Additive Manufacturing Technologies and Their Applications in Dentistry: A Systematic Literature Review
Abstract
1. Introduction
- RQ1: What are the most common applications of additive manufacturing in dentistry?
- RQ2: Which technologies and materials are most prevalent in dental applications?
- RQ3: Which parameters affect the quality and safe usage of 3D-printed dental applications?
2. A Systematic Literature Review
3. Initial Analysis of the Selected Articles
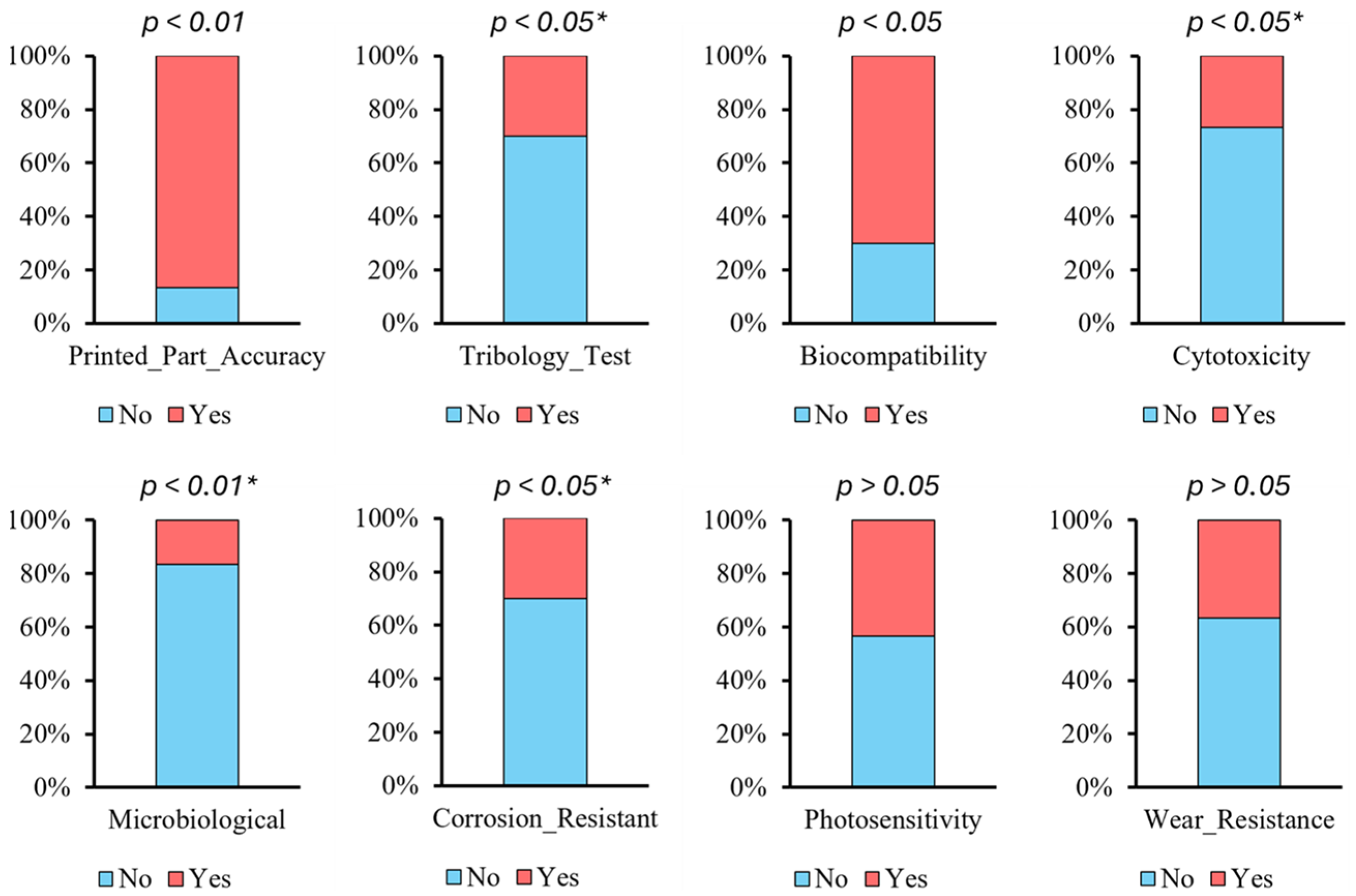
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Additive Manufacturing |
| SLR | Systematic Literature Review |
| RQ | Research Question |
| PRISMA | Preferred Reporting of Items for Systematic Reviews and Meta-Analyses |
| FDM | Fused Deposition Modeling |
| SLA | Stereolithography |
| DLP | Digital Light Processing |
| SLS | Selective Laser Sintering |
| LDED | Laser-Directed Energy Deposition |
| CNC | Computer Numerical Control |
| SLM | Selective Laser Melting |
| DMLS | Direct Metal Laser Sintering |
| FFF | Fused Filament Fabrication |
| PMMA | Polymethyl Methacrylate |
| CAD/CAM | Computer-Aided Design and Computer-Aided Manufacturing |
| WoS | Web of Science |
| EC | Exclusion Criteria |
| EC-NENG | Exclusion Criteria—Not English |
| EC-NART | Exclusion Criteria—Not Article |
| FGC | Fluorapatite Glass-Ceramic |
| YSZ | Yttria-Stabilized Zirconia |
| LP-DED | Laser Powder-Directed Energy Deposition |
| LPBF | Laser Powder Bed Fusion |
| LCM | Lithography-Based Ceramic Manufacturing |
| DIW | Direct Ink Writing |
| FGAM | Functionally Graded Additive Manufacturing |
| FP | Feldspathic Porcelain |
| PETG | Polyethylene Terephthalate Glycol |
| PCTG | Polycyclohexylene Dimethylene Terephthalate Glycol-Modified |
| FTIR | Fourier Transform Infrared |
| LDED-PF | Laser Directed Energy Deposition via Powder Feeding |
| LWT | Lost Wax Technique |
| PLA | Polylactic Acid |
| DPP | Daylight Polymer Printing |
| LCD | Liquid Crystal Display |
| UV | Ultraviolet |
References
- Dimitrova, M.; Capodiferro, S.; Vlahova, A.; Kazakova, R.; Kazakov, S.; Barile, G.; Corsalini, M. Spectrophotometric analysis of 3D Printed and conventional denture base resin after immersion in different colouring agents—An in vitro study. Appl. Sci. 2022, 12, 12560. [Google Scholar] [CrossRef]
- Sulaiman, M.M.; Fatalla, A.A.; Haider, J. the impact of incorporating grapefruit seed skin particles into 3D-printed acrylic resin on mechanical properties. Prosthesis 2024, 6, 1420–1436. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Agathopoulos, S. Selective laser melting-sintering technology: From dental Co-Cr alloys to dental ceramic materials. Sol. St. Phen. 2022, 339, 115–122. [Google Scholar] [CrossRef]
- Rouf, S.; Malik, A.; Singh, N.; Raina, A.; Naveed, N.; Siddiqui, M.I.; Haq, M.I. Additive manufacturing technologies: Industrial and medical applications. Sustain. Oper. Comput. 2022, 3, 258–274. [Google Scholar] [CrossRef]
