The Influence of PEF, Pulsed Light, Microwave and Conventional Heat Treatments on Quality Parameters of Berry Fruit Juice Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents and Standards
2.3. Juices Production Technology and Composition
2.4. Juice Preservation Methods
2.5. Analytical Methods
2.5.1. Total Soluble Solids (TSS), Active Acidity (pH) and Total Titratable Acidity (TTA) Measurements
2.5.2. Nephelometric Turbidity (NT)
2.5.3. Total Polyphenols Content (TPC)
2.5.4. HPLC Analysis of Anthocyanins
2.5.5. Antioxidant Activity Measurements—DPPH Assay (AA)
2.5.6. Color Measurement
2.6. Statistical Analyses
3. Results and Discussion
3.1. Effect on Total Soluble Solids
3.2. Effect on Active and Total Titratable Acidity
3.3. Effect on Nephelometric Turbidity
3.4. Effect on Total Polyphenols Content
3.5. Effect on Anthocyanin Content
3.6. Effect on Antioxidant Activity
3.7. Effect on Color
3.8. PCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Antioxidant activity |
| Cy-3,5-O-diglc | Cyanidin-3,5-O-diglucoside |
| Cy-3-O-gal | Cyanidin-3-O-galactoside |
| Cy-3-O-glc | Cyanidin-3-O-glucoside |
| Cy-3-O-rut | Cyanidin-3-O-rutinoside |
| Cy-3-O-xyl | Cyanidin-3-O-xyloside |
| Dp-3-O-glc | Delphinidin-3-O-glucoside |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HB | Hot bottling |
| HPLC | High-pressure liquid chromatography |
| GAE | Gallic acid equivalents |
| GHC | Grape–blue honeysuckle–chokeberry juice |
| GHS | Grape–blue honeysuckle–strawberry juice |
| MP | Flow microwave pasteurization |
| n.d. | Non detected |
| NT | Nephelometric turbidity |
| PEF | Pulsed electric field |
| Pg-3-O-ara | Pelargonidin-3-O-arabinoside |
| pH | Active acidity |
| Pg-3-O-glc | Pelargonidin-3-O-glucoside |
| Pn-3-O-glc | Peonidin-3-O-glucoside |
| PL | Pulsed light |
| PT | Pasteurization |
| SHC | Strawberry–blue honeysuckle–chokeberry juice |
| TE | Trolox |
| TEAC | Trolox equivalent antioxidant capacity |
| TPC | Total polyphenol content |
| TSS | Total soluble solids |
| TTA | Total titratable activity |
Appendix A
| a* | b* | TAC | AA | TPC | |
|---|---|---|---|---|---|
| a* | - | 0.80 | 0.43 | 0.28 | 0.20 |
| b* | 0.80 | - | 0.72 | 0.59 | 0.56 |
| TAC | 0.43 | 0.72 | - | 0.88 | 0.84 |
| AA | 0.28 | 0.59 | 0.88 | - | 0.91 |
| TPC | 0.20 | 0.56 | 0.84 | 0.91 | - |
References
