Predicting Attachment Class Using Coherence Graphs: Insights from EEG Studies on the Secretary Problem
Abstract
1. Introduction
2. Methods
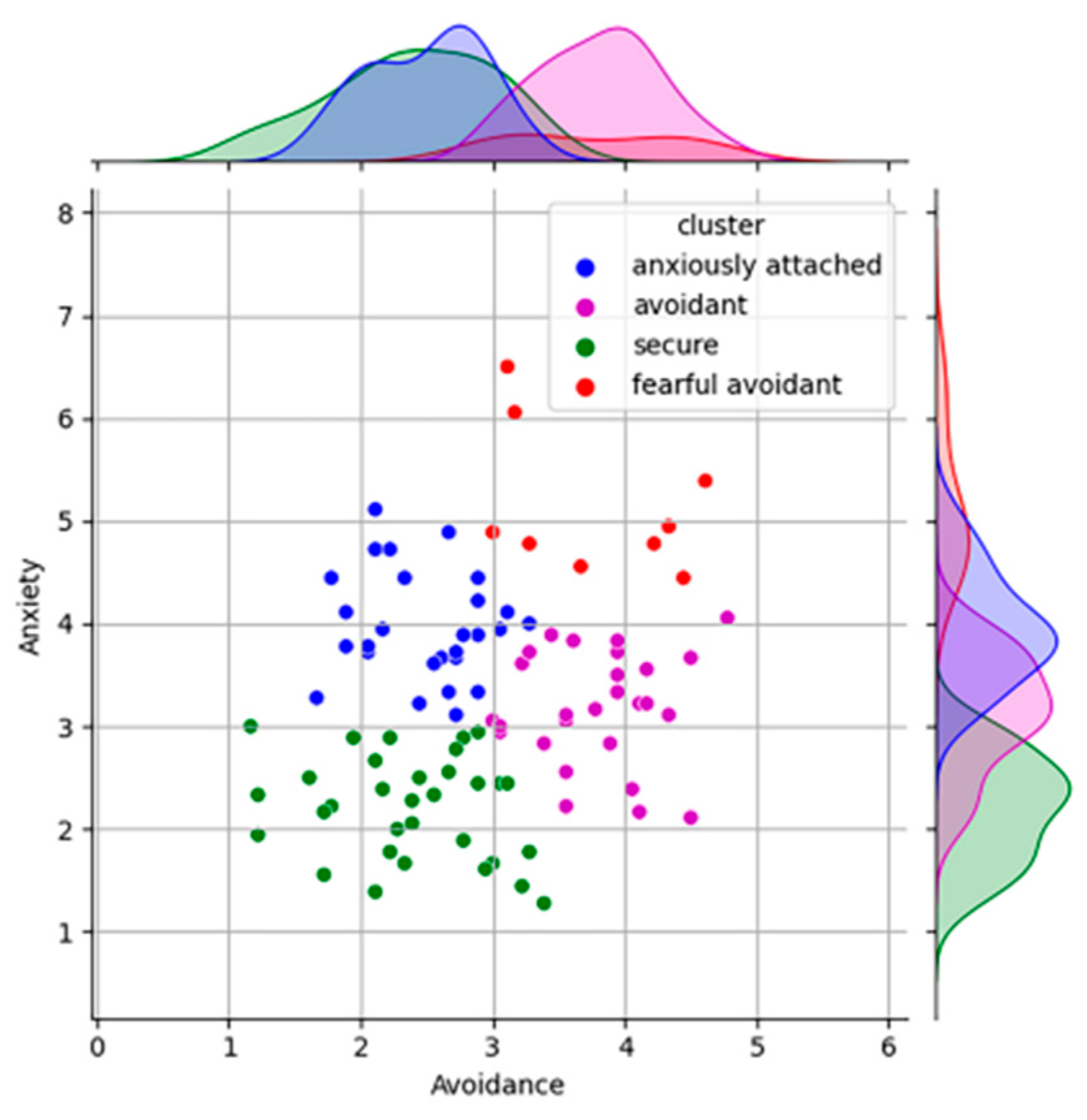
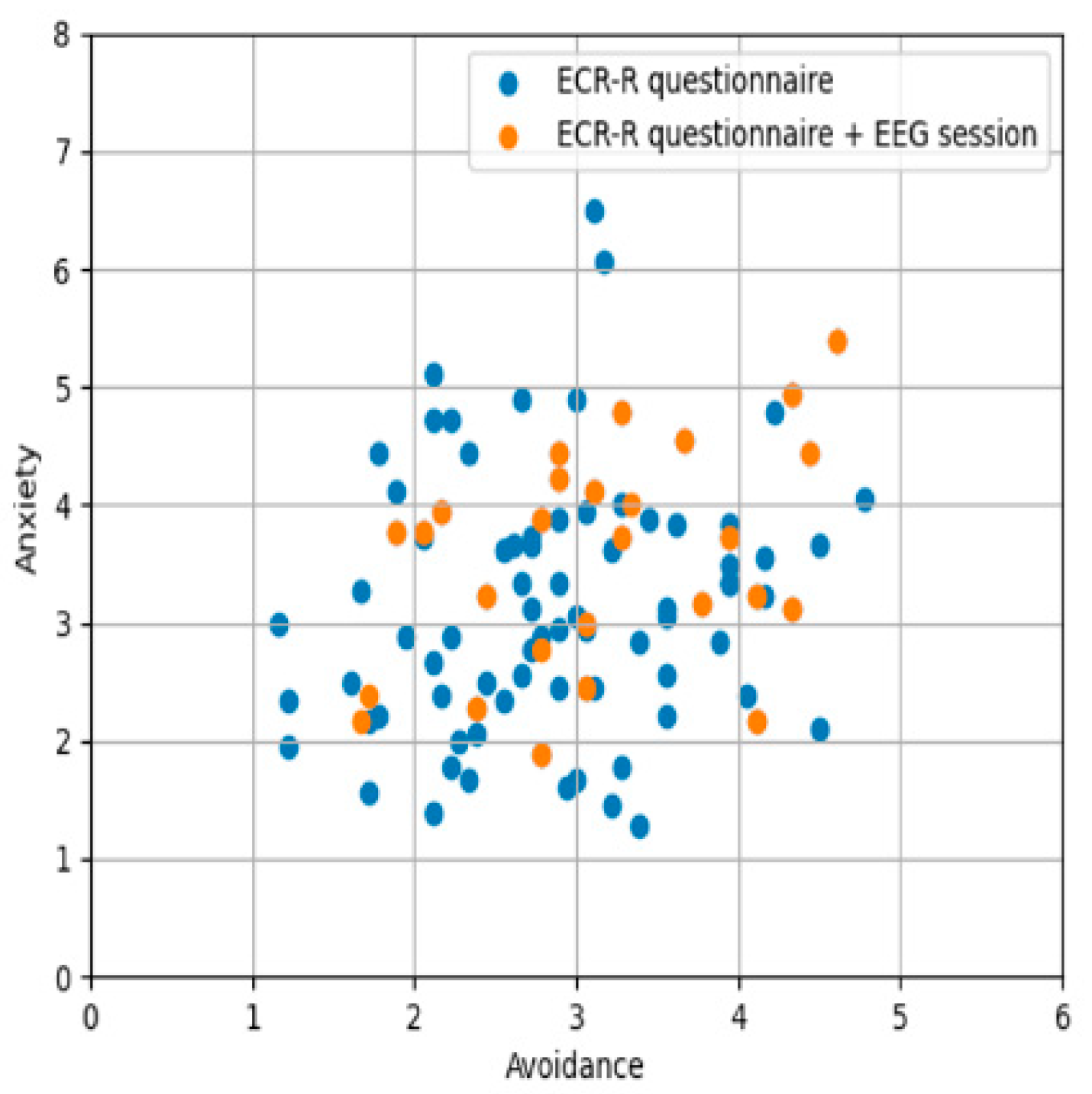
