Abstract
Microplastic (MP) particles are ubiquitous in the environment and pose a growing threat to ecosystem stability. As concern over their ecological impact increases, biotests and ecotoxicological approaches using plant species have become valuable tools for research. This study aimed to evaluate the effects of varying concentrations of low-density polyethylene (LDPE) MP on seed germination, root development, and shoot growth of white mustard (Sinapis alba L.) under controlled laboratory and pot experiment conditions. For the seven-day laboratory experiment, concentrations of 0.01% and 1% w/w were used, whereas concentrations of 1% and 5% w/w were applied in the ten-day pot experiment. Results indicated no statistically significant effects of LDPE MP on germination rate or germination speed index (GSI) in either setting. However, shoot length data suggest that the 5% LDPE treatment may have a slight stimulatory effect compared to the control, though this trend was marginally significant. These findings highlight the complex and context-dependent nature of MP–plant interactions. Further research is needed to better understand the mechanisms driving these responses and to support the development of mitigation strategies for MP contamination in terrestrial ecosystems.
1. Introduction
Microplastic (MP) particles are widely dispersed and accumulate in various ecosystems, leading to significant environmental imbalances. The majority of MP pollution originates from terrestrial sources [1] and is primarily introduced into the environment through wastewater discharge and industrial effluents [2]. Additional sources include municipal sewage, sewage sludge, household waste, agricultural activities (e.g., irrigation, pesticide and fertilizer application, plastic sheeting, and greenhouse materials), improper waste disposal, and road traffic [3]. Due to the low availability of light and oxygen in the soil, MP particles can persist for decades without significant degradation, retaining their chemical composition over time [1]. The continuous accumulation of MP in soils disrupts ecosystem functioning and biodiversity, as these particles readily infiltrate soil systems and food chains due to their small size [4]. While substantial research has focused on MP pollution and its impacts in aquatic ecosystems, studies on terrestrial environments remain relatively scarce [5]. Existing terrestrial research has primarily examined MP accumulation in agricultural soils [6,7,8], its interactions with soil biota [9,10] and physicochemical properties [11,12,13], and potential effects on plant growth and nutrient cycling [4,5,14,15]. However, estimates suggest that soil environments contain 4 to 23 times more MP than aquatic systems [16], underscoring the urgent need for further investigation in this field.
Soil ecotoxicity is assessed using various biological tests and diagnostic methods, including in situ bioindication monitoring and laboratory ecotoxicity assessment of samples. An indicator of ecotoxicity is the degree of change in certain parameters, which is determined by biophysical and biochemical methods or by visual counting [17]. The bioassays used in soil ecotoxicology were standardized by the OECD and ISO [18,19] in the early 2020s and are based on the consideration of mortality and reproduction rates of soil organisms and the ability of plants to respond to changes in environmental conditions [20]. The test organisms used for bioassays should be widely distributed under natural conditions and available in large quantities throughout the year, represent a population that is as homogeneous as possible and have a high sensitivity to toxicants. The list of recommended plants includes 23 dicotyledonous and 9 monocotyledonous plants, all of which are cultivated plants [18,19]. The test parameters include the germination rate of the seeds, the length of the shoots and their biomass, the length of the root, and the total length of the plant with the root [18].
The experimental plant in this study is white mustard (Sinapis alba), family Brassicaceae, a fast-growing annual plant that is cultivated worldwide and is of great economic importance. It is characterized by rapid germination, low soil requirements and high biomass production and is often used as a cover crop to reduce soil erosion and control soil pests [21]. It has been the most commonly used biological model in ecotoxicological and phytotoxicological studies on bioaccumulation of potentially toxic elements, including chromium, thallium, arsenic, zinc, copper, cadmium, and nickel [22,23,24,25,26,27]. The phytotoxicity of leachate from the tire fraction was demonstrated by an inhibition of root growth of Sinapis alba by up to 47% [28]. A study on the effects of antibiotics on the development of antibiotic resistance genes in the environment showed a direct toxicity of antibiotics on root development of Sinapis alba only at high concentrations [29]. White mustard was also a reliable indicator of the phytotoxicity of municipal solid waste leachate and showed that the growth and CO2 assimilation of Sinapis alba responded positively to low leachate levels, while root growth was strongly inhibited upon direct exposure to a >70% leachate solution [30].
Biotests and ecotoxicological methods to assess the risk of MP to the environment are becoming increasingly useful, and a number of plant species are being used. For example, seed germination and root growth of cress (Lepidium sativum) showed a size-dependent response to MP, but only in the first 24 h, which was attributed to physical clogging of the pores in the seed capsule by MP, while MP accumulated in the root tips at later stages [5]. In common bean [31], maize [32], and onions [33], limited or no effects of polyethylene MP (LDPE or PEHD) on growth parameters were reported, possibly due to the fact that the chemical structure of polyethylene MP (C2H4)n, which is structurally stable and does not contain nutrients that could affect soil nutrient dynamics, has less pronounced effects on changes in soil properties. Negative effects were observed with the use of biodegradable MP (PBAT, PLA, starch-based MP), as evidenced by reduced root and shoot biomass of beans, maize, and wheat. In their work, Ref. [34] suggested that the altered bacterial communities in the rhizosphere and the increase in volatile compounds such as dodecanal could be responsible for the decline in total biomass. The intermediate and final metabolites degraded by PLA-MPs could have direct or indirect effects on soil properties, soil biota, and nutrient availability [34]. PBAT could increase the growth of rhizobacteria in the soil and thus compete with plant roots for nutrients [35].
Polyester microfibers at concentrations of 0.1% and 1% (w/w) acted as a stressor on Sinapis alba by altering chlorophyll fluorescence levels, reducing the number of flowers and decreasing the pod-to-seed ratio [23]. The addition of polypropylene (PP) plastic, which is often used for the production of bottles and packaging, led to contradictory results in plant growth parameters. Negative effects were observed for the germination of tomatoes [36] and garden cress [37], as well as for the shoot growth of pumpkin (Cucurbita pepo) [38], while PP contamination of the soil significantly increased the root biomass and length of green onion as well as the total biomass of wild carrot (Daucus carota) [33,39]. Under hydroponic conditions, PP-MP had no effect on seed germination, but increased shoot and root elongation of tomato and cherry tomato seedlings. Nutrient uptake was also affected by MP, but the effect varied depending on the element and plant species as well as the duration of MP exposure, suggesting that long-term exposure to MPs may lead to nutrient imbalance in plants [40].
