, G. Study of Fibrous Microplastic and Natural Microfiber Levels in Branded Milk Samples from Italy. Ital. J. Food Saf. 2025, 14, 13523. [Google Scholar] [CrossRef] [PubMed]
- Banica, A.L.; Radulescu, C.; Dulama, I.D.; Bucurica, I.A.; Stirbescu, R.M.; Stanescu, S.G. Microplastics, Polycyclic Aromatic Hydrocarbons, and Heavy Metals in Milk: Analyses and Induced Health Risk Assessment. Foods 2024, 13, 3069. [Google Scholar] [CrossRef]
- Badwanache, P.; Dodamani, S. Qualitative and Quantitative Analysis of Microplastics in Milk Samples. Indian J. Health Sci. Biomed. Res. KLEU 2024, 17, 150–154. [Google Scholar] [CrossRef]
- Banica, A.L.; Radulescu, C.; Stirbescu, R.M.; Dulama, I.D.; Bucurica, I.A.; Stanescu, S.G.; Stirbescu, N.M. Microplastics Contamination of Dairy Products with High-Fat Content-Occurrence and Associated Risks. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2024, 86, 2024. [Google Scholar]
- Chakraborty, T.K.; Hasan, M.J.; Netema, B.N.; Rayhan, M.A.; Asif, S.M.H.; Biswas, A.; Sarker, S.; Ahmmed, M.; Nice, M.S.; Islam, K.R.; et al. Microplastics in the Commercially Available Branded Milk in Bangladesh: An Emerging Threat for Human Health. J. Hazard. Mater. 2024, 477, 135374. [Google Scholar] [CrossRef] [PubMed]
- Banica, A.L.; Radulescu, C.; Dulama, I.D.; Bucurica, I.A.; Stirbescu, R.M.; Stanescu, S.G. Microplastic Debris in Yogurt: Occurrence, Characterization, and Implications for Human HealthHEALTH. J. Sci. Arts 2024, 24, 223–248. [Google Scholar] [CrossRef]
- Basaran, B.; Özçifçi, Z.; Akcay, H.T.; Aytan, Ü. Microplastics in Branded Milk: Dietary Exposure and Risk Assessment. J. Food Compos. Anal. 2023, 123, 105611. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Jiang, Y.; Zhang, Y.; Fan, Y.; Rao, W.; Qian, X. Microplastics in Infant Milk Powder. Environ. Pollut. 2023, 323, 121225. [Google Scholar] [CrossRef] [PubMed]
- Buyukunal, S.; Rbaibi Zipak, S.; Muratoglu, K. Microplastics in a Traditional Turkish Dairy Product: Ayran. Pol. J. Food Nutr. Sci. 2023, 73, 139–150. [Google Scholar] [CrossRef]
- Rbaibi Zipak, S.; Muratoglu, K.; Buyukunal, S.K. Evaluation of Microplastic Presence in Yogurt Production Process. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 633–641. [Google Scholar] [CrossRef]
