Battery-Free Innovation: An RF-Powered Implantable Microdevice for Intravesical Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
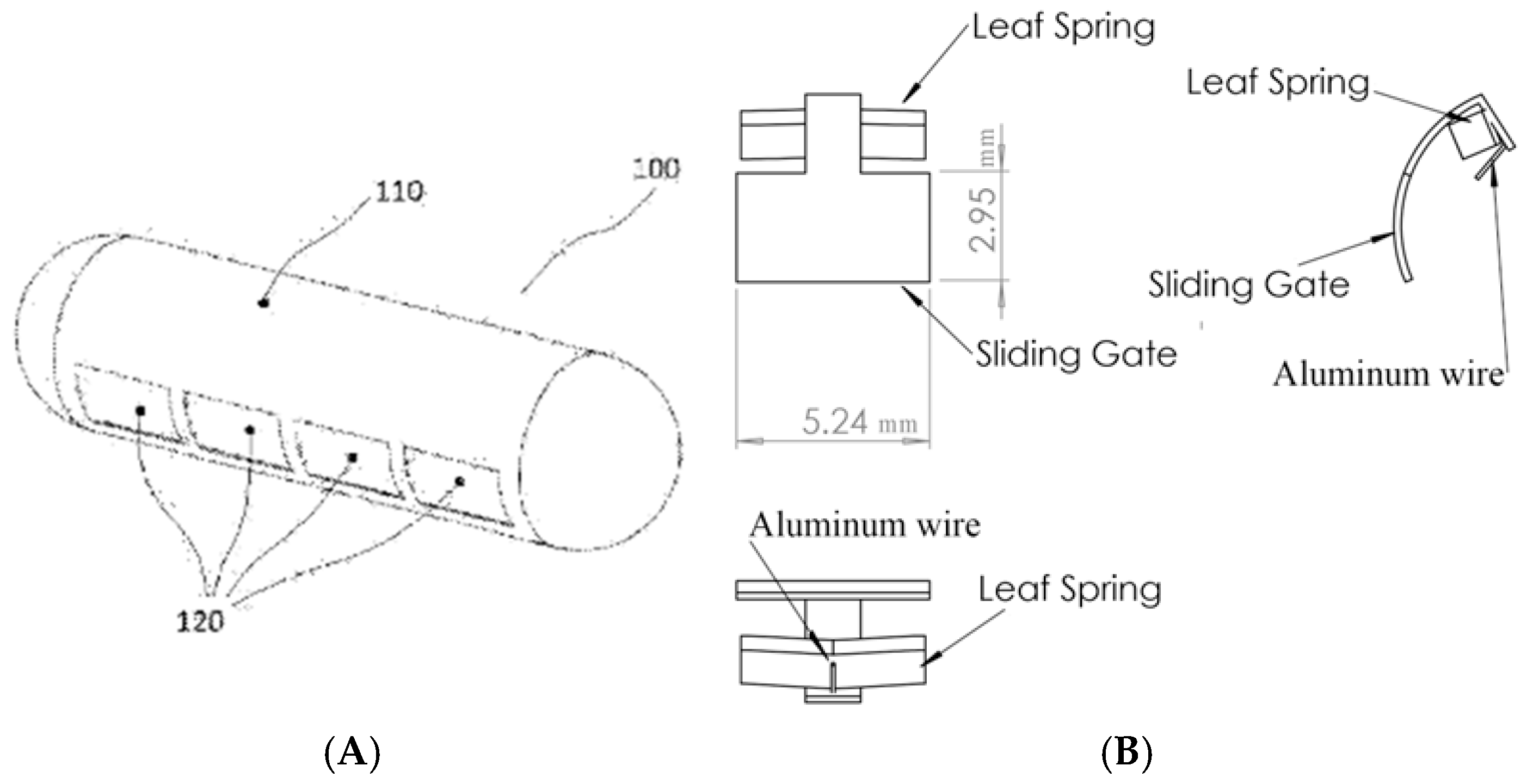
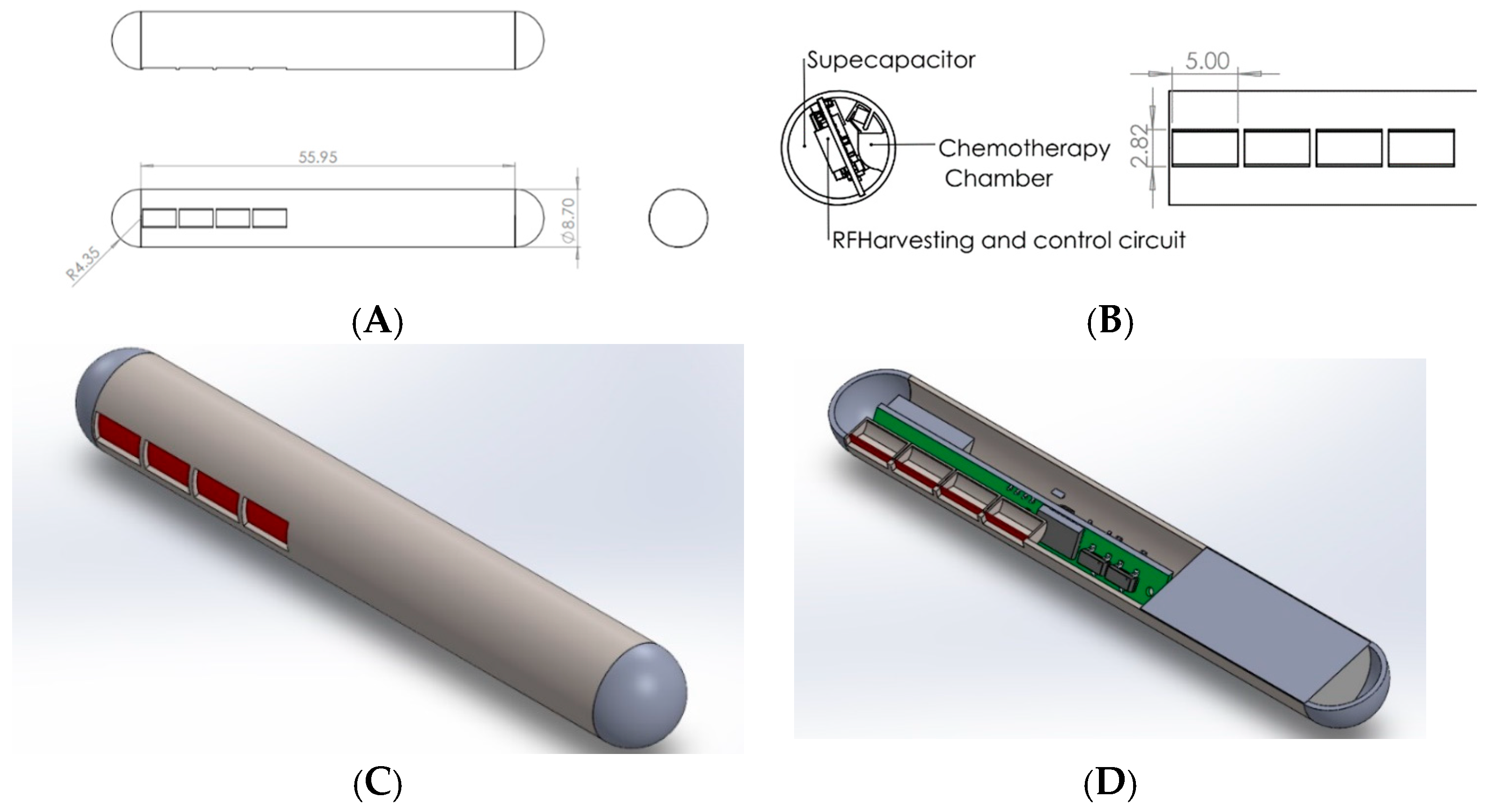
2.1. Mechanical Design
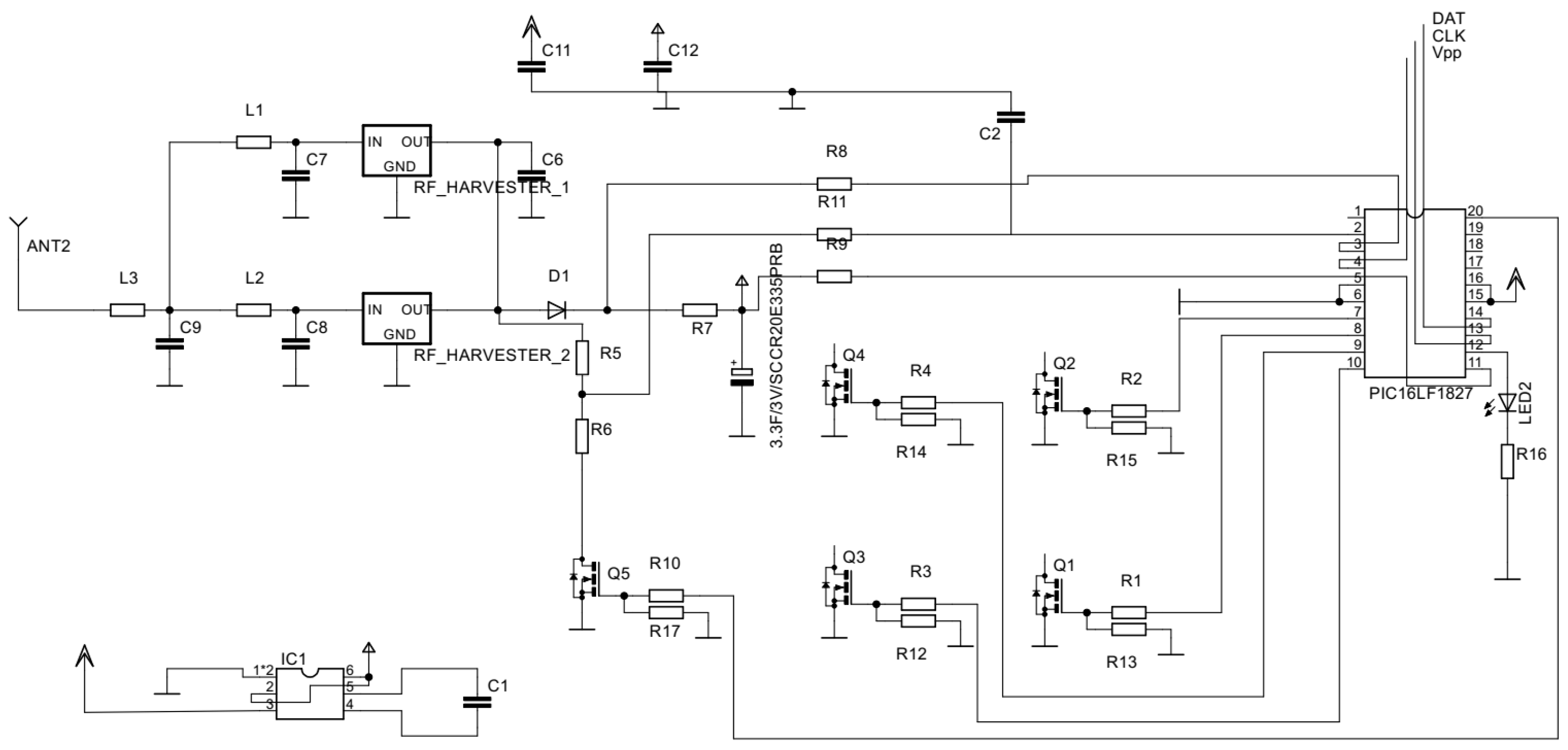
2.2. Electrical Design
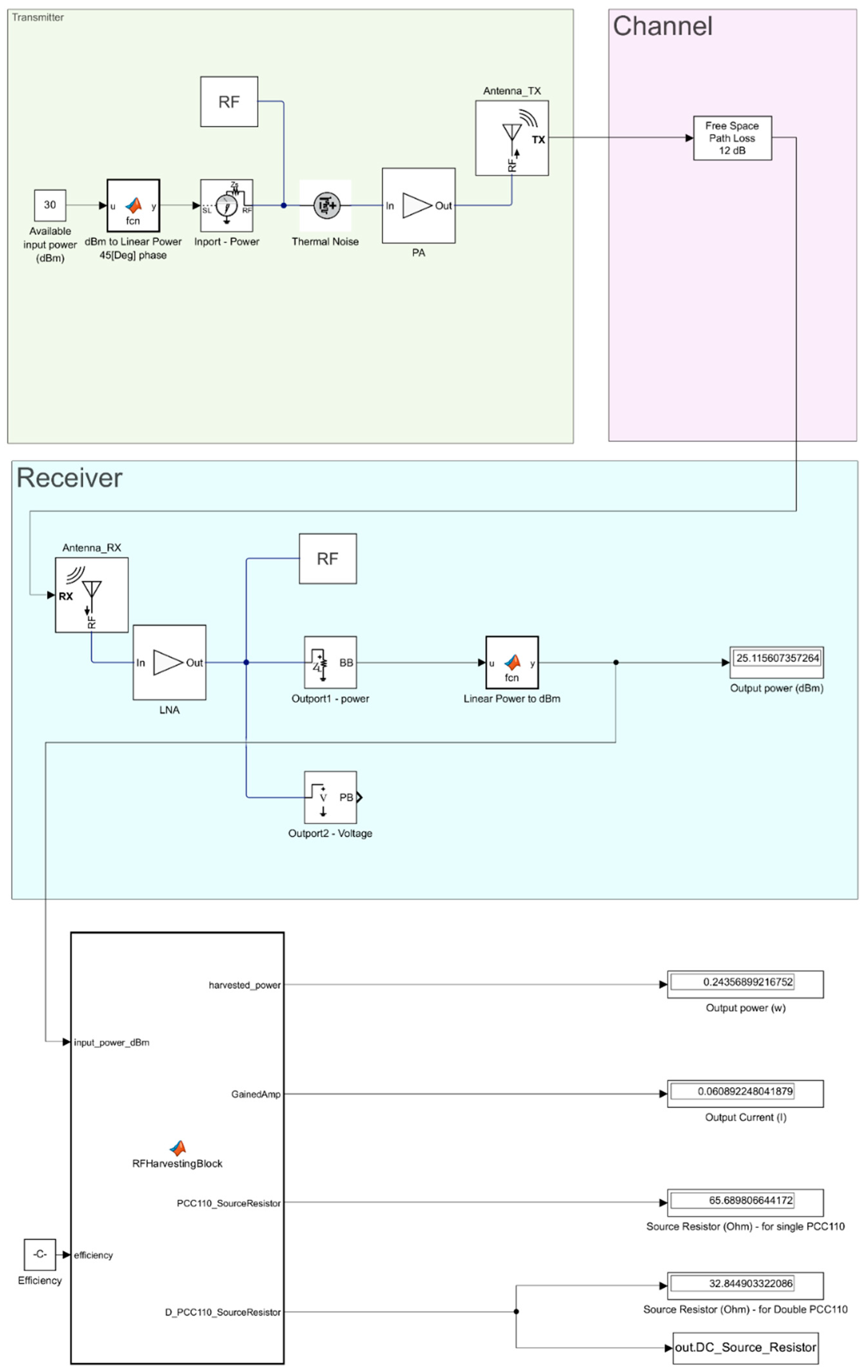
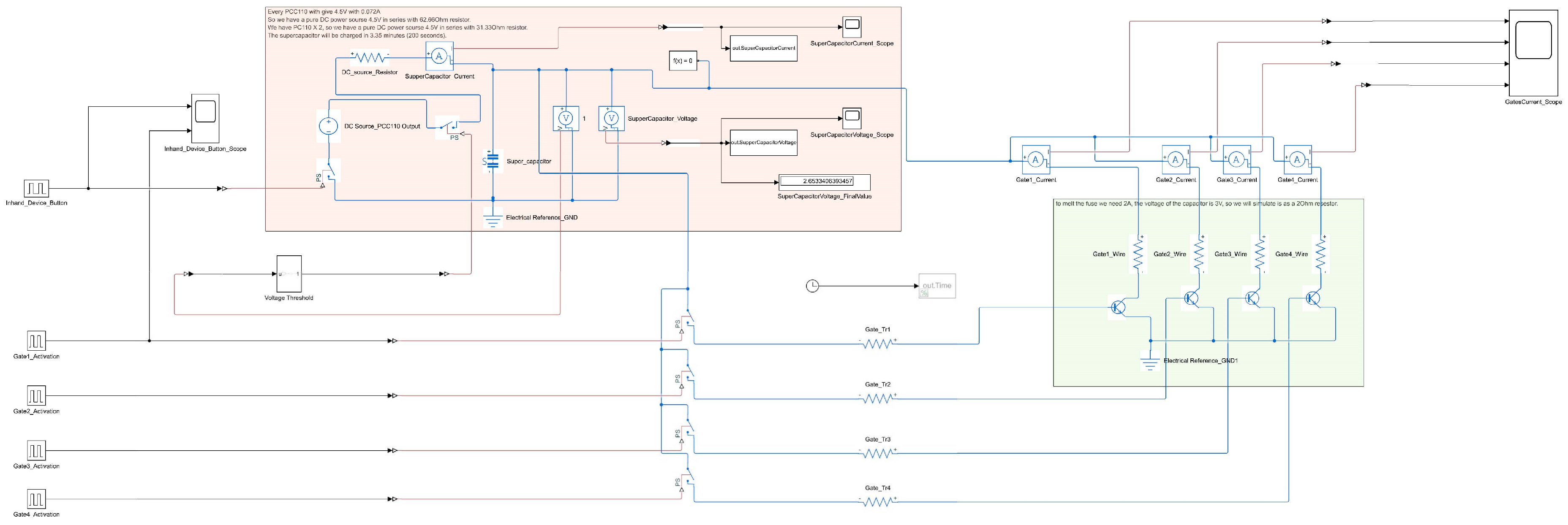
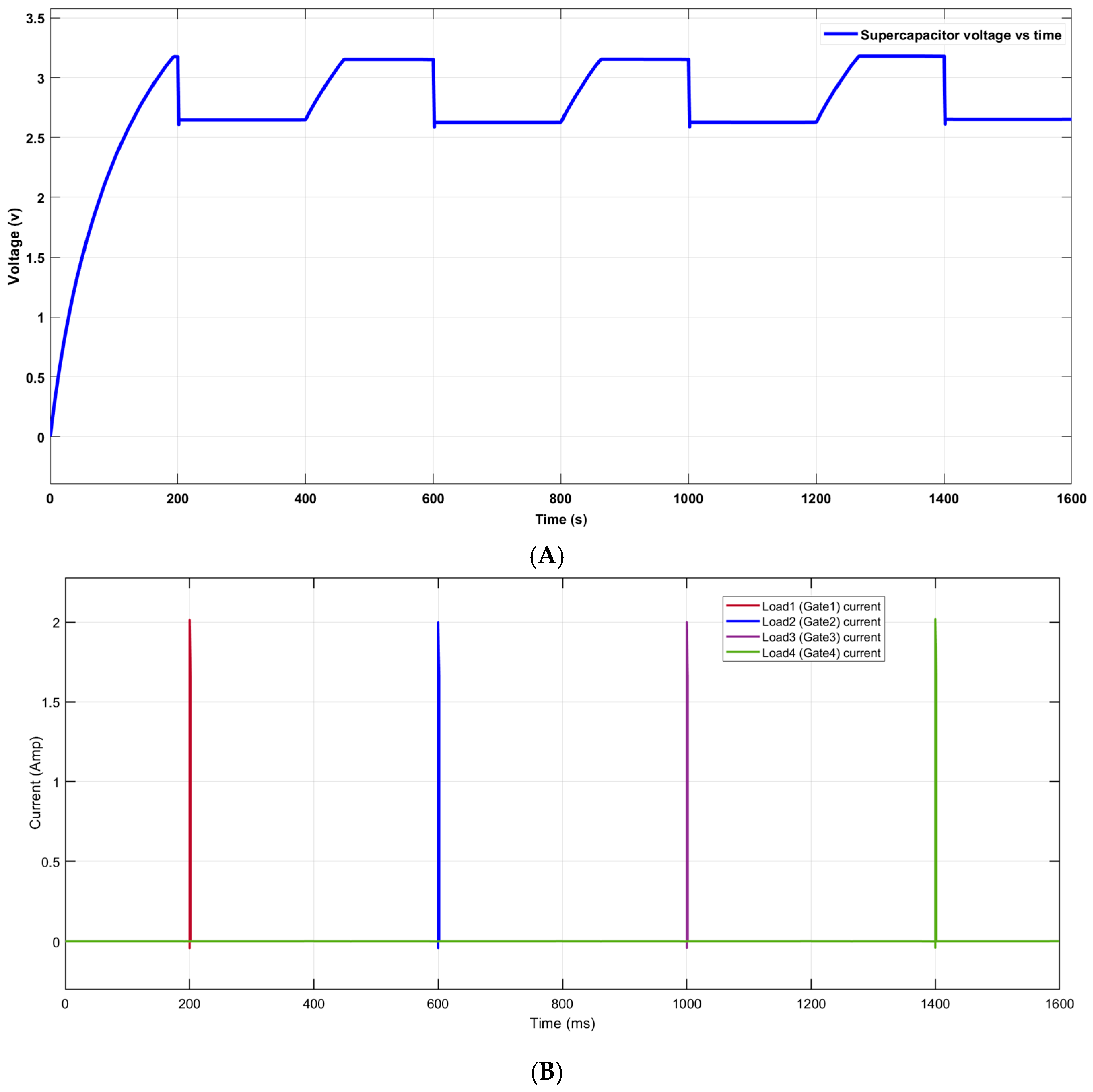
2.3. System Simulation
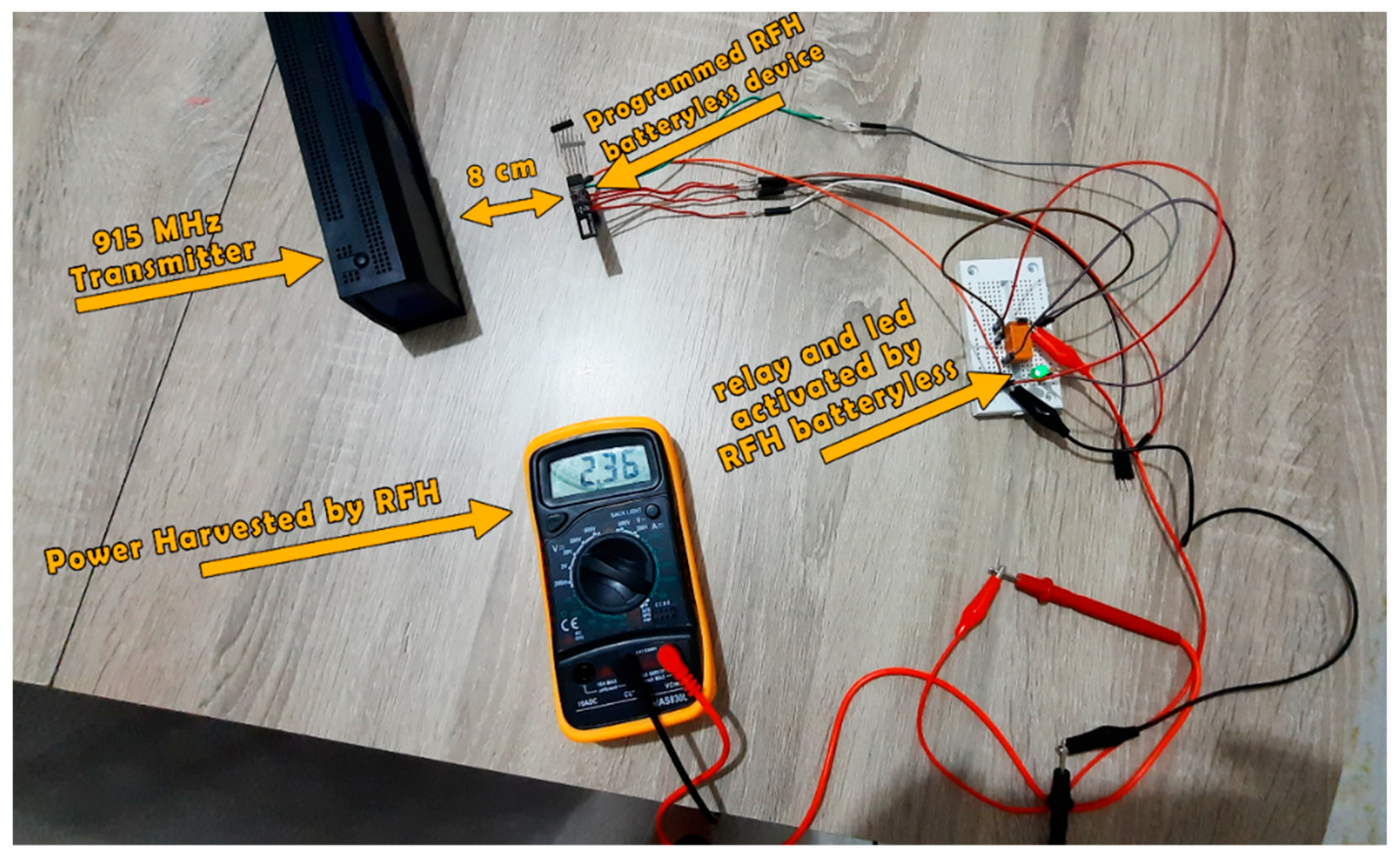
2.4. Practical Realization
3. Results
3.1. Mechanical and Electrical Design Results
3.2. System Simulation Results
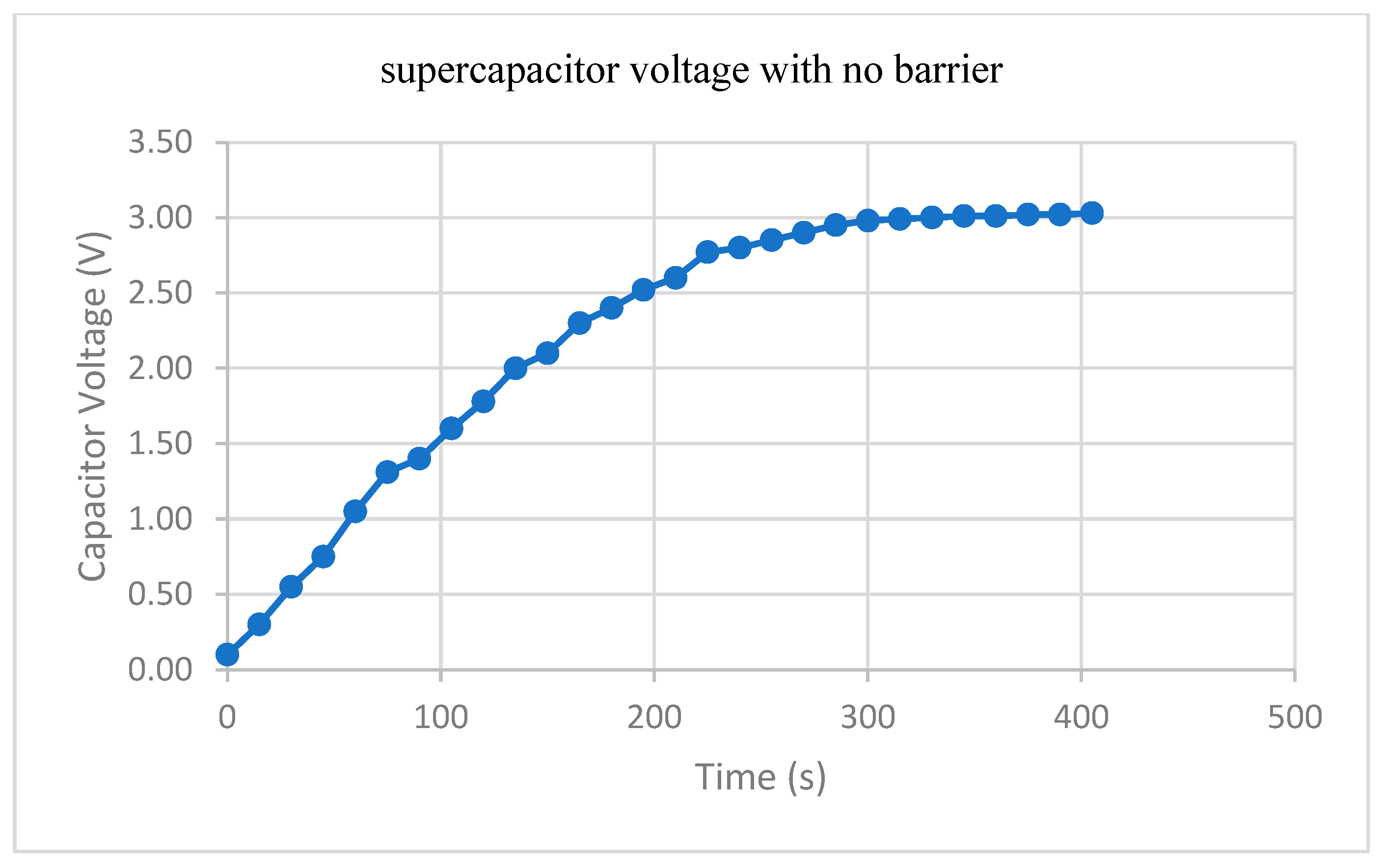
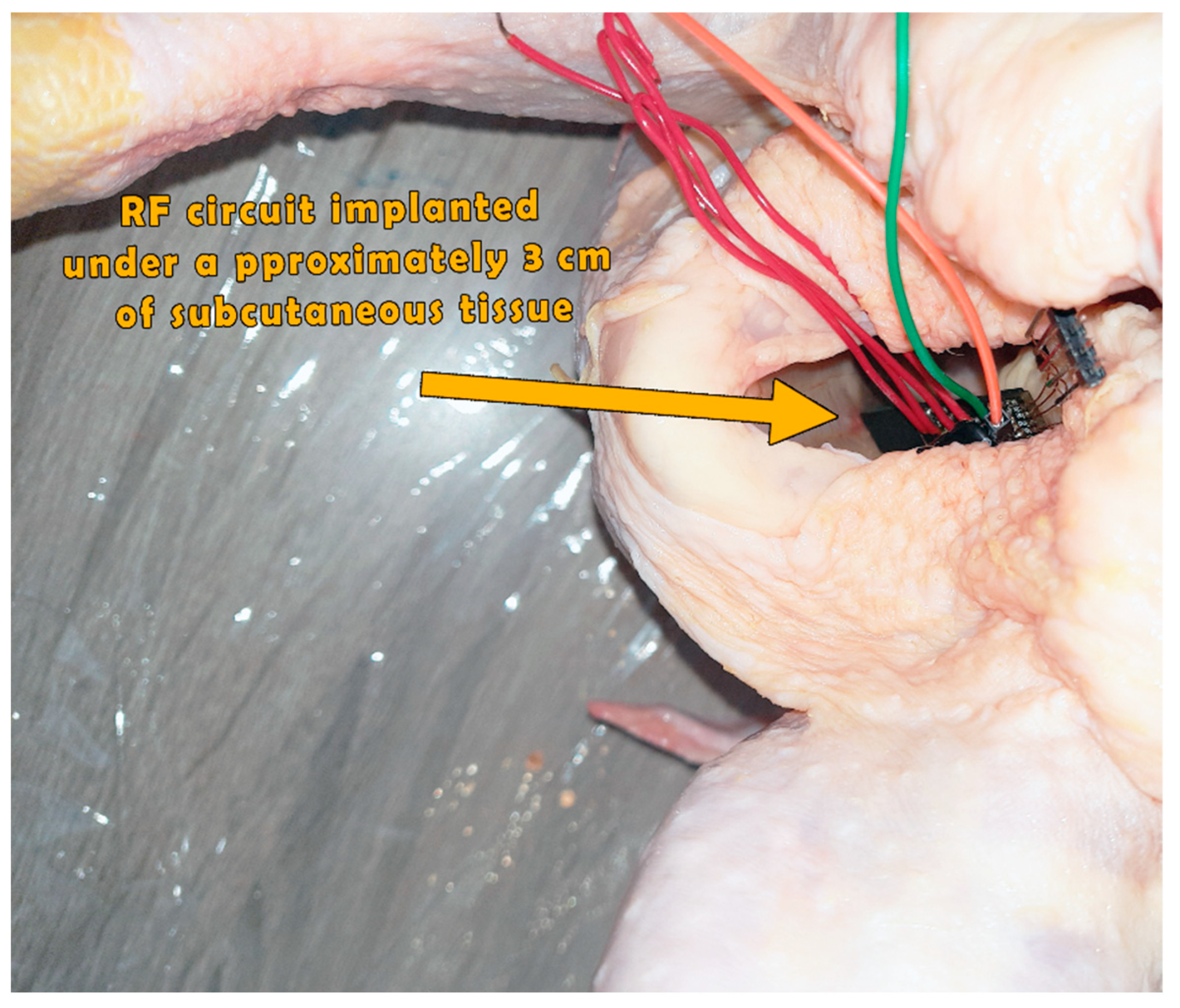
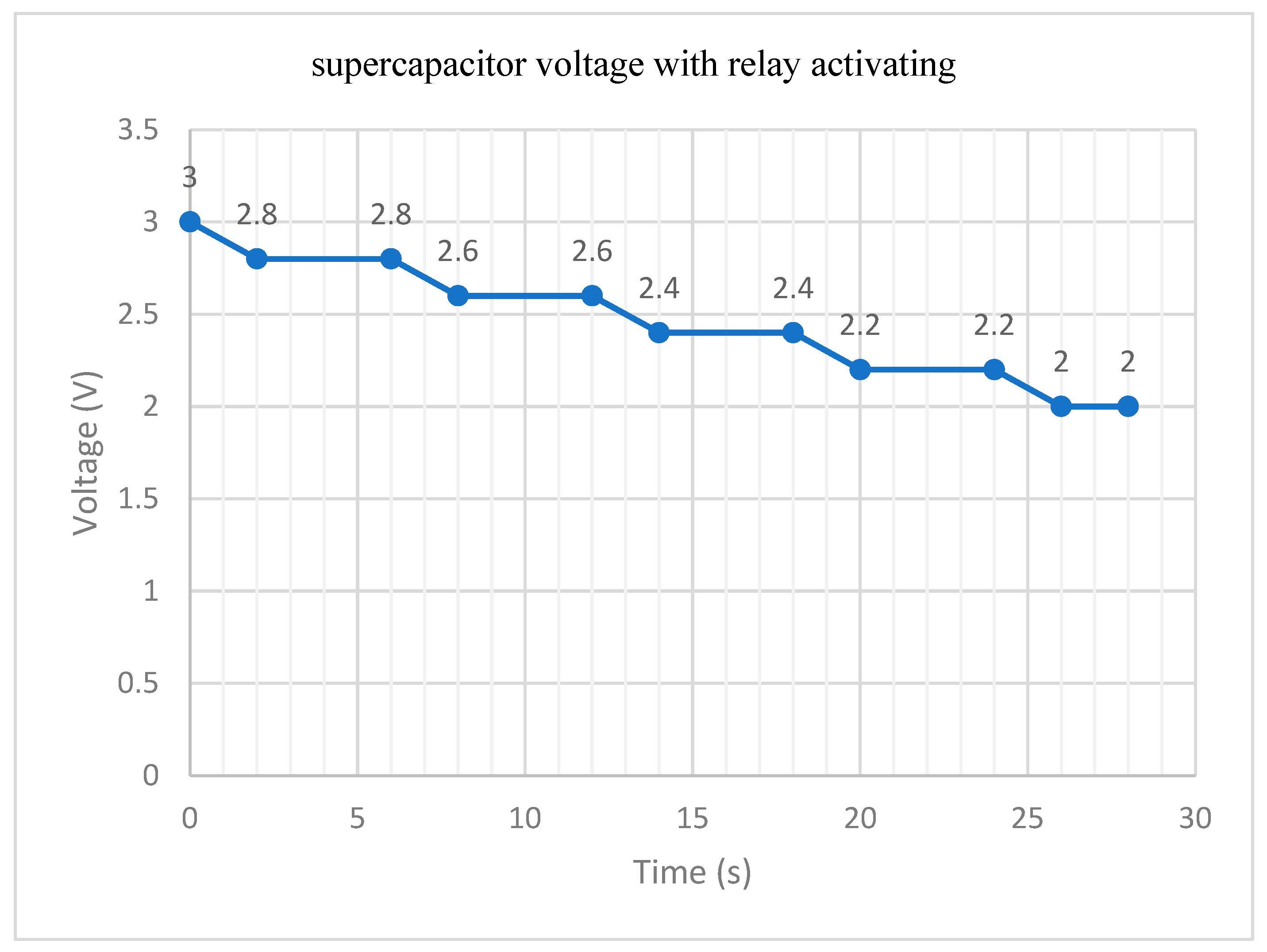
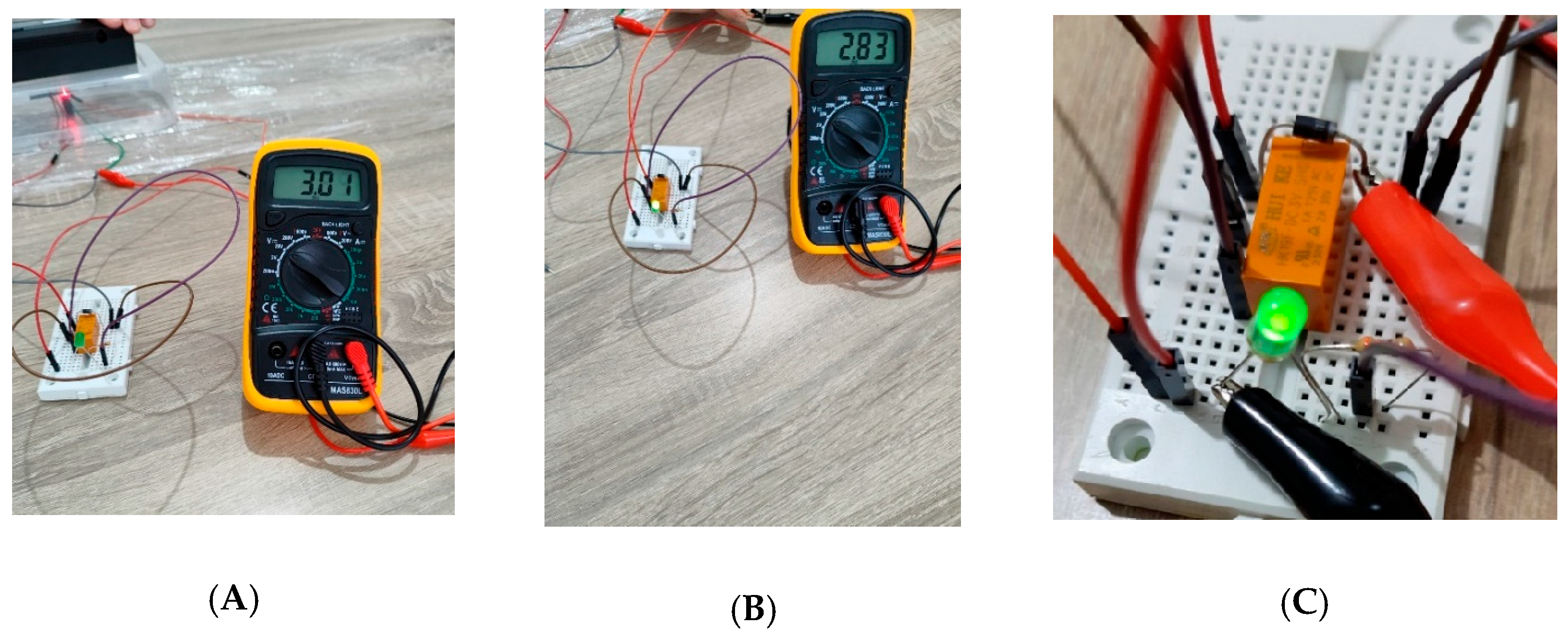
3.3. Practical Realization Results
4. Discussion
Intravesical Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Youssef, S.F.; Buanz, A.B. Intravesical Combination Therapies for Non-Muscle Invasive Bladder Cancer: Recent Advances and Future Directions. Eur. J. Pharmacol. 2022, 926, 175024. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and Progression of Disease in Non–Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Qu, F.; Darji, S.; Thompson, D.H. Recent Advances in Drug Delivery Strategies for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: A Brief Review from 2018 to 2024. Pharmaceutics 2024, 16, 1154. [Google Scholar] [CrossRef]
- Herr, H.W.; Donat, S.M. Quality Control in Transurethral Resection of Bladder Tumours. BJU Int. 2008, 102, 1242–1246. [Google Scholar] [CrossRef]