The objective of this study was to investigate the effects of different concentrations of low-density polyethylene (LDPE) MP on the germination and growth of a model plant species. Specifically, the impact on seed germination, root development, and shoot growth of white mustard (Sinapis alba L.) was assessed. LDPE dominates the market due to its durability, puncture resistance, tensile strength, and stability under UV radiation and low temperatures [41], making it ideal for protecting crops and enhancing soil conditions [42,43]. However, the poor end-of-life management of LDPE films often leads to their fragmentation and incorporation into soils during ploughing, contributing to microplastic accumulation [44]. Given its widespread use, environmental persistence, and high hydrophobicity, LDPE was selected in this study as a representative conventional plastic to assess its potential impacts on seed germination and shoot growth. Based on previous findings on MP-induced stress in plants [5,14,15,45,46,47], the following hypotheses were proposed: (i) exposure to LDPE microplastics would reduce seed germination and inhibit early seedling growth of Sinapis alba L. in a concentration-dependent manner under laboratory conditions; (ii) LDPE MPs would impair root and shoot development of S. alba L. under controlled pot experiment conditions. Both laboratory and pot experiments were conducted using white mustard seeds and LDPE MP particles. The experiments were carried out in a growth chamber under controlled and uniform environmental conditions. Each experiment was evaluated independently to ensure robust analysis.
2. Materials and Methods
For the purposes of this study, two separate experiments were conducted. A laboratory petri dish experiment and a pot experiment, both designed to assess the effects of different concentrations of LDPE MPs on plant development.
Both experiments utilized white mustard (Sinapis alba L.) seeds and LDPE MPs of the same origin. The growth conditions remained consistent across both experiments, with all experimental parts conducted in a controlled growth chamber at the Department of Agronomy, Biotechnical Faculty, University of Ljubljana, Slovenia (46°02′59.1″ N; 14°28′21.9″ E). The chamber was maintained at 20–22 °C, with a 12 h light/dark cycle (LED illumination: 150 µmol m−2 s−1) and 40% relative humidity.
The LDPE MPs were produced by milling commercially available agricultural mulch film. Smaller pieces of film were ground using a cryogenic milling process to prevent melting and aggregation. The morphology, size distribution, and shape of the resulting particles were examined using scanning electron microscopy (SEM). The milling process generated a broad size distribution of particles up to 250 μm. For the experiment, particles with sizes ≤250 μm were used. The density of the LDPE particles was 0.93 g/cm3. The treatment concentrations selected for both the Petri dish (0.01% and 1% w/w) and pot experiments (1% and 5% w/w) are consistent with concentrations frequently applied in previous studies investigating microplastic effects in soil–plant systems [16,34,48,49]. While these concentrations exceed typical levels currently observed in most agricultural soils—where MP contamination generally remains below 0.1% w/w [50]—they enable the evaluation of potential effects of future pollution scenarios. Furthermore, they facilitate comparability with the existing body of literature and contribute to a broader understanding of concentration-dependent responses. Concentrations approaching 1% w/w have been reported in soils subjected to intensive plastic use or near urbanized areas [51], highlighting the relevance of including such levels in experimental designs.
Commercial white mustard (Sinapis alba L.) seeds were obtained from Autrosaat, distributed by RWA Slovenia, and stored at room temperature. As the seeds were clean and calibrated, no further sterilization was performed before use.
2.1. Petri Dish Experiment
The Petri dish experiment was conducted under laboratory conditions and included three LDPE MP treatments: 0% (w/w) (control), 0.01% (w/w), and 1% (w/w). Each treatment was replicated five times, resulting in a total of 15 Petri dish samples (3 treatments × 5 replicates). The Petri dishes were randomly assigned to positions on two trays (Figure 1).
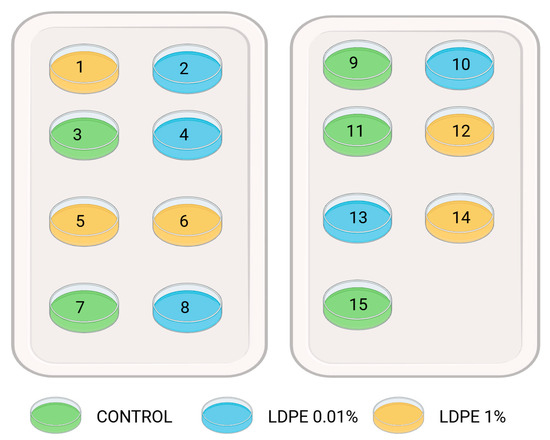
Figure 1.
Scheme of tray 1 (left) and tray 2 (right) with randomly positioned Petri dishes.
Each Petri dish was lined with five layers of filter paper, which were moistened with 10 mL of distilled water. The respective LDPE MP concentration was then evenly distributed over the moistened filter paper using a fine brush. The brushing technique ensured an even and uniform distribution of the microplastic particles across the filter paper surface. The particles adhered to the filter paper, eliminating the need for surfactants or wetting agents.
After the application of MP particles, 10 seeds of white mustard (Sinapis alba L.) were carefully placed on the filter paper using tweezers. The prepared Petri dishes were then positioned on the trays according to the predetermined layout (Figure 2). The trays containing the samples were transferred to a growth chamber and incubated under controlled conditions for seven days, in the period between 26 February and 4 March 2024. To minimize positional bias, the trays were rotated daily within the chamber. Seed germination was recorded every 24 h by counting the number of germinated seeds in each Petri dish. At the end of the experiment, germination percentage (G%) and germination speed index (GSI) were calculated.
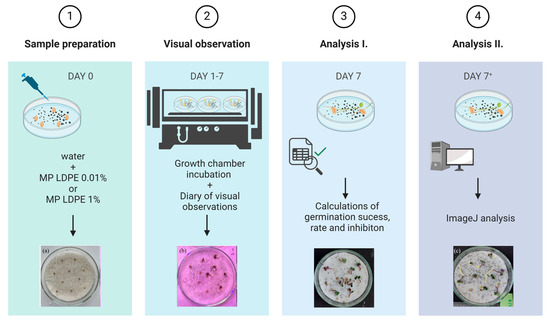
Figure 2.
Schematic presentation of the laboratory Petri dish experiment workflow.