- Kiruba, R.; Preethi, M.; Aganasteen, R.; Rithick Raj, M.; Hannah Thabitha, C.; Monica, P.; Sakthivel, J.; Levince, C.; Naseera Banu, I. Identification of Microplastics as Emerging Contaminant in Branded Milk of Tamil Nadu State, India. Asian J. Biol. Life Sci. 2022, 11, 181–187. [Google Scholar] [CrossRef]
- Da Costa Filho, P.A.; Andrey, D.; Eriksen, B.; Peixoto, R.P.; Carreres, B.M.; Ambühl, M.E.; Descarrega, J.B.; Dubascoux, S.; Zbinden, P.; Panchaud, A.; et al. Detection and Characterization of Small-Sized Microplastics (≥ 5 Μm) in Milk Products. Sci. Rep. 2021, 11, 24046. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Ibeto, C.N.; Enyoh, C.E.; Ofomatah, A.C.; Oguejiofor, L.A.; Okafocha, T.; Okanya, V. Microplastics Pollution Indices of Bottled Water from South Eastern Nigeria. Int. J. Environ. Anal. Chem. 2023, 103, 8176–8195. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Rakib, M.R.J. Application of Index Models for Assessing Freshwater Microplastics Pollution. World News Nat. Sci. 2021, 38, 37–48. [Google Scholar]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, J.E.; Stapleton, P.A. Nanopolystyrene Translocation and Fetal Deposition After Acute Lung Exposure during Late-stage Pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631, 449–458. [Google Scholar] [CrossRef]
- Mallapaty, S. Microplastics Block Blood Flow in the Brain, Mouse Study Reveals. Nature 2015, 638, 20. [Google Scholar] [CrossRef]
- Niu, H.; Liu, S.; Jiang, Y.; Hu, Y.; Li, Y.; He, L.; Xing, M.; Li, X.; Wu, L.; Chen, Z.; et al. Are Microplastics Toxic? A Review from Eco-toxicity to Effects on the Gut Microbiota. Metabolites 2023, 13, 739. [Google Scholar] [CrossRef]
- Yön Ertuğ, N.D.; Koçak, Ş.; Bağdatli, S.; Dinç, T.; Ikican, K.; Canik, M.; Kayhan, F.E. Recent Advances of Microplastics Toxicity and Fate on Zebrafish-a Review. Int. J. Environ. Sci. Technol. 2025, 22, 5143–5158. [Google Scholar] [CrossRef]
- Fard, N.J.H.; Jahedi, F.; Khaksar, M.A.; Shenavar, B. Systematic Review of Pulmonary Toxicity Induced by Microplastics and Nanoplastics: Insights from in Vivo and in Vitro Studies. Toxicol. Anal. Et Clin. 2025, 37, 223–241. [Google Scholar] [CrossRef]
