Aseptic Loosening in Total Hip Arthroplasty: Pathophysiology, Biomarkers, and Preventive Treatment Strategies
Abstract
1. Introduction
2. Methodology
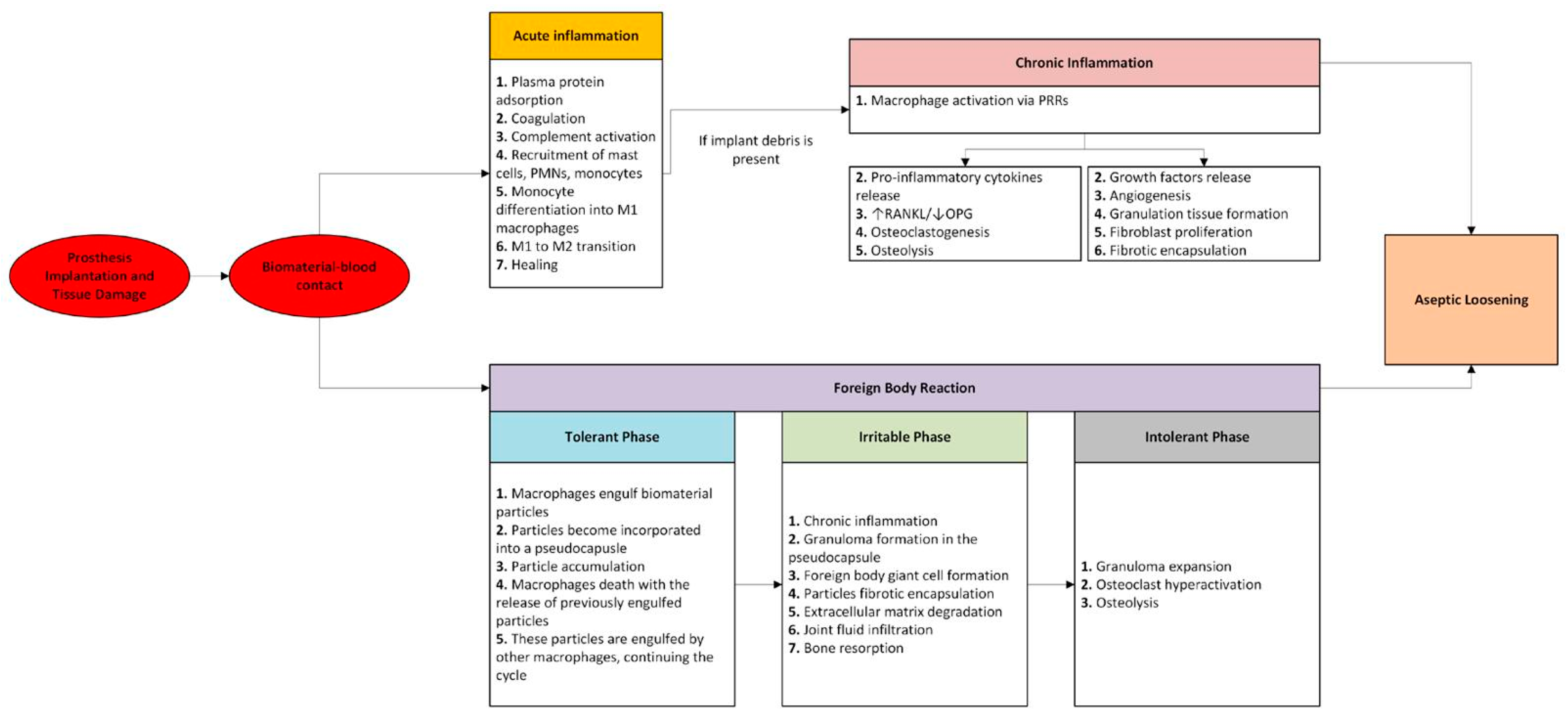
3. Immune Response to Biomaterial
3.1. Acute Inflammation
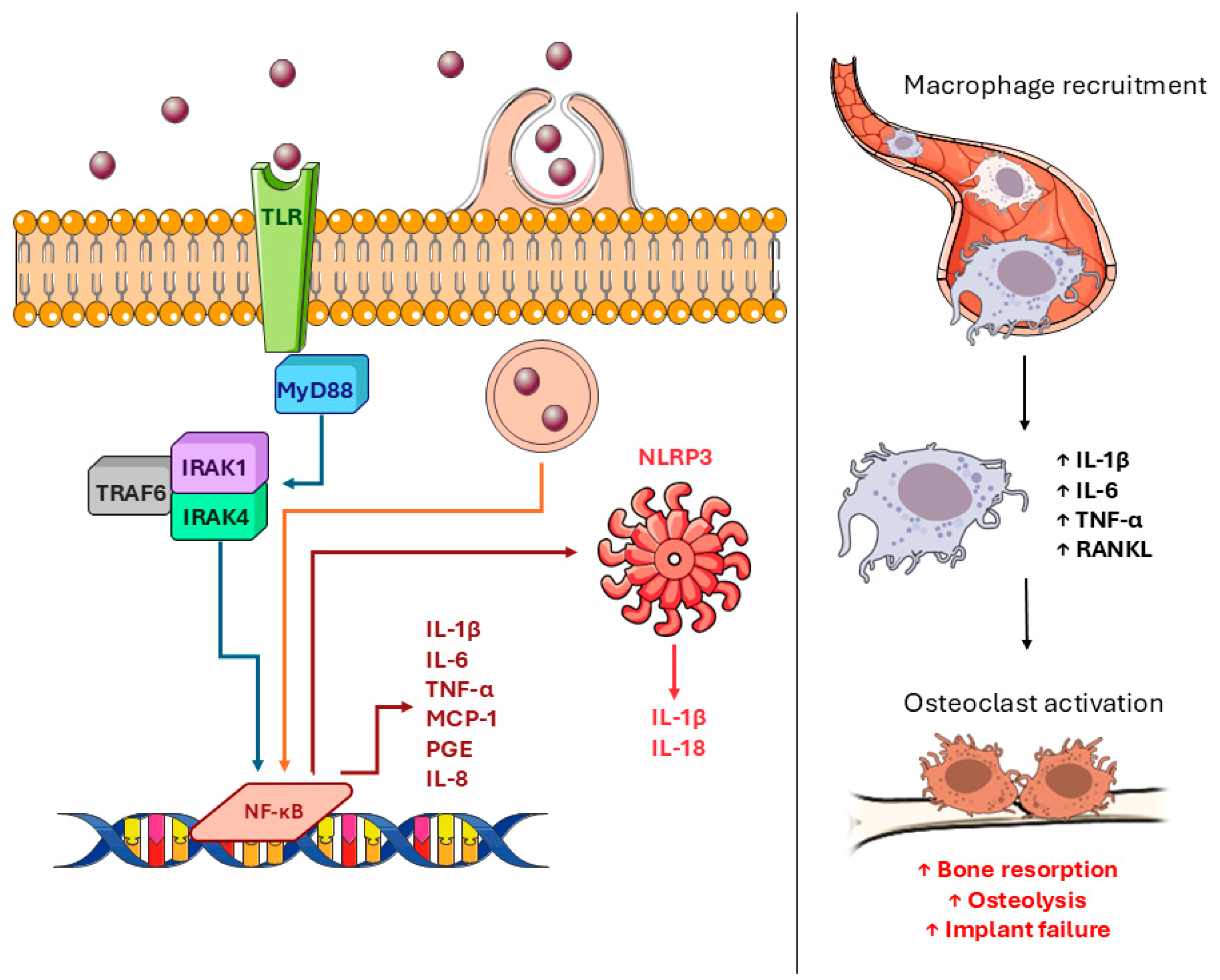
3.2. Chronic Inflammation
3.3. Granulation Tissue and Fibrosis
3.4. Foreign Body Reaction
3.4.1. Tolerant Phase
3.4.2. Irritable Phase
3.4.3. Intolerant Phase
3.4.4. Clinical Progression and Illustrative Scenarios
3.5. Inflammatory Response to Different Implant Materials
3.5.1. Polyethylene Wear Particles
3.5.2. Polymethylmethacrylate Wear Particles
3.5.3. Metallic Wear Debris
3.5.4. Ceramic Wear Debris
4. Biomarkers
Diagnostic Accuracy and Critical Evaluation of Biomarkers in Aseptic Loosening
5. Prevention and Treatment of Aseptic Loosening
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AL | aseptic loosening; |
| FXII | factor XII |
| TF | TF |
| IL | interleukin |
| MCP-1/CCL2 | monocyte chemotactic protein-1/chemokine CC motif ligand 2 |
| MIP-1 | macrophage inflammatory protein-1 |
| PMN | polymorphonuclear leukocyte |
| FBR | foreign body reaction |
| MSC | mesenchymal stem cell |
| PPOL | periprosthetic osteolysis |
| FBGC | foreign body giant cell |
| PRR | pattern recognition receptor |
| TNF | tumor necrosis factor |
| PGE | prostaglandin E |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| MIP-1α | macrophage inflammatory protein-1α |
| TLR | Toll-like receptors |
| OPG | osteoprotegerin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAPK | mitogen-activated protein kinase |
| PDGF | platelet-derived growth factor |
| FGF | fibroblast growth factor |
| TGF | transforming growth factor |
| EGF | epidermal growth factor |
| ROS | reactive oxygen species |
| PE | polyethylene |
| UHMWPE | ultra-high molecular weight polyethylene |
| XLPE | cross-linked polyethylene |
| PMMA | polymethylmethacrylate |
| ARMD | adverse reactions to metal debris |
References
- Varacallo, M.A.; Luo, T.D.; Johanson, N.A. Total Hip Arthroplasty Techniques. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507864/ (accessed on 9 August 2025).
- Li, C.; Schmid, S.; Mason, J. Effects of pre-cooling and pre-heating procedures on cement polymerization and thermal osteonecrosis in cemented hip replacements. Med. Eng. Phys. 2003, 25, 559–564. [Google Scholar] [CrossRef]
- Nugent, M.; Young, S.W.; Frampton, C.M.; Hooper, G.J. The lifetime risk of revision following total hip arthroplasty. Bone Jt. J. 2021, 103-B, 479–485. [Google Scholar] [CrossRef]
- Sadoghi, P.; Koutp, A.; Prieto, D.P.; Clauss, M.; Kayaalp, M.E.; Hirschmann, M.T. The projected economic burden and complications of revision hip and knee arthroplasties: Insights from national registry studies. Knee Surg. Sports Traumatol. Arthrosc. 2025; early view. [Google Scholar] [CrossRef]
- van Otten, T.J.; van Loon, C.J. Early aseptic loosening of the tibial component at the cement-implant interface in total knee arthroplasty: A narrative overview of potentially associated factors. Acta Orthop. Belg. 2022, 88, 103–111. [Google Scholar] [CrossRef]
- Yao, Z.; Lin, T.-H.; Pajarinen, J.; Sato, T.; Goodman, S. Chapter 12—Host Response to Orthopedic Implants (Metals and Plas-tics). In Host Response to Biomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 315–373. [Google Scholar] [CrossRef]
- Gibon, E.; Takakubo, Y.; Zwingenberger, S.; Gallo, J.; Takagi, M.; Goodman, S.B. Friend or foe? Inflammation and the foreign body response to orthopedic biomaterials. J. Biomed. Mater. Res. Part A 2024, 112, 1172–1187. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, Y.; Yuan, T.; Zhang, Z.; Ge, J.; Meng, Q.; Li, Z.; Sun, S. Macrophages in aseptic loosening: Characteristics, functions, and mechanisms. Front. Immunol. 2023, 14, 1122057. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial–cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1. [Google Scholar] [CrossRef]
- Andersson, J.; Ekdahl, K.N.; Larsson, R.; Nilsson, U.R.; Nilsson, B. C3 Adsorbed to a polymer surface can form an initiating alternative pathway convertase. J. Immunol. 2002, 168, 5786–5791. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Sperling, C.; Werner, C. Synergistic effect of hydrophobic and anionic surface groups triggers blood coagulation in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 931–937. [Google Scholar] [CrossRef]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Sperling, C.; Tengvall, P.; Werner, C. The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials 2010, 31, 2498–2507. [Google Scholar] [CrossRef]