2.1. First Phase—ECR-R Questionnaire
2.2. Second Phase—EEG Recording in the Secretary Game
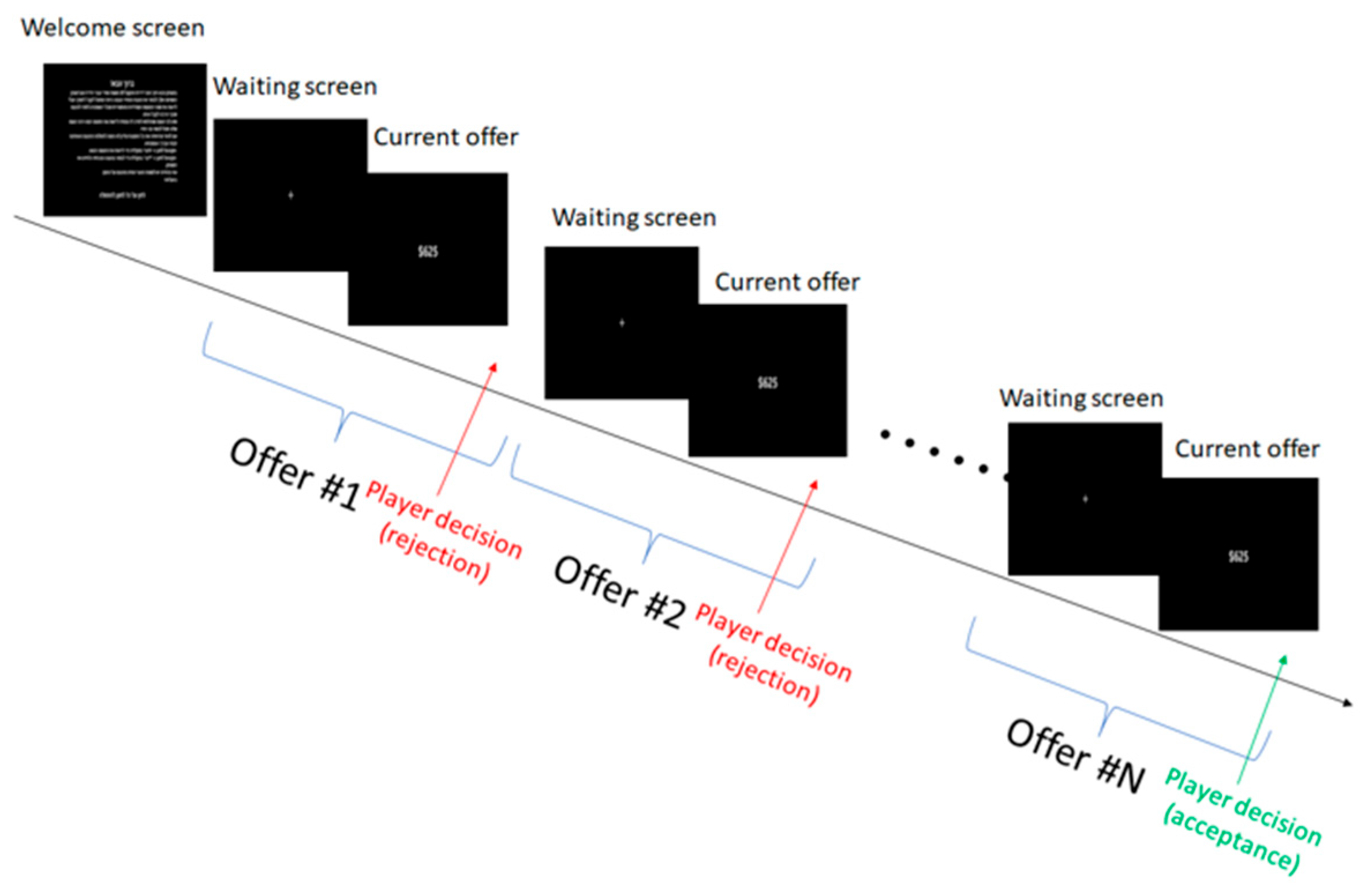
2.3. Experiment Procedure
2.4. Data Processing and Analysis
- Compute coherence between EEG channels and construct a 16 × 16 brain network (coherence graph) for each epoch.
- Add a virtual central node to the coherence graph and apply the node2vec algorithm to embed the graph into a feature vector representation [27].
