Deep Learning-Powered Super Resolution Reconstruction Improves 2D T2-Weighted Turbo Spin Echo MRI of the Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Magnetic Resonance Imaging
2.3. Qualitative Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Scan Time
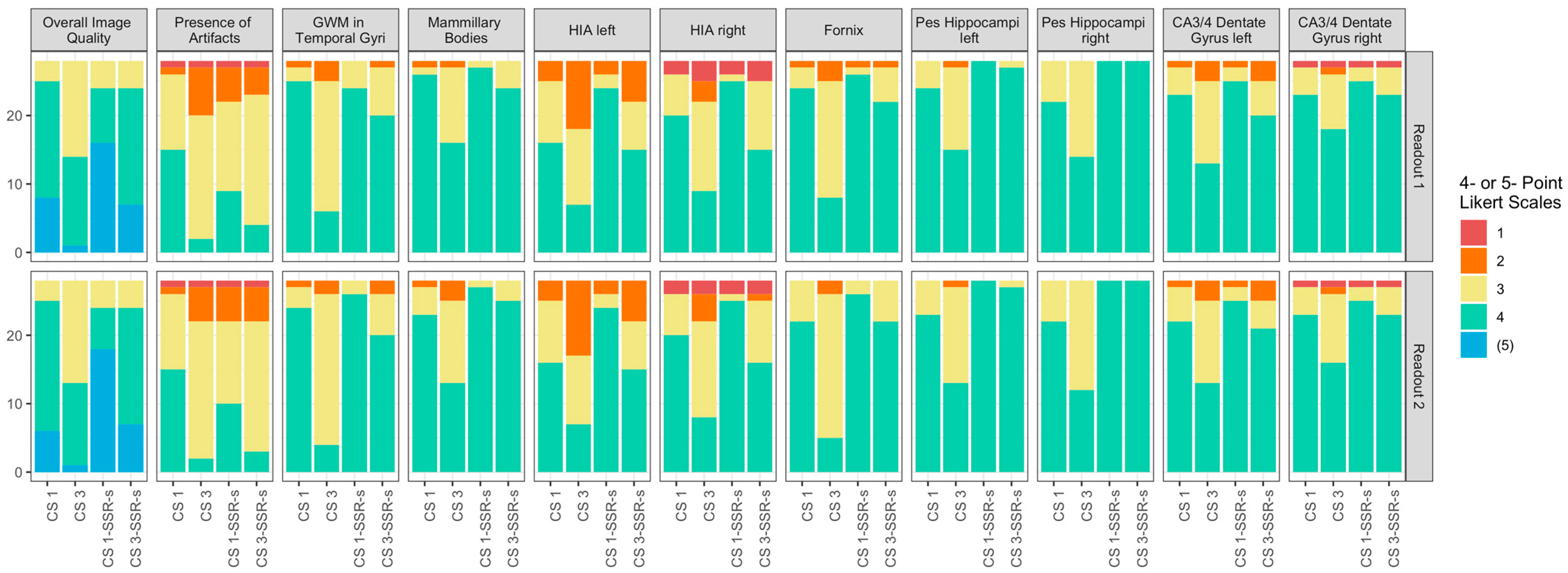
3.2. Image Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| T2w | T2-weighted |
| 2D | 2-dimensional |
| 3D | 3-dimensional |
| TSE | Turbo Spin Echo |
| PI | Parallel Imaging |
| CS | Compressed Sensing/Compressed Sense |
| AI | Artificial Intelligence |
| DL | Deep Learning |
| IRB | Institutional Review Board |
| ISTA | Iterative Shrinkage–Thresholding Algorithm |
| CNN | Convolutional Neural Network |
| SD | Standard Deviation |
| SNR | Signal-to-Noise Ratio |
| GAN | Generative Adversarial Network |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| ROI | Region of Interest |
| SRR | Super Resolution Reconstruction |
References
- Ver Hoef, L.W.; Williams, F.B.; Kennedy, R.E.; Szaflarski, J.P.; Knowlton, R.C. Predictive value of hippocampal internal architecture asymmetry in temporal lobe epilepsy. Epilepsy Res. 2013, 106, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.D.; Berkovic, S.F.; Duncan, J.S.; Connelly, A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am. J. Neuroradiol. 1993, 14, 753–762. [Google Scholar] [PubMed]
- Ver Hoef, L.W.; Paige, A.L.; Riley, K.O.; Cure, J.; Soltani, M.; Williams, F.B.; Kennedy, R.E.; Szaflarski, J.P.; Knowlton, R.C. Evaluating hippocampal internal architecture on MRI: Inter-rater reliability of a proposed scoring system. Epilepsy Res. 2013, 106, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.P.; Welch, E.B.; Niederhauser, B.D.; Whetsell, W.O.; Anderson, A.W.; Gore, J.C.; Avison, M.J.; Creasy, J.L. High-resolution 7T MRI of the human hippocampus in vivo. J. Magn. Reson. Imaging 2008, 28, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Ver Hoef, L.; Deshpande, H.; Cure, J.; Selladurai, G.; Beattie, J.; Kennedy, R.E.; Knowlton, R.C.; Szaflarski, J.P. Clear and Consistent Imaging of Hippocampal Internal Architecture With High Resolution Multiple Image Co-registration and Averaging (HR-MICRA). Front. Neurosci. 