- Henson, P.M.; Johnston, R.B. Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J. Clin. Investig. 1987, 79, 669–674. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Ganz, T.; Selsted, M.E.; Babior, B.M.; Curnutte, J.T. Neutrophils and host defense. Ann. Intern. Med. 1988, 109, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology. Neutrophils in human diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Lowell, C.A.; Berton, G. Integrin signal transduction in myeloid leukocytes. J. Leukoc. Biol. 1999, 65, 313–320. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Complement C3 participation in monocyte adhesion to different surfaces. Proc. Natl. Acad. Sci. USA 1994, 91, 10119–10123. [Google Scholar] [CrossRef]
- Tang, L. Mechanisms of fibrinogen domains: Biomaterial interactions. J. Biomater. Sci. Polym. Ed. 1998, 9, 1257–1266. [Google Scholar] [CrossRef]
- Zdolsek, J.; Eaton, J.W.; Tang, L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef]
- Hidaka, Y.; Ito, M.; Mori, K.; Yagasaki, H.; Kafrawy, A.H. Histopathological and immunohistochemical studies of membranes of deacetylated chitin derivatives implanted over rat calvaria. J. Biomed. Mater. Res. 1999, 46, 418–423. [Google Scholar] [CrossRef]
- VandeVord, P.J.; Matthew, H.W.T.; DeSilva, S.P.; Mayton, L.; Wu, B.; Wooley, P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002, 59, 585–590. [Google Scholar] [CrossRef]
- Park, C.J.; Gabrielson, N.P.; Pack, D.W.; Jamison, R.D.; Johnson, A.J.W. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials 2009, 30, 436–444. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Voyich, J.M.; Burlak, C.; DeLeo, F.R. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. 2005, 53, 505–517. [Google Scholar]
- Yamashiro, S.; Kamohara, H.; Wang, J.-M.; Yang, D.; Gong, W.-H.; Yoshimura, T. Phenotypic and functional change of cytokine-activated neutrophils: Inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J. Leukoc. Biol. 2001, 69, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004, 3, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Gallo, J.; Takagi, M. Macrophage Polarization and the Osteoimmunology of Periprosthetic Osteolysis. Curr. Osteoporos. Rep. 2022, 20, 43–52. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Kon, T.; Cho, T.-J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB Ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Xing, Z.; Lu, C.; Hu, D.; Yu, Y.; Wang, X.; Colnot, C.; Nakamura, M.; Wu, Y.; Miclau, T.; Marcucio, R.S. Multiple roles for CCR2 during fracture healing. Dis. Model. Mech. 2010, 3, 451–458. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Jain, M.; Alimperti, S. Bone Microvasculature: Stimulus for Tissue Function and Regeneration. Tissue Eng. Part B Rev. 2021, 27, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: Cause, effect or both. Nat. Rev. Rheumatol. 2023, 19, 200–211. [Google Scholar] [CrossRef]
- Couto, M.; Vasconcelos, D.P.; Sousa, D.M.; Sousa, B.; Conceição, F.; Neto, E.; Lamghari, M.; Alves, C.J. The Mechanisms Underlying the Biological Response to Wear Debris in Periprosthetic Inflammation. Front. Mater. 2020, 7, 274. [Google Scholar] [CrossRef]
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef]
- Green, T.R.; Fisher, J.; Matthews, J.B.; Stone, M.H.; Ingham, E. Effect of size and dose on bone resorption activity of macrophages byin vitro clinically relevant ultra high molecular weight polyethylene particles. J. Biomed. Mater. Res. 2000, 53, 490–497. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Essner, A.; Stark, C.; Dumbleton, J. Comparison of the size and morphology of UHMWPE wear debris produced by a hip joint simulator under serum and water lubricated conditions. Biomaterials 1996, 17, 865–871. [Google Scholar] [CrossRef]
- Hallab, N.J.; McAllister, K.; Brady, M.; Jarman-Smith, M. Macrophage reactivity to different polymers demonstrates particle size- and material-specific reactivity: PEEK-OPTIMA®particles versus UHMWPE particles in the submicron, micron, and 10 micron size ranges. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 480–492. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef]
- Chiu, R.; Ma, T.; Smith, R.L.; Goodman, S.B. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J. Biomed. Mater. Res. Part A 2009, 89, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages—Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. Part A 2013, 101, 3033–3045. [Google Scholar] [CrossRef] [PubMed]
- Athanasou, N.A. The pathobiology and pathology of aseptic implant failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef]