- Fruit Juice—Nutrition & Health. An IFU Scientific Review; International Fruit and Vegetable Juice Association: Paris, France, 2017.
- Bezerra, M.; Ribeiro, M.; Cosme, F.; Nunes, F.M. Overview of the Distinctive Characteristics of Strawberry, Raspberry, and Blueberry in Berries, Berry Wines, and Berry Spirits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13354. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Ł etowska, A.; Kucharska, A.Z. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An Update on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515. [Google Scholar] [CrossRef]
- Kersh, D.M.E.; Hammad, G.; Donia, M.S.; Farag, M.A. A Comprehensive Review on Grape Juice Beverage in Context to its Processing and Composition with Future Perspectives to Maximize its Value. Food Bioprocess Technol. 2023, 16, 1–23. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and Relative Stabilities of Acylated and Nonacylated Anthocyanins in Beverage Systems. J. Food Sci. Technol. 2022, 59, 831–845. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimut Agenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Chen, Y.; Belwal, T.; Xu, Y.; Ma, Q.; Li, D.; Li, L.; Xiao, H.; Luo, Z. Updated Insights into Anthocyanin Stability Behavior from Bases to Cases: Why and Why Not Anthocyanins Lose during Food Processing. Crit. Rev. Food Sci. Nutr. 2023, 63, 8639–8671. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Wang, Z.; Feng, Y.; Han, Y. Advances in the Preparation, Stability, Metabolism, and Physiological Roles of Anthocyanins: A Review. Foods 2023, 12, 3969. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: A Review of Plant Sources, Extraction, Stability, Content Determination and Modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Basak, S.; Mahale, S.; Chakraborty, S. Changes in Quality Attributes of Pulsed Light and Thermally Treated Mixed Fruit Beverages during Refrigerated Storage (4 °C) Condition. Innov. Food Sci. Emerg. Technol. 2022, 78, 103025. [Google Scholar] [CrossRef]
- Ptak, S.; Żarski, A.; Kapuśniak, J. Technological, Economic and Health Aspects of Application of Microwave Radiation in Food Processing. Zywnosc Nauka Technol. Jakosc 2020, 27, 47–62. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Cardozo, T.S.F.; Couto, S.M.