Germination Percentage (G%) and Germination Speed Index (GSI)
Germination was defined as the opening of the seed coat accompanied by the emergence of a primary root. In both experiments, the following parameters were assessed:
The germination percentage (G%) was determined by calculating the level of cumulative seed germination percentage. It was calculated using Equation (1) (adapted from [52]):
The germination speed index (GSI) was determined using Equation (2) (adapted from [52]). It is expressed as the germination in terms of the total number of seeds germinating in a time interval:
where N1–N7 represent the number of germinated seeds per day (from D1 to D7), and D1–D7 represent day of germination.
2.2. Pot Experiment
The pot experiment included three LDPE MP treatments: 0% (w/w) (control), 1% (w/w), and 5% (w/w). Each treatment was replicated five times, resulting in a total of 15 pot samples (3 treatments × 5 replicates). Pots (size 15 cm × 11 cm × 4.5 cm) were randomly assigned to positions on two trays (Figure 3).
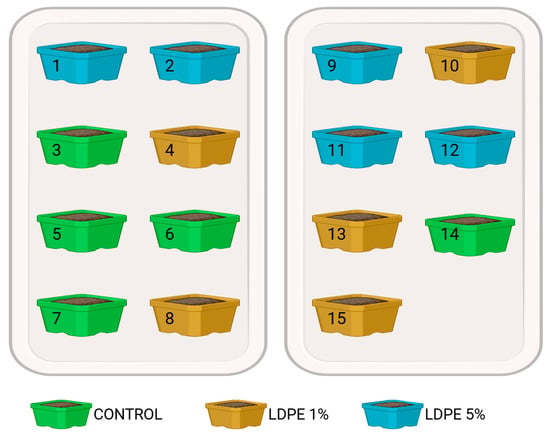
Figure 3.
Scheme of tray 1 (left) and tray 2 (right) with randomly positioned pots included in the experiment.
A mass of approximately 223 g of substrate was added to each pot (equivalent to ~248 ml). The substrate used in the pot experiment consisted of 62.8% sand, 24.6% silt, and 12.6% clay, classifying it within the sandy-clay texture class. It contained 3.1% organic matter and had a measured density of 0.93 g/cm³. Based on the volume and the mass of the substrate, the mass of LDPE MPs at the target concentration were calculated.
The pre-weighed LDPE MPs were carefully transferred into a mixing bowl using a brush and thoroughly mixed to ensure uniform distribution. Mixing was performed in a standardized manner, using the following sequence: 10 stirs clockwise, 10 stirs counter-clockwise, and 10 stirs back and forth. This procedure was applied consistently across all replicates, including the control, to minimize variability and ensure methodological consistency. Pots were then placed in a larger container, allowing the substrate to reach adequate moisture levels through water absorption. The prepared pots were left to stabilize for one hour.
Subsequently, thirty seeds of white mustard (Sinapis alba L.) were sown in each pot. The pots were arranged on trays according to the pre-established experimental layout. The trays containing the samples were transferred to a growth chamber and incubated under controlled conditions for 10 days, in the period between 26 February and 6 March 2024. To minimize positional bias, the trays were rotated daily within the chamber. Seed germination and seedling growth was recorded every 24 h (Figure 4). At the end of the experiment, germination percentage (G%) and germination speed index (GSI) were calculated, as presented in Equations (1) and (2).
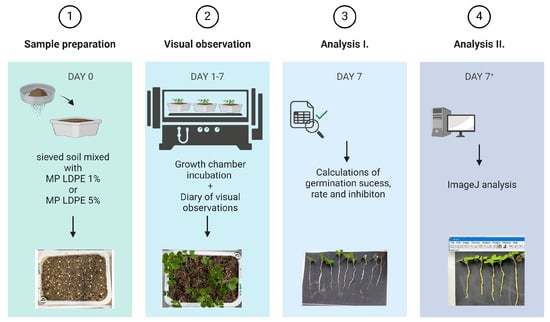
Figure 4.
Schematic presentation of the pot experiment workflow.
2.3. ImageJ Analysis
At the end of each experiment, collected germinated seeds from Petri dishes and seedlings from the pot experiment were analyzed using ImageJ software (Version 1.54p) [53]. With this open-source image processing software, the seed length of the germ and the lengths of the stem and root parts of the seedling were estimated.
2.4. Statistical Analysis
Data analysis was performed using R statistical software (Version 4.2) [54]. The Rcmdr package (R Commander interface) was used to perform a descriptive analysis based on the means of each germination index. Results are expressed as mean ± standard error, calculated using the numSummary() function from the RcmdrMisc package. Prior to conducting analysis of variance (ANOVA) on the data related to sprout length (Petri dish experiment) and sprout and root length (pot experiment), the assumptions of ANOVA were tested. The normality of residuals was assessed using the Shapiro–Wilk test, and homogeneity of variances was evaluated using Levene’s test. Both tests indicated that the assumptions were satisfied. Subsequently, a one-way ANOVA was performed using the aov() function from the stats package. When the ANOVA showed statistically significant effects, pairwise comparisons between treatment groups were conducted using Duncan’s test, implemented via the duncan.test() function from the agricolae package. Significance levels were reported as follows: p < 0.001 (***); p < 0.01 (**); p < 0.05 (*); p < 0.1 (.); and p ≥ 0.1 (ns, not significant). Data were illustrated graphically using the boxplot() function from the graphics package [54].
3. Results
3.1. Petri Dish Experiment
3.1.1. Germination Percentage (G%) and Germination Speed Index (GSI) Calculations
Germination as a test parameter in the Petri dish experiment was calculated for three LDPE MP concentrations: 0.01%, 1%, and control with 0% LDPE MP. After seven days of exposure, the germination percentage was 92% for Control, 94% for LDPE 0.01%, and 90% for 1% LDPE concentration (Figure 5).
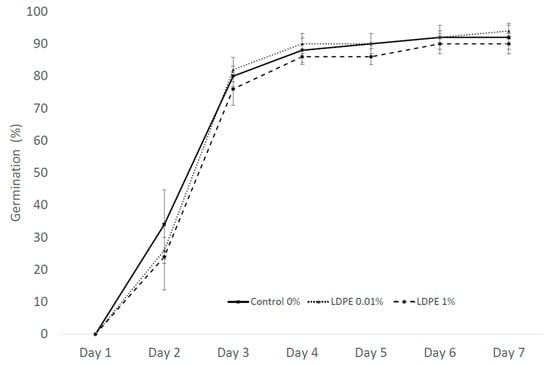
Figure 5.
Effect on germination percentage of S. alba exposed to different LDPE MP concentrations in the substrate-free Petri dish experiment exposure after 7 days. Values are presented as mean ± standard error (SE).
