1. Introduction
Hepatocellular carcinoma is the sixth most common cancer worldwide and represents the third leading cause of cancer-related mortality [
1]. It is mainly associated with chronic liver diseases, particularly hepatitis B, hepatitis C, and cirrhosis [
2]. When anatomical conditions permit, hepatic resection constitutes one of the few curative options, offering favorable survival outcomes [
3]. However, this intervention remains extremely complex due to the dense vascular architecture of the liver, interindividual variability, and the necessity to remove tumor lesions without compromising residual liver function [
4].
To optimize this procedure, precise preoperative planning is essential. Conventional imaging examinations, such as computed tomography (CT), although essential, require a mental reconstruction in three dimensions that is often difficult to master, even for experienced surgeons [
5]. In this context, 3D physical models derived from printing allow for direct anatomical visualization, realistic tactile feedback, and facilitate the spatial understanding of hepatic structures [
6,
7]. These models prove particularly useful for surgical training, personalized surgical planning, and reducing human errors, especially during complex procedures [
8,
9,
10].
Recently, 3D printing in medicine has undergone rapid development [
11]. Recent systematic reviews have highlighted the growing impact of this technology in areas ranging from orthopedic surgery to oncological planning, including medical training [
12,
13]. Li et al. emphasized that the integration of patient-specific 3D-printed models into preoperative training enables surgeons to realistically simulate the planned procedure, thereby improving surgical planning, reducing operative time, and helping anticipate intraoperative complications [
14]. Similarly, reviews such as those by Wu et al. (2023) and Jiang et al. (2024) have consistently ranked 3D printing among the most promising tools for the development of low-cost surgical simulators in resource-limited and developing countries [
15,
16]. This is primarily due to its ability to faithfully replicate human anatomy from standard medical imaging while significantly reducing production costs [
15,
16].
Despite their interest, the high production costs of anatomical models hinder their widespread use. Indeed, certain printing technologies, such as PolyJet or stereolithography (SLA), allow for outstanding precision and the possibility of using multi-color and multi-texture materials. However, they are expensive, require industrial printers and a complex post-processing environment, which makes them difficult to access for institutions with limited resources [
17,
18].
Other methods, such as Selective Laser Sintering (SLS), offer the possibility of producing support-free structures, but they remain poorly suited for simulating soft tissues due to high surface roughness and the limitation of compatible materials [
19]. In contrast, more affordable technologies like Fused Deposition Modeling (FDM) and Liquid Crystal Display (LCD) represent effective and accessible alternatives. FDM allows for the creation of solid and functional parts made from PLA (polylactic acid), a rigid, biocompatible, and economical material. As for LCD technology, it allows for higher resolution, suitable for printing complex structures such as blood vessels or tumors, using photopolymer resins [
20,
21].
To realistically simulate the consistency of liver parenchyma, soft materials such as food-grade gelatin or silicone are commonly used. These materials reproduce the mechanical and tactile properties of liver tissue, making them ideal for realistic surgical simulations, particularly in a training or preoperative context [
22,
23].
This work presents a method for fabricating a 3D-printed liver model specifically designed to support preoperative planning and surgical training in resource-limited clinical settings. The proposed approach leverages open-source tools and combines cost-effective 3D printing technologies (FDM and LCD) with soft materials such as gelatin to realistically replicate hepatic anatomy at a low cost. The process involves segmenting liver structures from CT images, creating molds and internal components using a reproducible workflow, and assembling the final model using gelatin to simulate liver tissue texture. A preliminary evaluation conducted with healthcare professionals highlighted the model’s educational value, particularly for surgical simulation in low-resource environments.