- Gu, Q.; Shi, Q.; Yang, H. The role of TLR and chemokine in wear particle-induced aseptic loosening. J. Biomed. Biotechnol. 2012, 2012, 596870. [Google Scholar] [CrossRef]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Shiratori, T.; Kyumoto-Nakamura, Y.; Kukita, A.; Uehara, N.; Zhang, J.; Koda, K.; Kamiya, M.; Badawy, T.; Tomoda, E.; Xu, X.; et al. IL-1β Induces Pathologically Activated Osteoclasts Bearing Extremely High Levels of Resorbing Activity: A Possible Pathological Subpopulation of Osteoclasts, Accompanied by Suppressed Expression of Kindlin-3 and Talin-1. J. Immunol. 2018, 200, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Tamaki, Y.; Pajarinen, J.; Waters, H.A.; Woo, D.K.; Yao, Z.; Goodman, S.B. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014, 10, 1–10. [Google Scholar] [CrossRef]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A review of UHMWPE wear-induced osteolysis: The role for early detection of the immune response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef] [PubMed]
- Sukur, E.; Akman, Y.E.; Ozturkmen, Y.; Kucukdurmaz, F. Particle Disease: A Current Review of the Biological Mechanisms in Periprosthetic Osteolysis After Hip Arthroplasty. Open Orthop. J. 2016, 10, 241–251. [Google Scholar] [CrossRef]
- Purdue, P.E.; Koulouvaris, P.; Potter, H.G.; Nestor, B.J.; Sculco, T.P. The cellular and molecular biology of periprosthetic osteolysis. Clin. Orthop. Relat. Res. 2007, 454, 251–261. [Google Scholar] [CrossRef]
- Anderson, J.M. Inflammation, Wound Healing, and the Foreign-Body Response. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Beck, R.T.; Illingworth, K.D.; Saleh, K.J. Review of periprosthetic osteolysis in total joint arthroplasty: An emphasis on host factors and future directions. J. Orthop. Res. 2012, 30, 541–546. [Google Scholar] [CrossRef]
- Choi, M.G.; Koh, H.S.; Kluess, D.; O’Connor, D.; Mathur, A.; Truskey, G.A.; Rubin, J.; Zhou, D.X.F.; Sung, K.-L.P. Effects of titanium particle size on osteoblast functions in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 4578–4583. [Google Scholar] [CrossRef]
- Takagi, M. Neutral proteinases and their inhibitors in the loosening of total hip prostheses. Acta Orthop. Scand. 1996, 67 (Suppl. S271), 3–29. [Google Scholar] [CrossRef]
- Yang, F.; Wu, W.; Cao, L.; Huang, Y.; Zhu, Z.; Tang, T.; Dai, K. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials 2011, 32, 9159–9167. [Google Scholar] [CrossRef] [PubMed]
- Renò, F.; Sabbatini, M.; Massè, A.; Bosetti, M.; Cannas, M. Fibroblast apoptosis and caspase-8 activation in aseptic loosening. Biomaterials 2003, 24, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Anil, U.; Singh, V.; Schwarzkopf, R. Diagnosis and Detection of Subtle Aseptic Loosening in Total Hip Arthroplasty. J. Arthroplast. 2022, 37, 1494–1500. [Google Scholar] [CrossRef]
- Santavirta, S.; Konttinen, Y.T.; Bergroth, V.; Eskola, A.; Tallroth, K.; Lindholm, T.S. Aggressive granulomatous lesions associ-ated with hip arthroplasty. Immunopathological studies. J. Bone Joint Surg. Am. 1990, 72, 252–258. [Google Scholar] [CrossRef]
- Solovieva, S.A.; Čeponis, A.; Konttinen, Y.T.; Takagi, M.; Suda, A.; Eklund, K.K.; Sorsa, T.D.; Santavirta, S. Mast cells in loosening of totally replaced hips. Clin. Orthop. Relat. Res. 1996, 322, 158–165. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chang, T.-K.; Lin, T.-C.; Yeh, S.-T.; Fang, H.-W.; Huang, C.-H.; Huang, C.-H. The potential role of herbal extract Wedelolactone for treating particle-induced osteolysis: An in vivo study. J. Orthop. Surg. Res. 2022, 17, 335. [Google Scholar] [CrossRef]
- Shet, S.S.; Kakish, E.; Murphy, S.C.; Roopnarinesingh, R.; Power, S.P.; Maher, M.M.; Ryan, D.J. Imaging evaluation of periprosthetic loosening: A primer for the general radiologist. World J. Radiol. 2025, 17, 102373. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Geng, L.; Li, Q.; Qi, E.; Shi, Y.; Wang, Y.; Zheng, Q.; Zhang, G.; Chen, J.; et al. Different uptake patterns of 68Ga-FAPI in aseptic loosening and periprosthetic joint infection of hip arthroplasty: A case series and literature review. Front. Med. 2022, 9, 1014463. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Anttila, A.; Konttinen, Y.T.; Lappalainen, R.; Goodman, S.B.; Nordsletten, L.; Santavirta, S. Benign response to particles of diamond and SiC: Bone chamber studies of new joint replacement coating materials in rabbits. Biomaterials 1996, 17, 807–812. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Jasty, M.; Harris, W.H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J. Bone Joint Surg. Am. 1992, 74, 849–863. [Google Scholar] [CrossRef]
- Manley, M.T.; D’antonio, J.; Capello, W.N.; Edidin, A.A. Osteolysis: A Disease of access to fixation interfaces. Clin. Orthop. Relat. Res. 2002, 405, 129–137. [Google Scholar] [CrossRef]