2021, 15, 546312. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.S.; Coutinho, A.M.N.; Vieira, A.P.F.; Rocha, B.P.; Passos, U.L.; Gonçalves, V.T.; Silva, P.D.S.; Zhan, M.X.; Pinho, P.C.; Delgado, D.S.; et al. Imaging Aspects of the Hippocampus. RadioGraphics 2022, 42, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Sartoretti, T.; Binkert, C.; Najafi, A.; Schwenk, Á.; Hinnen, M.; van Smoorenburg, L.; Eichenberger, B.; Sartoretti-Schefer, S. Reduction of procedure times in routine clinical practice with Compressed SENSE magnetic resonance imaging technique. PLoS ONE 2019, 14, e0214887. [Google Scholar] [CrossRef] [PubMed]
- Knoll, F.; Zbontar, J.; Sriram, A.; Muckley, M.J.; Bruno, M.; Defazio, A.; Parente, M.; Geras, K.J.; Katsnelson, J.; Chandarana, H.; et al. fastMRI: A Publicly Available Raw k-Space and DICOM Dataset of Knee Images for Accelerated MR Image Reconstruction Using Machine Learning. Radiol. Artif. Intell. 2020, 2, e190007. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pan, X.; Zhu, K.; Xiao, Y.; Yue, X.; Peng, P.; Zhang, X.; Huang, J.; Chen, J.; Yuan, Y.; et al. Accelerated 3D high-resolution T2-weighted breast MRI with deep learning constrained compressed sensing, comparison with conventional T2-weighted sequence on 3.0 T. Eur. J. Radiol. 2022, 156, 110562. [Google Scholar] [CrossRef] [PubMed]
- Akçakaya, M.; Moeller, S.; Weingärtner, S.; Uğurbil, K. Scan-specific robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction: Database-free deep learning for fast imaging. Magn. Reson. Med. 2019, 81, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, L.M.; Peeters, J.M.; Weinhold, L.; Krausewitz, P.; Ellinger, J.; Katemann, C.; Isaak, A.; Weber, O.M.; Kuetting, D.; Attenberger, U.; et al. Deep Learning Super-Resolution Reconstruction for Fast and Motion-Robust T2-weighted Prostate MRI. Radiology 2023, 308, e230427. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, N.; Yousefi, S.; Elmahdy, M.S.; Van Gemert, J.H.F.; Schuelke, C.; Doneva, M.; Nielsen, T.; Kastryulin, S.; Lelieveldt, B.P.F.; Van Osch, M.J.P.; et al. An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction. IEEE Access 2020, 8, 204825–204838. [Google Scholar] [CrossRef]
- Pezzotti, N.; de Weerdt, E.; Yousefi, S.; Elmahdy, M.S.; van Gemert, J.; Schülke, C.; Doneva, M.; Nielsen, T.; Kastryulin, S.; Lelieveldt, B.P.F.; et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arXiv 2019, arXiv:1912.12259. [Google Scholar] [CrossRef]
- Foreman, S.C.; Neumann, J.; Han, J.; Harrasser, N.; Weiss, K.; Peeters, J.M.; Karampinos, D.C.; Makowski, M.R.; Gersing, A.S.; Woertler, K. Deep learning–based acceleration of Compressed Sense MR imaging of the ankle. Eur. Radiol. 2022, 32, 8376–8385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, W.; Xiao, Y.; Ming, Y.; Ma, K.; Hu, S.; Zeng, W.; Zeng, L.; Liang, Z.; Zhang, X.; et al. Rapid 3D breath-hold MR cholangiopancreatography using deep learning–constrained compressed sensing reconstruction. Eur. Radiol. 2023, 33, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Harder, F.N.; Weiss, K.; Amiel, T.; Peeters, J.M.; Tauber, R.; Ziegelmayer, S.; Burian, E.; Makowski, M.R.; Sauter, A.P.; Gschwend, J.E.; et al. Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer. Cancers 2022, 14, 5741. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Sartoretti, T.; Bertulli, L.; Golshani, S.; Alfieri, A.; Hoh, T.; Maurer, A.; Mannil, M.; Binkert, C.A.; Sartoretti-Schefer, S. Deep learning constrained compressed sensing reconstruction improves high-resolution three-dimensional (3D) T2-weighted turbo spin echo magnetic resonance imaging (MRI) of the lumbar spine. Clin. Radiol. 2024, 79, e1514–e1521. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.S.; Fang, Z.; Kogan, F.; Wood, J.; Stevens, K.J.; Gibbons, E.K.; Lee, J.H.; Gold, G.E.; Hargreaves, B.A. Super-resolution musculoskeletal MRI using deep learning. Magn. Reson. Med. 2018, 80, 2139–2154. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Loy, C.C.; He, K.; Tang, X. Image Super-Resolution Using Deep Convolutional Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 38, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sixou, B.; Peyrin, F. A Review of the Deep Learning Methods for Medical Images Super Resolution Problems. IRBM 2021, 42, 120–133. [Google Scholar] [CrossRef]
- Kravchenko, D.; Isaak, A.; Mesropyan, N.; Peeters, J.M.; Kuetting, D.; Pieper, C.C.; Katemann, C.; Attenberger, U.; Emrich, T.; Varga-Szemes, A.; et al. Deep learning super-resolution reconstruction for fast and high-quality cine cardiovascular magnetic resonance. Eur. Radiol. 2024, 35, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Terzis, R.; Dratsch, T.; Hahnfeldt, R.; Basten, L.; Rauen, P.; Sonnabend, K.; Weiss, K.; Reimer, R.; Maintz, D.; Iuga, A.-I.; et al. Five-minute knee MRI: An AI-based super resolution reconstruction approach for compressed sensing. A validation study on healthy volunteers. Eur. J. Radiol. 2024, 175, 111418. [Google Scholar] [CrossRef] [PubMed]
- Jurka, M.; Macova, I.; Wagnerova, M.; Capoun, O.; Jakubicek, R.; Ourednicek, P.; Lambert, L.; Burgetova, A. Deep-learning-based reconstruction of T2-weighted magnetic resonance imaging of the prostate accelerated by compressed sensing provides improved image quality at half the acquisition time. Quant. Imaging Med. Surg. 2024, 14, 3534–3543. [Google Scholar] [CrossRef] [PubMed]
