Abstract
Due to the degradative effect of the traditional pasteurization process related to the long exposure of high temperatures to the food matrix, alternative methods of food preservation are being investigated. In the case of liquid fruit products, unconventional thermal and non-thermal methods can be used for this purpose. The aim of the study was to evaluate the effect of various preservation methods: conventional pasteurization (PT), microwave pasteurization (MP), hot bottling (HB), pulsed electric field (PEF) and pulsed light (PL) on selected quality parameters of mixed juices. In the studied samples, extract (TTS), active acidity (pH), titratable acidity (TTA), nephelometric turbidity (NT), total polyphenol content (TPC), color parameters and antioxidant activity (AA) were determined. Qualitative and quantitative chromatographic analysis of anthocyanins was also performed. The different influence of the preservation methods and the raw materials used on the individual characteristics was demonstrated. The TTS and TTA changes did not exceed 4%, while no changes in pH were observed. Thermal methods increased turbidity significantly, with HB increasing it to the greatest extent. Non-thermal methods caused greater degradation of TPC, anthocyanins, and AA, while they caused significantly less color change. The microwave pasteurization resulted in an increase in TPC in two out of three studied juice blends. Based on the obtained results, it can be concluded that thermal methods allowed for the preservation of a greater amount of bioactive compounds, which translates into a potentially greater health-promoting value of the produced juice blends.
1. Introduction
Nowadays, the consumer is looking for products with high nutritional value. Plant raw materials and plant-based products, including juices, provide many compounds needed for the daily functioning of the body and the prevention of civilization diseases. Juices in their composition contain mainly water (80–90%) essential for the human body, but most importantly, they contain numerous phytonutrients such as vitamins, carotenoids, polyphenols, minerals, organic acids, ellagitannins and more [1]. Food products with high nutritional content, which at the same time the consumer can keep for a longer period of time, are becoming essential.
Fruits such as blue honeysuckle berry, black chokeberry, strawberries and grapes are a valuable and desirable raw material for juice production, due to their distinctive and desirable taste qualities, as well as their health-promoting benefits. Blue honeysuckle berries, chokeberries or strawberries are good sources of vitamin C and polyphenolic compounds, mainly anthocyanins [2,3,4]. Grapes are also a valuable source of polyphenols, especially resveratrol [5]. Long-term consumption of products rich in polyphenolic compounds has a proven disease-mitigating effect: protecting against type 2 diabetes, neurodegenerative diseases, cardiovascular diseases, osteoporosis and other conditions [3].
Of particular interest are anthocyanins, which not only have health benefits but also act as colorants in the product. They are a group of phenolic compounds commonly found in berries, such as those used in this study. They can change color, ranging from red through purple to blue, depending on pH. They are derivatives of anthocyanidins such as cyanidin, pelargonidin, petunidin, peonidin, delphinidin and malvidin. Anthocyanins are sensitive to various factors, such as light, temperature, oxygen, enzymes, metal ions and others, hence the importance of selecting appropriate processing methods [6,7,8,9]. They have numerous health-promoting properties—antioxidant, anti-inflammatory, anti-obesity, anti-cancer and antibacterial properties. They support vision and cardiovascular health, and also improve brain function and skin health. The pharmacological action of anthocyanins also includes an effect on gastric mutation, hypotensive, nonalcoholic fatty liver and Alzheimer’s syndrome [9,10].
Optimal preservation methods are being searched to obtain safe, durable and attractive products. In the range of interest are modern preservation methods, which, thanks to innovative physical phenomena, degrade beneficial compounds in food to a lesser extent in comparison to traditional heat-based preservation methods, commonly used in industry, such as hot bottling (HB) and high temperature-short time (HTST) or ultra-high temperature (UHT). Among these optimal innovative preservation methods, the following are of interest to the food industry: pulsed electric field (PEF), pulsed light (PL) or microwave flow pasteurization (MP).
PEF, PL and MP are processing methods that can affect small molecules differently, such as polyphenols and vitamins, as well as their degradation kinetics, in a varied matrix set [11,12,13]. Juice blend from apples, carambola and black grapes treated with PL contained less vitamin C and polyphenols than non-processed samples by 4–22% and 10–17%, respectively. However, the literature reports also that these methods can enhance the biological value of products by extracting compounds from the food matrix. Martins et al. [14] and Bansal et al. [15] showed that microwave-pasteurized orange juice–milk drink and PEF-treated gooseberry juice, respectively, contained more polyphenols than the unprocessed samples. The bidirectional effect of MP depending on the food matrix was shown by Piasek et al. [16]. MP caused an increase in polyphenol content in blue honeysuckle berry juice, while a decrease in black chokeberry juice. Also, Marszałek et al. [17] showed an increase in polyphenol content in strawberry puree after MP preservation.
To date, there is a gap in the literature regarding studies comparing the effects of conventional heat treatment (hot bottling HB and pasteurization PT), PEF, MP and PL methods on the quality of blended fruit juices. In addition, there are only a few scientific reports regarding the possibility of using PL and MP to preserve fruit juices. Considering the situation that the same preservation methods can affect other food matrices differently, it becomes important to analyze all products and customize the best processes and conditions to obtain the best quality.
The aim of the study was to investigate the effect of different methods (hot bottling, conventional heat pasteurization, flow microwave pasteurization, pulsed electric field, pulsed light) on the quality of three blended juices made from strawberry, blue honeysuckle berry, chokeberry and grapes. The scope of the study included obtaining the juices and their blending, processing and then analyzing key physicochemical parameters and the content of selected bioactive compounds, as well as antioxidant activity to help find the most optimal preservation method and most promising formulation of juice.
2. Materials and Methods
2.1. Materials
The fruits of the blue honeysuckle berry (L. caerulea L. var. kamtschatica Sevast., Wojtek variety), black chokeberry (Aronia melanocarpa [Michx.] Elliott, Nero variety), dark grape (Vitis vinifera L., Nero variety) and strawberry (Fragaria x ananassa Duchesne, Rumba variety) were obtained from the plantations in central Poland. The fruits were collected from farmland, cleaned of inedible parts and impurities, next immediately frozen and stored at −23 °C until juice’s production.
2.2. Reagents and Standards
Folin–Ciocalteu reagent, sodium carbonate, sodium hydroxide, gallic acid, formic acid, Trolox and DPPH were reagent grade. Acetonitrile and methanol used in the study were of HPLC grade. All chemicals were purchased from one vendor (Merck KGaA, Darmstadt, Germany). For HPLC analysis ultra-pure water of 18.2 MΩ cm resistivity was used (Milli-Q® system, Merck Millipore, Darmstadt, Germany).
2.3. Juices Production Technology and Composition
Raw juices were obtained by cold pressing on the HP 14 compact hydraulic laboratory press BUCHER Unipectin AG. Three blends of juices based on previously performed organoleptic evaluation were composed in the following variants: SHC—60% strawberry juice, 30% blue honeysuckle juice, 10% chokeberry juice; GHS—50% grape juice, 25% blue honeysuckle juice, 25% strawberry juice; GHC—50% grape juice, 25% blue honeysuckle juice, 25% chokeberry juice.
Next, prepared juices were preserved using the following conventional and unconventional methods: hot bottling, pasteurization, flow microwave pasteurization, pulsed electric fields and pulsed light.
2.4. Juice Preservation Methods
Hot bottling (HB) was performed at 95 °C. Briefly, mixed juices were heated on Thermomix Vorwerk device and then sterile jars were filled immediately with hot juice and twisted, then set aside to cool down.
To pasteurize the juices (PT), sealed jars filled with juice at room temperature were heated for 10 min in a 90 °C water bath. Then, jars were cooled to 20 °C in the water bath.