- Gobena, S.T.; Woldeyohannes, A.D. Assessments and investigation of process parameter impacts on surface roughness, microstructure, tensile strength, and porosity of 3D printed polyetherether ketone (PEEK) materials. Results Eng. 2024, 24, 103317. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzański, L.B.; Dobrzańska-Danikiewicz, A.D.; Dobrzańska, J.; Rudziarczyk, K.; Achtelik-Franczak, A. Non-antagonistic contradictoriness of the progress of advanced digitized production with SARS-CoV-2 virus transmission in the area of dental engineering. Processes 2020, 8, 1097. [Google Scholar] [CrossRef]
- Wechkunanukul, N.; Klomjit, K.; Kumtun, T.; Jaikumpun, P.; Kengtanyakich, S.; Katheng, A. Comparison of mechanical and surface properties between conventional and CAD/CAM provisional restorations. Eur. J. Dent. 2025, 19, 697–703. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kumar, R.; Saini, R.S.; Vyas, R.; Bai, S.; Vaddamanu, S.K. Assessment of marginal fit and accuracy of crowns fabricated using CADCAM milling and 3D printing technology. J. Pharm. Bioallied Sci. 2024, 16, S3509–S3511. [Google Scholar] [CrossRef]
- Quezada, M.M.; Fernandes, C.; Montero, J.; Correia, A.; Salgado, H.; Fonseca, P. A Different approach to analyzing the surface roughness of prosthetic dental acrylic resins. Appl. Sci. 2024, 14, 619. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Vu, T.T.; Nguyen, Q.N. Advanced digital 3D technology in the combined surgery-first orthognathic and clear aligner orthodontic therapy for dentofacial deformity treatment. Processes 2021, 9, 1609. [Google Scholar] [CrossRef]
- Lassila, L.; Mangoush, E.; He, J.; Vallittu, P.K.; Garoushi, S. Effect of post-printing conditions on the mechanical and optical properties of 3d-printed dental resin. Polymers 2024, 16, 1713. [Google Scholar] [CrossRef]
- Dobrzański, L.B. Advanced engineering materials and materials processing technologies in dental implant and prosthetic treatment with clinical cases. Achiev. Mater. Manuf. Eng. 2023, 121, 7–45. [Google Scholar] [CrossRef]
- No-Cortes, J.; Attard, B.; Mifsud, D.P.; Ferreira Lima, J.; Markarian, R.A.; Ayres, A.P.; Cassar, G.; Cortes, A.R.; Attard, N.J. Comparison of 3D-printed single crown outcomes among different computer-aided design software programs. Int. J. Prosthodont. 2024, 37, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B. Transforming healthcare: A review of additive manufacturing applications in the healthcare sector. Eng. Proc. 2024, 72, 2. [Google Scholar] [CrossRef]
- Barbur, I.; Opris, H.; Crisan, B.; Cuc, S.; Colosi, H.A.; Baciut, M.; Opris, D.; Prodan, D.; Moldovan, M.; Crisan, L.; et al. Statistical comparison of the mechanical properties of 3D-printed resin through triple-jetting technology and conventional PMMA in orthodontic occlusal splint manufacturing. Biomedicines 2023, 11, 2155. [Google Scholar] [CrossRef] [PubMed]
- Nezir, M.; Özcan, S.; Atilla, A.O.; Evis, Z. A review, zirconia based dental materials. J. Aust. Ceram. Soc. 2025, 61, 235–249. [Google Scholar] [CrossRef]