- Mostafid, H.; Kamat, A.M.; Daneshmand, S.; Palou, J.; Taylor, J.A.; McKiernan, J.; Catto, J.; Babjuk, M.; Soloway, M. Best Practices to Optimise Quality and Outcomes of Transurethral Resection of Bladder Tumours. Eur. Urol. Oncol. 2021, 4, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, H.; Zhang, X.; Chen, G. Transurethral Resection of Bladder Tumor: Novel Techniques in a New Era. Bladder 1970, 10, e21200009. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Zeng, X.-T.; Liu, T.-Z.; Bai, Z.-M.; Dou, Z.-L.; Ding, D.-G.; Fan, Z.-L.; Han, P.; Huang, Y.-R.; Huang, X.; et al. Treatment and Surveillance for Non-Muscle-Invasive Bladder Cancer: A Clinical Practice Guideline (2021 Edition). Mil. Med. Res. 2022, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Laukhtina, E.; Abufaraj, M.; Al-Ani, A.; Ali, M.R.; Mori, K.; Moschini, M.; Quhal, F.; Sari Motlagh, R.; Pradere, B.; Schuettfort, V.M.; et al. Intravesical Therapy in Patients with Intermediate-Risk Non–Muscle-Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis of Disease Recurrence. Eur. Urol. Focus 2022, 8, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Lin, T. Emerging Strategies for the Improvement of Chemotherapy in Bladder Cancer: Current Knowledge and Future Perspectives. J. Adv. Res. 2022, 39, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.H.C.; Patel, M.I. Transurethral Resection of Bladder Tumour (TURBT). Transl. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef]