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Pimentel, T.C.; Freitas, M.Q.; Raices, R.S.L.; et al. Effects of Microwave Heating on the Chemical Composition and Bioactivity of Orange Juice-Milk Beverages. Food Chem. 2021, 345, 128746. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L.; Kim, K.H. Influence of Pulsed Electric Field and Heat Treatment on Emblica officinalis Juice Inoculated with Zygosaccharomyces bailii. Food Bioprod. Process. 2015, 95, 146–154. [Google Scholar] [CrossRef]
- Piasek, A.; Kusznierewicz, B.; Grzybowska, I.; Malinowska-Pańczyk, E.; Piekarska, A.; Azqueta, A.; Collins, A.R.; Namieśnik, J.; Bartoszek, A. The Influence of Sterilization with EnbioJet® Microwave Flow Pasteurizer on Composition and Bioactivity of Aronia and Blue-Berried Honeysuckle Juices. J. Food Compos. Anal. 2011, 24, 880–888. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. Effect of Continuous Flow Microwave and Conventional Heating on the Bioactive Compounds, Colour, Enzymes Activity, Microbial and Sensory Quality of Strawberry Purée. Food Bioproc Tech. 2015, 8, 1864–1876. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic Pigment Determination in Red Fruit Juices, Concentrated Juices and Syrups Using Liquid Chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Kruszewski, B.; Domian, E.; Nowacka, M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules 2023, 28, 2018. [Google Scholar] [CrossRef]
- Mannozzi, C.; Fauster, T.; Haas, K.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Role of Thermal and Electric Field Effects during the Pre-Treatment of Fruit and Vegetable Mash by Pulsed Electric Fields (PEF) and Ohmic Heating (OH). Innov. Food Sci. Emerg. Technol. 2018, 48, 131–137. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of High Pressure and Thermal Processing on Shelf Life and Quality of Strawberry Purée and Juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of Fruit Juices and Effect of Pasteurization and Storage Conditions on Their Microbial, Physicochemical, and Nutritional Quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mahale, S.; Dhar, R.; Basak, S. Development of a Mixed Fruit Beverage and Pulsed Light Treatment Thereof to Obtain a Microbially Safe and Enzymatically Stable Product. Food Biosci. 2022, 45, 101508. [Google Scholar] [CrossRef]
- Bhagat, B.; Chakraborty, S. Potential of Pulsed Light Treatment to Pasteurize Pomegranate Juice: Microbial Safety, Enzyme Inactivation, and Phytochemical Retention. LWT 2022, 159, 113215. [Google Scholar] [CrossRef]