- Arossi, G.A.; Abdou, N.A.; Hung, B.; Garcia, I.M.; Zimmer, R.; Melo, M.A. Safety of 3D-printed acrylic resins for prosthodontic appliances: A comprehensive cytotoxicity review. Appl. Sci. 2024, 14, 8322. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical applications of titanium alloys: A comprehensive review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Rezaie, F.; Farshbaf, M.; Dahri, M.; Masjedi, M.; Maleki, R.; Amini, F.; Wirth, J.; Moharamzadeh, K.; Weber, F.E.; Tayebi, L. 3D printing of dental prostheses: Current and emerging applications. J. Compos. Sci. 2023, 7, 80. [Google Scholar] [CrossRef]
- Palanisamy, S. Exploring the horizons of four-dimensional printing technology in dentistry. Cureus 2024, 16, e58572. [Google Scholar] [CrossRef]
- Pordeus, M.D.; Junior, J.F.; Venante, H.S.; da Costa, R.M.; Chocano, A.P.; Porto, V.C. Computer-aided technology for fabricating removable partial denture frameworks: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 128, 331–340. [Google Scholar] [CrossRef]
- Rus, F.; Neculau, C.; Imre, M.; Duica, F.; Popa, A.; Moisa, R.M.; Voicu-Balasea, B.; Radulescu, R.; Ripszky, A.; Ene, R.; et al. Polymeric materials used in 3DP in dentistry—Biocompatibility testing challenges. Polymers 2024, 16, 3550. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Teixeira, A.B.; dos Reis, A.C. Organic and inorganic antimicrobials incorporated into acrylic resin: Antimicrobial efficacy and cytotoxicity: A systematic review. Polym. Bull. 2024, 81, 13391–13418. [Google Scholar] [CrossRef]
- Dimitrova, M.; Vlahova, A.; Kalachev, Y.; Zlatev, S.; Kazakova, R.; Capodiferro, S. Recent advances in 3D printing of polymers for application in prosthodontics. Polymers 2023, 15, 4525. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.K.; Mansoor, N.S.; Mohammed, N.H.; Qasim, S.S. Subtractive and additive technologies in fixed dental restoration: A systematic review. J. Tech. 2023, 5, 162–167. [Google Scholar] [CrossRef]
- Cai, H.; Xu, X.; Lu, X.; Zhao, M.; Jia, Q.; Jiang, H.-B.; Kwon, J.-S. Dental materials applied to 3D and 4D printing technologies: A review. Polymers 2023, 15, 2405. [Google Scholar] [CrossRef]
- Chuchulska, B.; Dimitrova, M.; Vlahova, A.; Hristov, I.; Tomova, Z.; Kazakova, R. Comparative analysis of the mechanical properties and biocompatibility between CAD/CAM and conventional polymers applied in prosthetic dentistry. Polymers 2024, 16, 877. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A review on the biocompatibility of PMMA-based dental materials for interim prosthetic restorations with a glimpse into their modern manufacturing techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Moosabeiki, V.; Rajaai, S.M.; Zhou, J.; Zadpoor, A.A. Additive manufacturing of biomaterials—Design principles and their implementation. Materials 2022, 15, 5457. [Google Scholar] [CrossRef]
- Saha, S.; Roy, S. Metallic dental implants wear mechanisms, materials, and manufacturing processes: A literature review. Materials 2023, 16, 161. [Google Scholar] [CrossRef]