2. Materials and Methods
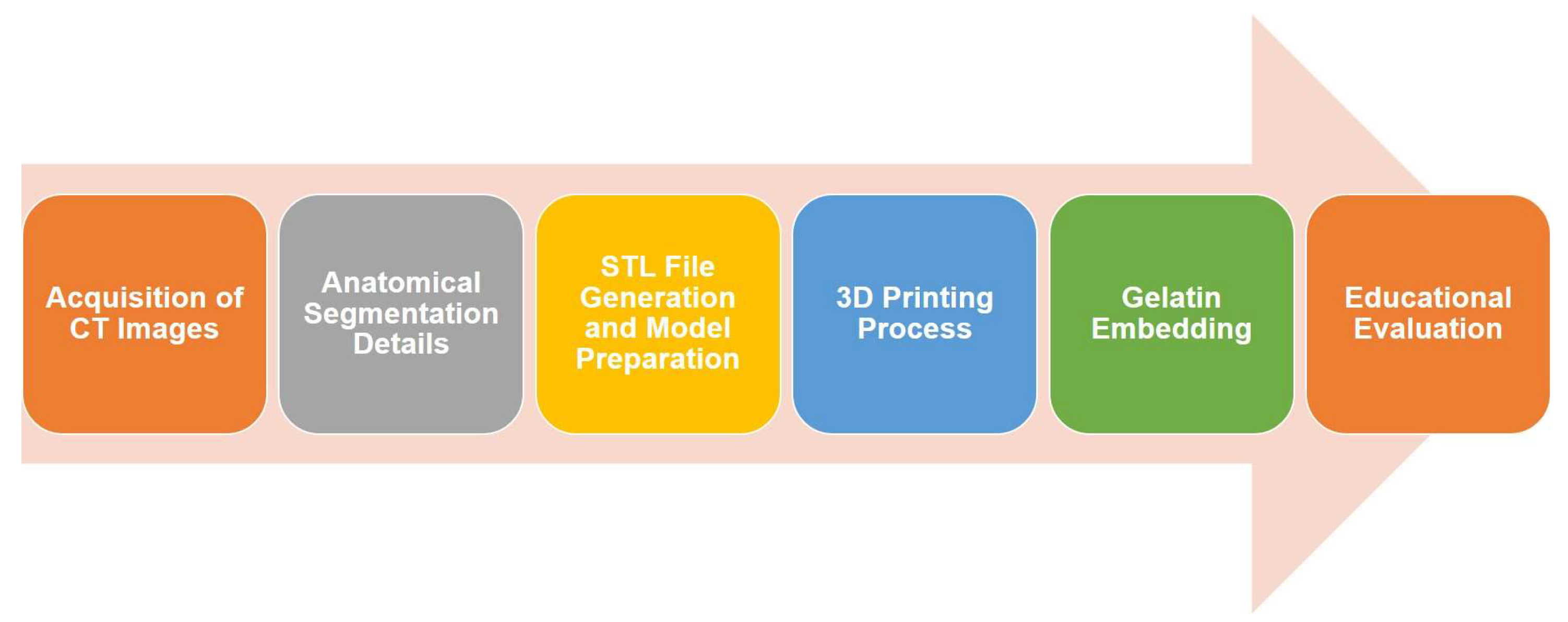
The methodology followed in this study is illustrated in
Figure 1 and consists of six main steps: (i) CT images were reviewed and pre-processed to identify key anatomical structures, including the hepatic parenchyma, vascular network, and lesions; (ii) these structures were segmented using open-source software, allowing for accurate 3D reconstructions that were exported in STL format; (iii) the STL files were processed using slicing software to define the appropriate printing parameters for each technology—FDM was used to fabricate the external molds, and LCD printing was employed for the finer vascular structures; (iv) the model components were then printed and assembled; (v) the assembled molds were filled with food-grade gelatin to simulate the texture and consistency of liver tissue; and (vi) finally, a qualitative evaluation was carried out by a panel of surgeons to assess the anatomical accuracy and educational relevance of the model.
2.1. Acquisition of CT Images
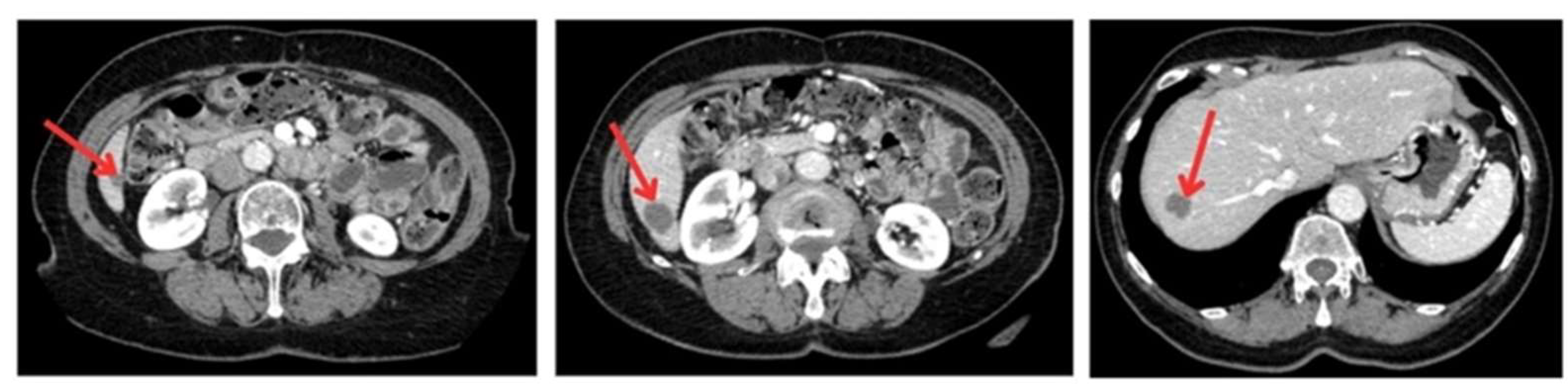
To generate a 3D liver phantom with three metastases, high-resolution CT images (512 × 512 pixels, slice thickness and interslice spacing of 1.25 mm) were acquired in both portal and arterial phases using a GE Healthcare Bright Speed Elite scanner. The acquisition period was 3 min and 45 s, with a tube voltage of 120 kVp and a current of 35 mA. These phases are essential for accurately characterizing liver tumors and visualizing the vascular architecture. The images were recorded in DICOM format to preserve essential acquisition metadata and ensure compatibility with medical imaging software. The anonymized images, providing optimal spatial resolution, were sourced from the Medical Segmentation Decathlon open-access dataset, ensuring data quality and reproducibility for segmentation and 3D modeling (
Figure 2).