- Brummelhuis, I.S.G.; Wimper, Y.; Witjes-van Os, H.G.J.M.; Arends, T.J.H.; Van Der Heijden, A.G.; Witjes, J.A. Long-Term Experience with Radiofrequency-Induced Hyperthermia Combined with Intravesical Chemotherapy for Non-Muscle Invasive Bladder Cancer. Cancers 2021, 13, 377. [Google Scholar] [CrossRef]
- Abyzova, E.; Dogadina, E.; Rodriguez, R.D.; Petrov, I.; Kolesnikova, Y.; Zhou, M.; Liu, C.; Sheremet, E. Beyond Tissue Replacement: The Emerging Role of Smart Implants in Healthcare. Mater. Today Bio 2023, 22, 100784. [Google Scholar] [CrossRef]
- Holmes-Martin, K.; Zhu, M.; Xiao, S.; Arab Hassani, F. Advances in Assistive Electronic Device Solutions for Urology. Micromachines 2022, 13, 551. [Google Scholar] [CrossRef]
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless Technologies for Implantable Devices. Sensors 2020, 20, 4604. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Q.; Lee, C. Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials 2022, 12, 1366. [Google Scholar] [CrossRef]
- Brown, W. Experiments Involving a Microwave Beam to Power and Position a Helicopter. IEEE Trans. Aerosp. Electron. Syst. 1969, AES-5, 692–702. [Google Scholar] [CrossRef]
- CSEI: International Conference on Computer Science, Electronics and Industrial Engineering (CSEI): Advances and Applications in Computer Science, Electronics and Industrial Engineering. Proceedings of the Conference on Computer Science, Electronics and Industrial Engineering (CSEI 2022); Garcia, M.V., Gordón-Gallegos, C., Eds.; Lecture Notes in Networks and Systems; Springer Nature: Cham, Switzerland, 2023; Volume 678, ISBN 978-3-031-30591-7. [Google Scholar]