- Rokaya, D.; Skallevold, H.E.; Srimaneepong, V.; Marya, A.; Shah, P.K.; Khurshid, Z.; Zafar, M.S.; Sapkota, J. Shape memory polymeric materials for biomedical applications: An update. J. Compos. Sci. 2023, 7, 24. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric denture base materials: A review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef]
- Quirosa-Galán, I.; García-Bravo, S.; Fabara-Rodríguez, A.C.; Rodríguez-Pérez, M.P.; Huertas-Hoyas, E.; Pérez-Corrales, J.; Fernández-Gómez, G.; Donovan, M.; García-Bravo, C. Video games in rehabilitation programs for people with Parkinson’s disease: A systematic review. Appl. Sci. 2025, 15, 311. [Google Scholar] [CrossRef]
- Panda, A.; Dyadyura, K.; Ivakhniuk, T.; Hrebenyk, L.; Primova, L. Nano-materials in dental implants-understanding composition and biofilm dynamics. MM Sci. J. 2024, 6, 8050–8057. [Google Scholar] [CrossRef]
- Li, C.; Shen, W.; Wang, S.; Kang, J.; Zhang, Y.; Wang, G. Design and 3D printing of glass-ceramic/zirconia composite ceramics for dental application. Ceram. Int. 2024, 50, 42593–42606. [Google Scholar] [CrossRef]
- Saha, S.; Grandhi, M.; Kiran, K.U.; Liu, Z.; Roy, S. Investigating the effect of select alloying elements in additively manufactured Co-Cr alloy for dental prosthetics. J. Mater. Process. Tech. 2024, 329, 118434. [Google Scholar] [CrossRef]
- Lu, C.; Chen, J.; Ma, T.; Chen, Y.; Zeng, D.; Gan, Y.; Yang, Y. Microstructure development of Ti scaffold by laser powder bed fusion with chemical polishing and its mechanical properties, biocompatibility. Biosurf. Biotribol. 2024, 10, 52–62. [Google Scholar] [CrossRef]
- Schweiger, J.; Edelhoff, D.; Schubert, O. 3D printing of ultra-thin veneers made of lithium disilicate using the LCM method in a digital workflow: A feasibility study. J. Esthet. Restor. Dent. 2024, 36, 588–594. [Google Scholar] [CrossRef]
- Stravinskas, K.; Shahidi, A.; Kapustynskyi, O.; Matijošius, T.; Vishniakov, N.; Mordas, G. Characterization of SLA-printed ceramic composites for dental restorations. Lith. J. Phys. 2024, 64, 203–213. [Google Scholar] [CrossRef]
- Balasubramainian, N.K.; Kothandaraman, L.; Sathish, T.; Giri, J.; Ammarullah, M.I. Optimization of process parameters to minimize circularity error and surface roughness in fused deposition modelling (FDM) using Taguchi method for biomedical implant fabrication. Adv. Manuf.-Polym. Compos. Sci. 2024, 10, 2406156. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Baciu, D.; Koltsakidis, S.; Tzetzis, D.; Garmpi, E.; Roussi, E.; Kitsou, I.; Tsetsekou, A.; Andreouli, C.D. Additive manufacturing of zirconia-based pastes for dental prosthesis via robocasting method. J. Mater. Eng. Perform. 2025, 34, 4735–4749. [Google Scholar] [CrossRef]
- Lee, J.M.; Son, K.; Lee, K.B. Evaluation of photopolymer resins for dental prosthetics fabricated via the stereolithography process at different polymerization temperatures—Part I: Conversion rate and mechanical properties. J. Prosthet. Dent. 2024, 131, 166.e1–166.e9. [Google Scholar] [CrossRef]
- Sutejo, I.A.; Kim, J.; Zhang, S.; Gal, C.W.; Choi, Y.J.; Park, H.; Yun, H.S. Fabrication of color-graded feldspathic dental prosthetics for aesthetic and restorative dentistry. Dent. Mater. 2023, 39, 568–576. [Google Scholar] [CrossRef]