2.2. Anatomical Segmentation Details
Anatomical segmentation was performed using the open-source software 3D Slicer (version 5.6.2; Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; available at:
https://www.slicer.org), which allowed for semi-automatic extraction of three-dimensional liver structures through the region-growing technique and built-in segmentation tools. A comparative evaluation of several methods of automatic segmentation and semi-automatic approaches, including region growing, thresholding, and contour interpolation, with manual segmentation as the gold standard, was conducted to determine the most accurate and reliable approach. Among these, the region growing method proved to be the most effective, yielding the highest Dice similarity coefficient and the lowest Hausdorff distance, as well as minimal RMS and standard deviation, thus indicating strong agreement with manual segmentation. While automatic segmentation was the fastest, it showed substantial inaccuracies and variability. Thresholding and contour interpolation provided intermediate results, superior to automatic methods but less precise and consistent than region growing.
Based on this evaluation, region growing was selected for all anatomical segmentations due to its optimal balance of precision, execution time, and reliability, enabling the creation of a 3D liver model closely resembling the actual anatomical structures.
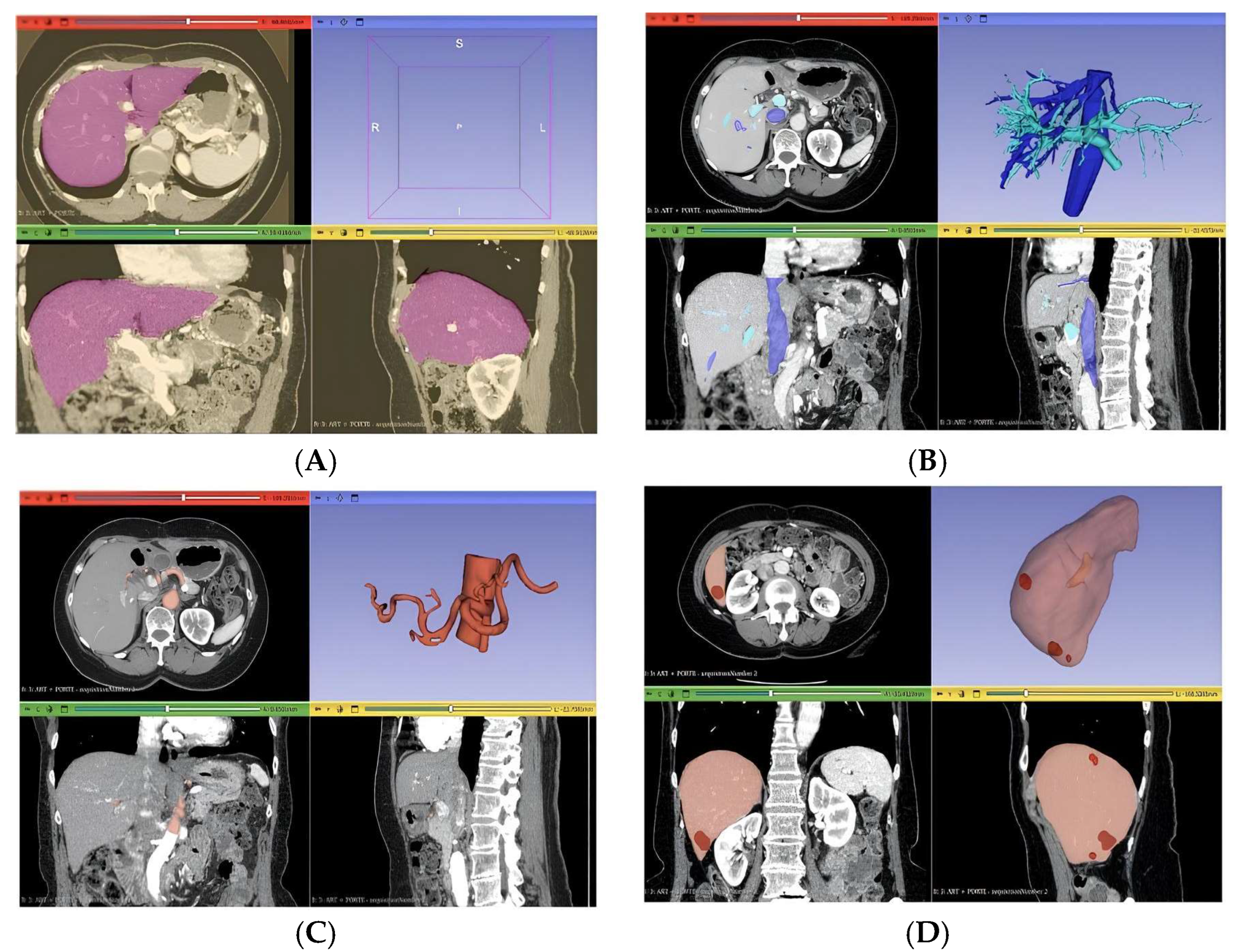
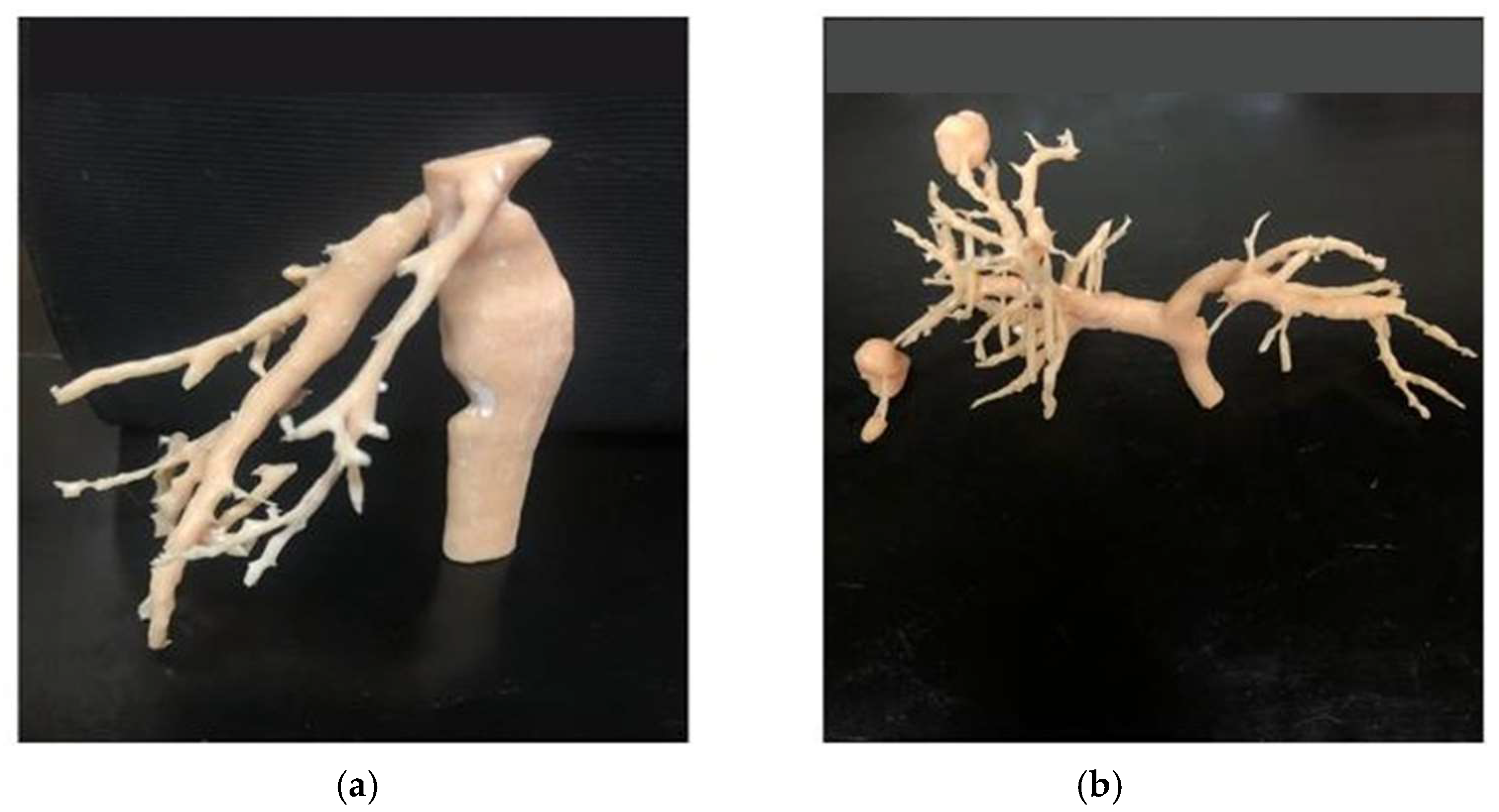
Three distinct anatomical segmentations were carried out to generate the final 3D liver model: lesion segmentation, vascular segmentation, and segmentation of the entire liver volume (
Figure 3). The lesion segmentation focused on identifying three metastases, with quantitative descriptors extracted for each, including maximum 2D diameter, volume, and short-axis length. Vascular segmentation was divided into two components: one targeting the portal and hepatic veins, and the other focusing on the hepatic artery.