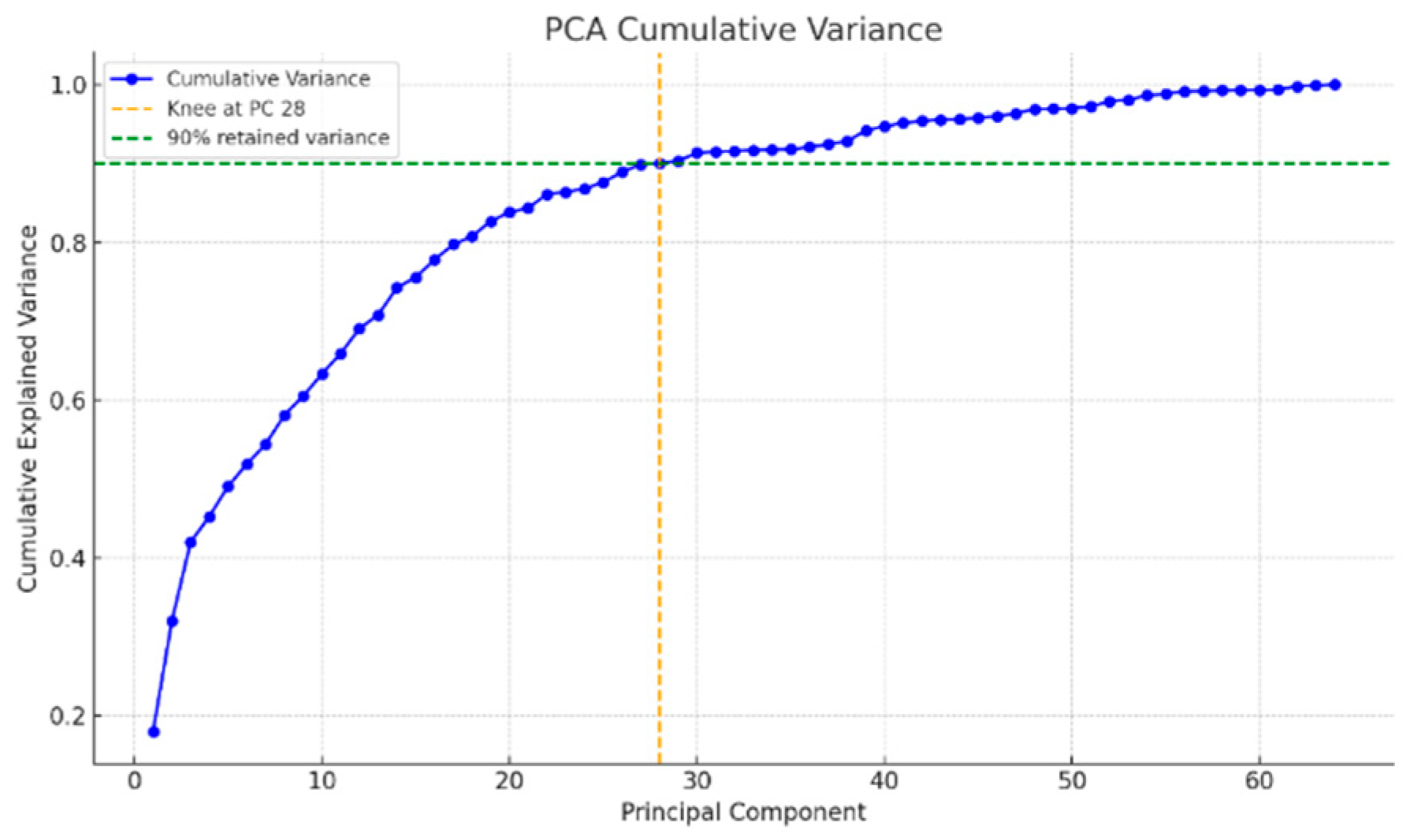
- Reduce the dimensionality of the resulting feature vectors using Principal Component Analysis (PCA) to retain the most important features [39].
- Use the resulting features as input to train an XGBoost ensemble decision-tree classification model to predict attachment class [33].
- Evaluate the model using nested 5-fold cross-validation at the participant level to tune hyperparameters and assess performance while preventing overfitting.