- Libonati, A.; Di Taranto, V.; Gallusi, G.; Dolci, A.; Montemurro, E.; Campanella, V. Evaluation of the marginal and internal fit of provisional crown fabricated with two different 3D printing technologies. J. Biol. Reg. Homeos. Ag. 2022, 36, 49–55. [Google Scholar] [CrossRef]
- Barro, Ó.; Arias-González, F.; Lusquiños, F.; Comesaña, R.; del Val, J.; Riveiro, A.; Badaoui, A.; Gómez-Baño, F.; Pou, J. Improved commercially pure titanium obtained by laser directed energy deposition for dental prosthetic applications. Metals 2021, 11, 70. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Dobrzański, L.B.; Achtelik-Franczak, A.; Dobrzańska, J. Application solid laser-sintered or machined Ti6Al4V alloy in manufacturing of dental implants and dental prosthetic restorations according to Dentistry 4.0 concept. Processes 2020, 8, 664. [Google Scholar] [CrossRef]
- Balasubramani, G.; Nisitha, S.; Pradeep, P.J. 3D dental prosthetic tooth crown: Re-modeling of customized tooth crown using additive manufacturing and synthesis of bio-compactible tooth filling. Int. J. Innov. Sci. Res. Technol. 2023, 8, 759–766. [Google Scholar] [CrossRef]
- Moraru, E.; Dontu, O.; Rizescu, C.; Spanu, A.; Ciobanu, R.; Draghici, C. Laser-assisted additive technologies for the execution of dental restorative prostheses. IOP Conf. Ser.-Mater. Sci. Eng. 2020, 997, 012050. [Google Scholar] [CrossRef]
- Noh, M.; Kim, J. A comparison of internal, marginal, and incisal gaps in zirconia laminates fabricated using subtractive manufacturing and 3D printing methods. Biomimetics 2024, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Effects of surface preparation methods on the color stability of 3D-printed dental restorations. J. Funct. Biomater. 2023, 14, 257. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Vohra, F.; Albogami, S.M.; Alkhammash, N.M.; Alnashwan, M.A.; Almutairi, N.S.; Aali, K.A.; Alrabiah, M.; Abduljabbar, T. Marginal misfit of 3D-printed (selective laser sintered), CAD-CAM and lost wax technique cobalt chromium copings with shoulder and chamfer finish lines: An in-vitro study. Medicina 2022, 58, 1313. [Google Scholar] [CrossRef] [PubMed]
- Uriciuc, W.A.; Vermesan, H.; Tiuc, A.E.; Ilea, A.; Bosca, A.B.; Popa, C.O. Casting over metal method used in manufacturing hybrid cobalt-chromium dental prosthetic frameworks assembles. Materials 2021, 14, 539. [Google Scholar] [CrossRef] [PubMed]
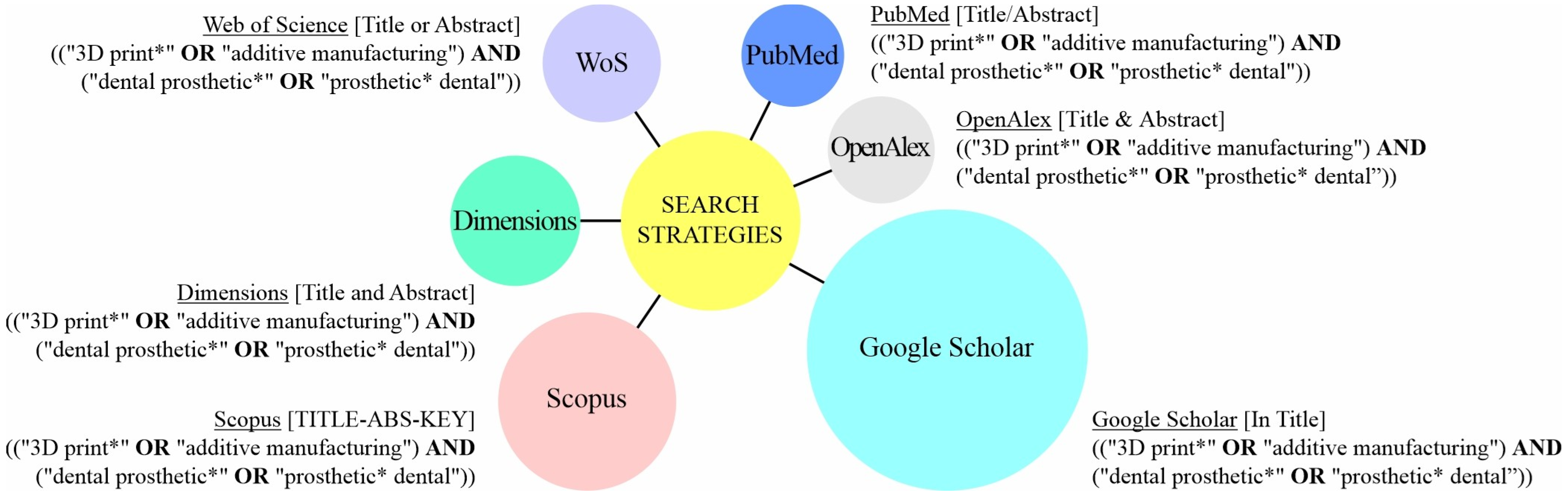
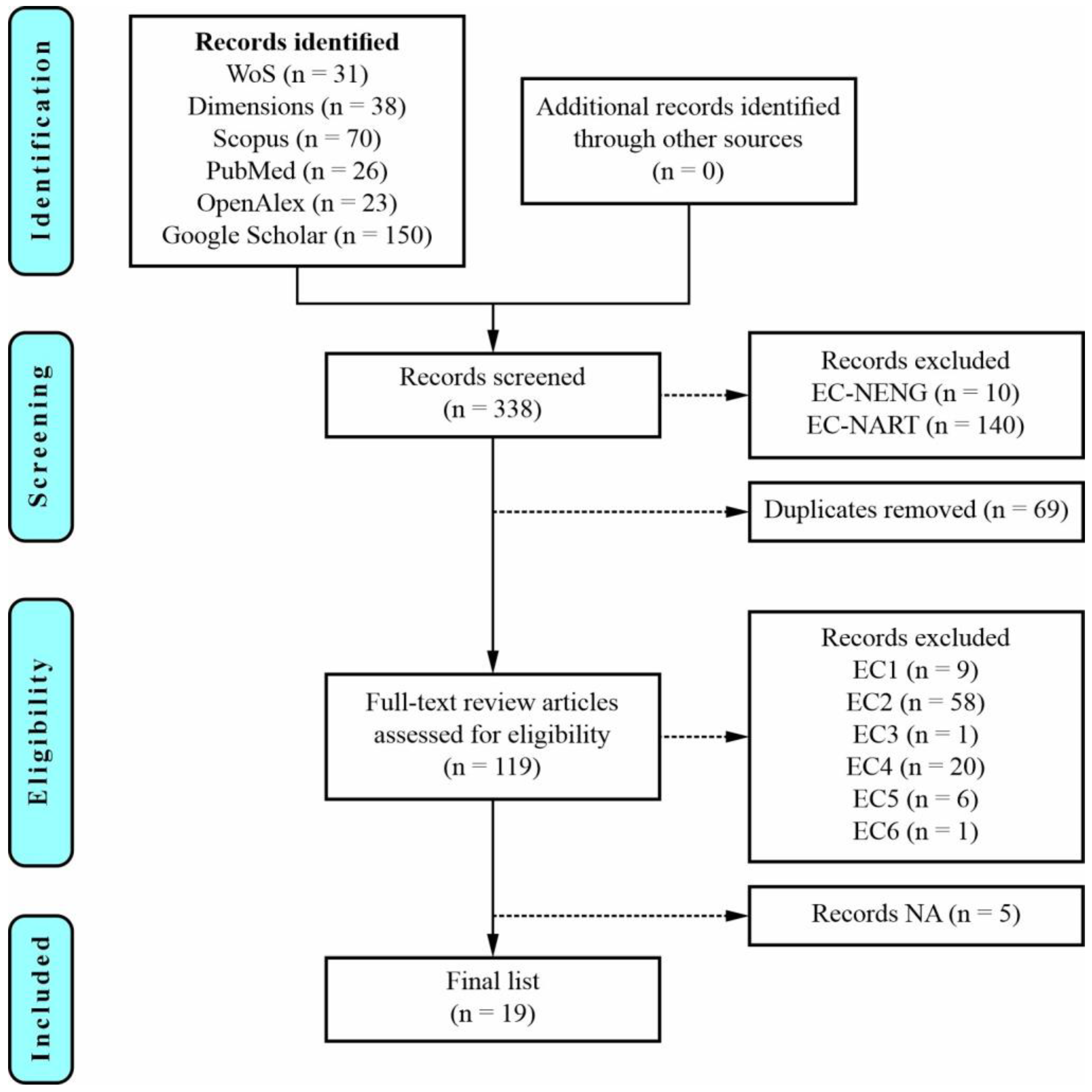

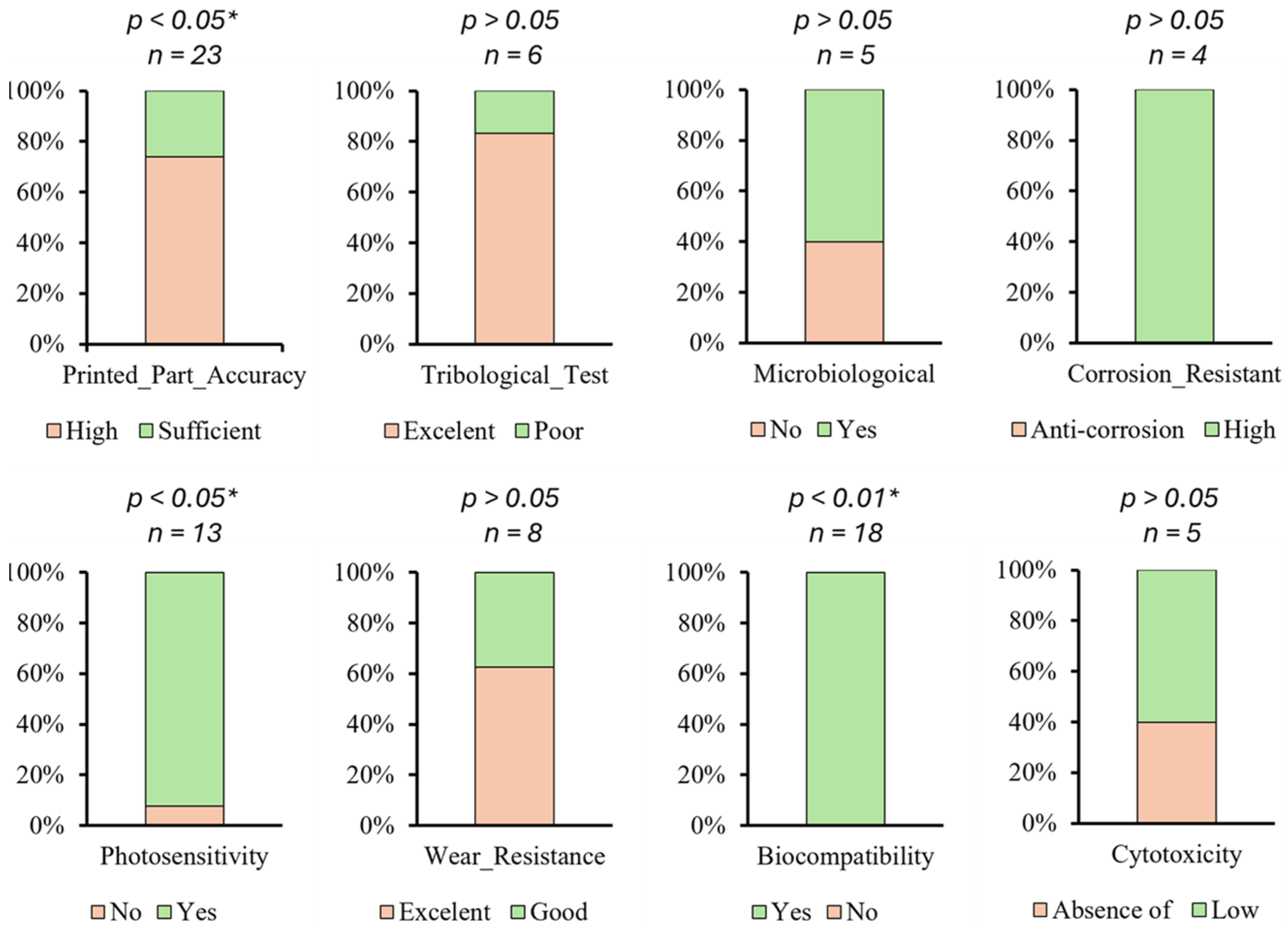
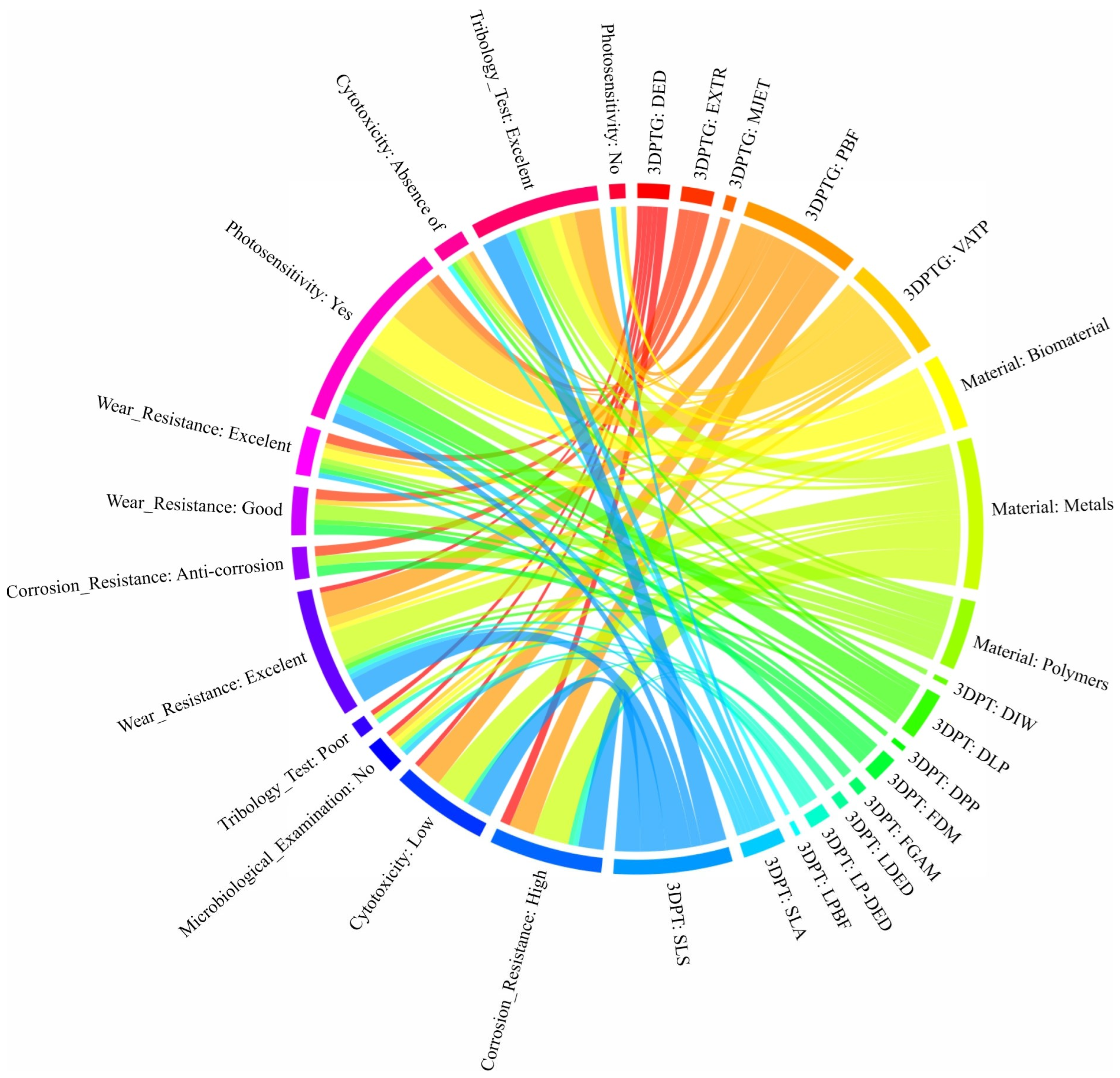
| Database | Language | Article | Review | Conf. Paper | Book | Book Chapter | Letter | Editorial | Patent | PhD Thesis | MSc Thesis | BSc Thesis | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WoS | all | 22 | 9 | / | / | / | / | / | / | / | / | / | 31 |
| English | 21 | 9 | / | / | / | / | / | / | / | / | / | 30 | |
| Dimensions | all | 20 | 6 | / | / | 5 | / | / | 7 | / | / | / | 38 |