- Zhang, D.; Li, Q.; Fang, Y.; Bai, P.; Liu, L.; Guo, J.; Wang, G.; Zhou, Y.; Ma, R. High-Performance Wood-Based Thermoelectric Sponges for Thermal Energy Harvesting and Smart Buildings. Nano Res. 2024, 17, 5349–5357. [Google Scholar] [CrossRef]
- Daneshvar, S.H.; Yuce, M.R.; Redouté, J.-M. Design of Miniaturized Variable-Capacitance Electrostatic Energy Harvesters; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-90251-3. [Google Scholar]
- Hussian, A.H.; Ghoni, R.; Ibrahim, M.T.; Wahid, S.R.; Hat, A.N.M.; Sulaiman, M.A.; Ibrahim, M.F. Mathematical Modelling of Electromagnetic Transduction for Optimal Power Harvesting from Induction Motors. In Proceedings of the 4th International Conference on Electronics, Biomedical Engineering, and Health Informatics, Surabaya, Indonesia, 4–5 October 2023; Triwiyanto, T., Rizal, A., Caesarendra, W., Eds.; Lecture Notes in Electrical Engineering. Springer Nature: Singapore, 2024; Volume 1182, pp. 103–116, ISBN 978-981-97-1462-9. [Google Scholar]
- Zgaren, M.; Moradi, A.; Wang, G.; Sawan, M. Low-Power, High-Data Rate 915 MHz Transceiver with Fully Passive Wake-Up Receiver for Biomedical Implants. In Proceedings of the 2015 IEEE International Conference on Ubiquitous Wireless Broadband (ICUWB), Montreal, QC, Canada, 4–7 October 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Iqbal, A.; Sura, P.R.; Al-Hasan, M.; Mabrouk, I.B.; Denidni, T.A. Wireless Power Transfer System for Deep-Implanted Biomedical Devices. Sci. Rep. 2022, 12, 13689. [Google Scholar] [CrossRef]