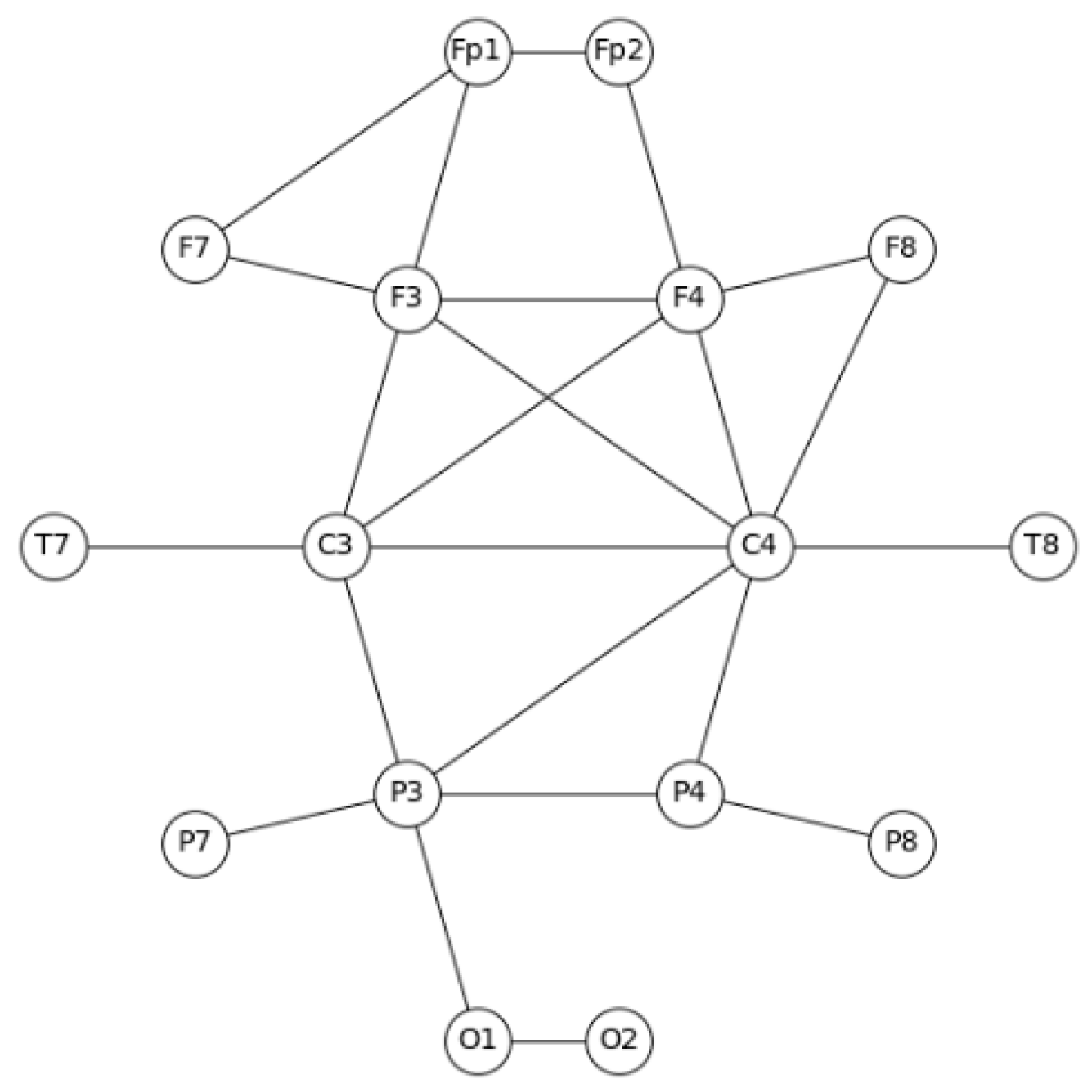
2.4.1. EEG Coherence
2.4.2. Transforming the Coherence Graph into a Feature Vector
2.4.3. Classification Model (XGBoost)
2.4.4. Model Training and Validation
- Outer loop: In each of five folds, data from ~5–6 participants were held out as the external test set; the remaining participants formed the training set.
- Inner loop: A second 5-fold split on the training participants tuned XGBoost hyperparameters (learning rate, tree depth, subsample ratio.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bretherton, I. Attachment theory: Retrospect and prospect. Monogr. Soc. Res. Child Dev. 1985, 50, 3–35. [Google Scholar] [CrossRef]
- Hazan, C.; Shaver, P. Romantic love conceptualized as an attachment process. J. Personal. Soc. Psychol. 1987, 52, 511. [Google Scholar] [CrossRef]
- Fearon, R.P.; Roisman, G.I. Attachment theory: Progress and future directions. Curr. Opin. Psychol. 2017, 15, 131–136. [Google Scholar] [CrossRef]
- Mikulincer, M.; Shaver, P.R. Applications of attachment theory and research: The blossoming of relationship science. In Applications of Social Psychology; Routledge: Abingdon, UK, 2020; pp. 187–206. [Google Scholar]
- Sutton, T.E. Review of attachment theory: Familial predictors, continuity and change, and intrapersonal and relational outcomes. Marriage Fam. Rev. 2019, 55, 1–22. [Google Scholar] [CrossRef]
- Van Petegem, S.; Beyers, W.; Brenning, K.; Vansteenkiste, M. Exploring the association between insecure attachment styles and adolescent autonomy in family decision making: A differentiated approach. J. Youth Adolesc. 2013, 42, 1837–1846. [Google Scholar] [CrossRef]
- Bolat, N.; Odacı, H. High school final year students’ career decision-making self-efficacy, attachment styles and gender role orientations. Curr. Psychol. 2017, 36, 252–259. [Google Scholar] [CrossRef]
- Deniz, M. An investigation of decision making styles and the five-factor personality traits with respect to attachment styles. Educ. Sci. Theory Pract. 2011, 11, 105–113. [Google Scholar]
- Gander, M.; Buchheim, A. Attachment classification, psychophysiology and frontal EEG asymmetry across the lifespan: A review. Front. Hum. Neurosci. 2015, 9, 79. [Google Scholar] [CrossRef]
- Ravitz, P.; Maunder, R.; Hunter, J.; Sthankiya, B.; Lancee, W. Adult attachment measures: A 25-year review. J. Psychosom. Res. 2010, 69, 419–432. [Google Scholar] [CrossRef]
- Sibley, C.G.; Fischer, R.; Liu, J.H. Reliability and validity of the revised experiences in close relationships (ECR-R) self-report measure of adult romantic attachment. Personal. Soc. Psychol. Bull. 2005, 31, 1524–1536. [Google Scholar] [CrossRef]
- Cho, Y.J.; Ryu, J.S.; Seok, J.-H.; Kim, E.; Oh, J.; Kim, B.-H. Machine Learning Prediction of Attachment Type From Bio-Psychological Factors in Patients With Depression. Psychiatry Investig. 2025, 22, 412. [Google Scholar] [CrossRef]
- Verbeke, W.J.M.I.; Pozharliev, R.; Strien, J.W.V.; Belschak, F.; Bagozzi, R.P. “I am resting but rest less well with you.” The moderating effect of anxious attachment style on alpha power during EEG resting state in a social context. Front. Hum. Neurosci. 2014, 8, 486. [Google Scholar] [CrossRef]