The germination speed index (GSI) expresses the percentage of germination on each day during the seven-day germination period. Higher GSI values indicate higher and faster germination of the seeds [52]. Calculated germination rate indexes of S. alba in the Petri dish experiment were 3.74 ± 0.22 for Control, 3.43 ± 0.11 for 0.01% LDPE MP, and 3.25 ± 0.24 for 1% LDPE MP.
3.1.2. Sprout Length Analysis
Results on sprout length (Figure 6) show the distribution of sprout length across three analyzed treatment groups, Control with 0% MP, LDPE 0.01%, and LDPE at 1% concentrations in the Petri dish experiment. Both LDPE 0.01% and LDPE 1% treatments exhibit slightly lower median sprout lengths compared to the Control, indicating a potential negative influence of LDPE on early sprout development. However, the observed differences are relatively modest.
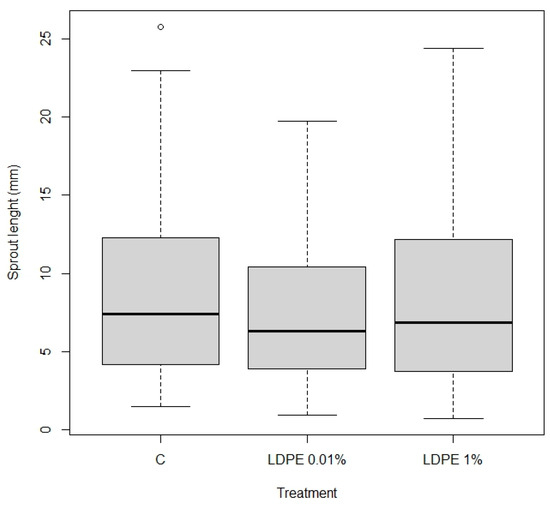
Figure 6.
Boxplot illustration of the distribution of length (in mm) of sprouts from the Petri dish experiment across three treatments, Control (C), LDPE 0.01%, and LDPE 1%, with a p-value of 0.812 indicating no significant difference. The central bold line within each box represents the median sprout length, while the box itself spans the interquartile range (25th to 75th percentile). Whiskers indicate the minimum and maximum values within 1.5 times the interquartile range. The dot represents outliers, where for each treatment, some individual measurements were much lower or higher than the majority of the data.
The overall variability is comparable across treatments, only the LDPE 1% group shows slightly greater dispersion, suggesting increased variability in response at this concentration.
The results presented in Table 1 show that there are no statistically significant differences in sprout height between the treatments in the Petri dish experiment. Specifically, the comparison between LDPE 0.01% and the Control has an estimate of −0.08234, with a confidence interval ranging from −0.36323 to 0.19854 and a p-value of 0.812, indicating no significant difference between these two treatments.

Table 1.
Duncan test for differences in sprout length between treatments in the Petri dish experiment.
Similarly, the comparison between LDPE 1% and the Control shows an estimate of −0.02403, with a confidence interval from −0.30966 to 0.26161 and a p-value of 0.974, which also suggests no significant effect of LDPE 1% on sprout height relative to the Control. Finally, the comparison between LDPE 1% and LDPE 0.01% shows an estimate of 0.05831, with a confidence interval ranging from −0.22732 to 0.34395 and a p-value of 0.930, further confirming that there is no significant difference in sprout height between these two LDPE treatments.
In summary, the data suggest that the different concentrations of LDPE do not have a statistically significant impact on sprout height in the Petri dish experiment.
3.2. Pot Experiment
3.2.1. Germination Percentage (G%) and Germination Speed Index (GSI)
Germination in the pot experiment was calculated for three LDPE MP concentrations: 1%, 5%, and Control with 0% LDPE MP. After ten days of exposure, the germination percentage was 63.3% for Control, 69.3% for LDPE 1%, and 52.6% for 5% LDPE concentration (Figure 7).
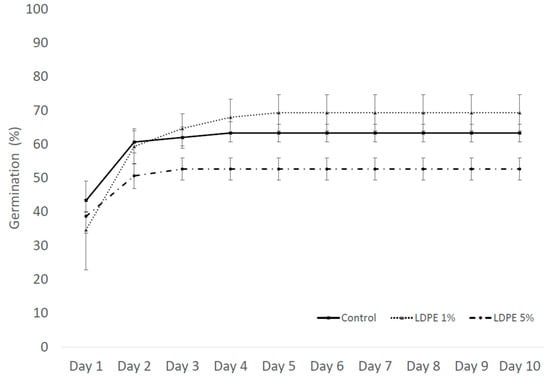
Figure 7.
Effect on germination percentage of S. alba exposed to different LDPE MP concentrations in the pot experiment exposure after 10 days. Values are presented as mean ± standard error (SE).
The germination speed index (GSI) expresses the percentage of germination on each day during the seven-day germination period. Higher GSI values indicate higher and faster germination of the seeds [52]. Calculated germination rate indexes of S. alba in pot experiment were 15.83 ± 0.46 for Control, 14.36 ± 1.03 for 1% LDPE MP, and 14.30 ± 0.98 for 5% LDPE MP.
3.2.2. Sprout Length
The boxplot in Figure 8 illustrates the distribution of sprout length across the three treatment groups. Both LDPE 1% and LDPE 5% treatments exhibit slightly higher median sprout lengths compared to the Control, indicating a potential positive influence of LDPE on early sprout development. However, the observed differences are relatively modest.
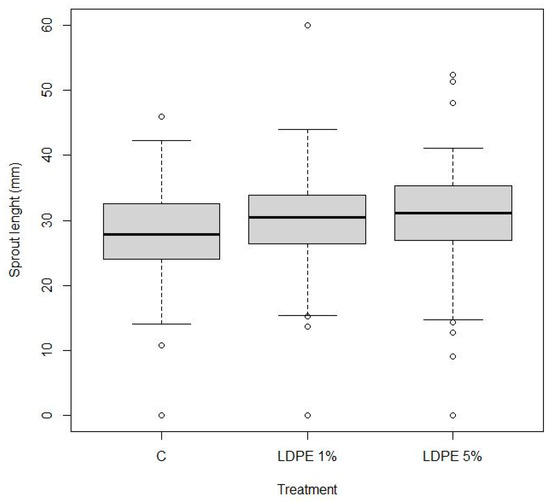
Figure 8.