- Khan, N.U.; Khan, F.U.; Farina, M.; Merla, A. RF Energy Harvesters for Wireless Sensors, State of the Art, Future Prospects and Challenges: A Review. Phys. Eng. Sci. Med. 2024, 47, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; O’Brien, J.M.; Bensel, T.; Cleveland, C.; Booth, L.; Smith, B.R.; Langer, R.; Traverso, G. Wireless Power Transfer to Millimeter-Sized Gastrointestinal Electronics Validated in a Swine Model. Sci. Rep. 2017, 7, 46745. [Google Scholar] [CrossRef]
- Kim, H.; Rigo, B.; Wong, G.; Lee, Y.J.; Yeo, W.-H. Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring. Nano-Micro Lett. 2024, 16, 52. [Google Scholar] [CrossRef]
- Raghav, K.S.; Bansal, D. Power Controlled System for Self-Sustained RF Energy Harvesting Sensors. Analog. Integr. Circ. Sig Process 2022, 113, 73–79. [Google Scholar] [CrossRef]
- Hosain, M.K.; Kouzani, A.Z.; Samad, M.F.; Tye, S.J. A Miniature Energy Harvesting Rectenna for Operating a Head-Mountable Deep Brain Stimulation Device. IEEE Access 2015, 3, 223–234. [Google Scholar] [CrossRef]
- Kiourti, A.; Lee, C.W.L.; Chae, J.; Volakis, J.L. A Wireless Fully Passive Neural Recording Device for Unobtrusive Neuropotential Monitoring. IEEE Trans. Biomed. Eng. 2016, 63, 131–137. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.G.; Qazi, R.; Shin, G.; Li, S.; Ikram, M.H.; Jang, K.-I.; Liu, Y.; Al-Hasani, R.; Bruchas, M.R.; Jeong, J.-W.; et al. Preparation and Implementation of Optofluidic Neural Probes for in Vivo Wireless Pharmacology and Optogenetics. Nat. Protoc. 2017, 12, 219–237. [Google Scholar] [CrossRef]