- Janiga-MacNelly, A.; Hoang, T.C.; Lavado, R. Comparative Toxicity of Microplastics Obtained from Human Consumer Products on Human cell-based Models. Food Chem. Toxicol. 2025, 196, 115194. [Google Scholar] [CrossRef]
- Mummaleti, G.; Feng, J.; Mohan, A.; Suh, J.; Kong, Z.L.; Kong, F. Microplastics Interactions and Transformations during in Vitro Digestion with Milk. Food Res. Int. 2024, 197, 115247. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Sogut, E.; Uysal-Unalan, I.; Corredig, M. Quantification of Polystyrene Microplastics in Water, Milk, and Coffee Using Thermogravimetry Coupled with Fourier Transform Infrared Spectroscopy (TGA-FTIR). Chemosphere 2024, 368, 143777. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Cheng, J.; Yao, W.; Qian, H.; Ding, D.; Yu, Z.; Xie, Y.; Yang, F. Identification and Visualization of Polystyrene Microplastics/Nanoplastics in Flavored Yogurt by Raman Imaging. Toxics 2024, 12, 330. [Google Scholar] [CrossRef]
- Katsara, K.; Kenanakis, G.; Viskadourakis, Z.; Papadakis, V.M. Polyethylene Migration from Food Packaging on Cheese Detected by Raman and Infrared (ATR/FT-IR) Spectroscopy. Materials 2021, 14, 3872. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Viskadourakis, Z.; Kenanakis, G.; Papadakis, V.M. Microplastic Migration from Food Packaging on Cheese. Microplastics 2025, 4, 17. [Google Scholar] [CrossRef]
- Di Fiore, C.; Carriera, F.; Iannone, A.; Paris, E.; Gallucci, F.; Avino, P. First Approach for Defining an Analytical Protocol for the Determination of Microplastics in Cheese Using Pyrolysis–Gas Chromatography–Mass Spectrometry. Appl. Sci. 2024, 14, 5621. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Dongare, P.R.; Shimada, Y.; Gore, A.H. A Critical Review on Current Challenges in the Analysis of Microplastics in Food Samples. ACS Food Sci. Technol. 2023, 3, 2001–2017. [Google Scholar] [CrossRef]