Juices were also preserved using a flow microwave pasteurizer (MP) model W0314 (WEINDICH Sp. z o.o., Chorzów, Poland) using parameters: 85 °C, generator power 2400 W, flowrate 0.5 L1/min, with implemented tubular heat exchanger. After the MP process, the juice was transferred aseptically to jars in laminar chamber FT83 Sterile Filling System (Armfield Ltd., Ringwood, United Kingdom) equipped with HEPA filters.
Another method of juice blends preservation was the pulsed electric fields (PEF) process performed on PEFPilot™ Dual System (Elea Vertriebs und Vermarktungsgesellschaft mbH, Quakenbrück, Germany). Juice samples were placed in a chamber that included two parallel stainless-steel electrodes. The parameters of the PEF were electric field strength was 6 kV/cm, pulse frequency was at 20 Hz, and the pulse width took the value of 7 µs. Different numbers of pulses and the amount of specific energy were used for individual juice blends, respectively, for SHC—52 and 114.25 kJ/kg, GHS—61 and 105.63 kJ/kg, GHC—61 and 109.40 kJ/kg. During this experiment, the temperature of samples was below 25 °C. Next jars were filled immediately and twisted.
Samples of juices on Petri dishes were preserved using the XENON™ X-1100 High-Intensity Pulsed Light System (USA) (PL). The parameters of the pulsed light were 35 pulses, optical energy of a single pulse 1 J/cm2 and a voltage 2000 V. Next, jars were filled immediately and twisted. The juice blend temperatures after processing were 27.8 °C for SHC, 28.3 °C for GHS and 32.2 °C for GHC.
2.5. Analytical Methods
2.5.1. Total Soluble Solids (TSS), Active Acidity (pH) and Total Titratable Acidity (TTA) Measurements
Total soluble solids (TSS) were determined using digital refractometer Refracto 30PX (Mettler Toledo, Columbus, OH, USA) according to device manual, and expressed as °Brix. Active acidity (pH) and total titratable acidity (TTA) were measured using the titrator TitroLine® 5000 (Xylem Analytics Germany Sales GmbH & Co. KG., Weilheim in Oberbayern, Germany). For titration, the 0.1 M sodium hydroxide solution was used, and a final pH of 8.1 was always reached. The results of TTA were expressed as g of citric acid per 100 mL of juice.
2.5.2. Nephelometric Turbidity (NT)
The 2100Q portable turbidimeter was used for nephelometric turbidity (NT) determination. The juice samples were placed in a 15 mL glass cuvette up to the mark, and the measurement at 20 °C was made. Direct turbidity was expressed in nephelometric turbidity units (°NTU). Before measurement, the device was calibrated against formazin turbidity standards.
2.5.3. Total Polyphenols Content (TPC)
The Folin–Ciocalteu reagent’s method for measurement of total phenolic content (TPC) in samples was used [18]. Dilutions of the juices were made so that the absorbance fell within the determined standard curve. The reaction mixture was composed of 0.1 mL of sample solution, 0.2 mL of Folin–Ciocalteu reagent, 1 mL of 15% sodium carbonate and 2 mL of redistilled water. The reaction mixture was incubated for 60 min in a dark place, and after that, the absorbance was measured using UV–Vis spectrophotometer (UV1650PC, Shimadzu Corp., Kyoto, Japan) at wavelength λ = 765 nm against a mixed reagent. The TPC content in samples was expressed as mg gallic acid equivalents (GAE) per 100 mL of juice.
2.5.4. HPLC Analysis of Anthocyanins
For anthocyanin determination, the HPLC with PDA detector (Shimadzu, Japan) was used [19]. Juices were centrifuged (MPW-350R, MPW Med. Instruments, Warsaw, Poland) in Eppendorf tubes under 23,384× g, 10 min, at 4 °C. For analysis, the supernatant was taken, previously filtered through 0.45 µm PTFE filters (Phenomenex, Torrance, CA, USA). We used a Luna 5 μm C18(2) 250 × 4.6 mm column (Phenomenex LTD Deutschland, Aschaffenburg, Germany), conditioned at 25 °C. The mobile phase was a mixture of water, acetonitrile and formic acid in the ratio 81:9:10 (v/v/v), working in a continuous flow of 1 mL/min. A single analysis lasted 30 min. Identification of anthocyanin compounds was performed on the basis of standard chromatograms. All chromatograms were recorded at λ = 520 nm. The individual anthocyanin content was expressed as mg anthocyanin per 100 mL of juice. Limit of quantification (LOQ) for all anthocyanin monomers was 0.15 mg/100 mL. The total amount of anthocyanins (TAC) was expressed as mg cyaniding-3-O-glucoside per 100 mL sample.
2.5.5. Antioxidant Activity Measurements—DPPH Assay (AA)
Antioxidant activity (AA) of juices was determined with DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals [7]. The reaction mixture was made with 1 mL of sample dilution, 3 mL of methanol, and 1 mL of DPPH radicals solution. After 10 min of incubation in a dark place, the absorbance at λ = 517 nm was measured (UV1650PC, Shimadzu, Japan). The AA was expressed as µmol of Trolox equivalents (TE) per 100 mL of juice.
2.5.6. Color Measurement
Color parameters in the CIE L*, a* and b* scale were determined using 3600-d device (Konica Minolta, Tokyo, Japan) in the mode of transmitting light. The illuminant was D65, the observer 10° and the port size 25.4 mm. Moreover, the total color differences between treated and non-treated samples were calculated using equation
2.6. Statistical Analyses
All measurements were carried out in triplicate. Statistical analysis was performed in Statgraphics Centurion 19 program using multivariate ANOVA and Tukey’s test in order to check the significance of the differences between preservation methods and juice’s variants, with the significance level α = 0.05. Moreover, the Spearman’s rank correlation test (R) was used to assess the correlation between the content of polyphenols and anthocyanins, or between the content of bioactive components and color parameters and antioxidant activity. The obtained data were used in the principal component analysis (PCA) performed in Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA, USA). PCA was performed in order to comprehensively show the changes in physicochemical and bioactive profiles in all three juice blends processed with different thermal and non-thermal methods. The gathered data were qualified for PCA analysis based on a correlation score with the first or second principal component (PC) of at least 0.6 [20]. As a result of this classification, turbidity (NT) values were rejected (correlation factor 0.5 and 0.4 with the first and second PC).
3. Results and Discussion
3.1. Effect on Total Soluble Solids
The preservation method was shown to have a slight effect on TSS value (Table 1). The only repeatable observed trend was a slight decrease in the TSS of juices processed by the MP method. Other heat-based methods like HB and PT caused a slight increase in the TSS parameter, which could be due to the extraction of water-soluble compounds under heat. In the case of the PL method, the slight increase in TSS of the juices may have been related to the large surface area of the sample on Petri dishes, which may have contributed to greater water loss and sample thickening.

Table 1.
Total titratable acidity (TTA), pH, total soluble solids (TSS) and turbidity (NT) in raw and preserved juices.
Mannozzi et al. [21] and Aaby et al. [22] reported that heating carrot, apple and strawberry juices significantly increased their extract. The researchers claim that the increase in TSS value may be due to the structural breach of the raw material’s tissues as a result of heating, and thus a greater release of organic acids and sugars, which are the main components of the extract. However, Mandha et al. [23] studying watermelon and mango juices, did not observe significant changes in TSS content at different pasteurization times (1–15 min). This creates the need for further research to justify obtaining different results in various studies.
Chakraborty et al. [24] showed that there was no significant effect of conventional pasteurization and pulsed light on the TSS of a mixed fruit beverage. Bhagat and Chakraborty [25], studying pomegranate juice, also noted the lack of change in TSS value due to the effects of PL with different parameters. De Souza et al. [26] and Biancaniello et al. [27] report that UV-C light treatment also did not significantly affect the extract of lemonade, citrus juice and green cold-pressed juices. At the same time, they indicate that the thickness of the layer of juice subjected to processing, as well as the time of exposure to PL, influences the direction of changes in the extract, causing it to decrease, increase or stay constant.