- Pap, G.; Machner, A.; Rinnert, T.; Hörler, D.; Gay, R.E.; Schwarzberg, H.; Neumann, W.; Michel, B.A.; Gay, S.; Pap, T. De-velopment and characteristics of a synovial-like interface membrane around cemented tibial hemiarthroplasties in a novel rat model of aseptic prosthesis loosening. Arthritis Rheum. 2001, 44, 956–963. [Google Scholar] [CrossRef]
- Banerjee, S.; Pivec, R.; Issa, K.; Kapadia, B.H.; Khanuja, H.S.; Mont, M.A. Large-diameter femoral heads in total hip arthroplasty: An evidence-based review. Am. J. Orthop. 2014, 43, 506–512. [Google Scholar] [PubMed]
- Oparaugo, P.C.; Clarke, I.C.; Malchau, H.; Herberts, P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: A survey of 8 reports in the literature. Acta Orthop. Scand. 2001, 72, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhu, W.; Liang, S.; Li, H.; Li, S. Cross-Linked Versus Conventional Polyethylene for Long-Term Clinical Outcomes After Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Investig. Surg. 2021, 34, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Neut, D.; van der Veen, H.C.; Bulstra, S.K. Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: A review of the current literature. Int. Orthop. 2019, 43, 1549–1557. [Google Scholar] [CrossRef]
- McKellop, H.; Shen, F.-W.; Lu, B.; Campbell, P.; Salovey, R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. A hip-simulator study. J. Bone Joint Surg. Am. 2000, 82, 1708–1725. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Puertolas, J.-A.; Munuera, L.; Konttinen, Y.T. Update on UHMWPE research from the bench to the bedside. Acta Orthop. 2008, 79, 832–840. [Google Scholar] [CrossRef]
- Oral, E.; Muratoglu, O.K. Vitamin E diffused, highly crosslinked UHMWPE: A review. Int. Orthop. 2011, 35, 215–223. [Google Scholar] [CrossRef]
- Oral, E.; Rowell, S.L.; Muratoglu, O.K. The effect of α-tocopherol on the oxidation and free radical decay in irradiated UHMWPE. Biomaterials 2006, 27, 5580–5587. [Google Scholar] [CrossRef]
- Valladares, R.D.; Nich, C.; Zwingenberger, S.; Li, C.; Swank, K.R.; Gibon, E.; Rao, A.J.; Yao, Z.; Goodman, S.B. Toll-like receptors-2 and 4 are overexpressed in an experimental model of particle-induced osteolysis. J. Biomed. Mater. Res. Part A 2014, 102, 3004–3011. [Google Scholar] [CrossRef]
- Maitra, R.; Clement, C.C.; Scharf, B.; Crisi, G.M.; Chitta, S.; Paget, D.; Purdue, P.E.; Cobelli, N.; Santambrogio, L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 2009, 47, 175–184. [Google Scholar] [CrossRef]
- Vermes, C.; Chandrasekaran, R.; Jacobs, J.J.; Galante, J.O.; Roebuck, K.A.; Glant, T.T. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J. Bone Joint Surg. Am. 2001, 83, 201–211. [Google Scholar] [CrossRef]
- Hodrick, J.T.; Severson, E.P.; McAlister, D.S.; Dahl, B.; Hofmann, A.A. Highly crosslinked polyethylene is safe for use in total knee arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 2806–2812. [Google Scholar] [CrossRef]
- Bichara, D.A.; Malchau, E.; Sillesen, N.H.; Cakmak, S.; Nielsen, G.P.; Muratoglu, O.K. Vitamin E-diffused highly cross-linked UHMWPE particles induce less osteolysis compared to highly cross-linked virgin UHMWPE particles in vivo. J. Arthroplast. 2014, 29, 232–237. [Google Scholar] [CrossRef]
- Chen, W.; Bichara, D.A.; Suhardi, J.; Sheng, P.; Muratoglu, O.K. Effects of vitamin E-diffused highly cross-linked UHMWPE particles on inflammation, apoptosis and immune response against S. aureus. Biomaterials 2017, 143, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Renò, F.; Bracco, P.; Lombardi, F.; Boccafoschi, F.; Costa, L.; Cannas, M. The induction of MMP-9 release from granulocytes by Vitamin E in UHMWPE. Biomaterials 2004, 25, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Hamasaki, M.; Takahashi, D.; Ota, M.; Kadoya, K.; Yutani, T.; Uetsuki, K.; Asano, T.; Irie, T.; Arai, R.; et al. Transcriptional profile of human macrophages stimulated by ultra-high molecular weight polyethylene particulate debris of orthopedic implants uncovers a common gene expression signature of rheumatoid arthritis. Acta Biomater. 2018, 65, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; Joyner, C.; Triffitt, J.; Athanasou, N. Polymethylmethacrylate-induced inflammatory macrophages resorb bone. J. Bone Joint Surg. Brit. Vol. 1992, 74-B, 652–658. [Google Scholar] [CrossRef]
- Sabokbar, A.; Pandey, R.; Quinn, J.M.; Athanasou, N.A. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch. Orthop. Trauma Surg. 1998, 117, 136–140. [Google Scholar] [CrossRef]
- Pearl, J.I.; Ma, T.; Irani, A.R.; Huang, Z.; Robinson, W.H.; Smith, R.L.; Goodman, S.B. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials 2011, 32, 5535–5542. [Google Scholar] [CrossRef] [PubMed]