- Mesropyan, N.; Katemann, C.; Leutner, C.; Sommer, A.; Isaak, A.; Weber, O.M.; Peeters, J.M.; Dell, T.; Bischoff, L.; Kuetting, D.; et al. Accelerated High-resolution T1- and T2-weighted Breast MRI with Deep Learning Super-resolution Reconstruction. Acad. Radiol. 2025, 32, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Hahnfeldt, R.; Terzis, R.; Dratsch, T.; Basten, L.M.; Rauen, P.; Oppermann, J.; Grevenstein, D.; Janßen, J.P.; Zeid, N.E.-H.A.; Sonnabend, K.; et al. Is a 3-Minute Knee MRI Protocol Sufficient for Daily Clinical Practice? A SuperResolution Reconstruction Approach Using AI and Compressed Sensing. Diagnostics 2025, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Safaie, T.; Bischoff, L.M.; Katemann, C.; Peeters, J.M.; Kravchenko, D.; Mesropyan, N.; Beissel, L.D.; Dell, T.; Weber, O.M.; Pieper, C.C.; et al. Fast and Robust Single-Shot Cine Cardiac MRI Using Deep Learning Super-Resolution Reconstruction. Investig. Radiol. 2025. published ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Dimitri, D.; Heyn, C.; Kiehl, T.-R.; Mikulis, D.; Valiante, T. Histologically Confirmed Hippocampal Structural Features Revealed by 3T MR Imaging: Potential to Increase Diagnostic Specificity of Mesial Temporal Sclerosis. Am. J. Neuroradiol. 2010, 31, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.G.; Stables, L.; Du, A.T.; Schuff, N.; Truran, D.; Cashdollar, N.; Weiner, M.W. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol. Aging 2007, 28, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.; Avants, B.; Pluta, J.; Das, S.; Minkoff, D.; Mechanichamilton, D.; Glynn, S.; Pickup, S.; Liu, W.; Gee, J. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. NeuroImage 2009, 44, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Suh, P.S.; Park, J.E.; Roh, Y.H.; Kim, S.; Jung, M.; Koo, Y.S.; Lee, S.-A.; Choi, Y.; Kim, H.S. Improving Diagnostic Performance of MRI for Temporal Lobe Epilepsy with Deep Learning-Based Image Reconstruction in Patients with Suspected Focal Epilepsy. Korean J. Radiol. 2024, 25, 374. [Google Scholar] [CrossRef] [PubMed]