For the portal and hepatic veins, a threshold mask was applied within the intensity range [137.67; 1472]. Due to the presence of surrounding artifacts, an additional mask was generated using the “Keep Selected Islands” function to isolate relevant vascular structures. The “Region Growing” tool was then applied to differentiate hepatic veins from the portal vein. Hepatic artery segmentation began with a manual volume cropping to localize the arterial region, which served as a refined mask for subsequent region growth. Unrelated structures were removed, ensuring that only the hepatic artery remained. This process was performed specifically on arterial-phase CT images to enhance vascular contrast and segmentation fidelity.
2.3. STL File Generation and Model Preparation
After the segmentation was complete, a three-dimensional visualization was carried out to validate the segmented structures. The 3D reconstruction was performed in 3D Slicer using the Marching Cubes algorithm to generate surface meshes from the segmented volumes. The models that were found to be satisfactory were then exported from 3D Slicer as mesh-type files in the stereolithography (STL) format for further processing and 3D printing. The STL files were generated using optimized mesh parameters suitable for surgical 3D printing. A chordal deviation of 0.05 mm and an angular deviation of 15° were applied to ensure high geometric fidelity while maintaining manageable file sizes. These settings were selected to preserve the anatomical accuracy of the liver and lesion structures while avoiding excessive surface simplification or large file sizes.
We used a free program called Meshmixer (version 3.5.474; Autodesk, Inc., San Rafael, CA, USA) to finish the mesh models. The program did the following things: (1) It checked and removed artifacts that were overlapping vessel walls; (2) it made the manifold (watertight) model; and (3) it made the liver mold, which is needed for 3D printing.
To transfer the 3D models from the segmentation into a print-ready STL file, each segment is inserted. This operation is repeated for all the necessary segments. Then, we combined the models into a single file for 3D printing, as illustrated in
Figure 4. The 3D printing technology compatible with this model is material extrusion 3D printing technology. Thus, it uses a wide range of materials and even allows for the simultaneous combination of several (each stored in separate print heads). It allows for the creation of objects composed of multiple materials and different colors.
The advantage of our proposal lies in the unavailability and high cost of 3D printing technology by material deposition. We are focusing on creating several parts ready to print in STL format (lesions, vascular segments, and liver volume) of the same object using the hepatic parenchyma technique to assemble the model in a mold within the same coordinate system, casting it inside using gelatin, and then subsequently disassembling the external parts.
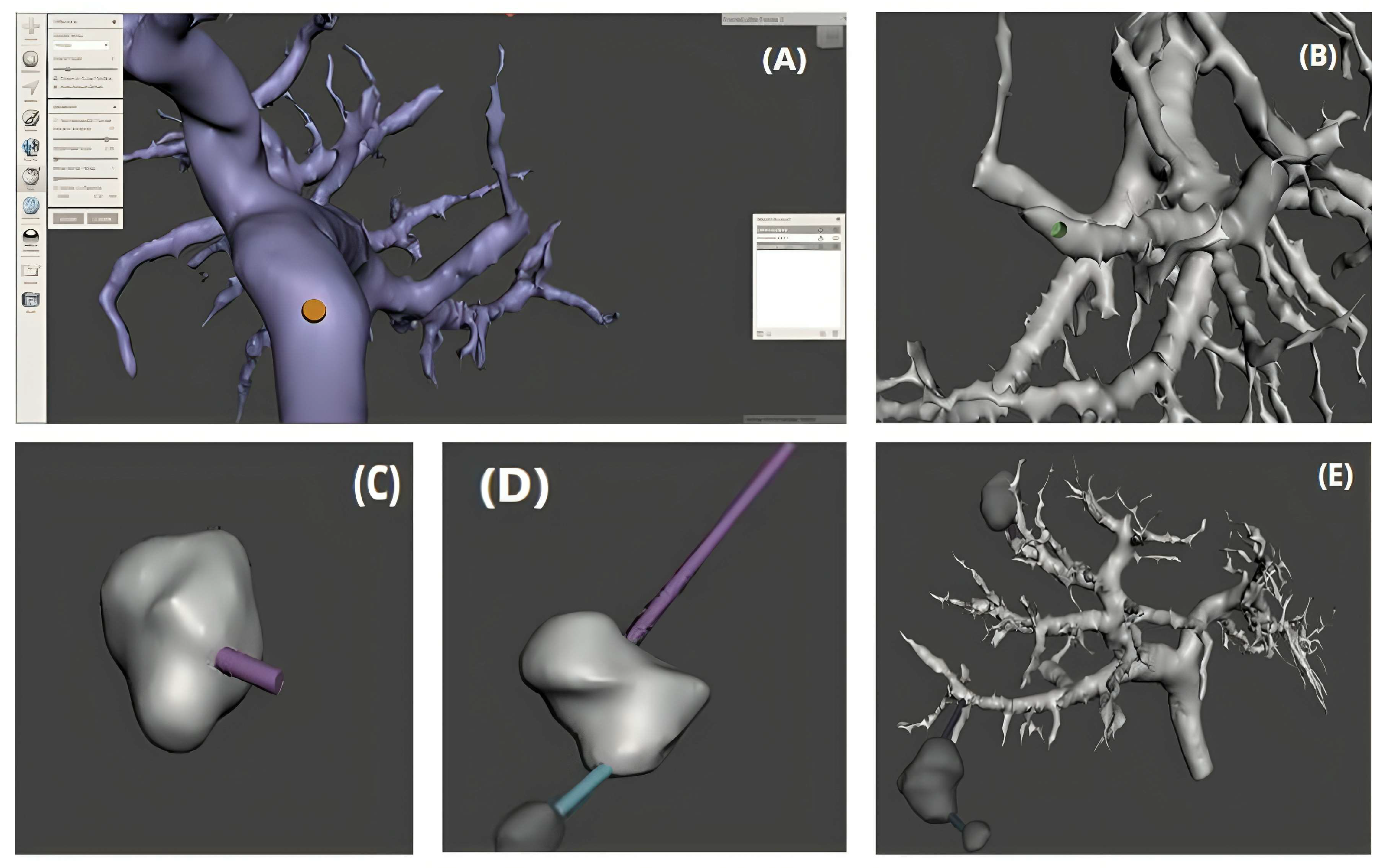
The model preparation process first involves generating holes on the portal veins in MeshMixer to insert a support for the tumors. Using the modeling tools, we create a cylinder at the desired location, then apply Boolean operations to subtract this cylinder from the portal vein, thereby creating the hole. Then, we position support 45 in this hole and attach the tumor to it. Since the tumors are not connected to any vessels and are independent, additional supports are created and strategically positioned to secure them firmly. These supports are then merged with the tumors using Boolean functions. This process ensures a solid and precise connection between the elements, ready for 3D printing (