- Sridhar, A.; Kannan, D.; Kapoor, A.; Prabhakar, S. Extraction and Detection Methods of Microplastics in Food and Marine Systems: A Critical Review. Chemosphere 2022, 286, 131653. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for Sampling and Detection of Microplastics in Water and Sediment: A Critical Review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A Small-Scale, Portable Method for Extracting Microplastics from Marine Sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in Seafood: Benchmark Protocol for Their Extraction and Characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Schmitt, S.W.; Holtmannspötter, H.; Christiansen, S.H.; Dicke, W. Development of an Optimal Filter Substrate for the Identification of Small Microplastic Particles in Food by Micro-Raman Spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4099–4109. [Google Scholar] [CrossRef]
- Baruah, A.; Sharma, A.; Sharma, S.; Nagraik, R. An Insight into Different Microplastic Detection Methods. Int. J. Environ. Sci. Technol. 2022, 19, 5721–5730. [Google Scholar] [CrossRef]
- Giardino, M.; Balestra, V.; Janner, D.; Bellopede, R. Automated Method for Routine Microplastic Detection and Quantification. Sci. Total Environ. 2023, 859, 160036. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar] [CrossRef]
- Saraluck, A.; Techarang, T.; Bunyapipat, P.; Boonchuwong, K.; Pullaput, Y.; Mordmuang, A. Detection of Microplastics in Human Breast Milk and Its Association with Changes in Human Milk Bacterial Microbiota. J. Clin. Med. 2024, 13, 4029. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the İmpact of Certain Plastic Products on the Environment (Text with EEA Relevance). 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019L0904 (accessed on 14 July 2025).
- Kossalbayev, B.D.; Belkozhayev, A.M.; Abaildayev, A.; Kadirshe, D.K.; Tastambek, K.T.; Kurmanbek, A.; Toleutay, G. Biodegradable Packaging from Agricultural Wastes: A Comprehensive Review of Processing Techniques, Material Properties, and Future Prospects. Polymers 2025, 17, 2224. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An Overview of Biodegradable Packaging in Food Industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
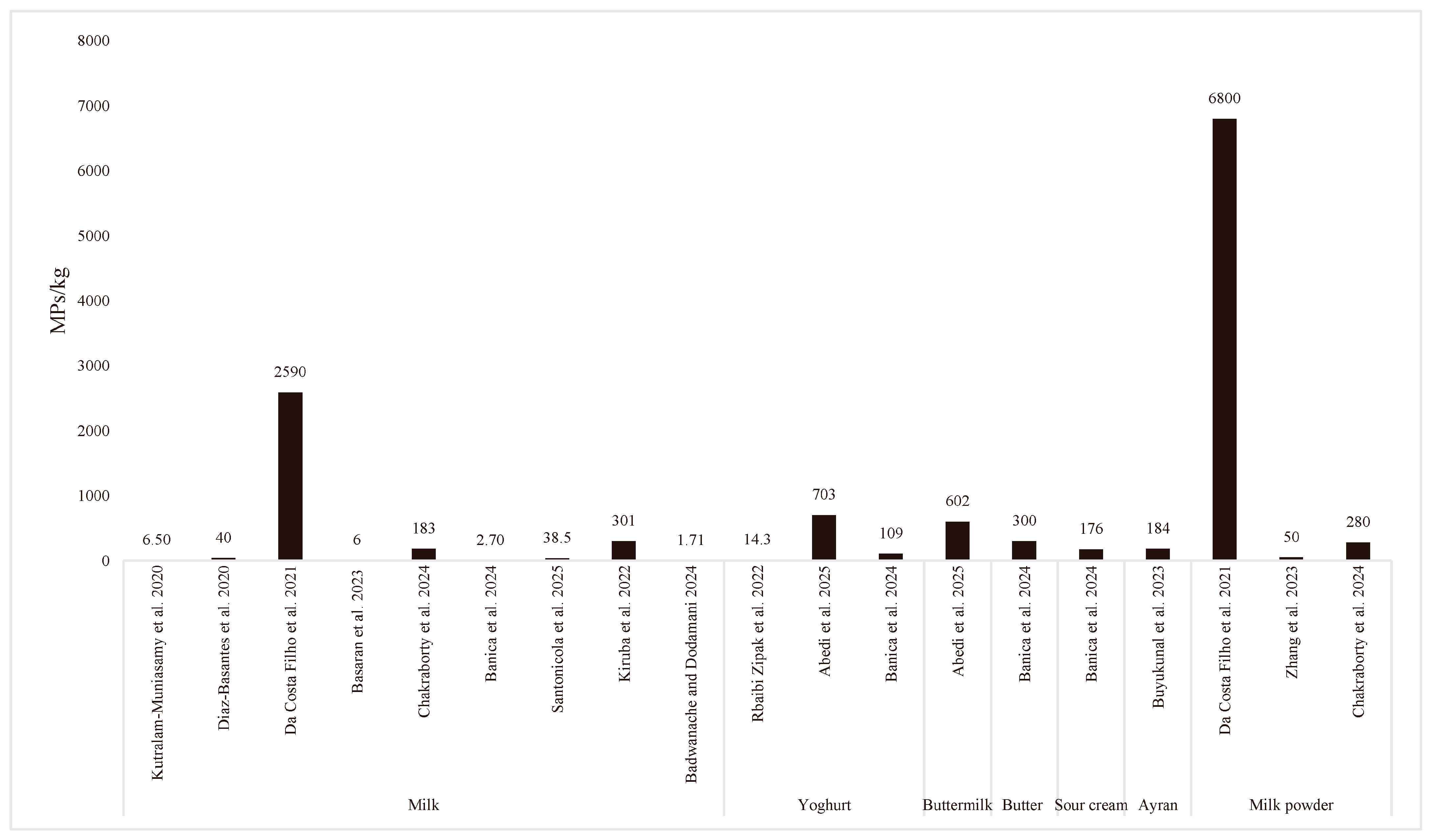
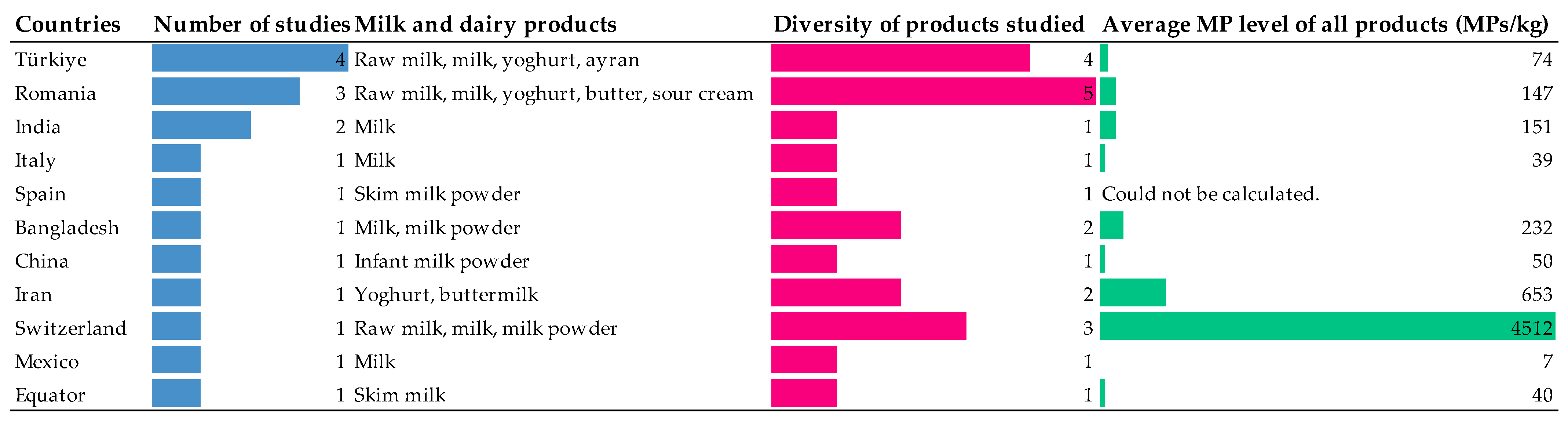
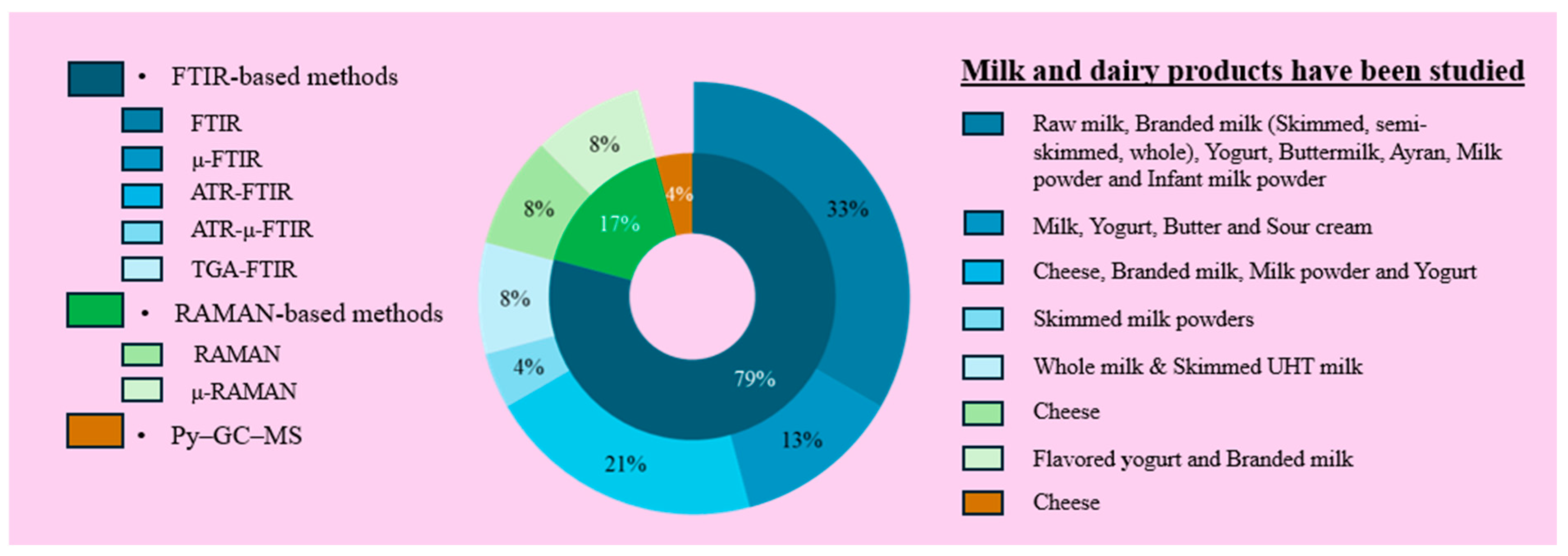
| Dairy Product | Amount of MP | Physical | Chemical | Method | Country | References | ||
|---|---|---|---|---|---|---|---|---|