- Wisse, L.E.M.; Daugherty, A.M.; Olsen, R.K.; Berron, D.; Carr, V.A.; Stark, C.E.L.; Amaral, R.S.C.; Amunts, K.; Augustinack, J.C.; Bender, A.R.; et al. A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals?: A Harmonized Hippocampal Subfield Protocol: Key Goals and Impact. Hippocampus 2017, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Santos, J.E.; Carvalho, L.E.D.D.; Kandratavicius, L.; Diniz, P.R.B.; Scandiuzzi, R.C.; Coras, R.; Blümcke, I.; Assirati, J.A.; Carlotti, C.G.; Matias, C.C.M.S.; et al. Manual Hippocampal Subfield Segmentation Using High-Field MRI: Impact of Different Subfields in Hippocampal Volume Loss of Temporal Lobe Epilepsy Patients. Front. Neurol. 2018, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Hayman, L.A.; Fuller, G.N.; Cavazos, J.E.; Pfleger, M.J.; Meyers, C.A.; Jackson, E.F. The hippocampus: Normal anatomy and pathology. Am. J. Roentgenol. 1998, 171, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Derix, J.; Yang, S.; Lüsebrink, F.; Fiederer, L.D.J.; Schulze-Bonhage, A.; Aertsen, A.; Speck, O.; Ball, T. Visualization of the amygdalo–hippocampal border and its structural variability by 7T and 3T magnetic resonance imaging. Hum. Brain Mapp. 2014, 35, 4316–4329. [Google Scholar] [CrossRef] [PubMed]
- Altahawi, F.F.; Blount, K.J.; Morley, N.P.; Raithel, E.; Omar, I.M. Comparing an accelerated 3D fast spin-echo sequence (CS-SPACE) for knee 3-T magnetic resonance imaging with traditional 3D fast spin-echo (SPACE) and routine 2D sequences. Skeletal Radiol. 2017, 46, 7–15. [Google Scholar] [CrossRef] [PubMed]
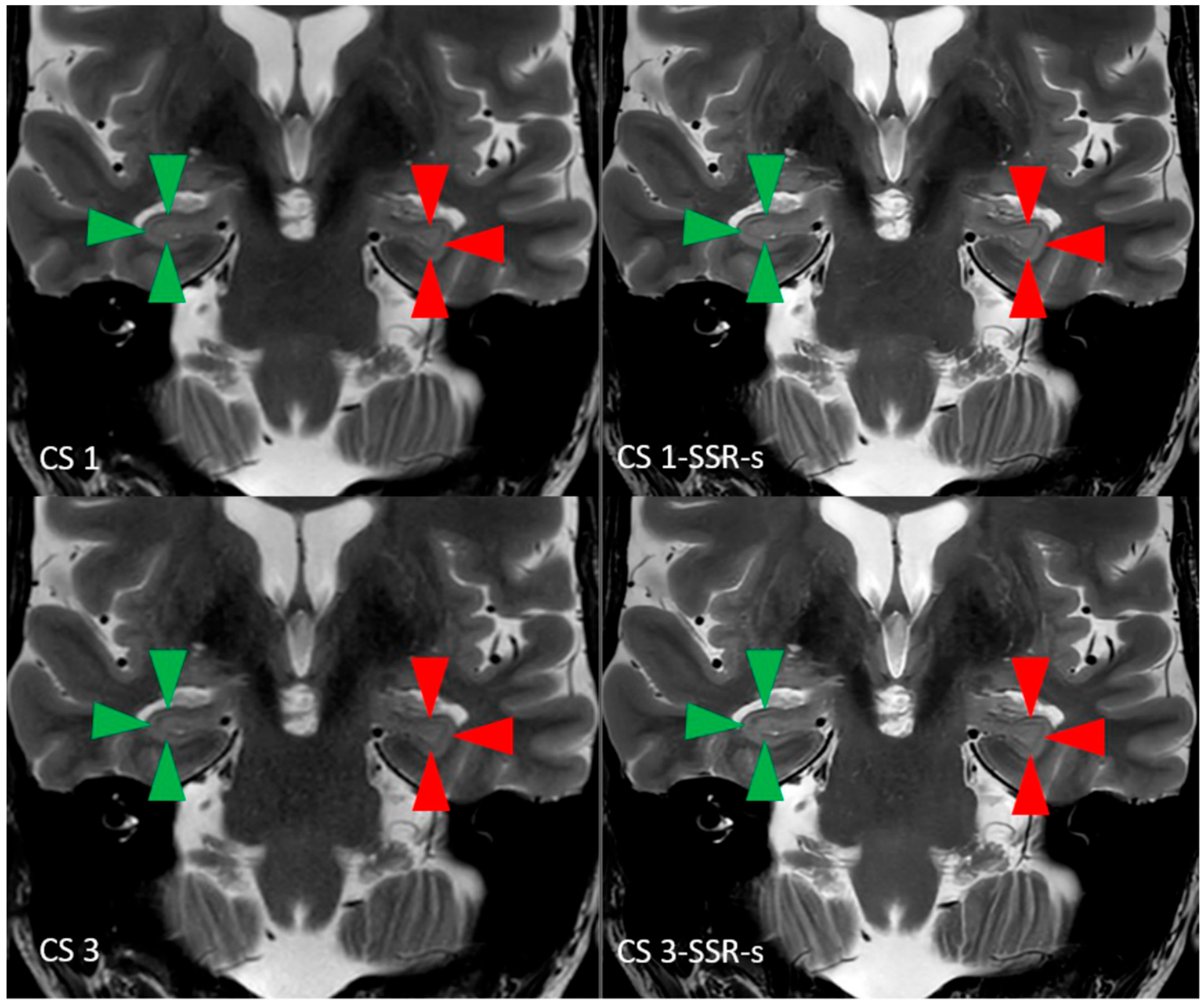
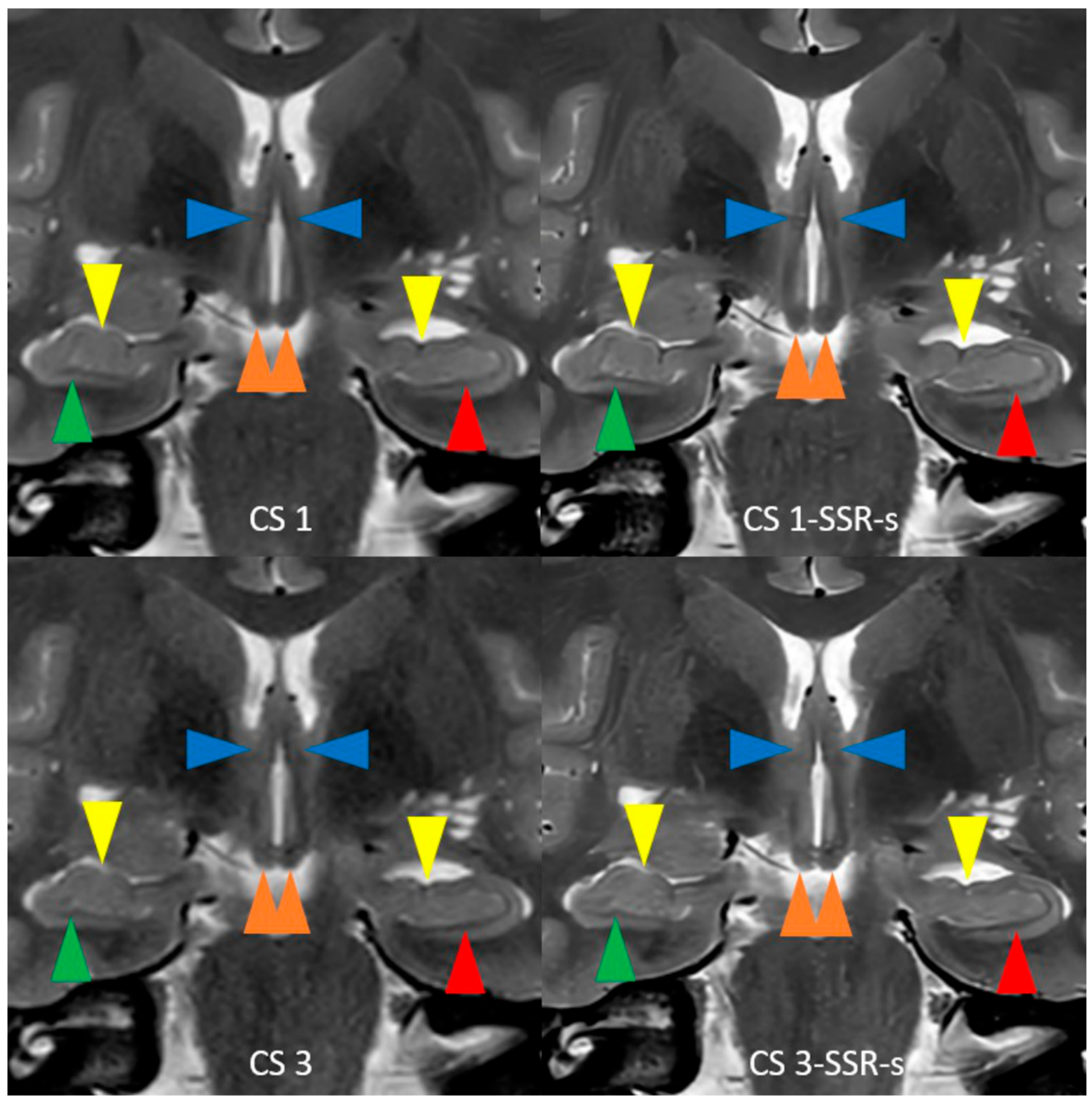
| 2D T2w TSE CS 1/CS 3 | |
|---|---|
| Acquisition | coronal |
| Drive pulse | no |
| Repetition time TR in ms | 3668 |
| Echo time TE in ms | 90 |
| Flip angle | 90° |
| Echo train length ETL | 15 |
| Number of echoes | 1 |
| FOV | 230 × 184 × 88 |
| Slice thickness/gap | 2 mm/0.2 mm |
| Acquired voxel size | 0.6 mm |
| Reconstructed voxel size | 0.4 mm |
| Number of slices | 40 |
| Acceleration | CS factor 1/CS factor 3 |
| Number of signal averages NSA | 2 |