Boxplot illustration of the distribution of length (in mm) of sprouts from the pot experiment across three treatments: Control (C), LDPE 1%, and LDPE 5%. The central bold line within each box represents the median sprout length, while the box itself spans the interquartile range (25th to 75th percentile). Whiskers indicate the minimum and maximum values within 1.5 times the interquartile range. The dots represent outliers, where for each treatment, some individual measurements were much lower or higher than the majority of the data. A marginally significant difference was observed between LDPE 5% and Control (p = 0.0607), while other comparisons were not significant (p > 0.05).
The overall variability is comparable across treatments, although the LDPE 5% group shows slightly greater dispersion and a higher number of upper outliers, suggesting increased variability in response at this concentration.
ANOVA indicated there is an overall statistically significant difference among group means, but it did not reveal where those differences occur. Therefore, Duncan’s test was applied as a post hoc analysis to perform all possible pairwise comparisons between treatments.
The results presented in Table 2 indicate a marginally significant difference (p = 0.0607) between the LDPE 5% treatment and the Control. Specifically, the sprout lengths from the LDPE 5% treatment were between 0.01 mm and 0.54 mm greater than those from the Control treatment. However, since the p-value is slightly above the conventional significance level of 0.05, this difference should be interpreted with caution.

Table 2.
Duncan’s test for differences in sprout length between treatments in the pot experiment.
In contrast, the comparison between LDPE 1% and the Control shows an estimate of 0.22712 with a p-value of 0.1068, which is not statistically significant. Similarly, the comparison between LDPE 5% and LDPE 1% showed an estimate of 0.03619 with a p-value of 0.9464, indicating no significant difference in sprout length between these two treatments. Overall, the findings suggest that the LDPE 5% treatment may have a slight positive effect on sprout length compared to the Control, but the difference is marginally significant.
3.2.3. Root Length
The boxplot in Figure 9 presents the distribution of root length across the three treatment groups: Control (C), LDPE 1%, and LDPE 5%. The median root lengths appear similar across all treatments, suggesting no notable differences in root growth due to the presence of LDPE. All three treatments display a comparable range and spread of values, and several outliers are observed in each group. Additional differences were analyzed using Duncan’s test.
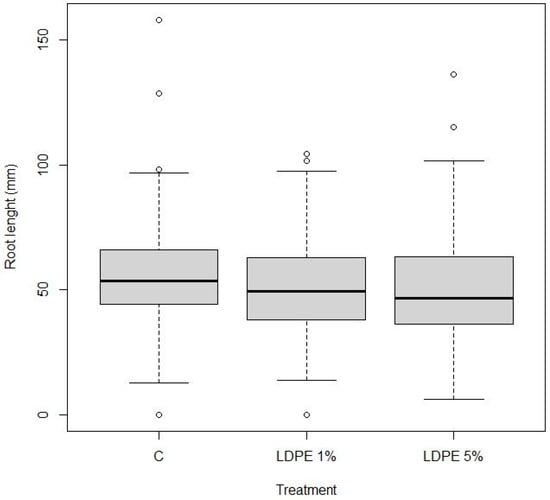
Figure 9.
Boxplot illustration of the distribution of root length (in mm) across three treatments, Control (C), LDPE 1%, and LDPE 5%, with a p-value of 0.295 indicating no significant difference. The central bold line within each box represents the median root length, while the box itself spans the interquartile range (25th to 75th percentile). Whiskers indicate the minimum and maximum values within 1.5 times the interquartile range. The dots represent outliers, where for each treatment, some individual measurements were much lower or higher than the majority of the data.
Duncan’s test was conducted to compare the root length means between different treatment groups. As shown in Table 3, there are no statistically significant differences between the root length means across treatments.

Table 3.
Duncan’s test for differences in root length between treatments in the pot experiment.
In particular, the comparison between LDPE 1% and the Control shows an estimate of −0.3114, with a 95% confidence interval ranging from −10.608 to 0.4380. Similarly, the comparison between LDPE 5% and the Control has an estimate of −0.4473, with a confidence interval from −12.219 to 0.3273. Finally, the comparison between LDPE 5% and LDPE 1% shows an estimate of −0.1359, with a confidence interval from −0.9029 to 0.6311. Since the confidence intervals for all these comparisons span zero, it can be concluded that the root length means are not significantly different between treatments.
4. Discussion
Numerous studies have shown that the effects of MPs on plants are highly variable—ranging from negative to neutral or even positive—depending on factors such as particle size, shape, polymer type, degree of aging, and the presence of additives [24,35,36]. MPs, particularly those shaped as films and fibers, have been shown to negatively impact seed germination rates and overall shoot height [37,38]. However, other studies suggest that certain types of MPs may actually enhance seed germination synchrony. This highlights a complex and species-specific interaction, where both positive and negative effects can occur depending on the characteristics of the MP and the plant species involved [49,55,56].
4.1. Sinapis Alba L. Response to LDPE Microplastic: Petri Dish Experiment
In our study, the Petri dish experiment seed germination of S. alba was evaluated across three treatment groups, Control (0% LDPE) and two concentrations of LDPE MPs (0.01% and 1%). After seven days of exposure, the germination percentage was 92% for Control, 94% for LDPE 0.01% treatment, and 90% for 1% LDPE treatment. The higher GSI values in this experiment were calculated in the Control treatment, followed by 0.01% LDPE MP and 1% LDPE MP treatments. In sprout height, no statistically significant differences of LDPE MP between the two concentration treatments in the Petri dish experiment were found.
Plant responses to microplastic exposure appear to be highly species-specific, with studies reporting a range of physiological effects. For example, wheat (Triticum aestivum) has exhibited reduced germination rates in the presence of MPs, while cress (Lepidium sativum) showed delayed germination, attributed to MP particles obstructing water uptake through essential seed capsule pores [5,23]. The effect of microplastics on the germination process is also time-dependent. A significant decrease in germination was observed in the first 8 h after exposure of L. sativum seeds to larger (4800 nm) particles and higher concentrations (107) of MPs, compared to particles of 50 nm and a concentration of 103. A negative effect of MPs on germination rate disappeared in the following 24 h. A delay in germination in the first 8 h was probably due to physical obstruction. Although MP particles were observed on the surface of cress roots and root hairs, this had no effect on root growth [5], which is in line also with our study where particles were observed on the surface of roots and roots hair. In addition, other influencing factors such as seed quality, room temperature, and errors in the preparation of the experiment may also affect seed germination, GSI, and sprout development. In other research, the type and size of microplastics significantly influence their impact on germination rates. The authors in [57] report that MPs with a particle size of 100 nm significantly reduced seed germination in wheat (T. aestivum L.) compared to larger particles measuring 5 µm. Additionally, the concentration of MPs in soil plays a critical role, with studies indicating a dose-dependent relationship, where higher MP levels were linked with more noticeable negative effects. In a study on lettuce (Lactuca sativa L.), increasing MP concentrations led to reductions in plant weight, height, root length, and leaf number [15]. Effects of 1% concentration of three MP polymers on rice (Oryza sativa L.) growth over a 15-day period revealed inhibited root elongation, reduced shoot height, and decreased rice fresh weight [45].