| Size (µm) | Shape | Color | Polymers | |||||
| Yoghurt (n = 16) | 0–1660 MPs/kg | <500: 20%, 500–1000: 30%, >1000: 50% | Fibers | Transparent, red, brown, black, gray, blue and green | PA, PET, PVC, PE, PC, PMMA, PU | FT-IR | Iran | [39] |
| Buttermilk (n = 14) | 0–2000 MPs/kg | <500: 13%, 500–1000: 36%, >1000: 51% | Transparent, red, brown, black, gray, blue and green | PA, PET, PVC, PC, PMMA, PU | ||||
| Milk (skimmed, semi-skimmed, and whole UHT) (n = 20) | 10–270 MPs/kg | <350: 23%, 350–1000: 37%, >1000: 40% | Fibers | Blue, black, red, pink, violet, yellow, green, orange, transparent, blue sky | PE, polyester | FT-IR | Italy | [51] |
| Skim-milk powder (n = 16) | 466–5765 MPs/kg | <99: 80%, 99–1444: 20% | Fibers, sphere, fragments | Black, blue, brown, fuchsia, green, and gray | PEVA, PE, CPE, PP, PVC, PA, PC, polyisoprene, polyvinylidene fluoride | FT-IR | Spain | [40] |
| Raw milk (n = 588) | 84–128 MPs/kg | <500: 56%, 500–1000: 15%, >1000: 29% | Fibers, fragments, film, Sphere | Black, blue, brown, grey, green, orange, pink, purple, red, transparent, and yellow | PEA, EPC, HNBR, PAM, PARA, CR, PTFE | FT-IR | Türkiye | [47] |
| Raw milk (n = 4) | 7–23 MPs/kg | 115–798 | Fibers, fragments | Black, blue, red, brown, yellow | PMMA, PA | µ-FT-IR | Romania | [52] |
| Organic milk (n = 6) | 10–99 MPs/kg | 68–2152 | Fibers, fragments | Black, blue, red, brown, gray, yellow, green | PMMA, PA, PU, polyester | |||
| Conventional milk (n = 10) | 2–36 MPs/kg | 76–1507 | Fibers, fragments | Black, blue, red, brown, gray, golden, turquoise | PMMA, PA, PE, PU, polyester | |||
| Milk (n = 21) | Not available | Not available | Fibers, fragments | Blue, red, violet, and green | PS, PP, PVC | FT-IR | India | [53] |
| Butter (n = 8) | 375–1500 MPs/kg | 41–1444 | Irregular, fiber oval, square, film, triangle, trapezoid, rectangular and diamond | Black, blue, red, brown, yellow, gray, purple | PA, acrylic | μ-FT-IR | Romania | [54] |
| Sour cream (n = 7) | 400–1200 MPs/kg | 47–1748 | Irregular, film, fiber, oval, triangle | Black, blue, green, yellow, gray, purple | PA, acrylic | |||