Figure 5). We utilized the 3D model of the liver and a partially assembled tile to produce the mold through the application of the Boolean difference operation. The liver model was initially placed within the tile, followed by the application of the “Boolean Difference” operation to remove the liver from the tile, thus forming the mold cavity that corresponds to the liver’s shape. Subsequently, to guarantee a secure connection between the two sections of the mold, male and female fasteners were affixed to the edges of the mold. This ensures that the two halves of the mold remain securely positioned during the filling and assembly process, guaranteeing a precise and stable 3D print.
2.4. Three-Dimensional Printing Process
Two 3D printing technologies were used to fabricate the different components of the liver model. The external mold was designed as two separate halves, each printed using an Anycubic Vyper desktop FDM printer with PLA+ filament (Esun, Shenzhen, China). Polylactic acid (PLA) was chosen for its low cost, wide availability, and ease of use, making it particularly suitable for low-resource medical environments and educational applications. The filament was deposited layer by layer with a defined layer thickness of 0.15 mm to ensure adequate precision. Printing was carried out at a nozzle temperature between 200 and 220 °C and a bed temperature of 60 °C to ensure proper adhesion. As is often the case with FDM printing, the mold surfaces exhibited moderate roughness due to the successive deposition of layers. To improve surface quality, the printed parts were manually sanded with abrasive paper after the supports were removed. This step helped reduce irregularities and produce smoother contact surfaces for molding. Finally, the two mold halves were aligned and assembled using integrated male and female connectors, and their fit was carefully verified to ensure proper sealing and stability during the molding process (
Figure 6).
The models were sliced using Cura (version 5.6.0; Ultimaker B.V., Utrecht, The Netherlands; available at:
https://ultimaker.com/software/ultimaker-cura, accessed on 26 June 2025), an open-source software developed by Ultimaker (Netherlands). The total printing time varied depending on model size, number of parts, resolution, and printer type. In this case, printing both mold sections took approximately 52 h, divided into two print jobs, and the final fitting was precise.
The inferior vena cava, portal vein, and tumor lesions were printed using UNIZ IBEE LCD printing technology with PLA Pro resin. This method offers high resolution and fine detail reproduction, essential for accurately replicating complex vascular structures. The prepared models were sliced using the UnizMaker software (version 1.3.0.11; Uniz, San Diego, CA, USA; available at:
https://www.uniz.com/software/uniz-maker, accessed on 26 June 2025) linked to the machine. The average printing time was 7 h and 30 min for the portal vein and tumors, and 8 h and 40 min for the inferior vena cava, with a precision rate of 0.05 mm in each layer. After printing, the parts were carefully detached from the platform, the supports were removed, and a post-processing protocol was applied, including a 5 min rinse in 99% isopropyl alcohol, air drying, and 10 min of photopolymerization at 25 °C under 405 nm light (
Figure 7).
2.5. Gelatin Embedding
The choice of food-grade gelatin to fill the liver mold was made due to its flexibility, which allows for the creation of a realistic and manageable model. This flexibility is beneficial for surgical simulations, allowing practitioners to perform resections realistically. By using gelatin, we obtain a model that can be easily manipulated and adjusted, thus facilitating training and preoperative planning. The gelatin was poured into the assembled model. There are several approaches to execute this step: a tube or funnel can be placed in the opening of the mold and secured against leaks, or a “chimney” can be made at the top of the mold, even for beginners, which avoids silicone leakage issues. The silicone was cured for 72 h at room temperature or at a temperature of 4 °C for 24 h.