| English | 20 | 6 | / | / | 5 | / | / | 7 | / | / | / | 38 | |
| Scopus | all | 47 | 12 | 7 | 1 | 2 | 1 | / | / | / | / | / | 70 |
| English | 42 | 12 | 7 | 1 | 2 | 1 | / | / | / | / | / | 65 | |
| PubMed | all | 19 | 7 | / | / | / | / | / | / | / | / | / | 26 |
| English | 19 | 7 | / | / | / | / | / | / | / | / | / | 26 | |
| OpenAlex | all | 14 | 8 | / | / | / | 1 | / | / | / | / | / | 23 |
| English | 14 | 8 | / | / | / | 1 | / | / | / | / | / | 23 | |
| Google Scholar | all | 76 | 51 | / | 4 | 7 | / | 2 | / | 2 | 7 | 1 | 150 |
| English | 72 | 51 | / | 4 | 7 | / | 2 | / | 2 | 7 | 1 | 146 |
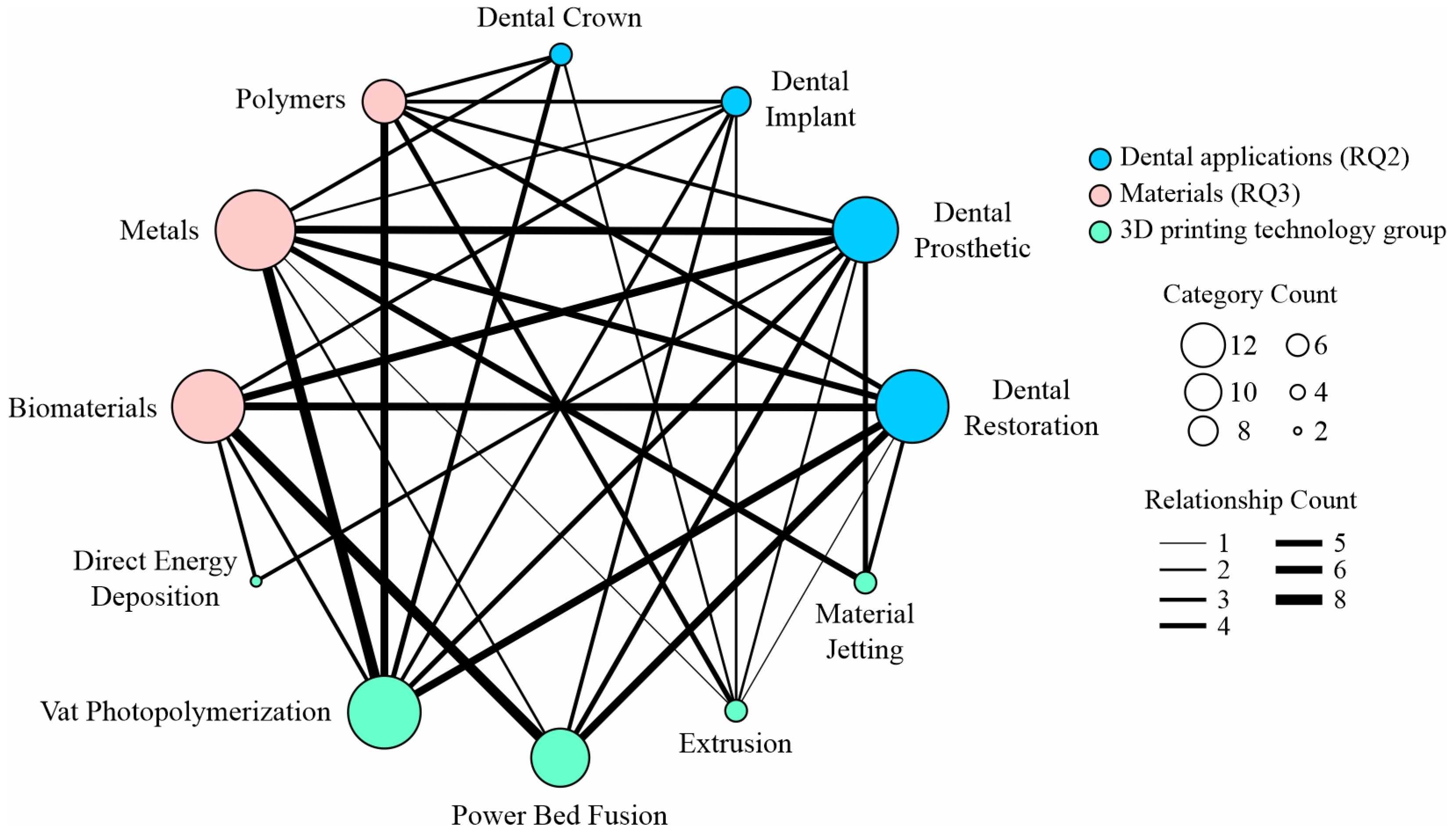
| 3D Printing Technology | Characteristics | Materials | Application | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Fused Deposition Modeling (FDM) | material extrusion through a nozzle; a heater installed in the printer head melts the filament, then pushes it (mechanically) through the nozzle and stacks it as a layer on the printing bed; | polylactic acid (PLA); Polyethylene Terephthalate Glycol (PETG); | dental implant; dental crown; dental restoration; | low-cost; easy to use; wide range of materials; minimal waste; design flexibility; | lower resolution and surface finish; slow for high-resolution or large parts; support removal can be difficult; parts with accuracy limitationsare weaker along the Z-axis; warping and cracking; | [40,47] |
| Stereolithography (SLA) | high-power pulsed laser light increases the temperature of specific areas to weld or sinter additional material on a three-axis moving base; | GC-YSZ composite ceramics; ceramic-composite resin Liqcreate Composite-X; ZMD-1000B; liquid resin; | dental restoration; dental prosthetic; dental crown; | high precision and accuracy; high-detail, smooth, and accurate printed parts; good for complex geometries; | material limitations; resins can be toxic; post-processing required; higher operating costs; | [35,39,42,44] |
| Lithography-based Ceramics Manufacturing (LCM) | uses a photosensitive ceramic suspension—a mixture of ceramic particles and a binder that is cured by light; it cures the material layer by layer using UV light (from a projector or laser), similar to SLA technology; after printing, the binder separation and sintering processes remove the polymer and fuse the ceramic particles into a dense, finished product; | lithium disilicate ceramics | dental prosthetic; dental restoration | high resolution and surface quality; usage of ceramics (Alumina Al2O3, Zirconia ZrO2, Silicon Nitride Si3N4, Silicon Carbide SiC); good for complex geometries; ideal for custom products; | high cost; post-processing; material limitations; final part shrinks during sintering; size constraints; | [38] |
| Digital Light Processing (DLP) | similar to SLA technology, but uses a digital projector to cure photopolymer resin layer by layer instead of a laser; | Tera Harz TC-85 resin—photopolymer; DruckWege Type D Dental resin; | dental implant; dental prosthetic; dental restoration; | high resolution and accuracy; faster than SLA technology; excellent surface finish; wide range of resins; | post-processing required; shrinkage; resins are toxic; resin is more expensive than FDM filament; | [34,48,49] |