- Khan, M.S.; Deng, H. Design and Implementation of a Highly Efficient UHF Energy Harvesting Antenna. In Proceedings of the 2016 IEEE International Symposium on Antennas and Propagation (APSURSI), Fajardo, PR, USA, 26 June–1 July 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 611–612. [Google Scholar]
- Liu, C.; Guo, Y.-X.; Sun, H.; Xiao, S. Design and Safety Considerations of an Implantable Rectenna for Far-Field Wireless Power Transfer. IEEE Trans. Antennas Propagat. 2014, 62, 5798–5806. [Google Scholar] [CrossRef]
- Bakogianni, S.; Koulouridis, S. A Dual-Band Implantable Rectenna for Wireless Data and Power Support at Sub-GHz Region. IEEE Trans. Antennas Propagat. 2019, 67, 6800–6810. [Google Scholar] [CrossRef]
- Agrawal, D.R.; Tanabe, Y.; Weng, D.; Ma, A.; Hsu, S.; Liao, S.-Y.; Zhen, Z.; Zhu, Z.-Y.; Sun, C.; Dong, Z.; et al. Conformal Phased Surfaces for Wireless Powering of Bioelectronic Microdevices. Nat. Biomed. Eng. 2017, 1, 0043. [Google Scholar] [CrossRef] [PubMed]
- Değirmenci, E.; Tek, M.; Erat, S.; Karabulut, Y.Y. A Local Treatment Kit in the Form of an Intra-Organ Capsule Allowing Local Treatment in Solid and Hollow Organ Cancers. Available online: https://patentimages.storage.googleapis.com/83/98/3b/35707742c51b2b/WO2023211394A1.pdf (accessed on 10 October 2024).