- Antonios, J.K.; Yao, Z.; Li, C.; Rao, A.J.; Goodman, S.B. Macrophage polarization in response to wear particles in vitro. Cell. Mol. Immunol. 2013, 10, 471–482. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, T.; Ren, P.; Smith, R.L.; Goodman, S.B. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2010, 94, 1264–1269. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Burton, L.; Paget, D.; Binder, N.B.; Bohnert, K.; Nestor, B.J.; Sculco, T.P.; Santambrogio, L.; Ross, F.P.; Goldring, S.R.; Purdue, P.E. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J. Orthop. Res. 2013, 31, 73–80. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9 (Suppl. S1), S6. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Samelko, L.; McAllister, K.; Jacobs, J.J.; Hallab, N.J. Increasing both CoCrMo-alloy Particle Size and Surface Irregularity Induces Increased Macrophage Inflammasome Activation In vitro Potentially through Lysosomal Destabilization Mechanisms. J. Orthop. Res. 2013, 31, 1633–1642. [Google Scholar] [CrossRef]
- Samelko, L.; Landgraeber, S.; McAllister, K.; Jacobs, J.; Hallab, N.J.; Ryffel, B. Cobalt Alloy Implant Debris Induces Inflammation and Bone Loss Primarily through Danger Signaling, Not TLR4 Activation: Implications for DAMP-ening Implant Related Inflammation. PLoS ONE 2016, 11, e0160141. [Google Scholar] [CrossRef]
- Oblak, A.; Pohar, J.; Jerala, R.; Haziot, A. MD-2 Determinants of Nickel and Cobalt-Mediated Activation of Human TLR. PLoS ONE 2015, 10, e0120583. [Google Scholar] [CrossRef] [PubMed]
- Panez-Toro, I.; Heymann, D.; Gouin, F.; Amiaud, J.; Heymann, M.-F.; Córdova, L.A. Roles of inflammatory cell infiltrate in periprosthetic osteolysis. Front. Immunol. 2023, 14, 1310262. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Holyoak, D.T.; Trebše, R.; Randau, T.M.; Porporati, A.A.; Siskey, R.L. Ceramic Wear Particles: Can They Be Retrieved In Vivo and Duplicated In Vitro? J. Arthroplast. 2023, 38, 1869–1876. [Google Scholar] [CrossRef]
- Catelas, I.; Petit, A.; Zukor, D.J.; Marchand, R.; Yahia, L.; Huk, O.L. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials 1999, 20, 625–630. [Google Scholar] [CrossRef]
- Bylski, D.; Wedemeyer, C.; Xu, J.; Sterner, T.; Löer, F.; von Knoch, M. Alumina ceramic particles, in comparison with titanium particles, hardly affect the expression of RANK-, TNF-α-, and OPG-mRNA in the THP-1 human monocytic cell line. J. Biomed. Mater. Res. Part A 2009, 89A, 707–716. [Google Scholar] [CrossRef]
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur. Cytokine Netw. 2004, 15, 339–346. [Google Scholar]
- Ha, Y.-C.; Kim, S.-Y.; Kim, H.J.; Yoo, J.J.; Koo, K.-H. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin. Orthop. Relat. Res. 2007, 458, 106–110. [Google Scholar] [CrossRef]
- Zywiel, M.G.; Sayeed, S.A.; Johnson, A.J.; Schmalzried, T.P.; Mont, M.A. Survival of hard-on-hard bearings in total hip arthroplasty: A systematic review. Clin. Orthop. Relat. Res. 2011, 469, 1536–1546. [Google Scholar] [CrossRef]
- Hasan, S.; van Schie, P.; Kaptein, B.L.; Schoones, J.W.; de Mheen, P.J.M.-V.; Nelissen, R.G.H.H. Biomarkers to discriminate between aseptic loosened and stable total hip or knee arthroplasties: A systematic review. EFORT Open Rev. 2024, 9, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, R.K.; Purdue, E.; Sculco, T.P.; Mandl, L.A. Elevation of serum tumor necrosis factor α in patients with periprosthetic osteolysis: A case-control study. Clin. Orthop. Relat. Res. 2014, 472, 584–589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hundrić-Hašpl, Ž.; Pecina, M.; Haspl, M.; Tomicic, M.; Jukic, I. Plasma cytokines as markers of aseptic prosthesis loosening. Clin. Orthop. Relat. Res. 2006, 453, 299–304. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Zhang, C.; Tang, T.; Ren, W.; Dai, K. Expansion of CD14+CD16+ peripheral monocytes among patients with aseptic loosening. Inflamm. Res. 2009, 58, 561–570. [Google Scholar] [CrossRef]
- He, T.; Wu, W.; Huang, Y.; Zhang, X.; Tang, T.; Dai, K. Multiple biomarkers analysis for the early detection of prosthetic aseptic loosening of hip arthroplasty. Int. Orthop. 2013, 37, 1025–1031. [Google Scholar] [CrossRef]
- Moreschini, O.; Fiorito, S.; Magrini, L.; Margheritini, F.; Romanini, L. Markers of connective tissue activation in aseptic hip prosthetic loosening. J. Arthroplast. 1997, 12, 695–703. [Google Scholar] [CrossRef]
- López-Anglada, E.; Collazos, J.; Montes, A.H.; Pérez-Is, L.; Pérez-Hevia, I.; Jiménez-Tostado, S.; Suárez-Zarracina, T.; Alvarez, V.; Valle-Garay, E.; Asensi, V. IL-1 β gene (+3954 C/T, exon 5, rs1143634) and NOS2 (exon 22) polymorphisms associate with early aseptic loosening of arthroplasties. Sci. Rep. 2022, 12, 18382. [Google Scholar] [CrossRef]