In our study, the PEF method did not affect the extract content of any juice. Studies of Wibowo et al. [28] on cloudy apple juice, Bansal et al. [15] on gooseberry juice, Aadil et al. [29] on grapefruit juice and Carbonell-Capella et al. [30] on fruit drink confirm our findings in this regard.
3.2. Effect on Active and Total Titratable Acidity
One of the most important parameters of fruit and vegetable products is pH, which enables quality control, including the selection of appropriate preservation parameters. In addition, it can affect the stability of various bioactive compounds like valuable pigments and antioxidants. In the conducted study, no significant effect of preservation method on pH was observed in all three juices (Table 1). Other studies [15,21,31] showed that PEF statistically does not change the pH of fruit and vegetable juices. Bhagat and Chakraborty [25] reported no significant effect of pasteurization and PL on the pH of pomegranate juice.
Minor changes in TTA were observed in all types of juices in most trials (Table 1). Small changes in TTA are beneficial from the tasters’ point of view—it allows the original flavor of the product to be preserved. The titratable acidity of a product is a feature closely related to the flavor profile of the product, and thus can affect consumer acceptance. Organic acids in a food product are also expected to participate in ensuring adequate shelf life and safety [32]. Also, other researchers [28,29,31] confirm the lack of significant effect of PEF on TTA in different juices. Likewise, PL as well as PT did not significantly affect TTA of different fruit juices according to the literature [25,33].
3.3. Effect on Nephelometric Turbidity
During fruit pressing, macromolecular compounds such as proteins or polysaccharides (for example cellulose, hemicellulose, starch, lignin, pectin substances), as well as polyphenolic compounds (especially tannins), or their condensation products enter the juice [34]. The polyphenols present in juices can react with proteins (through electrostatic, hydrophobic interactions, and hydrogen bonding) or polysaccharides (via non-covalent interactions—hydrophobic, hydrogen and ionic binding—but also dedicated to electrostatic interactions between oppositely charged fragments, i.e., positively charged hydroxyl groups of polyphenols and negatively charged groups of pectins) [34].
All the mentioned compounds are responsible for turbidity and sediment formation in juices. Seed fragments, peels or other parts of the raw material can also form a sediment layer [35]. As consumers prefer clear juices, it is worth studying the effect of preservation methods on the transformation of turbidity and the compounds responsible for it.
Thermal processes HB, PT and MP significantly increased turbidity of juices, in comparison to raw ones. The HB method increased turbidity to the greatest extent, followed by PT, then MP processing (Table 1). In each of these processes, the mechanism for the effect of preservation on turbidity can be considered to be similar (providing an adequate dose of heat). In each of the juices, there was a significant increase in turbidity due to thermic processes—in SHC by 10–49%, in GHS by 111–239% and in GHC by 126–175%. Such changes can lead to lower consumer acceptance of the finished product. Türkyılmaz et al. [36] studied the effect of pasteurization on pomegranate juices and observed that heating significantly increased turbidity. On the other hand, Riganakos et al. [37] indicated no significant change in turbidity after thermal preservation of carrot juice. The treatment temperatures used by these researchers were significantly lower (65 °C) than those used in our study (85–95 °C), hence the likely difference in results.
Among all the PEF or PL-treated juice variants analyzed, only the GHS juice showed a statistically significant increase in turbidity up to 24% of the initial value. In other cases, these non-thermal methods did not significantly increase the turbidity, which could be related to keeping the product temperature below 40 °C. This allowed reducing the degrading effects of heat on macromolecular compounds and polyphenols. However, other researchers [38] have examined the effects of PEF preservation on red bell pepper juice and observed an increase in turbidity up to 25–48%, depending on the amount of energy applied. Fabroni et al. [31] and Wibowo et al. [28] indicated that PEF has no significant effect on the turbidity of bloody orange juice and unclarified apple juice, respectively. Fabroni et al. [31] suggest that the associated electroporation and dielectric breakdown of the juice cells do not adversely affect the turbidity of the studied fruit juice. There are only a few references in the literature about the effect of PL on turbidity. Referring to the effect of PL, researchers have used a continuous flow UV system to determine its effect on white grape juices, which resulted in a small increase in turbidity, by less than 2% [39]. Fenoglio et al. [40] examining mixed orange-mandarin juice, and Riganakos et al. [37] examining carrot juice, found no significance of the effects of UV light and PL on juice turbidity.
3.4. Effect on Total Polyphenols Content
The highest content of total polyphenols (TPC) among raw juices was found in SHC juice (168 ± 1 mg/100 mL). GHC and GHS juices contained less polyphenols: 121 ± 2 and 101 ± 4 mg/100 mL, respectively.
TPC changes in juices are shown in Figure 1. In the case of SHC juice, all methods except MP resulted in a significant decrease in TPC—from 2% after PT processing, up to 23% after PL treatment. For grape-containing juices, thermal methods caused a small increase in TPC. In the case of GHS, this was an increase of 6%, 5% and 3% for HB, PT and MP methods, respectively, while in GHC juice, there were larger changes noted: 8.8%, 9.4% and 5.6%, respectively. Based on the observations, it is possible to conclude the relevance of the components of blended juices, and thus the quantity and quality of the present bioactive compounds. The level of the changes observed as a result of thermal preservation of juices incorporating strawberries are less favorable than those incorporating grapes. This indicates that the bioactive compounds of strawberry are less stable. In turn, comparing GHS and GHC, it can be concluded that blackcurrant share has a better effect on the preservation of polyphenols in the juice blend than strawberry share.
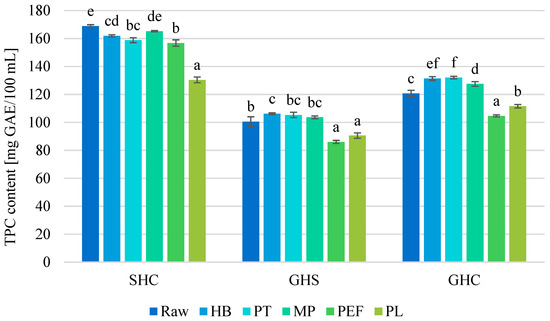
Figure 1.
Total polyphenolic content (TPC) [mg GAE/100 mL] in raw and preserved juices. SHC—strawberry–blue honeysuckle–chokeberry juice, GHS—grape–blue honeysuckle–strawberry juice, GHC—grape–blue honeysuckle–chokeberry juice; HB—hot bottling, PT—pasteurization, MP—flow microwave pasteurization, PEF—pulsed electric field, PL—pulsed light; small letters—homogenous groups depending on the preservation method in each of juices.
According to the literature, destruction of polyphenol structures, induction of isomerization, oxidation, and hydrolysis—overall chemical and physical processes—lead to the transformation of one compound into another, located in the same group of polyphenolic compounds. However, new polyphenolic compounds have different structures and properties [41]. This phenomenon is confirmed in our study by comparing the total content of polyphenols and anthocyanins in the juice samples. There was a noted increase or decrease in total polyphenol content depending on the matrix and preservation method, with total anthocyanin content always decreasing. Treatment, especially with heat, also leads to some level of enzyme inactivation such as peroxidase or polyphenol oxidase, resulting in the retention of more polyphenolic compounds in the food matrix [42]. The possibility of a positive effect of heat processing on TPC is confirmed by the results of our own studies for GHS and GHC juices. MP is the most promising technique in this respect. This could be related to the previously described possible structural transformation of phenolic compounds, but also to the extraction of these compounds from the food matrix (the samples were not completely clear; hence, the possible extraction of compounds from tissue residues in the juices). Therefore, in future studies, particularly those concerning MP, where literature data are limited, analyses should be expanded beyond TPC and chromatographic analysis of anthocyanins to include other classes of polyphenolic compounds in order to verify the possibility of degradation of some compounds and the formation of new complexes from their parts. Other researchers [43,44] also point to a positive effect of MP on TPC—they indicated an increase in TPC level by 127% in apple juice and by 20% in apple-based juices, respectively. In chokeberry juice, MP caused an increase in TAC and a decrease in TPC, while in blue honeysuckle berry juice, MP caused an increase in both TAC and TPC [16].