2.6. Educational Evaluation (Simulated Pedagogical Validation)
To evaluate the potential pedagogical value of the 3D-printed liver model, a preliminary simulation-based user study was conducted. Five medical trainees (3 senior medical students and 2 surgical residents) participated in a hands-on session using the printed liver phantom. Participants were first asked to review 2D CT images of a liver with metastases, followed by a physical manipulation of the 3D model.
After the session, participants completed a short questionnaire based on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree). The questionnaire assessed four domains: (1) anatomical comprehension, (2) perception of spatial relations, (3) confidence in preoperative planning, and (4) perceived realism of the model. The data were collected anonymously and used to evaluate the model’s pedagogical relevance.
In the future, this model could be used for more specific tasks such as the identification of complex vascular structures, the simulation of personalized liver resections, or even training in surgical planning.
3. Results
The final result of the process was a life-sized model of a liver. A red dye was added to the gelatin to give a parenchyma that almost resembles the shape of the liver. The inferior vena cava is colored differently from the portal vein and tumors using different dyes to help the surgeon distinguish each element during the simulation.
The liver tumors, represented in green, are visible and correctly positioned at the intended locations. This precision in tumor localization is crucial for surgical simulations and preoperative training.
The pliability of gelatin facilitates the development of a lifelike model that accurately replicates the texture and mobility of human liver tissue. This level of realism is essential for surgical simulations, as it allows practitioners to train in conditions close to reality. However, it is important to note that gelatin is slightly more sensitive than real liver, especially when inserting vessels inside. This difference increased sensitivity, making the model more prone to breaking, which can sometimes pose additional challenges (
Figure 8).
The production cost of the gelatin liver model finalized in this article is estimated to be less than USD 50 (
Table 1).
We estimate a total development time, from segmentation to the completed product, of approximately 128 h. The model is stored in the refrigerator at 4 °C to preserve the gelatine prior to being transferred to the surgical team, scheduled to do the operation three days before the surgery.
The 3D-printed liver model facilitated the precise and detailed visual identification of essential hepatic anatomical structures. Notably, it allowed for the clear localization and differentiation of tumor lesions, the portal vein, and the inferior vena cava. The use of distinct colors and translucent gelatin further enhanced the contrast between these components, thereby improving spatial comprehension and anatomical orientation. This level of detail is critical for planning complex liver resections, where understanding the relative positions of vascular and tumoral elements is essential to ensure safe and effective surgical outcomes. The model’s fidelity thus contributes not only to visualization but also to informed strategic surgical decision-making.
The faithfully reproduced spatial arrangement of these structures facilitated the development of surgical resection strategies adapted to the anatomical specifics of the simulated case. Manipulation of the model contributed to an enhanced understanding of intrahepatic vascular organization, supported by the translucency of the gelatin casting and the color-coded differentiation of internal components.
In addition to its visual realism, the soft texture of the simulated parenchyma reinforced the immersive experience by convincingly replicating the consistency of real liver tissue. These characteristics were particularly appreciated by users, as reflected in high satisfaction levels regarding anatomical understanding (4.6/5), spatial perception (4.4/5), confidence in preoperative planning (4.2/5), and overall realism of the model (4.4/5).
Surgeons also performed a visual and tactile assessment of the model, using it to guide discussions on surgical strategy. The model was further employed as a perioperative visual aid. These initial results highlight the model’s relevance as an effective educational and clinical tool for surgical planning (
Table 2).
4. Discussion
The main objective of this study was to develop a personalized, realistic, and low-cost hepatic model, intended for preoperative planning and surgical training, particularly suited for clinical environments with limited resources. This approach is part of a growing trend in research on the use of 3D-printed anatomical models to improve medical education and surgical preparation. Several studies have highlighted the effectiveness of physical models in enhancing anatomical understanding, particularly in contexts where access to advanced technologies is limited [
6,
7,
10]. Moreover, recent studies have shown that the integration of 3D-printed simulators into training programs helps improve surgical performance, practitioner confidence, and overall care quality [
12,
14,
15]. Furthermore, Luque et al. illustrated the efficacy of economical 3D-printed models for training in minor surgical procedures within primary care, emphasizing their educational significance, accessibility, and adaptability to the training requirements of general practitioners [
24]. Unlike expensive industrial solutions (PolyJet, SLA), our method relies on open-source tools and affordable materials to ensure broader adoption in healthcare institutions.