- Sloan, E.P.; Maunder, R.G.; Hunter, J.J.; Moldofsky, H. Insecure attachment is associated with the α-EEG anomaly during sleep. Biopsychosoc. Med. 2007, 1, 20. [Google Scholar] [CrossRef]
- Rognoni, E.; Galati, D.; Costa, T.; Crini, M. Relationship between adult attachment patterns, emotional experience and EEG frontal asymmetry. Personal. Individ. Differ. 2008, 44, 909–920. [Google Scholar] [CrossRef]
- Zuckerman, I.; Mizrahi, D.; Laufer, I. Attachment style, emotional feedback, and neural processing: Investigating the influence of attachment on the P200 and P400 components of event-related potentials. Front. Hum. Neurosci. 2023, 17, 1249978. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Ha, T.; Ebbert, A.M.; Tucker, D.M.; Dishion, T.J. Dynamic Responses in Brain Networks to Social Feedback: A Dual EEG Acquisition Study in Adolescent Couples. Front. Comput. Neurosci. 2017, 11, 46. [Google Scholar] [CrossRef]
- Labek, K.; Karch, S.; Taubner, S.; Kessler, H.; Kächele, H.; Cierpka, M.; Roth, G.; Chrobok, A.; Pogarell, O.; Buchheim, A. EPA-1756-EEG activity in the gamma band and late positive potential (LPP) during processing attachment-related stimuli. Eur. Psychiatry 2014, 29, 1. [Google Scholar] [CrossRef]
- Dhake, D.; Angal, Y. A Comparative Analysis of EEG-based Stress Detection Utilizing Machine Learning and Deep Learning Classifiers with a Critical Literature Review. Int. J. Recent Innov. Trends Comput. Commun. 2023, 11, 61–73. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, X.; Deng, L. EEG research based on the influence of different music effects. J. Phys. Conf. Ser. 2020, 1631, 012147. [Google Scholar] [CrossRef]
- Mizrahi, D.; Zuckerman, I.; Laufer, I. Exploring Cognitive Load in the Secretary Problem Using EEG Signals. In Proceedings of the Intelligent Systems Conference, Amsterdam, The Netherlands, 5–6 September 2024; Springer Nature: Cham, Switzerland, 2024; pp. 664–674. [Google Scholar]
- Mizrahi, D.; Laufer, I.; Zuckerman, I. Neurophysiological insights into sequential decision-making: Exploring the secretary problem through ERPs and TBR dynamics. BMC Psychol. 2024, 12, 245. [Google Scholar] [CrossRef]
- Seale, D.A.; Rapoport, A. Sequential decision making with relative ranks: An experimental investigation of the “secretary problem”. Organ. Behav. Hum. Decis. Process. 1997, 69, 221–236. [Google Scholar] [CrossRef]
- Mikulincer, M.; Shaver, P.R. Attachment in Adulthood: Structure, Dynamics, and Change; Guilford Publications: New York, NY, USA, 2010. [Google Scholar]
- Vrtička, P.; Andersson, F.; Grandjean, D.; Sander, D.; Vuilleumier, P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS ONE 2008, 3, e2868. [Google Scholar] [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Grover, A.; Leskovec, J. node2vec: Scalable feature learning for networks. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Li, S.; Zaidi, N.A.; Du, M.; Zhou, Z.; Zhang, H.; Li, G. Property graph representation learning for node classification. Knowl. Inf. Syst. 2024, 66, 237–265. [Google Scholar] [CrossRef]
- Freeman, P.R. The secretary problem and its extensions: A review. Int. Stat. Rev. Int. Stat. 1983, 51, 189–206. [Google Scholar] [CrossRef]
- Quiroz, G.; Espinoza-Valdez, A.; Salido-Ruiz, R.A.; Mercado, L. Coherence analysis of EEG in locomotion using graphs. Rev. Mex. Ing. Biomédica 2017, 38, 235–246. [Google Scholar] [CrossRef]