- de Souza, V.R.; Popović, V.; Bissonnette, S.; Ros, I.; Mats, L.; Duizer, L.; Warriner, K.; Koutchma, T. Quality Changes in Cold Pressed Juices after Processing by High Hydrostatic Pressure, Ultraviolet-c Light and Thermal Treatment at Commercial Regimes. Innov. Food Sci. Emerg. Technol. 2020, 64, 102398. [Google Scholar] [CrossRef]
- Biancaniello, M.; Popović, V.; Fernandez-Avila, C.; Ros-Polski, V.; Koutchma, T. Feasibility of a Novel Industrial-Scale Treatment of Green Cold-Pressed Juices by Uv-c Light Exposure. Beverages 2018, 4, 29. [Google Scholar] [CrossRef]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the Impact of High Pressure, Pulsed Electric Field and Thermal Pasteurization on Quality Attributes of Cloudy Apple Juice Using Targeted and Untargeted Analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined Effects of Sonication and Pulsed Electric Field on Selected Quality Parameters of Grapefruit Juice. LWT 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Influence of Pulsed Electric Field Processing on the Quality of Fruit Juice Beverages Sweetened with Stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Fabroni, S.; Platania, G.M.; Amenta, M.; Ballistreri, G.; Galvano, F.; Nges, I.A.; Timpanaro, N. Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice. Appl. Sci. 2024, 14, 7275. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Viacava, G.; Ponce, A.; Moreira, M.R. Enhancing Quality Attributes of Fiber-Enriched Strawberry Juice by Application of Vanillin or Geraniol. LWT 2016, 72, 90–98. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Sequential Pulsed Light and Ultrasound Treatments for the Inactivation of Saccharomyces cerevisiae and PPO and the Retention of Bioactive Compounds in Sweet Lime Juice. Foods 2024, 13, 1996. [Google Scholar] [CrossRef]
- Shirvani, A.; Mirzaaghaei, M.; Goli, S.A.H. Application of Natural Fining Agents to Clarify Fruit Juices. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4190–4216. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Kalisz, S. Effects of Various Polysaccharide Clarification Agents and Reaction Time on Content of Polyphenolic Compound, Antioxidant Activity, Turbidity and Colour of Chokeberry Juice. LWT 2018, 92, 347–360. [Google Scholar] [CrossRef]