- Beaty, H.W.; Fink, D. Standard Handbook for Electrical Engineers, 16th ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Demir, A.F.; Abbasi, Q.H.; Ankarali, Z.E.; Serpedin, E.; Arslan, H. Numerical Characterization of in Vivo Wireless Communication Channels. In Proceedings of the 2014 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-Bio2014), London, UK, 8–10 December 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–3. [Google Scholar]
- Kiourti, A.; Nikita, K.S. A Review of In-Body Biotelemetry Devices: Implantables, Ingestibles, and Injectables. IEEE Trans. Biomed. Eng. 2017, 64, 1422–1430. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The Dielectric Properties of Biological Tissues: I. Literature Survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The Dielectric Properties of Biological Tissues: II. Measurements in the Frequency Range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- IT’IS Foundation Tissue Properties Database V4.2 2024. Available online: https://itis.swiss/virtual-population/tissue-properties/downloads/database-v4-1/ (accessed on 1 June 2025).
- Nishio, T.; Kunieda, E.; Shirato, H.; Ishikura, S.; Onishi, H.; Tateoka, K.; Hiraoka, M.; Narita, Y.; Ikeda, M.; Goka, T. Dosimetric Verification in Participating Institutions in a Stereotactic Body Radiotherapy Trial for Stage I Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Trial (JCOG0403). Phys. Med. Biol. 2006, 51, 5409–5417. [Google Scholar] [CrossRef] [PubMed]




| Part Number | Description | Function |
|---|---|---|