In every juice tested, it was observed that innovative non-thermal methods preserved bioactive components to a significantly worse degree. This may be related to the degrading effects of oxygen and the lack of proper inactivation of enzymes responsible for the oxidative transformation of polyphenols. The most sensitive juice was GHS, where a 14% and 10% decrease was found after PEF and PL processing, respectively. The negative effect of PL was also reported by Chakraborty et al. [24]—there was a 10–17% decrease in TPC in mixed fruit beverage. Vollmer et al. [45], comparing pasteurization and PL with different parameters, discovered that heating leads to an increase in TPC in contrast to the non-thermal PL. Some studies on the effects of food matrix pH and PL parameters indicate that the right combination of these factors can increase TPCs, and the wrong combination can degrade them [46]. Numerous studies indicate a neutral or positive effect of PEF on the content of the mentioned bioactive compounds [11,15,21,47]. This underlines definitively the importance of the type of food matrix on the behavior during processing and ultimately on the final quality of the product. Also, the use of inappropriate preservation conditions can carry adverse effects on the quality of food. The experiments highlight the need for further research to select the most optimal methods and their parameters that could favorably influence the content of polyphenols as important bioactive components of berry juices.
3.5. Effect on Anthocyanin Content
The mixed juices were subjected to a detailed analysis of anthocyanin compounds due to their therapeutic significance and the fact that strawberries, blue honeysuckle berries, grapes and chokeberries are valuable sources of these compounds.
In all three juices, a total of nine anthocyanin compounds were identified (Figure 2). The cyanidin-3,5-O-diglucoside (cy-3,5-O-diglc), cyanidin-3-O-glucoside (cy-3-O-glc), cyanidin-3-O-rutoside (cy-3-O-rut), pelargonidin-3-O-glucoside (pg-3-O-glc) and peonidin-3-O-glucoside (pn-3-O-glc) were determined in all samples. The delphinidin-3-O-glucoside (dp-3-O-glc) was present only in juices with grapes as their characteristic anthocyanin. The cyanidin-3-O-galactoside (cy-3-O-gal) and cyanidin-3-O-xyloside (cy-3-O-xyl) were identified in SHC and GHC juices, while pelargonidin-3-O-arabinoside (pg-3-O-ara) was identified in SHC and GHS juices.
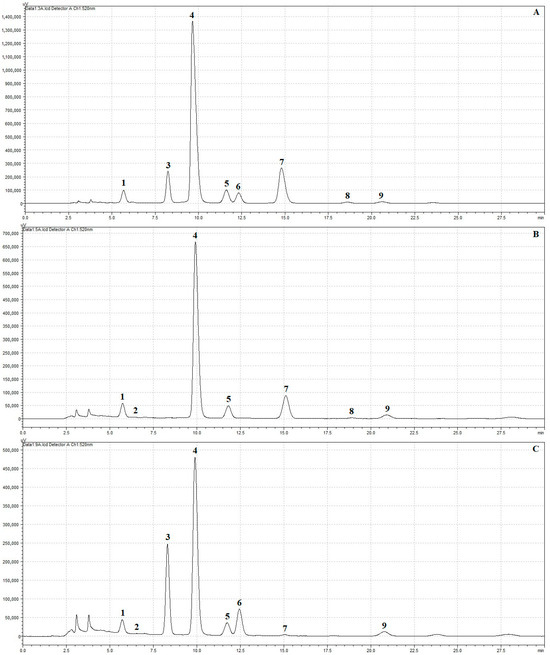
Figure 2.
HPLC chromatogram presenting the separation of anthocyanins (1—cyanidin-3,5-O-diglucoside, 2—delphinidin-3-O-glucoside, 3—cyanidin-3-O-galactoside, 4—cyanidin-3-O-glucoside, 5—cyanidin-3-O-rutinoside, 6—cyanidin-3-O-xyloside, 7—pelargonidin-3-O-glucoside, 8—pelargonidin-3-O-arabinoside, 9–peonidin-3-O-glucoside) in raw juices: (A) strawberry–honeysuckle berry–chokeberry juice (SHC), (B) grapefruit–honeysuckle berry–strawberry juice (GHS), (C) grapefruit–honeysuckle berry–chokeberry juice (GHC).
There was a high positive relationship (R = 0.84) between polyphenol and anthocyanin content in the analyzed juices (Table A1).
In raw SHC juice, anthocyanins accounted for 53% of the total number of polyphenols. Each preservation method (except MP) resulted in a neutral influence on the total anthocyanin content (TAC) share. In SHC juice, the cy-3-O-glc, pg-3-O-glc and cy-3-O-gal were present in the highest amounts (Table 2). These glycosides accounted for 61.1–62.2%, 15.6–16.0% and 7.1–8.2%, respectively, depending on the preservation method used.

Table 2.
Monomeric anthocyanin content [mg/100 mL juice] in raw and preserved juices.
In GHS juice, the anthocyanin profile was dominated by cy-3-O-glc, pg-3-O-glc and cy-3-O-rut. They accounted for 67.0–75.9%, 9.7–14.2% and 6.2–6.4% of the TAC, respectively, depending on processing method. Regardless of the preservation method, the share of anthocyanin compounds in the total pool of polyphenols in GHS juice decreased. In raw juice, they accounted for 65%. PL preservation yielded the largest decrease in the retention of anthocyanin compounds to 30% of total polyphenols, while HB resulted in the smallest decrease to 54.9% of the previous content.
Analyzing the composition of anthocyanins in GHC juice, it was revealed that cy-3-O-glc, cy-3-O-gal and cy-3-O-xyl predominated, contributing 52.2–55.9%, 21.2–22.8% and 9.5–10.1% of TAC, respectively, depending on the preservation method. The largest decrease in GHC juice occurred when PL was applied (to 22% of total polyphenols), while the smallest decrease occurred as a result of MP (to 55% of total polyphenols).
Comparing the changes in individual anthocyanin components, the preservation methods had different effects on different juice blends. In addition, it should be noted that in blends containing grape juice (GHS and GHC), the transformations of individual anthocyanins due to the performed methods were greater than in the case of strawberry-based juice (SHC). For example, the HB, PT, MP, PEF and PL methods caused degradation of cy-3,5-O-diglu in SHC by 8%, 6%, 14%, 2% and 2%, in GHS by 23%, 30%, 25%, 46% and 50%, while in GHC by 26%, 33%, 28%, 53% and 61%, respectively. For some of the anthocyanins (cy-3-O-gal, cy-3-O-glu, cy-3-O-xyl, pg-3-O-ara), the trend of changes varied depending on the type of juice. As a result of HB and MP processing, the content of cy-3-O-xyl decreased by 3% and 14% in SHC juice, while it increased by 4% in GHC juice.
For each juice variant, preservation methods never resulted in an increase in TAC (Figure 3). As polyphenolic compounds, anthocyanins are subject to various chemical and physical reactions leading to a change in their profile. SHC anthocyanins were the most stable of all the juices tested. Only MP processing resulted in a significant decrease in TAC of 12%. For GHS and GHC juices, the only significant changes came from using PEF and PL methods (a decrease in the range of 48–56% and 53–66%, respectively), while the best-preserved anthocyanin compounds were by HB. The results show that not only the juice components themselves, but also the method of production or preservation, determine the quality of the product.