- Türkyılmaz, M.; Hamzaoğlu, F.; Özkan, M. Effects of Pasteurization and Storage on Turbidity and Copigmentation in Pomegranate Juices Clarified with Various Hydrocolloid Combinations. Food Chem. 2021, 358, 129803. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and Thermal Treatments for the Preservation of Carrot Juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The Impact of Pulsed Electric Field Pretreatment of Bell Pepper on the Selected Properties of Spray Dried Juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S. Processing of Clear and Turbid Grape Juice by a Continuous Flow UV System. Innov. Food Sci. Emerg. Technol. 2016, 33, 282–288. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. UV-C Light Inactivation of Single and Composite Microbial Populations in Tangerine-Orange Juice Blend. Evaluation of Some Physicochemical Parameters. Food Bioprod. Process. 2019, 117, 149–159. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Kruszewski, B. High-Temperature Short-Time and Ultra-High-Temperature Processing of Juices, Nectars and Beverages: Influences on Enzyme, Microbial Inactivation and Retention of Bioactive Compounds. Appl. Sci. 2024, 14, 8978. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; dos Santos Funcia, E.; Pires, M.N.; Gut, J.A.W. Modeling of Time-Temperature History and Enzymatic Inactivation of Cloudy Apple Juice in Continuous Flow Microwave Assisted Pasteurization. Food Bioprod. Process. 2018, 111, 45–53. [Google Scholar] [CrossRef]
- Kosiński, J.; Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Jasińska, U.T.; Woźniak, Ł.; Kaniewska, B.; Marszałek, K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Appl. Sci. 2024, 14, 662. [Google Scholar] [CrossRef]
- Wójcik, M.; Szczepańska-Stolarczyk, J.; Woźniak, Ł.; Jasińska, U.T.; Trych, U.; Cywińska-Antonik, M.; Kosiński, J.; Kaniewska, B.; Marszałek, K. Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale. Appl. Sci. 2024, 14, 6008. [Google Scholar] [CrossRef]
- Vollmer, K.; Chakraborty, S.; Prakash Bhalerao, P.; Carle, R.; Frank, J.; Björn Steingass, C. Effect of Pulsed Light Treatment on Natural Microbiota, Enzyme Activity, and Phytochemical Composition of Pineapple (Ananas comosus [L.] Merr.) Juice. Food Bioprocess Technol. 2020, 13, 1095–1109. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Effect of PH and Total Fluence on Microbial and Enzyme Inactivation in Sweet Lime (Citrus limetta) Juice during Pulsed Light Treatment. J. Food Process. Preserv. 2022, 46, e16749. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Brzozowska, A.; Szwengiel, A.; Gramza-Michałowska, A. The Effect of Plant Additives on the Stability of Polyphenols in Cloudy and Clarified Juices from Black Chokeberry (Aronia melanocarpa). Antioxidants 2020, 9, 801. [Google Scholar] [CrossRef]
- Stübler, A.S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of Pilot-Scale Processing (Thermal, PEF, HPP) on the Stability and Bioaccessibility of Polyphenols and Proteins in Mixed Protein- and Polyphenol-Rich Juice Systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Duhan, S.; Kar, A. Optimization of Process Parameter Combinations for Pasteurization of Sugarcane (Saccharum officinarum) Juice Using Continuous Flow Microwave System. Indian J. Agric. Sci. 2018, 88, 1253–1257. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of Pulsed Electric Field Treatment on Shelf Life and Nutritional Value of Apple Juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of Ultrasonication and Pulsed Light Treatments on Phenolics Concentration and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. LWT 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Hać-Szymańczuk, E.; Kruszewski, B. Impact of Conventional Pasteurization, High Temperature Short Time, Ultra-High Temperature, and Storage Time on Physicochemical Characteristics, Bioactive Compounds, Antioxidant Activity, and Microbiological Quality of Fruit Nectars. Foods 2024, 13, 3963. [Google Scholar] [CrossRef] [PubMed]
- Perez-Magarino, S.; Gonzalez-Sanjose, M.L. Application of Absorbance Values Used in Wineries for estimating CIELAB Parameters in Red Wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
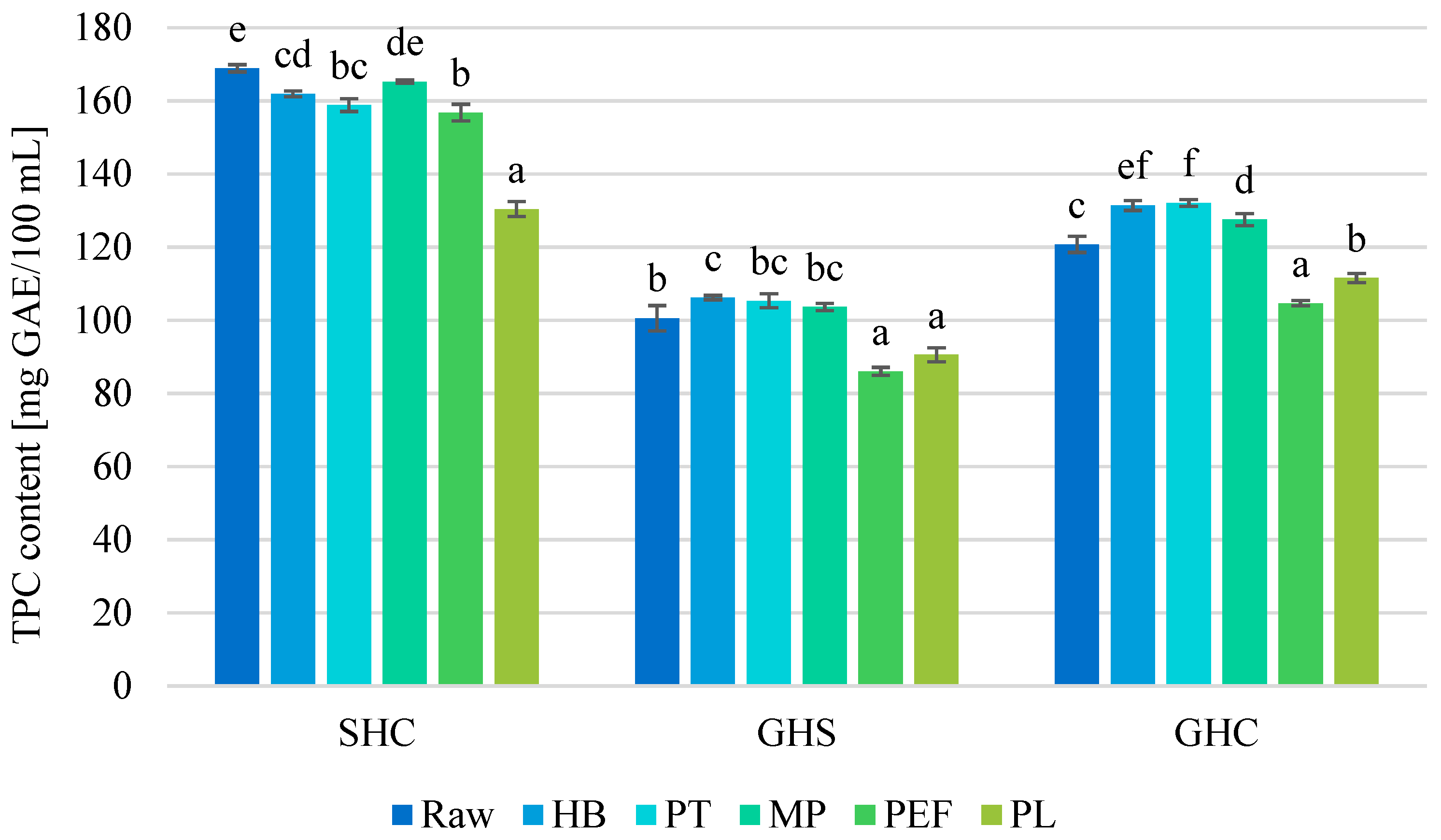
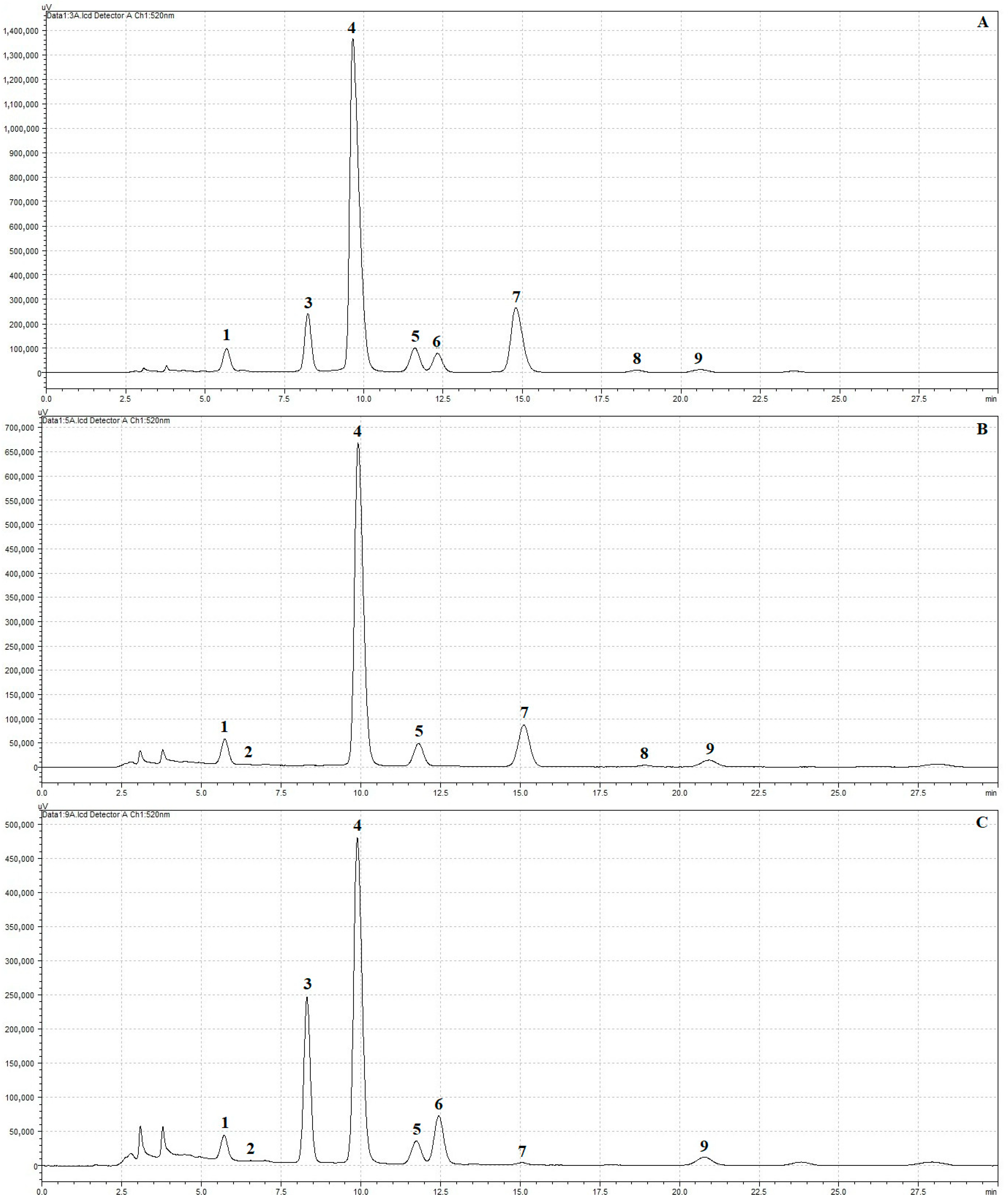
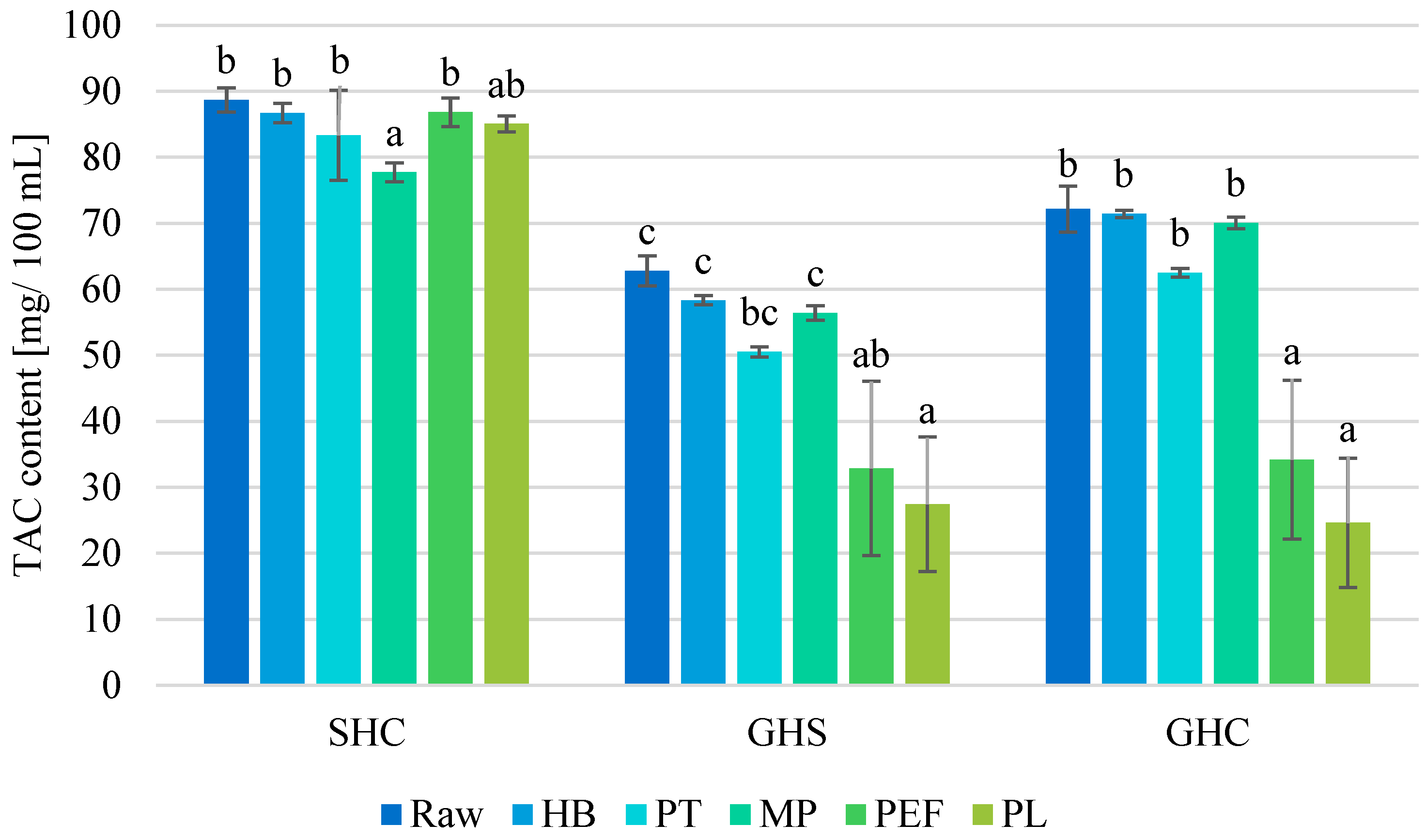
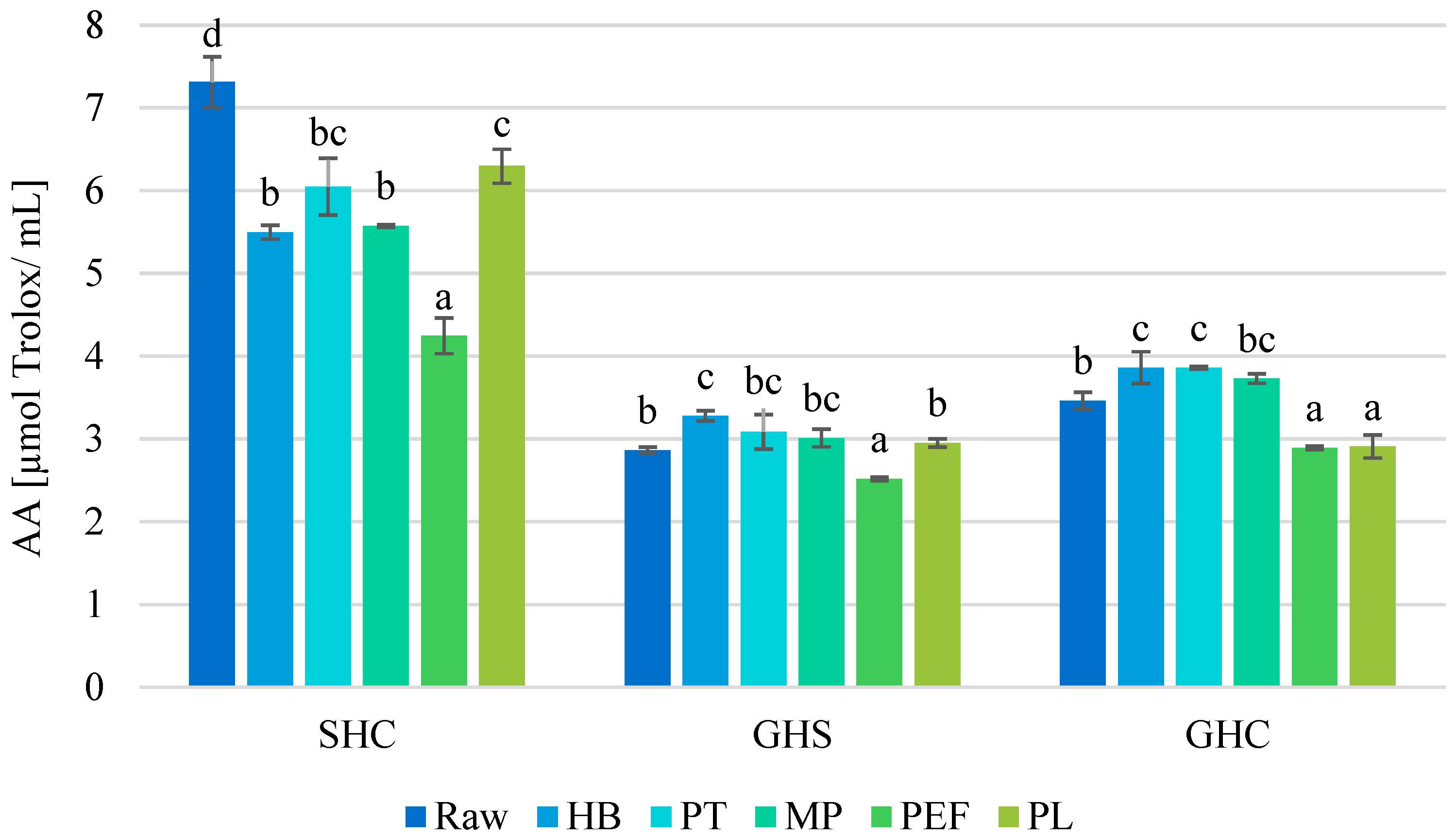
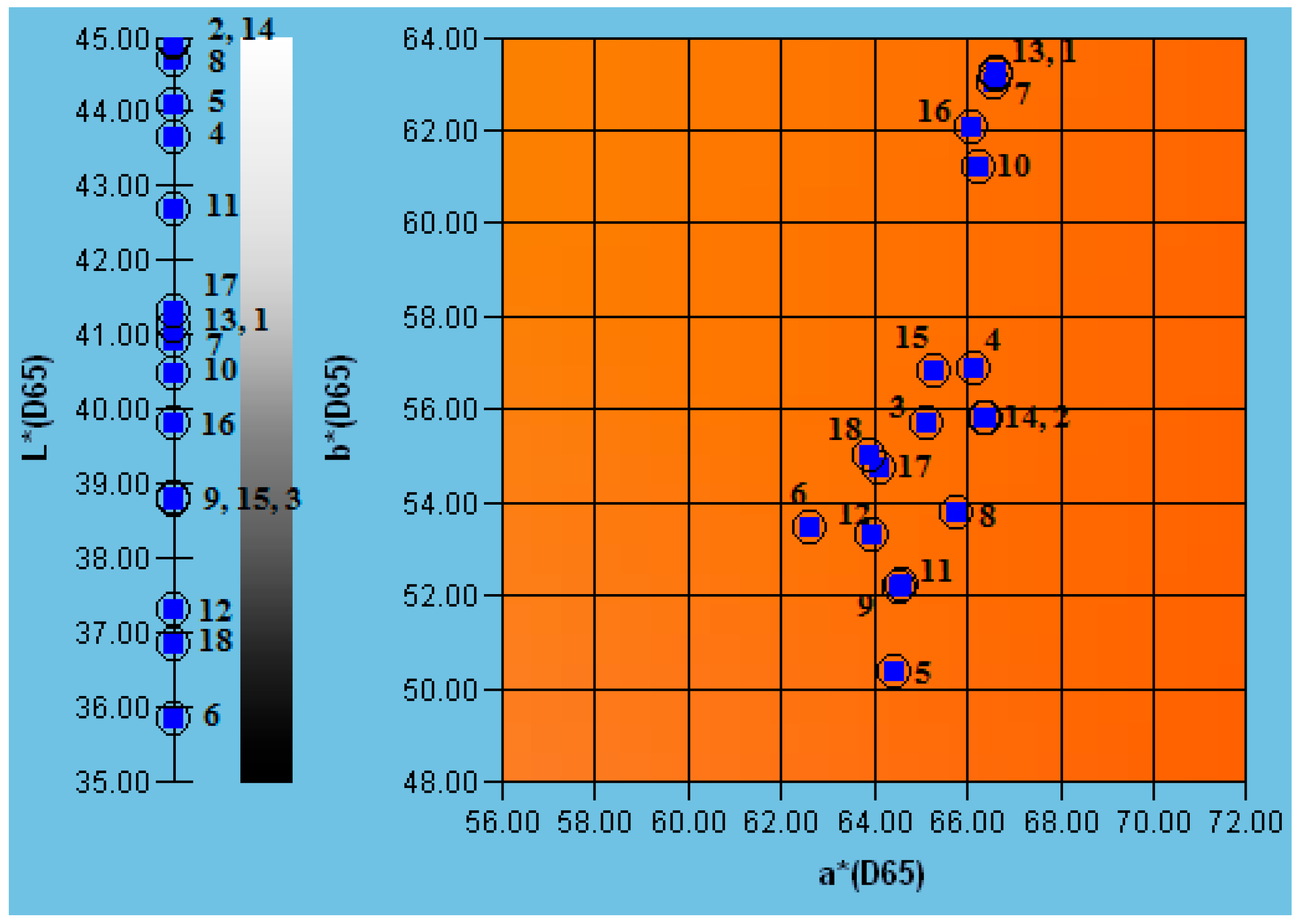
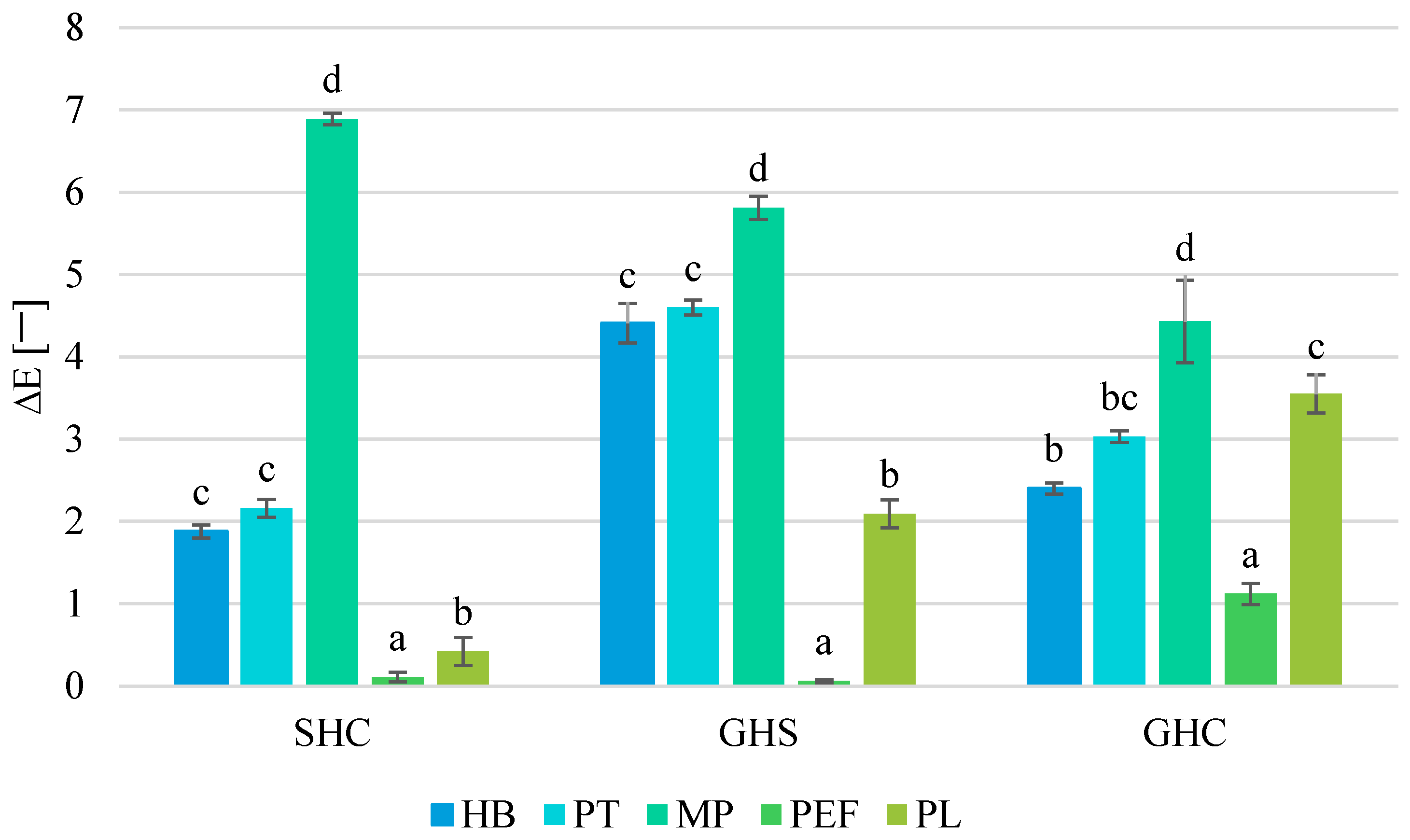
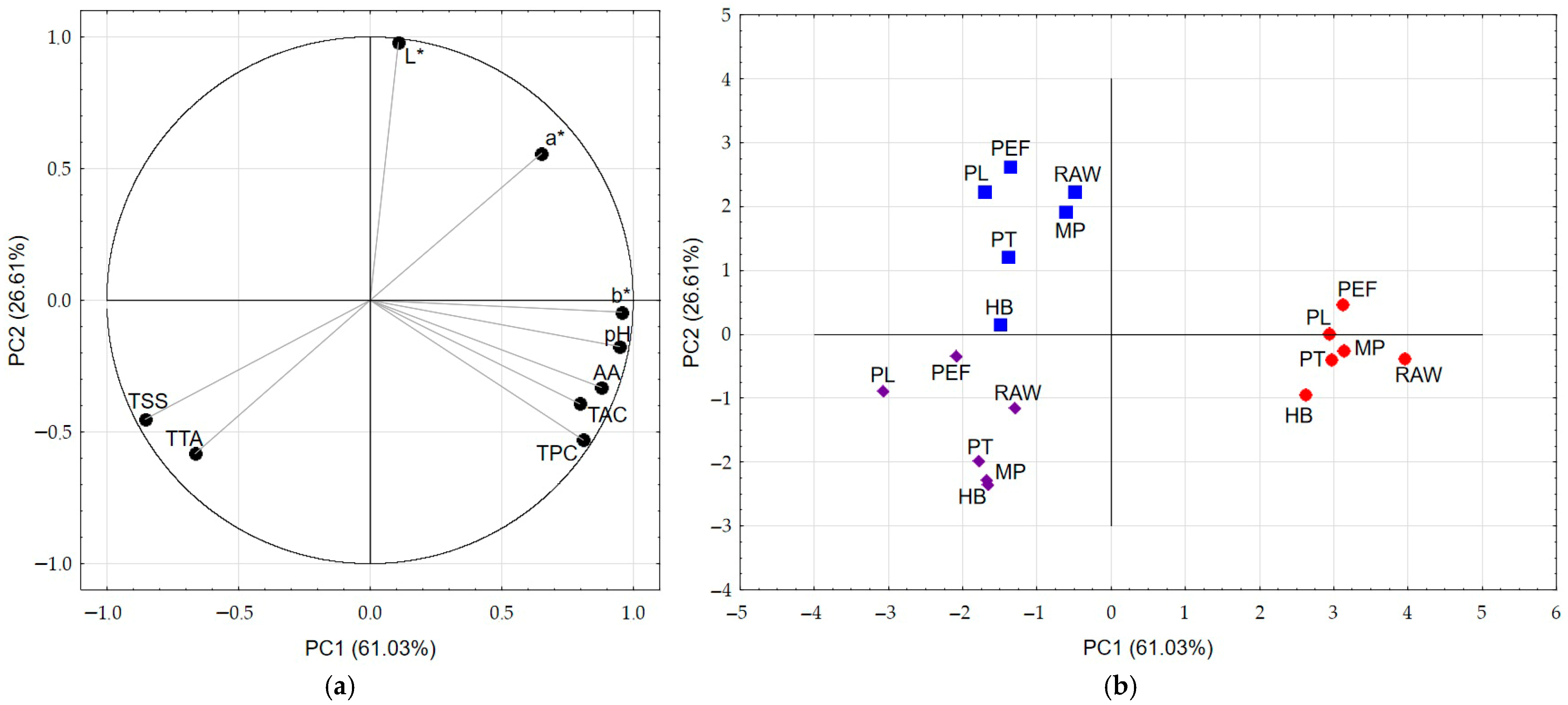
| Juice | Process | TTA [% Citric Acid] | pH [−] | TSS [°Brix] | NT [°NTU] |
|---|---|---|---|---|---|
| SHC | Raw | 1.28 ± 0.01 ab | 3.66 ± 0.00 a | 10.3 ± 0.1 c | 387 ± 3 b |
| HB | 1.33 ± 0.00 b | 3.65 ± 0.00 a | 10.7 ± 0.0 d | 577 ± 19 e | |