| Acquisition time | 270 s (4 min 39 s)/103 s (1 min 43 s) |
| CS 1 | CS 3 | CS 1-SRR-s | CS 3-SRR-s | |
|---|---|---|---|---|
| Overall Image Quality | S1: 4 (4,5); 4.2 ± 0.6 S2: 4 (4,4); 4.1 ± 0.6 | S1: 3.5 (3,4); 3.5 ± 0.6 ** S2: 3 (3,4); 3.5 ± 0.6 ** | S1: 5 (4,5); 4.4 ± 0.7 * S2: 5 (4,5); 4.5 ± 0.7 ** | S1: 4 (4,4.25); 4.1 ± 0.6 S2: 4 (4,4.25); 4.1 ± 0.6 |
| Presence of Artifacts | S1: 4 (3,4); 3.4 ± 0.7 S2: 4 (3,4); 3.4 ± 0.7 | S1: 3 (2,3); 2.8 ± 0.6 ** S2: 3 (3,3); 2.8 ± 0.6 ** | S1: 3 (3,4); 3.1 ± 0.8 ** S2: 3 (3,4); 3.1 ± 0.8 ** | S1: 3 (3,3); 2.9 ± 0.7 ** S2: 3 (3,3); 2.9 ± 0.7 ** |
| GWM in Temporal Gyri | S1: 4 (4,4); 3.9 ± 0.4 S2: 4 (4,4); 3.8 ± 0.4 | S1: 3 (3,3); 3.1 ± 0.6 *** S2: 3 (3,3); 3.1 ± 0.5 *** | S1: 4 (4,4); 3.9 ± 0.4 S2: 4 (4,4); 3.9 ± 0.3 | S1: 4 (3,4); 3.7 ± 0.5 S2: 4 (3,4); 3.6 ± 0.6 |
| Fornix | S1: 4 (4,4); 3.8 ± 0.5 S2: 4 (4,4); 3.8 ± 0.5 | S1: 3 (3,4); 3.2 ± 0.6 *** S2: 3 (3,2); 3.1 ± 0.5 *** | S1: 4 (4,4); 3.9 ± 0.4 S2: 4 (4,4); 3.9 ± 0.3 | S1: 4 (4,4); 3.8 ± 0.5 S2: 4 (4,4); 3.8 ± 0.4 |
| Hippocampal Internal Architecture Left | S1: 4 (3,4); 3.5 ± 0.7 S2: 4 (3,4); 3.5 ± 0.7 | S1: 3 (2,3.25); 2.9 ± 0.8 ** S2: 3 (2,3.25); 2.9 ± 0.8 ** | S1: 4 (4,4); 3.8 ± 0.6 ** S2: 4 (4,4); 3.8 ± 0.6 ** | S1: 4 (3,4); 3.3 ± 0.8 S2: 4 (3,4); 3.3 ± 0.8 |
| Hippocampal Internal Architecture Right | S1: 4 (3,4); 3.6 ± 0.8 S2: 4 (3,4); 3.6 ± 0.8 | S1: 3 (3,4); 3 ± 0.9 ** S2: 3 (3,4); 3 ± 0.9 ** | S1: 4 (4,4); 3.8 ± 0.8 S2: 4 (4,4); 3.8 ± 0.8 | S1: 4 (3,4); 3.3 ± 0.9 S2: 4 (3,4); 3.4 ± 0.9 |
| Mammillary Bodies | S1: 4 (4,4); 3.9 ± 0.5 S2: 4 (4,4); 3.8 ± 0.5 | S1: 4 (3,4); 3.5 ± 0.6 * S2: 3 (3,4); 3.4 ± 0.7 ** | S1: 4 (4,4); 4 ± 0.2 S1: 4 (4,4); 4 ± 0.2 | S1: 4 (4,4); 3.9 ± 0.4 S1: 4 (4,4); 3.9 ± 0.3 |
| Pes Hippocampi Left | S1: 4 (4,4); 3.9 ± 0.4 S2: 4 (4,4); 3.8 ± 0.4 | S1: 4 (3,4); 3.5 ± 0.6 * S2: 3 (3,4); 3.4 ± 0.6 ** | S1: 4 (4,4); 4 ± 0 S2: 4 (4,4); 4 ± 0 | S1: 4 (4,4); 4 ± 0.2 S2: 4 (4,4); 4 ± 0.2 |