- Zuckerman, I.; Mizrahi, D.; Laufer, I. EEG Pattern Classification of Picking and Coordination Using Anonymous Random Walks. Algorithms 2022, 15, 114. [Google Scholar] [CrossRef]
- Li, Y.; Tarlow, D.; Brockschmidt, M.; Zemel, R. Gated Graph Sequence Neural Networks. In Proceedings of the International Conference on Learning Representations, San Juan, Puerto Rico, 2–4 May 2016. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Xu, R.; Wunsch, D. Survey of clustering algorithms. IEEE Trans. Neural Netw. 2005, 16, 645–678. [Google Scholar] [CrossRef]
- Waters, E.; Merrick, S.; Treboux, D.; Crowell, J.; Albersheim, L. Attachment security in infancy and early adulthood: A twenty-year longitudinal study. Child Dev. 2000, 71, 684–689. [Google Scholar] [CrossRef]
- Laufer, I.; Mizrahi, D.; Zuckerman, I. Enhancing EEG-Based Attachment Style Prediction: Unveiling the Impact of Feature Domains. Front. Psychol. 2024, 15, 1326791. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Kemp, S. The effect of incentive structure on search in the secretary problem. Judgm. Decis. Mak. 2020, 15, 182–192. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Webb, S.J.; Greenson, J.; Dawson, G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol. Psychiatry 2007, 62, 270–273. [Google Scholar] [CrossRef]
- Basharpoor, S.; Heidar, F.; Molavi, P. EEG coherence in theta, alpha, and beta bands in frontal regions and executive functions. Appl. Neuropsychol. Adult 2021, 28, 310–317. [Google Scholar] [CrossRef]
- Rippon, G.; Brock, J.; Brown, C.; Boucher, J. Disordered connectivity in the autistic brain: Challenges for the ‘new psychophysiology’. Int. J. Psychophysiol. 2007, 63, 164–172. [Google Scholar] [CrossRef]
- Bocci, T.; Moretto, C.; Tognazzi, S.; Briscese, L.; Naraci, M.; Leocani, L.; Mosca, F.; Ferrari, M.; Sartucci, F. How does a surgeon’s brain buzz? An EEG coherence study on the interaction between humans and robot. Behav. Brain Funct. 2013, 9, 14. [Google Scholar] [CrossRef]
- Ahmadi, H.; Fatemizadeh, E.; Nasrabadi, A.M. A comparative study of the effect of weighted or binary functional brain networks in fMRI data analysis. Front. Biomed. Technol. 2020, 7. [Google Scholar] [CrossRef]
- Nicolini, C.; Bordier, C.; Bifone, A. Community detection in weighted brain connectivity networks beyond the resolution limit. Neuroimage 2017, 146, 28–39. [Google Scholar] [CrossRef]
- Bardella, G.; Pani, P.; Brunamonti, E.; Giarrocco, F.; Ferraina, S. The small scale functional topology of movement control: Hierarchical organization of local activity anticipates movement generation in the premotor cortex of primates. Neuroimage 2020, 207, 116354. [Google Scholar] [CrossRef]
- Bardella, G.; Giuffrida, V.; Giarrocco, F.; Brunamonti, E.; Pani, P.; Ferraina, S. Response inhibition in premotor cortex corresponds to a complex reshuffle of the mesoscopic information network. Netw. Neurosci. 2024, 8, 597–622. [Google Scholar] [CrossRef]
- Wang, F.; Tian, Y.-C.; Zhang, X.; Hu, F. An ensemble of Xgboost models for detecting disorders of consciousness in brain injuries through EEG connectivity. Expert Syst. Appl. 2022, 198, 116778. [Google Scholar] [CrossRef]