- Tang, F.; Liu, X.; Jiang, H.; Wu, H.; Hu, S.; Zheng, J.; Guo, H.; Yan, L.; Xu, C.; Lin, Y.; et al. Biomarkers for early diagnosis of aseptic loosening after total hip replacement. Int. J. Clin. Exp. Pathol. 2016, 9, 1954–1960. [Google Scholar]
- Trehan, S.K.; Zambrana, L.; Jo, J.E.; Purdue, E.; Karamitros, A.; Nguyen, J.T.; Lane, J.M. An Alternative Macrophage Activation Pathway Regulator, CHIT1, May Provide a Serum and Synovial Fluid Biomarker of Periprosthetic Osteolysis. HSS J. 2018, 14, 148–152. [Google Scholar] [CrossRef]
- Morakis, A.; Tournis, S.; Papakitsou, E.; Donta, I.; Lyritis, G.P. Decreased tibial bone strength in postmenopausal women with aseptic loosening of cemented femoral implants measured by peripheral quantitative computed tomography (pQCT). J Long Term Eff. Med. Implants 2011, 21, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Hamer, A.J.; Rogers, A.; Stockley, I.; Eastell, R. Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J. Orthop. Res. 2003, 21, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Huk, O.; Zukor, D.; Eyre, D.; Alini, M. Collagen crosslinked N-telopeptides as markers for evaluating particulate osteolysis: A preliminary study. J. Orthop. Res. 2000, 18, 64–67. [Google Scholar] [CrossRef]
- Savarino, L.; Avnet, S.; Greco, M.; Giunti, A.; Baldini, N. Potential role of tartrate-resistant acid phosphatase 5b (TRACP 5b) as a surrogate marker of late loosening in patients with total hip arthroplasty: A cohort study. J. Orthop. Res. 2010, 28, 887–892. [Google Scholar] [CrossRef]
- Lawrence, N.R.; Jayasuriya, R.L.; Gossiel, F.; Wilkinson, J.M. Diagnostic accuracy of bone turnover markers as a screening tool for aseptic loosening after total hip arthroplasty. HIP Int. 2015, 25, 525–530. [Google Scholar] [CrossRef]
- Ovrenovits, M.; Pakos, E.E.; Vartholomatos, G.; Paschos, N.K.; Xenakis, T.A.; Mitsionis, G.I. Flow cytometry as a diagnostic tool for identifying total hip arthroplasty loosening and differentiating between septic and aseptic cases. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 1153–1159. [Google Scholar] [CrossRef]
- Papagiannis, S.; Tatani, I.; Kyriakopoulos, G.; Kokkalis, Z.; Megas, P.; Stathopoulos, C.; Grafanaki, K.; Lakoumentas, J. Alterations in Small Non-coding MicroRNAs (miRNAs) and the Potential Role in the Development of Aseptic Loosening After Total Hip Replacement: Study Protocol for an Observational, Cross-Sectional Study. Cureus 2024, 16, e72179. [Google Scholar] [CrossRef]
- Noshahr, R.M.; Omrani, F.A.; Chari, A.Y.; Roudsari, M.S.; Madadi, F.; Jousheghan, S.S.; Manafi-Rasi, A. MicroRNAs in Aseptic Loosening of Prosthesis: Pathophysiology and Potential Therapeutic Approaches. Arch. Bone Jt. Surg. 2024, 12, 612–621. [Google Scholar] [CrossRef]
- Malik, M.H.A.; Jury, F.; Bayat, A.; Ollier, W.E.R.; Kay, P.R. Genetic susceptibility to total hip arthroplasty failure: A preliminary study on the influence of matrix metalloproteinase 1, interleukin 6 polymorphisms and vitamin D receptor. Ann. Rheum. Dis. 2007, 66, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Stołtny, T.; Dobrakowski, M.; Augustyn, A.; Rokicka, D.; Kasperczyk, S. The concentration of chromium and cobalt ions and parameters of oxidative stress in serum and their impact on clinical outcomes after metaphyseal hip arthroplasty with modular metal heads. J. Orthop. Surg. Res. 2023, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Suh, D.-H.; Jafari, M.S.; Mullan, A.; Purtill, J.J. Aseptic loosening of total hip arthroplasty: Infection always should be ruled out. Clin. Orthop. Relat. Res. 2011, 469, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.T.; Singh, J.A. Biomarkers in Arthroplasty: A Systematic Review. Open Orthop. J. 2011, 5, 92–105. [Google Scholar] [CrossRef]
- Hart, A.J.; Sabah, S.A.; Bandi, A.S.; Maggiore, P.; Tarassoli, P.; Sampson, B.; Skinner, J.A. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J. Bone Joint Surg. Br. Vol. 2011, 93-B, 1308–1313. [Google Scholar] [CrossRef]
- Ettinger, M.; Calliess, T.; Kielstein, J.T.; Sibai, J.; Brückner, T.; Lichtinghagen, R.; Windhagen, H.; Lukasz, A. Circulating Biomarkers for Discrimination Between Aseptic Joint Failure, Low-Grade Infection, and High-Grade Septic Failure. Clin. Infect. Dis. 2015, 61, 332–341. [Google Scholar] [CrossRef]
- Qin, L.; Du, C.; Yang, J.; Wang, H.; Su, X.; Wei, L.; Zhao, C.; Chen, C.; Chen, H.; Hu, N.; et al. Synovial Fluid Interleukin Levels Cannot Distinguish between Prosthetic Joint Infection and Active Rheumatoid Arthritis after Hip or Knee Arthroplasty. Diagnostics 2022, 12, 1196. [Google Scholar] [CrossRef]
- Alotaibi, H.F.; Perni, S.; Prokopovich, P. Nanoparticle-based model of anti-inflammatory drug releasing LbL coatings for uncemented prosthesis aseptic loosening prevention. Int. J. Nanomed. 2019, 14, 7309–7322. [Google Scholar] [CrossRef]