| Daylight Polymer Printing (DPP) | similar to liquid crystal display (LCD) technology, but it uses visible light (daylight spectrum) instead of ultraviolet (UV) to cure photopolymer resin; | commercial resins: Denture 3D+, Crowntec A3, and Crowntec A2; | dental restoration; | low cost of operation; scalability; uses daylight-spectrum light which is less harmful than UV; high detail and surface finish; | requires specific daylight-reactive resins; DPP printers were slower than DLP or SLA due to the nature of daylight curing and resin chemistry; lower mechanical strength; | [50] |
| Selective Laser Sintering (SLS) | layers of powder are applied sequentially, and each layer is selectively sintered using a laser beam along a predetermined path; the technique is then repeated layer by layer until the intended 3D object is finished; | Ti6AI4V alloy; CoCrW alloy; CoCrMo alloy; zirconia paste (ININI-CERA); | dental restoration; dental prosthetic; | high strength and functionality; excellent design freedom; good surface uniformity; no need for support structures; wide range of materials; | high cost; post-processing required (excavation, cleaning, depowdering); slow cooling times; powder handling requires proper ventilation and safety equipment; | [46,48] |
| Selective Laser Melting (SLM) | a powerful laser is used to completely melt and fuse metal powder layer by layer into a solid, dense metal part; | CoCr alloy; | dental restoration; dental prosthetic; | the usage of true metal parts; complex geometries; high density and accuracy; ideal for custom implants; | support structures required; very slow build speed; post-processing intensive (heat treatment, support removal, CNC machining, surface polishing); fine metal powders are combustible; very high cost; | [51,52] |
| Laser-Directed Energy Deposition (LDED) | a form of 3D metal printing that uses a laser to melt a metal raw material, either a powder or wire, as it is deposited onto a substrate; LDED technology builds or repairs metal parts layer by layer and is often used to print large-scale metal structures, repair expensive components, or add features to existing parts; | commercially pure Ti Grade 4 (CP-Ti Grade 4); | dental prosthetic; | lower material waste compared to subtractive methods; ideal for repairing damaged parts; large build volumes; high deposition rates; multi-material capability; | support structures required; high equipment cost; lower resolution and accuracy compared to SLM, DMLS, or LDED printers; requires precise synchronization of laser, powder flow, and motion systems; | [45] |