| Milk (n = 6) | 95–250 MPs/kg | <100: 35%, >100: 65% | Fibers, fragments, film | Black, red, blue, white, green, yellow, and violet | PE, PP, Nylon-6, PS, PA | ATR-FT-IR | Bangladesh | [55] |
| Milk powder (n = 25) | 120–570 MPs/kg | <100: 31%, >100: 69% | Fibers, fragments, film | Black, red, blue, white, green, yellow, and violet | PE, PP, PET, PA, PS, PA | |||
| Yoghurt (n = 17) | 693–1155 MPs/kg | Not available | Fibers, fragments | Black, blue, red, brown, grey, yellow, purple | PA, PE, PU, polyester | µ-FT-IR | Romania | [56] |
| UHT Milk (n = 14) | 3–48 MPs/kg | <500: 30%, 500–1000: 37%, >1000: 33% | Fibers, fragments | Black, red, green, blue, brown, and gray | PA, PET, PEVA, PP, PU | ATR-FT-IR | Türkiye | [57] |
| Infant milk powder (n = 13) | 0–180 MPs/kg | <50: 50% 50–608: 50% | Fibers, fragments, film | Not available | PE, PET, PP, PA, PVC | FT-IR | China | [58] |
| Ayran (traditional fermented dairy product) (n = 180) | 0–430 MPs/kg | <150: 37%, 151–1000: 37%, >1000: 26% | Fibers, fragments, film, sphere | Black, blue, brown, dark blue, grey, green, orange, pink, purple, red, transparent, and yellow | EP, PTFE, PA, NP, PP, PAM, PE | FT-IR | Türkiye | [59] |
| Yoghurt (n = 12) | 20–580 MPs/kg | <500: 70%, 500–1000: 11%, >1000: 19% | Fibers, fragments film, sphere | Black, blue, brown, gray, green, orange, pink, red, purple, reddish brown, and transparent white | PP, PS, PE, PVC | FT-IR | Türkiye | [60] |
| Milk (n = 10) | 164–427 MPs/kg | <500 | Fibers, fragments pellet | Pink, purple, and blue | PE, PP, PAM | FT-IR | India | [61] |
| Raw milk (n = 2) | 2040–6250 MPs/kg | ≥5 | Fibers, fragment | Not available | PE, PES, PP, PU, PA, PTFE | μ-Raman | Switzerland | [62] |
| Milk (n = 4) | 1720–3480 MPs/kg | PE, PS, PES, PVA, PTFE, PU, PP, PSU | ||||||
| Milk powder (n = 2) | 3560–10,040 MPs/kg | PE, PES, PP, PA, PTFE | ||||||
| Milk (n = 23) | 3–11 MPs/kg | <500: 40%, 500–1000: 28%, >1000: 32% | Fibers, Fragments | Blue, brown, red and pink | PES, PSU | μ-Raman | Mexico | [38] |
| Skim milk (n = 10) | 134–444 MPs/kg | 2.48–6742 | Fibers, fragments | Green, yellow, red, violet and blue | HDPE, LDPE, PAM, PP | FT-IR | Equator | [63] |
| Identification Methods | Spectroscopy Device Brand and Model | Imaging Methods | Filtration Methods Filter Type and Pore Size | Digestion | Reference |