- van Duren, B.H.; Firth, A.M.; Berber, R.; Matar, H.E.; James, P.J.; Bloch, B.V. Revision Rates for Aseptic Loosening in the Obese Patient: A Comparison Between Stemmed, Uncemented, and Unstemmed Tibial Total Knee Arthroplasty Components. Arthroplast. Today 2025, 32, 101621. [Google Scholar] [CrossRef] [PubMed]
- Layson, J.T.; Hameed, D.; Dubin, J.A.; Moore, M.C.; Mont, M.; Scuderi, G.R. Patients with Osteoporosis Are at Higher Risk for Periprosthetic Femoral Fractures and Aseptic Loosening Following Total Hip Arthroplasty. Orthop. Clin. N. Am. 2024, 55, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Dilley, J.E.; Carlone, A.; Deckard, E.R.; Meneghini, R.M.; Sonn, K.A. Effect of Tobacco Use on Radiolucent Lines in Modern Cementless Total Knee Arthroplasty Tibial Components. Arthroplast. Today 2023, 19, 101082. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Yi, C.; Krettek, C.; Jagodzinski, M.; Katoh, M. Smoking and Risk of Prosthesis-Related Complications after Total Hip Arthroplasty: A Meta-Analysis of Cohort Studies. PLoS ONE 2015, 10, e0125294. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Issa, K.; Pivec, R.; Bonutti, P.M.; Mont, M.A. Tobacco use may be associated with increased revision and complication rates following total hip arthroplasty. J. Arthroplast. 2014, 29, 777–780. [Google Scholar] [CrossRef]
- Khatod, M.; Cafri, G.; Namba, R.S.; Inacio, M.C.S.; Paxton, E.W. Risk Factors for Total Hip Arthroplasty Aseptic Revision. J. Arthroplast. 2014, 29, 1412–1417. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef]
- Safavi, S.; Yu, Y.; Robinson, D.L.; Gray, H.A.; Ackland, D.C.; Lee, P.V.S. Additively manufactured controlled porous orthopedic joint replacement designs to reduce bone stress shielding: A systematic review. J. Orthop. Surg. Res. 2023, 18, 42. [Google Scholar] [CrossRef]
- De Meo, F.; Cacciola, G.; Bellotti, V.; Bruschetta, A.; Cavaliere, P. Trabecular Titanium acetabular cups in hip revision surgery: Mid-term clinical and radiological outcomes. HIP Int. 2018, 28, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Giustra, F.; Bosco, F.; De Meo, F.; Bruschetta, A.; De Martino, I.; Risitano, S.; Sabatini, L.; Massè, A.; Cavaliere, P. Trabecular titanium cups in hip revision surgery: A systematic review of the literature. Ann. Jt. 2023, 8, 36. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lin, T.; Liu, A.; Shi, M.-M.; Hu, B.; Shi, Z.-L.; Yan, S.-G. Does hydroxyapatite coating have no advantage over porous coating in primary total hip arthroplasty? A meta-analysis. J. Orthop. Surg. Res. 2015, 10, 21. [Google Scholar] [CrossRef]
- Morano, C.; Garofalo, S.; Bertuccio, P.; Sposato, A.; Zappone, I.; Pagnotta, L. A Comprehensive Literature Review of Total Hip Arthroplasty (THA): Part 1—Biomaterials. J. Funct. Biomater. 2025, 16, 179. [Google Scholar] [CrossRef]
- Soliman, M.; Chowdhury, M.E.H.; Islam, M.T.; Musharavati, F.; Nabil, M.; Hafizh, M.; Khandakar, A.; Mahmud, S.; Nezhad, E.Z.; Shuzan, N.I.; et al. A Review of Biomaterials and Associated Performance Metrics Analysis in Pre-Clinical Finite Element Model and in Implementation Stages for Total Hip Implant System. Polymers 2022, 14, 4308. [Google Scholar] [CrossRef]
- Savin, L.; Pinteala, T.; Mihai, D.N.; Mihailescu, D.; Miu, S.S.; Sirbu, M.T.; Veliceasa, B.; Popescu, D.C.; Sirbu, P.D.; Forna, N. Updates on Biomaterials Used in Total Hip Arthroplasty (THA). Polymers 2023, 15, 3278. [Google Scholar] [CrossRef]
- Isaacson, B.M.; Jeyapalina, S. Osseointegration: A review of the fundamentals for assuring cementless skeletal fixation. Orthop. Res. Rev. 2014, 6, 55–65. [Google Scholar] [CrossRef]
- McCormick, K.L.; Mastroianni, M.A.; Kolodychuk, N.L.; Herndon, C.L.; Shah, R.P.; Cooper, H.J.; Sarpong, N.O. Complications and Survivorship After Aseptic Revision Total Hip Arthroplasty: Is There a Difference by Surgical Approach? J. Arthroplast. 2025, 40, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, V.; Garellick, G.; Kärrholm, J.; Wretenberg, P. The type of surgical approach influences the risk of revision in total hip arthroplasty: A study from the Swedish Hip Arthroplasty Register of 90,662 total hipreplacements with 3 different cemented prostheses. Acta Orthop. 2012, 83, 559–565. [Google Scholar] [CrossRef]