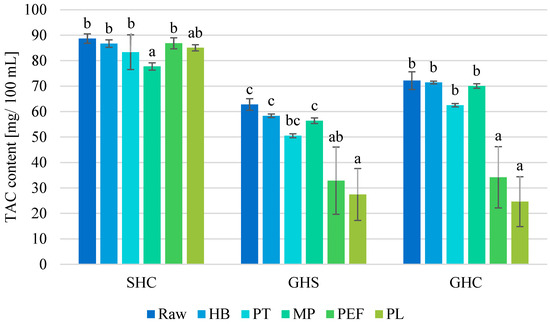
Figure 3.
Total anthocyanin content (TAC) [mg/100 mL] in raw and preserved juices. SHC—strawberry–blue honeysuckle–chokeberry juice, GHS—grape–blue honeysuckle–strawberry juice, GHC—grape–blue honeysuckle–chokeberry juice; HB—hot bottling, PT—pasteurization, MP—flow microwave pasteurization, PEF—pulsed electric field, PL—pulsed light; small letters—homogeneous groups depending on the preservation method in each of juices.
Study of clear and cloudy chokeberry juices, with or without ascorbic acid, cinnamon or clove extract, revealed that the amount of particular anthocyanin compounds, such as cy-3-O-ara, increased due to PT usage [48]. In our study, the application of PT reduced anthocyanin content by 6%, 20% and 13% in SHC, GHS and GHC juice, respectively. In the case of the MP technique, other researchers also observed a decrease in TAC but at only 10% of the baseline amount in apple–chokeberry juice [44]. PEF processing for SHC juice did not affect TAC. This is in line with the most frequently reported impact in a review published on this technology [11]. Other researchers have noticed a significant increase in total anthocyanins, explaining this as their extraction from tissue particles [49].
PL was the worst preservation method in terms of anthocyanin retention, although the magnitude of the impact varied depending on the composition of the juices. The aromatic acyl group of anthocyanins has the ability to absorb light energy and potentially release electrons due to the presence of the double bond. The main factors affecting the transformation of anthocyanin compounds associated with lighting are photolysis, oxygen-mediated quenching and also radical generation and attack. The proper presence of ascorbic acid could protect anthocyanins from photolysis under an air atmosphere. It is compared with the scavenging effects of this acid on the peroxyl radicals formed from anthocyanin compounds due to stress indicated by light. Lower pH also helps slow down the degradation associated with light exposure [6,8,9]. Our study, together with those available in the literature, highlights the need to research the impact of each preservation method on each new food matrix.
3.6. Effect on Antioxidant Activity
SHC juice had the highest antioxidant activity (AA) among raw juices (7.31 μmol TE/mL), while GHS had the lowest (2.87 μmol TE/mL). Considering the different juices and preservation methods, a varied effect on AA was observed (Figure 4). For SHC juice, there was a significant decrease in AA in all variants, while in GHS and GHC juices, thermal methods contributed to a partial increase in AA. For example, HB resulted in a 25% decrease in SHC juice, while in grape-based juices, GHS and GHC, the increase was 14% and 12%, respectively, was observed. The largest negative changes occurred as a result of PEF, where AA decreased by 42%, 12% and 16% in SHC, GHS and GHC juice, respectively.
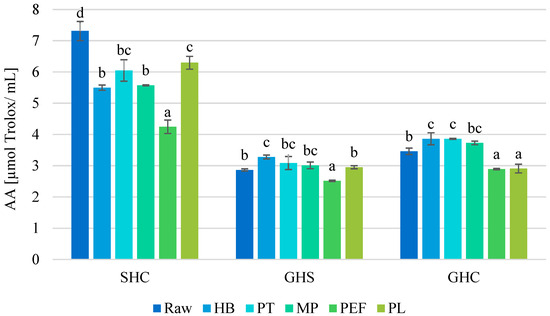
Figure 4.
Antioxidant activity (AA) [μmol Trolox/mL] in raw and preserved juices. SHC—strawberry–blue honeysuckle–chokeberry juice, GHS—grape–blue honeysuckle–strawberry juice, GHC—grape–blue honeysuckle–chokeberry juice; HB—hot bottling, PT—pasteurization, MP—flow microwave pasteurization, PEF—pulsed electric field, PL—pulsed light; small letters—homogeneous groups depending on the preservation method in each of the juices.
Significant positive correlations were found between TPC and AA (R = 0.91) and between TAC and AA (R = 0.88) (Table A1).
Based on the results, it can be stated that thermal methods variously affected AA depending on the food matrix. Diverse observations on the effect of heating methods on AA are presented in the literature. Duhan and Kar [50] indicated a decrease in AA due to both traditional and microwave pasteurization. Wójcik et al. [44] in apple–peach and apple–chokeberry juices showed a decrease in AA by 63% and 13%, respectively. Mandha et al. [23] reported no significant changes during 1–15 min pasteurization of fruit juices due to the lack of changes in polyphenol content. This may be related to thermal inactivation of enzymes responsible for the degradation of these compounds.
Considering non-thermal methods, other research also observed a decrease in AA at the level of 7–19% in apple juice preserved by PEF with different parameters [51]. Oziembłowski et al. [47] also observed a decrease in AA in juices from six different black chokeberry varieties, ranging from 3 to 29%. A significant increase in AA was measured in strawberry puree and kale juice, but there was no significant change in AA in the mix of these products [49].
PL differentially affected the antioxidant activity of juices in our study. In the SHC and GHC juices, a significant decrease of 14% and 16% was noted, respectively, while in GHS juice, a slight increase of 3% was measured. This phenomenon shows the influence of the type of matrix and the need to select the type and parameters of the preservation method based on the raw material used. That also shows published studies by other authors. Kwaw et al. [52] found that PL slightly increased the AA of lactic-acid-fermented mulberry juice by less than 4%. On the basis of pineapple juice analysis, it has been indicated that different PL parameters can lead to a reduction in AA by up to 14%, whereas standard pasteurization can reduce it by up to 27% [45]. UV-C light treatment caused no significant effect in citrus juice and lemonade, while in fruit–vegetable mixed juice, a significant decrease by 25% was observed [26].
3.7. Effect on Color
The color of food products is closely related to their content of pigments, coloring substances and also compounds whose transformations can affect this quality trait. Anthocyanins, flavonoids, carotenoids, betalains and other compounds contribute to fruit coloration. Berries are characterized by a significant content of anthocyanins—these compounds have colors ranging from red through purple to blue, depending on pH, as was mentioned before. In the literature can be found different values of color parameters for juices extracted from the same raw materials, which is directly related to the method of their extraction, the date of harvesting the fruit or other biotic and abiotic factors.
PT and HB have reduced the brightness (L*) of the juices (Figure 5). The observed darkening of the heated samples could have been due to the oxidoreductive enzymes or non-enzymatic browning processes, such as Maillard reactions, leading to the formation of brown-colored compounds [41]. We made a different observation in a previous study—PT with the same parameters caused a slight increase in L* in strawberry–blackcurrant and strawberry–chokeberry nectars [53]. Also, other authors have documented the brightening of mixed juice of carambola, apple and black grapes as a result of pasteurization [24]. In the same mixed juice, they showed less impact of PL on brightness than PT processing. The occurrence of color changes after PL application may be due to enzyme activity upon phenols caused by the lack of thermal inactivation. However, the absence of high temperatures does not lead to non-enzymatic browning reactions [24]. In our study, the temperature of the juices during PEF and PL did not exceed 33 °C, while during thermal methods, the juices reached at least 85 °C. Hence, there was less effect of PEF and PL on color changes.