| Pes Hippocampi Right | S1: 4 (4,4); 3.8 ± 0.4 S2: 4 (4,4); 3.8 ± 0.4 | S1: 3.5 (3,4); 3.5 ± 0.5 * S2: 3 (3,4); 3.4 ± 0.5 ** | S1: 4 (4,4); 4 ± 0 * S2: 4 (4,4); 4 ± 0 * | S1: 4 (4,4); 4 ± 0 * S2: 4 (4,4); 4 ± 0 * |
| CA3/4 Dentate Gyrus Left | S1: 4 (4,4); 3.8 ± 0.5 S2: 4 (4,4); 3.8 ± 0.5 | S1: 3 (3,4); 3.4 ± 0.7 ** S2: 3 (3,4); 3.4 ± 0.7 ** | S1: 4 (4,4); 3.9 ± 0.4 S2: 4 (4,4); 3.9 ± 0.4 | S1: 4 (3,4); 3.6 ± 0.7 S2: 4 (3.75,4); 3.6 ± 0.7 |
| CA3/4 Dentate Gyrus Right | S1: 4 (4,4); 3.8 ± 0.6 S2: 4 (4,4); 3.8 ± 0.6 | S1: 4 (3,4); 3.5 ± 0.7 S2: 4 (3,4); 3.5 ± 0.7 * | S1: 4 (4,4); 3.8 ± 0.6 S2: 4 (4,4); 3.8 ± 0.6 | S1: 4 (4,4); 3.8 ± 0.6 S2: 4 (4,4); 3.8 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartoretti, E.; Sartoretti, T.; Alfieri, A.; Hoh, T.; Maurer, A.; Mannil, M.; Binkert, C.A.; Sartoretti-Schefer, S. Deep Learning-Powered Super Resolution Reconstruction Improves 2D T2-Weighted Turbo Spin Echo MRI of the Hippocampus. Appl. Sci. 2025, 15, 8202. https://doi.org/10.3390/app15158202
Sartoretti E, Sartoretti T, Alfieri A, Hoh T, Maurer A, Mannil M, Binkert CA, Sartoretti-Schefer S. Deep Learning-Powered Super Resolution Reconstruction Improves 2D T2-Weighted Turbo Spin Echo MRI of the Hippocampus. Applied Sciences. 2025; 15(15):8202. https://doi.org/10.3390/app15158202
Chicago/Turabian StyleSartoretti, Elisabeth, Thomas Sartoretti, Alex Alfieri, Tobias Hoh, Alexander Maurer, Manoj Mannil, Christoph A. Binkert, and Sabine Sartoretti-Schefer. 2025. "Deep Learning-Powered Super Resolution Reconstruction Improves 2D T2-Weighted Turbo Spin Echo MRI of the Hippocampus" Applied Sciences 15, no. 15: 8202. https://doi.org/10.3390/app15158202
APA StyleSartoretti, E., Sartoretti, T., Alfieri, A., Hoh, T., Maurer, A., Mannil, M., Binkert, C. A., & Sartoretti-Schefer, S. (2025). Deep Learning-Powered Super Resolution Reconstruction Improves 2D T2-Weighted Turbo Spin Echo MRI of the Hippocampus. Applied Sciences, 15(15), 8202. https://doi.org/10.3390/app15158202