- Steiness, J.; Hägi-Pedersen, D.; Lunn, T.H.; Overgaard, S.; Brorson, S.; Graungaard, B.K.; Lindberg-Larsen, M.; Varnum, C.; Lundstrøm, L.H.; Beck, T.; et al. Non-opioid analgesic combinations following total hip arthroplasty (RECIPE): A randomised, placebo-controlled, blinded, multicentre trial. Lancet Rheumatol. 2024, 6, e205–e215. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Manzano-Moreno, F.; Ruiz, C. Repercussions of NSAIDS drugs on bone tissue: The osteoblast. Life Sci. 2015, 123, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.D.; Miller, C.; Jirjis, T.; Nasir, M.; Sharma, D. The effect of non-steroidal anti-inflammatory drugs on the osteogenic activity in osseointegration: A systematic review. Int. J. Implant. Dent. 2018, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Birrell, F.; Lohmander, S. Non-steroidal anti-inflammatory drugs after hip replacement. BMJ 2006, 333, 507–508. [Google Scholar] [CrossRef]
- Friedl, G.; Radl, R.; Stihsen, C.; Rehak, P.; Aigner, R.; Windhager, R. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. A randomized, double-blind, controlled trial. J. Bone Jt. Surg. 2009, 91, 274–281. [Google Scholar] [CrossRef]
| Category | Biomarkers | Levels in AL Patients | Role | Sample | References |
|---|---|---|---|---|---|
| Inflammatory Biomarkers | TNF-α, IL-1β, IL-6, IL-8, CD14+CD16+ monocytes, MCP-1, CCL18 | High | Indicative of an active inflammatory response that promotes the recruitment and activation of immune cells, contributing to bone destruction. | Blood, synovial fluid | [115,116,117,118,119,120,121,122,133,134] |
| C-reactive protein (CRP) | High | Can assist in clinical evaluation. Persistently elevated in the absence of infection may indicate ongoing periprosthetic inflammation or tissue damage. | |||
| Bone Metabolism | RANKL, CTX, NTX, TRAP5b, ICTP | High | Represent the altered balance between bone formation and resorption; increased resorption and reduced bone formation, typical of AL. | Blood, urine | [118,121,123,124,125,126,127] |
| Osteocalcin, PiCP | Low | ||||
| Matrix Degradation | Hyaluronic acid, CHIT1, CD18, CD11b, CD11c | High | Signals of extracellular matrix degradation and cellular activation, indicative of tissue damage and local immune response. | Blood, synovial fluid | [119,122,128] |
| MicroRNA | miR-21, miR-92a, miR-106b, miR-130, miR-135, miR-155 | High | Involved in the regulation of inflammatory processes and bone remodeling, contributing to the altered balance between osteoresorption and formation. | Blood | [129,130] |
| miR-29 | Low | ||||
| Genetic Factors | SNPs in NOS2 (AA genotype), IL-1β (TT genotype), and MMP1 | Associated with increased risk | Genetically predisposed to the establishment of an accentuated inflammatory response and aberrant bone remodeling, favoring the development of AL | Blood | [120,131] |
| Systemic Biomarkers | Whole-blood Cobalt (Co) and Chromium (Cr) levels | >5 μg/L | May indicate implant wear, ARMD, or imminent mechanical failure. Elevations can precede clinical or radiological signs and warrant further investigation. | Blood | [132] |
| Biomarker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Reference |
|---|---|---|---|---|---|
| ICTP | 91 | 69 | NA | NA | [127] |
| TRAP 5b | 100 | 31 | NA | NA | [127] |
| Osteocalcin | 69 | 65 | NA | NA | [127] |
| NTX | 82 | 87 | NA | NA | [127] |
| Cobalt (Co) and Chromium (Cr) ions | 63 | 86 | NA | NA | [135] |
| IL-6 | 80 | 87.7 | 69.6 | 92.6 | [136] |
| IL-1β | 94.6 | 86.2 | NA | NA | [137] |
| IL-8 | 86.1 | 100 | NA | NA | [137] |
| TNF-α | 35 | 86 | 46.7 | 79 | [136] |
| CRP | 80 | 64 | 37.2 | 92.3 | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, G.; Siracusano, L.; Micale, E.; Addorisio, V.; Ballato, M.; Donadio, D.; Tralongo, P.; Giuffrè, G.; Leonetti, D.; Martini, M.; et al. Aseptic Loosening in Total Hip Arthroplasty: Pathophysiology, Biomarkers, and Preventive Treatment Strategies. Appl. Sci. 2025, 15, 9156. https://doi.org/10.3390/app15169156
Ricciardi G, Siracusano L, Micale E, Addorisio V, Ballato M, Donadio D, Tralongo P, Giuffrè G, Leonetti D, Martini M, et al. Aseptic Loosening in Total Hip Arthroplasty: Pathophysiology, Biomarkers, and Preventive Treatment Strategies. Applied Sciences. 2025; 15(16):9156. https://doi.org/10.3390/app15169156
Chicago/Turabian StyleRicciardi, Gabriele, Lorenza Siracusano, Edoardo Micale, Vito Addorisio, Mariagiovanna Ballato, Domenico Donadio, Pietro Tralongo, Giuseppe Giuffrè, Danilo Leonetti, Maurizio Martini, and et al. 2025. "Aseptic Loosening in Total Hip Arthroplasty: Pathophysiology, Biomarkers, and Preventive Treatment Strategies" Applied Sciences 15, no. 16: 9156. https://doi.org/10.3390/app15169156
APA StyleRicciardi, G., Siracusano, L., Micale, E., Addorisio, V., Ballato, M., Donadio, D., Tralongo, P., Giuffrè, G., Leonetti, D., Martini, M., & Zampogna, B. (2025). Aseptic Loosening in Total Hip Arthroplasty: Pathophysiology, Biomarkers, and Preventive Treatment Strategies. Applied Sciences, 15(16), 9156. https://doi.org/10.3390/app15169156