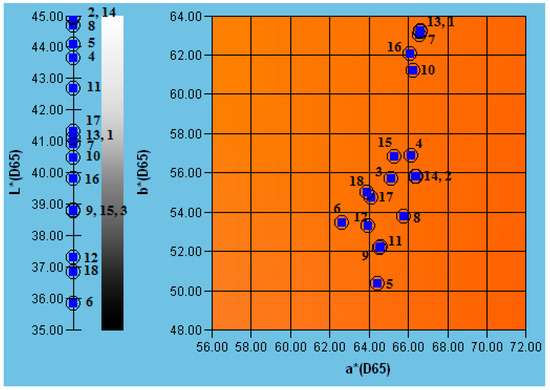
Figure 5.
Color parameters L*, a* and b* of raw and preserved juices. SHC—strawberry–blue honeysuckle–chokeberry juice, GHS—grape–blue honeysuckle–strawberry juice, GHC—grape–blue honeysuckle–chokeberry juice; HB—hot bottling, PT—pasteurization, MP—flow microwave pasteurization, PEF—pulsed electric field, PL—pulsed light. Numbers correspond to the juice codes: 1—SHC-raw, 2—GHS-raw, 3—GHC-raw, 4—SHC-MP, 5—GHS-MP, 6—GHC-MP, 7—SHC-PL, 8—GHS-PL, 9—GHC-PL, 10—SHC-P, 11—GHS-P, 12—GHC-P, 13—SHC-PEF, 14—GHS-PEF, 15—GHC-PEF, 16—SHC-HB, 17—GHS-HB, 18—GHC-HB.
a* is an important color parameter of food products responsible for the red color closely related to the presence of anthocyanin pigments in berries. Thermal methods like HB, PT and MP reduced the value of this parameter, while innovative non-thermal methods behave neutrally. An average positive correlation was shown between the anthocyanin content of juices and the value of the parameter a* (R = 0.43) (Table A1). A much stronger correlation was shown between the amount of anthocyanin pigments and the value of the parameter b* (R = 0.72) (Table A1). Bhagat and Chakraborty [25] indicated that PL-preserved pomegranate juice had a significantly higher a* value than the raw sample.
Based on the obtained L*, a* and b* results, the ΔE parameter (absolute color difference) was calculated for each method (Figure 6). In general, humans are able to discriminate between two colors when ΔE ≥ 1, but when observation is made through a glass, the human eye can discriminate between colors when ΔE ≥ 5 [54].
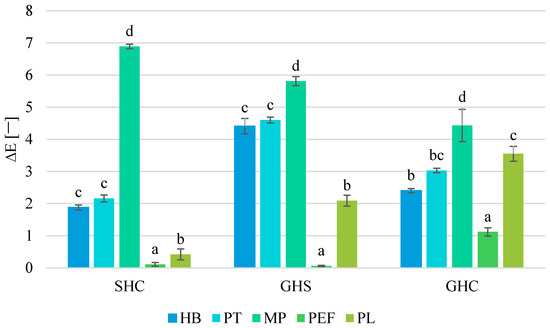
Figure 6.
Absolute color differences (ΔE) calculated for preserved juices. SHC—strawberry–blue honeysuckle–chokeberry juice, GHS—grape–blue honeysuckle–strawberry juice, GHC—grape–blue honeysuckle–chokeberry juice; HB—hot bottling, PT—pasteurization, MP—flow microwave pasteurization, PEF—pulsed electric field, PL—pulsed light; small letters—homogeneous groups depending on the preservation method in each of juices.
The largest significant changes were observed in samples preserved with MP, and the smallest with PEF in each juice. Thermal methods changed the color of the juices more than non-thermal processes. The direction and magnitude of color changes were affected not only by the preservation method, but also by the chemical composition of the juice—those with grapes were more susceptible to the degrading effects of processes. Mandha et al. [23] reported that ΔE following 10 min pasteurization of watermelon juice was 6.7, while in melon juice it was only 3.0. Other studies indicate that PL, depending on preservation parameters, results in a ΔE between 1.1 and 4.0 and may affect color less than thermal methods [25]. In lime juice preserved with PL, the ΔE was 1.7, while after pasteurization it was 12.4 [33]. As it emerges, it is important to choose carefully the parameters of each preservation method. Chakraborty et al. [24] observed that the ΔE of heated (4–5 min, 90 °C) mixed fruit beverage was 4.2, while samples treated with different PL variants ranged from 0.8 ≤ ΔE ≤ 7.0. Depending on whether the purpose of preservation (besides ensuring safety) is to maintain as much as possible the initial color of the product or to create new sensory characteristics without the use of food additives, it becomes reasonable to study the most appropriate processing methods.
3.8. PCA
Based on the first two principal components, which explained together 87.64% of the total variance, principal component analysis figures (Figure 7a,b) show classification of the study samples into groups according to the composition of juice blends with the visible influence of preservation methods.
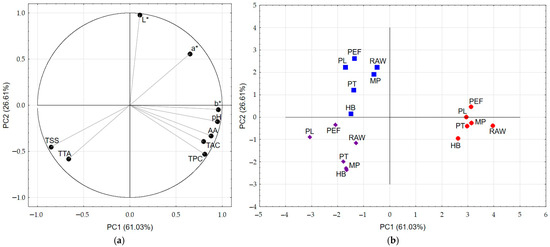
Figure 7.
PCA results: (a) Score plot, PC1 versus PC2 of data from determinations used as variables; (b) Score plot, PC1 versus PC2 of all samples, where red circles refer to SHC juice, blue squares refer to GHS juice, and violet rhombs refer to GHC juice.
The SHC juice blend constituted a distinct group due to the highest values of bioactive compounds such as TPC, TAC and antioxidant activity. SHC juice was also distinguished by its physicochemical properties (highest pH, highest b* color parameter value). Samples of the remaining two juice blends, GHS and GHC, showed similar characteristics but were separated due to differences in color and TSS and TTA values. GHC juice was the darkest and had the highest TTA and TSS among all tested juice blends.
The processing method also had an impact on the separation of samples within individual juice blends. With the exception of SHC juice, thermal methods were separated from non-thermal ones. MP in the flow was usually classified closest to the raw sample, suggesting the highest affinity through the lowest losses in TAC, TPC and AA. Among the thermal methods, the samples preserved using the HB method had the greatest difference in characteristics. In grape-based juices (GHS and GHC), the PL and PEF methods were clearly separated from the rest of the samples. They were most similar in color parameters to the raw juice blends samples, but showed significant variability in TAC, TPC content and AA value. Furthermore, the PL process showed the greatest changes in the physicochemical properties of the samples among all the methods studied. Based on PCA analysis, the PL method is not recommended for juice processing and preservation.
4. Conclusions
The use of different preservation methods of juice blends affected selected quality characteristics in a varied manner. Taking into account the results obtained, it can be concluded that non-thermal methods allowed better preservation of organoleptic characteristics, important from the consumer point of view. On the other hand, it can be stated that the thermal methods allowed higher retention of bioactive compounds in the juices, which translates into their potentially greater health-promoting value. It should be emphasized that MP allowed the increase in TPC in GHC and GHS juices. This phenomenon can be explained by the transformation and release of phenolic compounds from the food matrix due to the effect of heat, as well as microwave radiation. Therefore, due to the limited data available in the literature, our further scientific research will undertake the comparison of various parameters of microwave in flow treatment (MP) on the quality of food, including the impact on microbiological quality immediately after the process and during storage.
In general, the limitation of our research that we want to take into account in the future is microbiological and sensory testing of juices and other liquid products subjected to modern, innovative preservation methods.