These outcomes highlight the complexity of plant–MP interactions and suggest that the impact of MPs is not uniform across species. Consequently, there is a critical need for broader, species-diverse investigations to comprehensively assess the ecological implications of MP contamination [5,40].
4.2. Sinapis Alba L. Response to LDPE Microplastic: Pot Experiment
In the pot experiment, seed germination of S. alba was evaluated across three treatment groups: Control (0% LDPE) and two concentrations of LDPE MP (1% and 5%). After ten days, germination percentage was highest in the 1% LDPE treatment, followed by the Control and 5% LDPE. The GSI, reflecting daily germination dynamics over 10 days, showed slightly higher values in the control than in 1% and 5% LDPE treatments, indicating a minor delay in germination speed with LDPE presence. In both experiments, treatments with different MP concentrations resulted in a similar total germination percentage and GSI, indicating that the MP particles themselves probably have little or no effect on germination. A study on germination of L. multiflorum exposed to MP particles and leachates showed similar results for germination [58]. The same statistical results were shown for two tomato varieties whose seeds were exposed to PP-MP, although the germination rate of both varieties was slightly higher compared to the Control, indicating a possible influence of PP-MP on alpha-amylase activity, which leads to the degradation of starch readily available for germination [40].
The slightly greater sprout length observed in both LDPE treatments, particularly the high variability in the 5% LDPE treatment, suggests that microplastics may influence early plant development in a non-uniform manner. While ANOVA indicated an overall significant difference among treatments, the marginal effect revealed by Duncan’s test and the lack of clear significance between 1% LDPE and the Control or between 1% and 5% LDPE imply that the observed trends should be interpreted with caution. These results may reflect species-specific resilience of S. alba to LDPE particles at the tested concentrations, or they may be influenced by indirect factors such as altered substrate aeration or water retention caused by the presence of MPs [14,33]. The non-significant result of the effect of MPs on shoot growth [40] was probably attributed to the insufficient exposure time of the plants to MPs, which could also be the reason for the non-significance of the differences in our study. In their work, Ref. [5] demonstrated that Lepidium sativum exposed to high concentrations of polyethylene MPs exhibited reduced shoot length within the first 24–48 h of exposure. However, these effects were often transient and diminished after the initial period [5]. Over longer exposure periods (>7 days), plant responses to MPs can vary further. In some cases, plants recover, and sprout length differences become negligible, whereas in others cumulative negative effects emerge [14,40]. On the other hand, the literature reports [59] that the positive effect of MPs on plant biomass may be attributed to improved soil aeration, which can enhance root penetration and nutrient uptake, as well as the potential role of microplastics as a carbon source that stimulates soil microbial activity.
The relationship between MPs and plant development is further complicated by their interactions with soil properties. MPs can significantly alter physical and chemical properties of soil, thereby affecting factors such as water retention and nutrient availability—both of which are critical for seedling establishment and growth [11,60]. For instance, MPs can modify soil structure, resulting in changes to pore connectivity and shifts in microbial activity, which can either alleviate or intensify stress conditions experienced by plants [9,61].
Additionally, further literature [59] suggests that future research should encompass a wider range of plastic morphologies (e.g., fibers, foams, fragments) and polymer types (e.g., polystyrene, polyvinyl chloride) based on the vital impact of plastic particles on plant–soil systems. Investigating these variables across a spectrum of concentrations would better represent the gradient of MP pollution found in real environmental conditions. Additionally, examining the combined effects of multiple microplastic types could reveal potential synergistic interactions, as such mixtures frequently coexist in natural environments [59].
5. Conclusions
This study investigates the impact of varying concentrations of LDPE MPs on seed germination and early seedling development of Sinapis alba. Two independent laboratory experiments were conducted under controlled environmental conditions to assess the effects on germination rates and initial sprout growth.
The results of this study partially support the proposed hypotheses. While no statistically significant effects of LDPE MP were observed on seed germination rates and GSI in Sinapis alba L. under laboratory conditions, some impact on early seedling growth was detected. Specifically, sprout length showed a marginally significant increase at the highest LDPE concentration (5%), suggesting that exposure to LDPE MPs does not consistently inhibit early growth and may even have a slight stimulatory effect under certain conditions.
This study is limited to LDPE MP, a single polymer type; therefore, the findings may not fully represent the effects of other common MPs, such as tire wear particles or biodegradable MPs, which may exhibit different impacts. Further research should explore a broader range of MP types and assess long-term and multigenerational effects under more environmentally realistic conditions. Relevant recent studies not only on plants but also on organisms [49,62,63,64] highlight the importance of addressing these gaps and promoting further research. In conclusion, MPs pose complex and multifactorial challenges to seed germination and early seedling development. Their detrimental effects may arise from the disruption of key enzymatic activities, alterations in soil physical and chemical properties, and species-specific physiological responses. Given the variability and context-dependence of these impacts, further research is essential to elucidate the underlying mechanisms and to inform effective strategies for mitigating the ecological consequences of MP contamination in terrestrial ecosystems.