|---|---|---|---|---|---|
| FT-IR | Agilent Cary 630 (Santa Clara, CA, USA) | Binocular biological microscope, SEM–EDS | Vacuum filtration (1 μm) | Multi-enzymatic detergent | [47] |
| FT-IR | Not specified | Microscopy | Vacuum filtration (11 µm) | Density separation: NaCl (1.17 g/cm3) | [53] |
| µ-FT-IR | Vertex 80 v, Bruker (Ettlingen, Germany) | Optical Microscopy—SEM-EDS | Vacuum filtration Membrane filter (5–13 µm) | Ultrasonic bath | [52] |
| μ-Raman | Horiba (Longjumeau, France) | Epifluorescence microscope + SEM | Vacuum filtration (11 µm) | No | [38] |
| FT-IR | Not specified | Epifluorescence microscope | Vacuum filtration (11 µm) | No | [61] |
| FT-IR | PerkinElmer (Hopkinton, MA, USA) | Digital microscope | Vacuum filtration (10 µm) | No | [57] |
| FT-IR | Nicolet 6700, Thermo Fisher Scientific (Middleton, WI, USA) | FESEM | Filtration | Salivary digestion, gastric digestion and intestinal digestion | [76] |
| FT-IR | Not specified | Optical microscopy | Vacuum filtration Membrane filter (10 µm) | H2O2 (30%) | [63] |
| FT-IR | Nicolet iMX10, Thermo Fisher Scientific (Waltham, MA, USA) | Optical microscopy | Vacuum filtration Cellulose nitrate membrane filter (8 µm) | H2O2 (30%) | [51] |
| TGA- FT-IR | Mettler Toledo (Zurich, Switzerland) | No | No | No | [77] |
| ATR-FT-IR | FTIR- 4600, JASCO Inc. (Tokyo, Japan) | Microscopy | Vacuum filtration Glass microfiber filter | No | [55] |
| FT-IR | PerkinElmer (Milan, Italy) | Microscopy | Vacuum filtration Silver membrane microfilter (3 µm) | KOH (10%) | [40] |
| FT-IR | Hyperion 2000, Bruker (Ettlingen, Germany) | Not mentioned | Vacuum filtration, Polycarbonate filter (8 μm) | Pancreatic enzymes | [58] |
| µ-FT-IR | Vertex 80, Bruker (Billerica, MA, USA) | Optical microscopy | Vacuum filtration, Cellulose membrane filter (12–15 µm) | 1% mix solution of Sodium dodecyl sulfate & NaOH | [54] |
| FT-IR | Shimadzu AIM-9000 (Kyoto, Japan) | Binocular microscope, SEM-EDS | Vacuum filtration, Cellulose nitrate membrane filter (0.45 µm) | H2O2 (30%) | [39] |
| FT-IR | Agilent Cary 630 (Santa Clara, CA, USA) | Binocular microscope, SEM | Vacuum filtration, Microfiber filters (1 μm) | Multi-enzymatic detergent | [60] |