Our study also shows that food matrices such as juices can be complex, and their processing can cause degradation and extraction of components, as well as the formation of new compounds through chemical processes. The influence of the environment (enzyme activity, light or air access) is also crucial. It can be concluded that the obtained results indicate the importance of conducting trial studies and adjusting methods with their parameters to process/preserve juice and create a good-quality product.
Based on the quantitative results of our work in terms of the physicochemical and bioactive properties of the tested juice blends, we recommend two preservation methods: PEF and MP. PCA analysis showed that these are the two methods that best preserve the original characteristics of raw juices.
Our research also showed how important the fruit species included in the juice blend are in terms of nutritional value. Based on the analyzed bioactive compounds, the most health-promoting composition was found to be a combination of strawberry, blue honeysuckle berry, and chokeberry in a ratio of 60:30:10 v/v/v (the SHC juice).
Author Contributions
Conceptualization, N.P. and S.K.; methodology, N.P. and A.W.; software, N.P. and B.K.; validation, N.P.; formal analysis, N.P.; investigation, N.P.; resources, N.P., S.K., and A.W.; data curation, N.P. and B.K.; writing—original draft preparation, N.P.; writing—review and editing, N.P., S.K., and B.K.; visualization, B.K.; supervision, S.K. and B.K.; project administration, S.K.; funding acquisition, S.K. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Research for this publication was conducted using research equipment purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Antioxidant activity |
| Cy-3,5-O-diglc | Cyanidin-3,5-O-diglucoside |
| Cy-3-O-gal | Cyanidin-3-O-galactoside |
| Cy-3-O-glc | Cyanidin-3-O-glucoside |
| Cy-3-O-rut | Cyanidin-3-O-rutinoside |
| Cy-3-O-xyl | Cyanidin-3-O-xyloside |
| Dp-3-O-glc | Delphinidin-3-O-glucoside |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HB | Hot bottling |
| HPLC | High-pressure liquid chromatography |
| GAE | Gallic acid equivalents |
| GHC | Grape–blue honeysuckle–chokeberry juice |
| GHS | Grape–blue honeysuckle–strawberry juice |
| MP | Flow microwave pasteurization |
| n.d. | Non detected |
| NT | Nephelometric turbidity |
| PEF | Pulsed electric field |
| Pg-3-O-ara | Pelargonidin-3-O-arabinoside |
| pH | Active acidity |
| Pg-3-O-glc | Pelargonidin-3-O-glucoside |
| Pn-3-O-glc | Peonidin-3-O-glucoside |
| PL | Pulsed light |
| PT | Pasteurization |
| SHC | Strawberry–blue honeysuckle–chokeberry juice |
| TE | Trolox |
| TEAC | Trolox equivalent antioxidant capacity |
| TPC | Total polyphenol content |
| TSS | Total soluble solids |
| TTA | Total titratable activity |
Appendix A

Table A1.
The results of correlations between individual parameters.
Table A1.
The results of correlations between individual parameters.
| a* | b* | TAC | AA | TPC | |
|---|---|---|---|---|---|
| a* | - | 0.80 | 0.43 | 0.28 | 0.20 |
| b* | 0.80 | - | 0.72 | 0.59 | 0.56 |
| TAC | 0.43 | 0.72 | - | 0.88 | 0.84 |
| AA | 0.28 | 0.59 | 0.88 | - | 0.91 |
| TPC | 0.20 | 0.56 | 0.84 | 0.91 | - |
a*, b*—color parameters in CIEL*a*b* system, AA—antioxidant activity, TAC—total anthocyanins content, TPC—total polyphenols content.
References
- Fruit Juice—Nutrition & Health. An IFU Scientific Review; International Fruit and Vegetable Juice Association: Paris, France, 2017.
- Bezerra, M.; Ribeiro, M.; Cosme, F.; Nunes, F.M. Overview of the Distinctive Characteristics of Strawberry, Raspberry, and Blueberry in Berries, Berry Wines, and Berry Spirits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13354. [Google Scholar] [CrossRef]
- Gołba, M.; Sokół-Ł etowska, A.; Kucharska, A.Z. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An Update on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Kolniak-Ostek, J. The Influence of the Use of Different Polysaccharide Coatings on the Stability of Phenolic Compounds and Antioxidant Capacity of Chokeberry Hydrogel Microcapsules Obtained by Indirect Extrusion. Foods 2023, 12, 515. [Google Scholar] [CrossRef]
- Kersh, D.M.E.; Hammad, G.; Donia, M.S.; Farag, M.A. A Comprehensive Review on Grape Juice Beverage in Context to its Processing and Composition with Future Perspectives to Maximize its Value. Food Bioprocess Technol. 2023, 16, 1–23. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and Relative Stabilities of Acylated and Nonacylated Anthocyanins in Beverage Systems. J. Food Sci. Technol. 2022, 59, 831–845. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimut Agenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Chen, Y.; Belwal, T.; Xu, Y.; Ma, Q.; Li, D.; Li, L.; Xiao, H.; Luo, Z. Updated Insights into Anthocyanin Stability Behavior from Bases to Cases: Why and Why Not Anthocyanins Lose during Food Processing. Crit. Rev. Food Sci. Nutr. 2023, 63, 8639–8671. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Wang, Z.; Feng, Y.; Han, Y. Advances in the Preparation, Stability, Metabolism, and Physiological Roles of Anthocyanins: A Review. Foods 2023, 12, 3969. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: A Review of Plant Sources, Extraction, Stability, Content Determination and Modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Basak, S.; Mahale, S.; Chakraborty, S. Changes in Quality Attributes of Pulsed Light and Thermally Treated Mixed Fruit Beverages during Refrigerated Storage (4 °C) Condition. Innov. Food Sci. Emerg. Technol. 2022, 78, 103025. [Google Scholar] [CrossRef]
- Ptak, S.; Żarski, A.; Kapuśniak, J. Technological, Economic and Health Aspects of Application of Microwave Radiation in Food Processing. Zywnosc Nauka Technol. Jakosc 2020, 27, 47–62. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Cardozo, T.S.F.; Couto, S.M.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Pimentel, T.C.; Freitas, M.Q.; Raices, R.S.L.; et al. Effects of Microwave Heating on the Chemical Composition and Bioactivity of Orange Juice-Milk Beverages. Food Chem. 2021, 345, 128746. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L.; Kim, K.H. Influence of Pulsed Electric Field and Heat Treatment on Emblica officinalis Juice Inoculated with Zygosaccharomyces bailii. Food Bioprod. Process. 2015, 95, 146–154. [Google Scholar] [CrossRef]
- Piasek, A.; Kusznierewicz, B.; Grzybowska, I.; Malinowska-Pańczyk, E.; Piekarska, A.; Azqueta, A.; Collins, A.R.; Namieśnik, J.; Bartoszek, A. The Influence of Sterilization with EnbioJet® Microwave Flow Pasteurizer on Composition and Bioactivity of Aronia and Blue-Berried Honeysuckle Juices. J. Food Compos. Anal. 2011, 24, 880–888. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. Effect of Continuous Flow Microwave and Conventional Heating on the Bioactive Compounds, Colour, Enzymes Activity, Microbial and Sensory Quality of Strawberry Purée. Food Bioproc Tech. 2015, 8, 1864–1876. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic Pigment Determination in Red Fruit Juices, Concentrated Juices and Syrups Using Liquid Chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Kruszewski, B.; Domian, E.; Nowacka, M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules 2023, 28, 2018. [Google Scholar] [CrossRef]