Author Contributions
Conceptualization, Š.Ž. and N.K.M.; Data curation, Š.Ž.; Formal analysis, N.K.M.; Funding acquisition, M.P.; Investigation, Š.Ž. and N.K.M.; Methodology, Š.Ž. and N.K.M.; Resources, Š.Ž. and M.P.; Software, Š.Ž.; Supervision, M.P.; Validation, Š.Ž. and N.K.M.; Visualization, Š.Ž.; Writing—original draft, Š.Ž. and N.K.M.; Writing—review and editing, Š.Ž., N.K.M. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Union’s Horizon 2020 Programme for research and innovation: project MINAGRIS (grant agreement number 101000407).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the MINAGRIS project consortium for supplying the essential materials required for this study and Dara Bavdek, Student, BSC Biotechnology. Her help in the research contributed to the formation of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MP | Microplastic |
| LDPE | Low density polyethylene |
| CI | Confidence interval |
| ANOVA | Analysis of variance |
| GSI | Germination speed index |
References
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic Sources, Formation, Toxicity and Remediation: A Review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Additives of Plastics: Entry into the Environment and Potential Risks to Human and Ecological Health. J. Environ. Manag. 2023, 348, 119364. [Google Scholar] [CrossRef]
- Jia, L.; Liu, L.; Zhang, Y.; Fu, W.; Liu, X.; Wang, Q.; Tanveer, M.; Huang, L. Microplastic Stress in Plants: Effects on Plant Growth and Their Remediations. Front. Plant Sci. 2023, 14, 1226484. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and Occurrence of Micro(Nano)Plastics in Soils: Knowledge Gaps and Possible Risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Bansal, O.P.; Singh, A. A Review on Microplastic in the Soils and Their Impact on Soil Microbes, Crops and Humans. Int. J. Res.-Granthaalayah 2022, 10, 245–273. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Sintim, H.Y.; De Bruyn, J.M. Effects of Biodegradable Plastic Film Mulching on Soil Microbial Communities in Two Agroecosystems. PeerJ 2020, 8, e9015. [Google Scholar] [CrossRef]
- Špela, Ž.; Matic, N.; Vesna, Z.; Esperanza, H.L.; Damjana, D.; Marina, P. Impact of Conventional and Biobased Microplastics from Mulch Films on Soil Bulk Density, Hydraulic Conductivity and Water Retention in Two Different Soil Types under Wetting–drying Cycles. Results Eng. 2025, 25, 104455. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Hanif, M.N.; Aijaz, N.; Azam, K.; Akhtar, M.; Laftah, W.A.; Babur, M.; Abbood, N.K.; Benitez, I.B. Impact of Microplastics on Soil (Physical and Chemical) Properties, Soil Biological Properties/Soil Biota, and Response of Plants to It: A Review. Int. J. Environ. Sci. Technol. 2024, 21, 10277–10318. [Google Scholar] [CrossRef]
- Harrison, E.G.; Reiling, K.; Halfpenny, R.K.; Gwinnett, C. The Effects of Polyester Microfibres on the Development and Seed Yield of White Mustard (Sinapis alba L.). Front. Environ. Sci. 2024, 12, 1310310. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of Polystyrene Nanoplastics (PSNPs) on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Yu, J.; Adingo, S.; Liu, X.; Li, X.; Sun, J.; Zhang, X. Micro Plastics in Soil Ecosystem—A Review of Sources, Fate, and Ecological Impact. Plant Soil Environ. 2022, 68, 1–17. [Google Scholar] [CrossRef]
- Terekhova, V.A. Biotesting of Soil Ecotoxicity in Case of Chemical Contamination: Modern Approaches to Integration for Environmental Assessment (a Review). Eurasian Soil Sci. 2022, 55, 601–612. [Google Scholar] [CrossRef]
- Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. Available online: https://www.oecd.org/en/publications/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en.html (accessed on 21 March 2025).
- ISO 11269-2:2012(En); Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Contaminated Soil on the Emergence and Early Growth of Higher Plants. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11269:-2:ed-3:v1:en (accessed on 21 March 2025).
- Nikolaeva, O.V.; Terekhova, V.A. Improvement of Laboratory Phytotest for the Ecological Evaluation of Soils. Eurasian Soil Sci. 2017, 50, 1105–1114. [Google Scholar] [CrossRef]
- WHO. Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. Available online: https://www.who.int/publications/i/item/9789241594448 (accessed on 21 March 2025).
- Vasile, G.-G.; Tenea, A.-G.; Dinu, C.; Iordache, A.M.M.; Gheorghe, S.; Mureseanu, M.; Pascu, L.F. Bioavailability, Accumulation and Distribution of Toxic Metals (As, Cd, Ni and Pb) and Their Impact on Sinapis Alba Plant Nutrient Metabolism. Int. J. Environ. Res. Public Health 2021, 18, 12947. [Google Scholar] [CrossRef]
- Holubík, O.; Vaněk, A.; Mihaljevič, M.; Vejvodová, K. Higher Tl Bioaccessibility in White Mustard (Hyper-Accumulator) Grown under the Soil than Hydroponic Conditions: A Key Factor for the Phytoextraction Use. J. Environ. Manag. 2020, 255, 109880. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Asztemborska, M.; Golimowski, J.; Strusińska, K. Determination of Thallium Forms in Plant Extracts by Anion Exchange Chromatography with Inductively Coupled Plasma Mass Spectrometry Detection (IC-ICP-MS). J. Anal. At. Spectrom. 2008, 23, 1632–1635. [Google Scholar] [CrossRef]
- Fargašová, A. Plants as Models for Chromium and Nickel Risk Assessment. Ecotoxicology 2012, 21, 1476–1483. [Google Scholar] [CrossRef]
- Molnárová, M.; Fargašová, A. Relationship between Various Physiological and Biochemical Parameters Activated by Cadmium in Sinapis alba L. and Hordeum vulgare L. Ecol. Eng. 2012, 49, 65–72. [Google Scholar] [CrossRef]
- Fargašová, A. Phytotoxic Effects of Cd, Zn, Pb, Cu and Fe on Sinapis alba L. Seedlings and Their Accumulation in Roots and Shoots. Biol. Plant. 2001, 44, 471–473. [Google Scholar] [CrossRef]
- Šourková, M.; Adamcová, D.; Winkler, J.; Vaverková, M.D. Phytotoxicity of Tires Evaluated in Simulated Conditions. Environments 2021, 8, 49. [Google Scholar] [CrossRef]
- Timmerer, U.; Lehmann, L.; Schnug, E.; Bloem, E. Toxic Effects of Single Antibiotics and Antibiotics in Combination on Germination and Growth of Sinapis alba L. Plants 2020, 9, 107. Plants 2020, 9, 107. [Google Scholar] [CrossRef]