| PT | 1.30 ± 0.01 ab | 3.66 ± 0.01 a | 10.5 ± 0.1 c | 510 ± 14 d | |
| MP | 1.26 ± 0.01 a | 3.68 ± 0.00 a | 10.1 ± 0.0 a | 426 ± 9 c | |
| PEF | 1.26 ± 0.05 a | 3.66 ± 0.00 a | 10.3 ± 0.1 b | 390 ± 7 b | |
| PL | 1.29 ± 0.01 ab | 3.68 ± 0.00 a | 10.7 ± 0.0 d | 356 ± 2 a | |
| GHS | Raw | 1.29 ± 0.01 ab | 3.39 ± 0.00 a | 11.2 ± 0.1 c | 377 ± 7 a |
| HB | 1.33 ± 0.00 c | 3.38 ± 0.00 a | 11.4 ± 0.0 d | 1280 ± 24 e | |
| PT | 1.29 ± 0.01 ab | 3.40 ± 0.00 a | 11.2 ± 0.0 c | 992 ± 62 d | |
| MP | 1.26 ± 0.01 a | 3.42 ± 0.00 a | 11.0 ± 0.0 a | 795 ± 3 c | |
| PEF | 1.31 ± 0.03 bc | 3.40 ± 0.00 a | 11.1 ± 0.0 b | 468 ± 14 b | |
| PL | 1.31 ± 0.00 bc | 3.41 ± 0.00 a | 11.4 ± 0.0 d | 421 ± 4 ab | |
| GHC | Raw | 1.37 ± 0.01 b | 3.42 ± 0.00 a | 12.4 ± 0.0 b | 388 ± 5 a |
| HB | 1.39 ± 0.02 bc | 3.42 ± 0.00 a | 12.5 ± 0.1 c | 1067 ± 58 d | |
| PT | 1.37 ± 0.01 b | 3.43 ± 0.00 a | 12.5 ± 0.0 c | 878 ± 11 b | |
| MP | 1.33 ± 0.01 a | 3.43 ± 0.01 a | 12.2 ± 0.0 a | 961 ± 14 c | |
| PEF | 1.37 ± 0.00 b | 3.42 ± 0.00 a | 12.4 ± 0.0 b | 403 ± 7 a | |
| PL | 1.40 ± 0.00 c | 3.43 ± 0.00 a | 12.7 ± 0.0 d | 452 ± 8 a |
| Juice | Process | Cy-3,5-O-diglc | Dp-3-O-glc | Cy-3-O-gal | Cy-3-O-glc | Cy-3-O-rut | Cy-3-O-xyl | Pg-3-O-glc | Pg-3-O-ara | Pn-3-O-glc |
|---|---|---|---|---|---|---|---|---|---|---|
| SHC | Raw | 3.5 ± 0.1 b | n.d. | 6.6 ± 0.5 ab | 55.0 ± 1.0 b | 4.5 ± 0.1 b | 3.4 ± 0.1 b | 14.0 ± 0.1 b | 0.8 ± 0.0 a | 1.0 ± 0.0 b |
| HB | 3.2 ± 0.1 ab | n.d. | 7.1 ± 0.2 b | 53.3 ± 0.9 b | 4.3 ± 0.1 b | 3.3 ± 0.0 ab | 13.8 ± 0.2 b | 0.8 ± 0.0 a | 1.0 ± 0.0 b | |
| PT | 3.2 ± 0.2 ab | n.d. | 6.9 ± 0.6 ab | 50.9 ± 4.1 ab | 4.2 ± 0.4 ab | 3.2 ± 0.4 ab | 13.3 ± 1.1 ab | 0.8 ± 0.1 a | 0.9 ± 0.1 a | |
| MP | 3.0 ± 0.1 a | n.d. | 6.4 ± 0.1 ab | 48.0 ± 1.0 a | 3.8 ± 0.1 a | 3.0 ± 0.06 a | 12.1 ± 0.2 a | 0.7 ± 0.0 a | 0.9 ± 0.0 a | |
| PEF | 3.4 ± 0.0 b | n.d. | 6.8 ± 0.4 ab | 53.8 ± 1.4 b | 4.3 ± 0.1 b | 3.3 ± 0.1 b | 13.6 ± 0.3 b | 0.7 ± 0.0 a | 0.9 ± 0.0 a | |
| PL | 3.4 ± 0.1 b | n.d. | 6.1 ± 0.3 a | 52.9 ± 0.6 ab | 4.3 ± 0.1 ab | 3.3 ± 0.0 ab | 13.5 ± 0.2 b | 0.7 ± 0.0 a | 1.0 ± 0.0 b | |
| GHS | Raw | 3.8 ± 0.1 c | 0.5 ± 0.0 d | n.d. | 44.4 ± 1.7 b | 3.9 ± 0.2 c | n.d. | 7.9 ±0.2 d | 0.5 ± 0.1 b | 1.9 ± 0.1 c |
| HB | 2.9 ± 0.0 bc | 0.2 ± 0.0 ab | n.d. | 43.9 ± 0.6 b | 3.7 ± 0.0 c | n.d. | 5.9 ±0.1 c | 0.4 ± 0.0 a | 1.4 ± 0.0 b | |