The technical approach relies on the integration of several essential steps: semi-automatic anatomical segmentation using the “region growing” method in 3D Slicer, chosen for its precision (high Dice scores and low Hausdorff distance); optimized STL export with a chord deviation of 0.05 mm and an angular deviation of 15°, ensuring satisfactory geometric fidelity without file overload; mold printing using FDM (PLA+), suitable for manufacturing solid structures at low cost; and the printing of complex internal structures (vessels, tumors) using LCD (PLA Pro), ensuring high resolution and excellent detail rendering. The use of food-grade gelatin as a molding material allows for the simulation of human liver texture while remaining easily manageable. The resultant model is modular, anatomically precise, and facilitates a coherent assembly of the various printed components. Although gelatin is more fragile than real liver tissue, its properties are sufficient for educational use or preoperative simulation.
This model is designed for personalized preoperative simulation, not for generic student training. It is intended for single use, tailored to the surgical planning of a specific case, where long-term durability is not required. The use of medical-grade silicone, although more durable, is not economically justified for such one-time applications.
In contrast, food-grade gelatin provides a realistic reproduction of liver texture at a very low cost and with minimal production time, making it particularly well-suited for patient-specific models in resource-limited clinical settings. Unlike reusable silicone-based simulators designed for repeated training, this model supports individualized surgical planning, where anatomical realism, rapid production, and cost-efficiency take precedence over reusability [
25].
An exploratory pilot evaluation was conducted with five users (3 advanced medical students and 2 surgical residents) to assess the feasibility, pedagogical relevance, and technical usability of the developed model. A questionnaire based on a Likert scale (1 to 5) was used to gather qualitative and quantitative feedback on several dimensions: anatomical understanding (4.6), spatial perception (4.4), confidence in planning (4.2), perceived realism (4.4), and ease of handling the model (4.2). The users highlighted the clarity of the internal structures printed in LCD, the precision of the lesion positioning, and the overall good stability of the FDM-printed mold, despite some fragility during intensive handling. These preliminary results pave the way for further studies. A validation with rigorous statistical methods and a larger sample will confirm the usefulness, pedagogical validity, and reproducibility of the proposed model in various clinical contexts [
10,
13,
22]. A larger validation study with quantitative analyses is necessary to confirm these results. Although this validation is based on a limited sample, it should not limit the scope of the proposed approach but rather constitute a foundational step towards a more in-depth evaluation in various contexts.
Conventional methods based on industrial printers (Stratasys Connex, AGILISTA-3100) offer very precise models but cost between USD 560 and 1000. Thus, PolyJet technologies and, to a lesser extent, stereolithography (SLA) have been the most commonly used to produce anatomical models for presurgical preparation. PolyJet printers, which are used for industrial purposes, have costs ranging from USD 6000 for entry-level models to over USD 200,000 for advanced versions [
18]. These devices are capable of producing highly detailed models, but their accessibility remains limited to major hospital centers. In comparison, desktop FDM printers, like those used in this study, generally cost between a few hundred dollars and USD 5000, depending on the technical specifications [
5]. In contrast, our model can be produced for less than USD 50, with a total production time of approximately 128 h, of which less than 70 h are dedicated to printing the various parts. This time is comparable, even slightly higher, to that required for models made using PolyJet technology, whose average printing duration varies between 36 and 40 h depending on the complexity of the model [
9]. However, a large part of the time in our method is related to the stabilization of the gelatin and manual post-processing. Moreover, this delay can be significantly reduced through the simultaneous use of multiple FDM and LCD printers, which represents a logistical advantage for organizations looking to optimize their production chain. For example, by using two FDM and two LCD printers in parallel, the average printing time can be reduced to approximately 33 h, about 25 h for the mold and 8 h for the internal vascular structures, thus bringing the total production time closer to that of PolyJet-based workflows while maintaining a significantly lower cost. Watson et al. have demonstrated that a compromise between precision, cost, and simplicity is achievable [
20]. Other recent studies also confirm the value of low-cost 3D-printed models for education and surgical planning, particularly in developing countries or hospitals with limited resources. For example, Meyer-Szary et al. showed that 3D-printed anatomical simulators significantly improved the practical skills of medical students [
12]. Li et al. also emphasized the importance of open-source tools and economical materials to promote the integration of simulation into clinical curricula [
14]. Our method illustrates this possibility with a single-color, single-material model, accessible and reproducible in the majority of hospitals.
This model can be used in surgical planning (particularly for complex resections) and in anatomy and surgery training, as well as a communication tool with patients. It offers tangible support that facilitates the spatial understanding of hepatic structures, particularly in institutions lacking digital simulators or immersive technologies.
The main identified limitations are the small sample size for educational evaluation, the fragility of the gelatin model, and the absence of post-printing quantitative measurements for dimensional validation. It is recommended to extend the study to a larger sample, to compare the model with commercial or virtual solutions, and to explore other biosimilar materials to improve durability and mechanical realism.
This study constitutes a first step towards the democratization of personalized 3D printing for medical simulation and training. It highlights the technical feasibility, financial accessibility, and educational value of our hepatic model. The results obtained, although preliminary, suggest a strong potential for integration into various clinical contexts and justify the development of future large-scale evaluations. Thanks to its simplicity, low cost, and satisfactory accuracy, our method represents a relevant and adaptable alternative, particularly in resource-limited environments.