- Palm, E.R.; Nissim, W.G.; Adamcová, D.; Podlasek, A.; Jakimiuk, A.; Vaverková, M.D. Sinapis alba L. and Triticum aestivum L. as Biotest Model Species for Evaluating Municipal Solid Waste Leachate Toxicity. J. Environ. Manag. 2022, 302, 114012. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of Common Bean (Phaseolus vulgaris L.) Growth to Soil Contaminated with Microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of Microplastics and Cadmium on Plant Growth and Arbuscular Mycorrhizal Fungal Communities in an Agricultural Soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of Plastic Mulch Film Residues on Wheat Rhizosphere and Soil Properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Redondo-Hasselerharm, P.E.; Diepens, N.J.; Koelmans, A.A. Effects of Nanoplastics and Microplastics on the Growth of Sediment-Rooted Macrophytes. Sci. Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of Polystyrene, Polyethylene and Polypropylene Microplastics on Tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological Responses of Garden Cress (L. sativum) to Different Types of Microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of Microplastics on Growth, Photosynthesis and Essential Elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Onandia, G.; Maass, S.; Zhao, T.; Rillig, M.C. Effects of Microplastics and Drought on Soil Ecosystem Functions and Multifunctionality. J. Appl. Ecol. 2021, 58, 988–996. [Google Scholar] [CrossRef]
- Shorobi, F.M.; Vyavahare, G.D.; Seok, Y.J.; Park, J.H. Effect of Polypropylene Microplastics on Seed Germination and Nutrient Uptake of Tomato and Cherry Tomato Plants. Chemosphere 2023, 329, 138679. [Google Scholar] [CrossRef]
- Serrano Ruiz, H.; Eras, J.; Martín-Closas, L.; Pelacho, A.M. Compounds Released from Unused Biodegradable Mulch Materials after Contact with Water. Polym. Degrad. Stab. 2020, 178, 109202. [Google Scholar] [CrossRef]
- Liu, S.; Jin, R.; Li, T.; Yang, S.; Shen, M. Are Biodegradable Plastic Mulch Films an Effective Way to Solve Residual Mulch Film Pollution in Farmland? Plant Soil 2024, 494, 85–94. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Zhang, H.; Semple, K.T. Environmental Fate of Microplastics and Common Polymer Additives in Non-Biodegradable Plastic Mulch Applied Agricultural Soils. Environ. Pollut. 2024, 363, 125249. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–159. ISBN 978-3-030-56271-7. [Google Scholar]
- Iswahyudi, I.; Widodo, W.; Warkoyo, W.; Sutanto, A.; Garfansa, M.P.; Mujiyanti, W.A.; Sholeh, M.S. Investigating the Impact of Microplastics Type of Polyethylene, Polypropylene, and Polystyrene on Seed Germination and Early Growth of Rice Plants. Environ. Qual. Manag. 2024, 34, e22287. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E.; Kempf, M.; Kempf, P.; Tabor, S. Impact of Various Microplastics on the Morphological Characteristics and Nutrition of the Young Generation of Beech (Fagus sylvatica L.). Sci. Rep. 2024, 14, 19284. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ingraffia, R.; Schloter, M.; Brüggemann, N.; Rillig, M.C. Effects of Multiple Microplastic Types on Growth of Winter Wheat and Soil Properties Vary in Different Agricultural Soils. Plants People Planet 2025, 7, 194–203. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Caesaria, P.U.; Rillig, M.C. Microplastics of Different Shapes Increase Seed Germination Synchrony While Only Films and Fibers Affect Seed Germination Velocity. Front. Environ. Sci. 2022, 10, 1017349. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Landt, L.; Rillig, M.C. Plastic Particles and Their Additives Promote Plant Invasion through Physicochemical Mechanisms on Seed Germination. J. Ecol. 2025, 113, 275–288. [Google Scholar] [CrossRef]
- MINAGRIS—Launching the MINAGRIS Multi-Scale Experiment Protocol. Available online: https://minagris.eu/launching-the-minagris-multi-scale-experiment-protocol/ (accessed on 19 November 2024).
- Li, J.; Song, Y.; Cai, Y. Focus Topics on Microplastics in Soil: Analytical Methods, Occurrence, Transport, and Ecological Risks. Environ. Pollut. 2020, 257, 113570. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Sulek, A.; Mader, H.; Heo, J.; Noh, J.H.; Penttinen, O.-P.; Kim, Y.; Kim, S.; Esterhuizen, M. The Influence of New and Artificial Aged Microplastic and Leachates on the Germination of Lepidium sativum L. Plants 2020, 9, 339. Plants 2020, 9, 339. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.net/ij/ (accessed on 24 March 2025).
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 21 February 2022).
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of Plastic Pollution in Soil Properties and Growth of Grass Species in Semi-Arid Regions: A Laboratory Experiment. Environ. Sci. Pollut. Res. 2022, 29, 59118–59126. [Google Scholar] [CrossRef]
- Jadhav, B.; Medyńska-Juraszek, A. Microplastic and Nanoplastic in Crops: Possible Adverse Effects to Crop Production and Contaminant Transfer in the Food Chain. Plants 2024, 13, 2526. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Vikfors, S.; Penttinen, O.-P.; Kim, Y.J.; Pflugmacher, S. Lolium Multiflorum Germination and Growth Affected by Virgin, Naturally, and Artificially Aged High-Density Polyethylene Microplastic and Leachates. Front. Environ. Sci. 2022, 10, 964230. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Perlenfein, C.; Bernal, M.G.; Rillig, M.C. Disentangling Mechanisms by Which Microplastic Films Affect Plant-Soil Systems: Physical Effects of Particles Can Override Toxic Effects of Additives. Environ. Sci. Eur. 2024, 36, 198. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, L.; Li, J. Effect of Microplastics on the Allelopathic Effects of Native and Invasive Plants on Co-Occurring Invaders. Front. Plant Sci. 2024, 15, 1425815. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, G.; Ye, X.; Zhang, X.; Jiang, Y.; Han, Y.; Lu, L.; Liu, Z.; Zhang, H. Multigenerational Toxic Effects in Daphnia Pulex Are Induced by Environmental Concentrations of Tire Wear Particle Leachate. J. Hazard. Mater. 2025, 486, 136977. [Google Scholar] [CrossRef]
- Dolar, A.; Mušič, B.; Skalar, T.; Marolt, G.; Drobne, D.; Škapin, A.S.; Jemec Kokalj, A. Microplastics from Cigarette Filters: Comparative Effects on Selected Terrestrial and Aquatic Invertebrates. Environ. Pollut. 2025, 374, 126199. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, Y.; Zhang, J.; Wen, T.; Wang, H.; Qu, C.; Tan, W.; Xi, B.; Hui, K.; Tang, J. Specific Response of Soil Properties to Microplastics Pollution: A Review. Environ. Res. 2023, 232, 116427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).