| PT | 2.6 ± 0.0 ab | 0.2 ± 0.0 ab | n.d. | 37.9 ± 0.6 b | 3.2 ± 0.1 bc | n.d. | 5.1 ± 0.1 abc | 0.3 ± 0.0 a | 1.2 ± 0.0 ab | |
| MP | 2.8 ± 0.1 b | 0.2 ± 0.0 a | n.d. | 42.8 ± 0.8 b | 3.5 ± 0.1 c | n.d. | 5.5 ± 0.1 bc | 0.3 ± 0.0 a | 1.3 ± 0.0 b | |
| PEF | 2.0 ± 0.6 ab | 0.3 ± 0.0 bc | n.d. | 22.9 ± 10.6 a | 2.1 ± 0.8 ab | n.d. | 4.2 ± 1.0 ab | 0.3 ± 0.0 a | 1.0 ± 0.2 ab | |
| PL | 1.9 ± 0.5 a | 0.4 ± 0.1 c | n.d. | 18.4 ± 7.9 a | 1.7 ± 0.7 a | n.d. | 3.9 ± 0.9 a | 0.3 ± 0.0 a | 0.9 ± 0.2 a | |
| GHC | Raw | 4.0 ± 0.2 c | 0.7 ± 0.2 b | 16.0 ± 0.9 b | 38.6 ± 2.1 b | 3.5 ± 0.2 b | 6.9 ± 0.4 b | 0.6 ± 0.0 d | n.d. | 2.0 ± 0.1 d |
| HB | 3.0 ± 0.0 b | 0.3 ± 0.0 a | 16.2 ± 0.2 b | 39.8 ± 0.3 b | 3.3 ± 0.0 b | 7.1 ± 0.1 b | 0.4 ± 0.0 c | n.d. | 1.4 ± 0.0 c | |
| PT | 2.7 ± 0.0 b | 0.3 ± 0.0 a | 14.0 ± 0.2 b | 35.0 ± 0.5 b | 3.0 ± 0.0 b | 6.1 ± 0.1 b | 0.4 ± 0.0 bc | n.d. | 1.2 ± 0.1 bc | |
| MP | 2.9 ± 0.0 b | 0.2 ± 0.0 a | 16.0 ± 0.2 b | 39.0 ± 0.5 b | 3.2 ± 0.0 b | 7.1 ± 0.1 b | 0.4 ± 0.0 bc | n.d. | 1.3 ± 0.0 c | |
| PEF | 1.9 ± 0.5 a | 0.3 ± 0.1 a | 7.4 ± 2.8 a | 18.3 ± 6.7 a | 1.7 ± 0.6 a | 3.3 ± 1.2 a | 0.3 ± 0.0 a | n.d. | 0.9 ± 0.2 ab | |
| PL | 1.6 ± 0.4 a | 0.3 ± 0.1 a | 5.2 ± 2.2 a | 12.9 ± 5.4 a | 1.3 ± 0.5 a | 2.3 ± 1.0 a | 0.3 ± 0.1 a | n.d. | 0.8 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, N.; Kalisz, S.; Wiktor, A.; Kruszewski, B. The Influence of PEF, Pulsed Light, Microwave and Conventional Heat Treatments on Quality Parameters of Berry Fruit Juice Blends. Appl. Sci. 2025, 15, 9234. https://doi.org/10.3390/app15179234
Polak N, Kalisz S, Wiktor A, Kruszewski B. The Influence of PEF, Pulsed Light, Microwave and Conventional Heat Treatments on Quality Parameters of Berry Fruit Juice Blends. Applied Sciences. 2025; 15(17):9234. https://doi.org/10.3390/app15179234
Chicago/Turabian StylePolak, Natalia, Stanisław Kalisz, Artur Wiktor, and Bartosz Kruszewski. 2025. "The Influence of PEF, Pulsed Light, Microwave and Conventional Heat Treatments on Quality Parameters of Berry Fruit Juice Blends" Applied Sciences 15, no. 17: 9234. https://doi.org/10.3390/app15179234
APA StylePolak, N., Kalisz, S., Wiktor, A., & Kruszewski, B. (2025). The Influence of PEF, Pulsed Light, Microwave and Conventional Heat Treatments on Quality Parameters of Berry Fruit Juice Blends. Applied Sciences, 15(17), 9234. https://doi.org/10.3390/app15179234