| Laser Powder-Directed Energy Deposition (LP-DED) | a subtype of DED that uses a laser as the energy source and metal powder as the raw material to fabricate or repair parts; it is a high-precision process that combines laser melting with precise powder delivery to produce metallurgically bonded metal parts; | CoCrW alloy; CoCrMo alloy; | dental prosthetic; | high precision metal deposition; multi-material structures; large build volumes; minimum waste; | surface finish and resolution; high equipment and operational costs; thermal stresses and distortion can occur; metal powders are hazardous; | [36] |
| Laser Powder Bed Fusion (LPBF) | high-powered laser selectively melts fine metal powder, layer by layer, to create complex parts directly from a CAD file; | Ti powder (TiCP-grade 2); | dental restoration; | excellent mechanical properties; high precision; complex geometries; wide material compatibility; no tooling required; | slow build speeds; support structures needed; requires post-processing (heat treatment, machining, and surface finishing); high equipment and operational costs; | [37] |
| Direct Ink Writing (DIW) | viscous „ink” (a paste or gel) is extruded through a nozzle and deposited layer by layer to build 3D structures; the ink is usually a material loaded with particles, polymers, or biological cells, and solidifies or cures after printing; | TZ-3YS-E zirconia powder; | dental prosthetic; | material versatility; low cost; scalability; multi-material printing; direct fabrication scaffolds; | limited resolution; mechanical properties (lower strength); post-processing required (drying, curing, or sintering); slower than powder- or resin-based printing; | [41] |
| Functionally Graded Additive Manufacturing (FGAM) | technology that produces parts with gradual variations in material composition, microstructure, or properties throughout the volume of the part; | feldspathic porcelain (FP)/yttrium aluminum garnet cerium YAG:Ce (Y-FP); | dental restoration; dental prosthetic; | optimized mechanical strength, wear resistance, thermal conductivity, or biocompatibility where needed; reduced stress concentrations; design freedom; | materials must be compatible in terms of melting points and chemical bonding; complex process control; high cost; limited material combinations. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oros, D.; Penčić, M.; Orošnjak, M.; Kedziora, S. Additive Manufacturing Technologies and Their Applications in Dentistry: A Systematic Literature Review. Appl. Sci. 2025, 15, 8346. https://doi.org/10.3390/app15158346
Oros D, Penčić M, Orošnjak M, Kedziora S. Additive Manufacturing Technologies and Their Applications in Dentistry: A Systematic Literature Review. Applied Sciences. 2025; 15(15):8346. https://doi.org/10.3390/app15158346
Chicago/Turabian StyleOros, Dragana, Marko Penčić, Marko Orošnjak, and Slawomir Kedziora. 2025. "Additive Manufacturing Technologies and Their Applications in Dentistry: A Systematic Literature Review" Applied Sciences 15, no. 15: 8346. https://doi.org/10.3390/app15158346
APA StyleOros, D., Penčić, M., Orošnjak, M., & Kedziora, S. (2025). Additive Manufacturing Technologies and Their Applications in Dentistry: A Systematic Literature Review. Applied Sciences, 15(15), 8346. https://doi.org/10.3390/app15158346