| µ-FT-IR | Vertex 80 v, Bruker (Massachusetts, USA) | Optical microscopy | Vacuum filtration, Cellulose membrane filter (12–15 µm) | Ultrasonic bath | [56] |
| μ-Raman | Horiba (Longjumeau, France) | Optical microscopy | Microfiltration, Silicon (Si) filter (5 μm) | Multi-enzymatic and alkaline digestion | [62] |
| μ-Raman | Thermo Fisher Scientific (Wisconsin, USA) | Optical microscopy, SEM-TEM | Vacuum filtration, Glass fiber filter (0.22 µm) | Multi-enzymatic detergent and alkali | [78] |
| FT-IR | Agilent Cary 630 (Santa Clara, CA, USA) | Optical microscope, SEM | Vacuum filtration, Glass microfiber filter (1 µm) | Multi-enzymatic detergent | [59] |
| ATR-FT-IR & Raman | Vertex 70 v, Bruker (Rosenheim, Germany) & LabRAM HR, Horiba (Longjumeau, France) | Optical microscopy | No | No | [79] |
| ATR-FT-IR & Raman | Vertex 70 v, Bruker (Rosenheim, Germany) & LabRAM HR, Horiba (Longjumeau, France) | Optical microscopy | No | No | [80] |
| Py–GC–MS | Agilent 7890 A (Santa Clara, CA, USA) | Optical microscopy | Vacuum filtration, Glass fiber filter (1.6 µm) | HNO3 (65%), H2O2 (5.4 M), KOH (1 and 5 M), and Fenton’s reagent. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürmeriç, H.E.; Basaran, B. Microplastics in Dairy Products: Occurrence, Characterization, Contamination Sources, Detection Methods, and Future Challenges. Appl. Sci. 2025, 15, 9411. https://doi.org/10.3390/app15179411
Gürmeriç HE, Basaran B. Microplastics in Dairy Products: Occurrence, Characterization, Contamination Sources, Detection Methods, and Future Challenges. Applied Sciences. 2025; 15(17):9411. https://doi.org/10.3390/app15179411
Chicago/Turabian StyleGürmeriç, Hüseyin Ender, and Burhan Basaran. 2025. "Microplastics in Dairy Products: Occurrence, Characterization, Contamination Sources, Detection Methods, and Future Challenges" Applied Sciences 15, no. 17: 9411. https://doi.org/10.3390/app15179411
APA StyleGürmeriç, H. E., & Basaran, B. (2025). Microplastics in Dairy Products: Occurrence, Characterization, Contamination Sources, Detection Methods, and Future Challenges. Applied Sciences, 15(17), 9411. https://doi.org/10.3390/app15179411