| IRLML250GPbF | MOSFET transistor | To open the gates. |
| PIC16LF1827 | Microcontroller | To control the whole process. |
| PCC110 | Rf harvester | 915 radio frequency harvesting. |
| 2JE05 | Antenna | Capturing the 915 MHz radio frequency. |
| TX91503 | Power spot transmitter | Broadcasts radio waves in the unlicensed 915 MHz band. |
| DSF305Q3R0 | DSF 3 V super capacitor | Harvested power storage. |
| Components | Length (mm) | Width (mm) |
|---|---|---|
| Medical Device | 60.3 | 8.6 |
| Chambers Hole | 5.00 | 2.82 |
| Sliding Gate | 5.24 | 2.95 |
| Electronic Circuit | 55.95 | 8.5 |
| Aluminum Wire | 1.31 | 0.1 |
| Leaf Spring | 5.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, O.A.; Degirmenci, E. Battery-Free Innovation: An RF-Powered Implantable Microdevice for Intravesical Chemotherapy. Appl. Sci. 2025, 15, 9304. https://doi.org/10.3390/app15179304
Ali OA, Degirmenci E. Battery-Free Innovation: An RF-Powered Implantable Microdevice for Intravesical Chemotherapy. Applied Sciences. 2025; 15(17):9304. https://doi.org/10.3390/app15179304
Chicago/Turabian StyleAli, Obidah Alsayed, and Evren Degirmenci. 2025. "Battery-Free Innovation: An RF-Powered Implantable Microdevice for Intravesical Chemotherapy" Applied Sciences 15, no. 17: 9304. https://doi.org/10.3390/app15179304
APA StyleAli, O. A., & Degirmenci, E. (2025). Battery-Free Innovation: An RF-Powered Implantable Microdevice for Intravesical Chemotherapy. Applied Sciences, 15(17), 9304. https://doi.org/10.3390/app15179304