- Mannozzi, C.; Fauster, T.; Haas, K.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Role of Thermal and Electric Field Effects during the Pre-Treatment of Fruit and Vegetable Mash by Pulsed Electric Fields (PEF) and Ohmic Heating (OH). Innov. Food Sci. Emerg. Technol. 2018, 48, 131–137. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of High Pressure and Thermal Processing on Shelf Life and Quality of Strawberry Purée and Juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of Fruit Juices and Effect of Pasteurization and Storage Conditions on Their Microbial, Physicochemical, and Nutritional Quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mahale, S.; Dhar, R.; Basak, S. Development of a Mixed Fruit Beverage and Pulsed Light Treatment Thereof to Obtain a Microbially Safe and Enzymatically Stable Product. Food Biosci. 2022, 45, 101508. [Google Scholar] [CrossRef]
- Bhagat, B.; Chakraborty, S. Potential of Pulsed Light Treatment to Pasteurize Pomegranate Juice: Microbial Safety, Enzyme Inactivation, and Phytochemical Retention. LWT 2022, 159, 113215. [Google Scholar] [CrossRef]
- de Souza, V.R.; Popović, V.; Bissonnette, S.; Ros, I.; Mats, L.; Duizer, L.; Warriner, K.; Koutchma, T. Quality Changes in Cold Pressed Juices after Processing by High Hydrostatic Pressure, Ultraviolet-c Light and Thermal Treatment at Commercial Regimes. Innov. Food Sci. Emerg. Technol. 2020, 64, 102398. [Google Scholar] [CrossRef]
- Biancaniello, M.; Popović, V.; Fernandez-Avila, C.; Ros-Polski, V.; Koutchma, T. Feasibility of a Novel Industrial-Scale Treatment of Green Cold-Pressed Juices by Uv-c Light Exposure. Beverages 2018, 4, 29. [Google Scholar] [CrossRef]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the Impact of High Pressure, Pulsed Electric Field and Thermal Pasteurization on Quality Attributes of Cloudy Apple Juice Using Targeted and Untargeted Analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined Effects of Sonication and Pulsed Electric Field on Selected Quality Parameters of Grapefruit Juice. LWT 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Influence of Pulsed Electric Field Processing on the Quality of Fruit Juice Beverages Sweetened with Stevia rebaudiana. Food Bioprod. Process. 2017, 101, 214–222. [Google Scholar] [CrossRef]
- Fabroni, S.; Platania, G.M.; Amenta, M.; Ballistreri, G.; Galvano, F.; Nges, I.A.; Timpanaro, N. Pulsed Electric Field as a Mild Treatment for Extended Shelf-Life and Preservation of Bioactive Compounds in Blood Orange Juice. Appl. Sci. 2024, 14, 7275. [Google Scholar] [CrossRef]
- Cassani, L.; Tomadoni, B.; Viacava, G.; Ponce, A.; Moreira, M.R. Enhancing Quality Attributes of Fiber-Enriched Strawberry Juice by Application of Vanillin or Geraniol. LWT 2016, 72, 90–98. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Sequential Pulsed Light and Ultrasound Treatments for the Inactivation of Saccharomyces cerevisiae and PPO and the Retention of Bioactive Compounds in Sweet Lime Juice. Foods 2024, 13, 1996. [Google Scholar] [CrossRef]
- Shirvani, A.; Mirzaaghaei, M.; Goli, S.A.H. Application of Natural Fining Agents to Clarify Fruit Juices. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4190–4216. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Kalisz, S. Effects of Various Polysaccharide Clarification Agents and Reaction Time on Content of Polyphenolic Compound, Antioxidant Activity, Turbidity and Colour of Chokeberry Juice. LWT 2018, 92, 347–360. [Google Scholar] [CrossRef]
- Türkyılmaz, M.; Hamzaoğlu, F.; Özkan, M. Effects of Pasteurization and Storage on Turbidity and Copigmentation in Pomegranate Juices Clarified with Various Hydrocolloid Combinations. Food Chem. 2021, 358, 129803. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and Thermal Treatments for the Preservation of Carrot Juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. The Impact of Pulsed Electric Field Pretreatment of Bell Pepper on the Selected Properties of Spray Dried Juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Kaya, Z.; Unluturk, S. Processing of Clear and Turbid Grape Juice by a Continuous Flow UV System. Innov. Food Sci. Emerg. Technol. 2016, 33, 282–288. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. UV-C Light Inactivation of Single and Composite Microbial Populations in Tangerine-Orange Juice Blend. Evaluation of Some Physicochemical Parameters. Food Bioprod. Process. 2019, 117, 149–159. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Kruszewski, B. High-Temperature Short-Time and Ultra-High-Temperature Processing of Juices, Nectars and Beverages: Influences on Enzyme, Microbial Inactivation and Retention of Bioactive Compounds. Appl. Sci. 2024, 14, 8978. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; dos Santos Funcia, E.; Pires, M.N.; Gut, J.A.W. Modeling of Time-Temperature History and Enzymatic Inactivation of Cloudy Apple Juice in Continuous Flow Microwave Assisted Pasteurization. Food Bioprod. Process. 2018, 111, 45–53. [Google Scholar] [CrossRef]
- Kosiński, J.; Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Jasińska, U.T.; Woźniak, Ł.; Kaniewska, B.; Marszałek, K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Appl. Sci. 2024, 14, 662. [Google Scholar] [CrossRef]
- Wójcik, M.; Szczepańska-Stolarczyk, J.; Woźniak, Ł.; Jasińska, U.T.; Trych, U.; Cywińska-Antonik, M.; Kosiński, J.; Kaniewska, B.; Marszałek, K. Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale. Appl. Sci. 2024, 14, 6008. [Google Scholar] [CrossRef]
- Vollmer, K.; Chakraborty, S.; Prakash Bhalerao, P.; Carle, R.; Frank, J.; Björn Steingass, C. Effect of Pulsed Light Treatment on Natural Microbiota, Enzyme Activity, and Phytochemical Composition of Pineapple (Ananas comosus [L.] Merr.) Juice. Food Bioprocess Technol. 2020, 13, 1095–1109. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Effect of PH and Total Fluence on Microbial and Enzyme Inactivation in Sweet Lime (Citrus limetta) Juice during Pulsed Light Treatment. J. Food Process. Preserv. 2022, 46, e16749. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Trenka, M.; Czaplicka, M.; Maksimowski, D.; Nawirska-Olszańska, A. Selected Properties of Juices from Black Chokeberry (Aronia melanocarpa L.) Fruits Preserved Using the PEF Method. Appl. Sci. 2022, 12, 7008. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Brzozowska, A.; Szwengiel, A.; Gramza-Michałowska, A. The Effect of Plant Additives on the Stability of Polyphenols in Cloudy and Clarified Juices from Black Chokeberry (Aronia melanocarpa). Antioxidants 2020, 9, 801. [Google Scholar] [CrossRef]
- Stübler, A.S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of Pilot-Scale Processing (Thermal, PEF, HPP) on the Stability and Bioaccessibility of Polyphenols and Proteins in Mixed Protein- and Polyphenol-Rich Juice Systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Duhan, S.; Kar, A. Optimization of Process Parameter Combinations for Pasteurization of Sugarcane (Saccharum officinarum) Juice Using Continuous Flow Microwave System. Indian J. Agric. Sci. 2018, 88, 1253–1257. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of Pulsed Electric Field Treatment on Shelf Life and Nutritional Value of Apple Juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of Ultrasonication and Pulsed Light Treatments on Phenolics Concentration and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. LWT 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Hać-Szymańczuk, E.; Kruszewski, B. Impact of Conventional Pasteurization, High Temperature Short Time, Ultra-High Temperature, and Storage Time on Physicochemical Characteristics, Bioactive Compounds, Antioxidant Activity, and Microbiological Quality of Fruit Nectars. Foods 2024, 13, 3963. [Google Scholar] [CrossRef] [PubMed]
- Perez-Magarino, S.; Gonzalez-Sanjose, M.L. Application of Absorbance Values Used in Wineries for estimating CIELAB Parameters in Red Wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).