- Elgart, M.; Lyons, G.; Romero-Brufau, S.; Kurniansyah, N.; Brody, J.A.; Guo, X.; Lin, H.J.; Raffield, L.; Gao, Y.; Chen, H.; et al. Non-linear machine learning models incorporating SNPs and PRS improve polygenic prediction in diverse human populations. Commun. Biol. 2022, 5, 256. [Google Scholar] [CrossRef]
- Tiwari, A.; Chaturvedi, A. A multiclass EEG signal classification model using spatial feature extraction and XGBoost algorithm. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Macau, China, 4–8 November 2019. [Google Scholar]
- Deng, X.; Li, M.; Deng, S.; Wang, L. Hybrid gene selection approach using XGBoost and multi-objective genetic algorithm for cancer classification. Med. Biol. Eng. Comput. 2022, 60, 663–681. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. Explainable AI for trees: From local explanations to global understanding. arXiv 2019, arXiv:1905.04610. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, Y. Lasso Ridge based XGBoost and Deep_LSTM Help Tennis Players Perform better. arXiv 2024, arXiv:2405.07030. [Google Scholar] [CrossRef]
- Mitchell, R.; Frank, E. Accelerating the XGBoost algorithm using GPU computing. PeerJ Comput. Sci. 2017, 3, e127. [Google Scholar] [CrossRef]
- Varma, S.; Simon, R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. 2006, 7, 91. [Google Scholar] [CrossRef]
- Cawley, G.C.; Talbot, N.L. On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach. Learn. Res. 2010, 11, 2079–2107. [Google Scholar]
- Bartholomew, K.; Horowitz, L.M. Attachment styles among young adults: A test of a four-category model. J. Personal. Soc. Psychol. 1991, 61, 226. [Google Scholar] [CrossRef] [PubMed]
- Sweet, J. The Association Between Attachment Style and Decision Making Style. Honors Thesis, Union College, Schenectady, NY, USA, 2021. [Google Scholar]
- Blalock, D.V.; Franzese, A.T.; Machell, K.A.; Strauman, T.J. Attachment style and self-regulation: How our patterns in relationships reflect broader motivational styles. Personal. Individ. Differ. 2015, 87, 90–98. [Google Scholar] [CrossRef]
- Harms, P.D. Adult attachment styles in the workplace. Hum. Resour. Manag. Rev. 2011, 21, 285–296. [Google Scholar] [CrossRef]
- Mikulincer, M.; Orbach, I. Attachment styles and repressive defensiveness: The accessibility and architecture of affective memories. J. Personal. Soc. Psychol. 1995, 68, 917. [Google Scholar] [CrossRef]
- Fraley, R.C.; Waller, N.G.; Brennan, K.A. An item response theory analysis of self-report measures of adult attachment. J. Personal. Soc. Psychol. 2000, 78, 350. [Google Scholar] [CrossRef]
- Brennan, K.A.; Clark, C.L.; Shaver, P.R. Self-report measurement of adult attachment: An integrative overview. Attach. Theory Close Relatsh. 1998, 46, 70–100. [Google Scholar]
- Fraley, R.C.; Hudson, N.W.; Heffernan, M.E.; Segal, N. Are adult attachment styles categorical or dimensional? A taxometric analysis of general and relationship-specific attachment orientations. J. Personal. Soc. Psychol. 2015, 109, 354. [Google Scholar] [CrossRef]
- Levy, K.N.; Blatt, S.J.; Shaver, P.R. Attachment styles and parental representations. J. Personal. Soc. Psychol. 1998, 74, 407. [Google Scholar] [CrossRef]
- Hacker, C.; Rieck, B. On the surprising behaviour of node2vec. arXiv 2022, arXiv:2206.08252. [Google Scholar] [CrossRef]
- Dalmia, A.; Gupta, M. Towards interpretation of node embeddings. In Proceedings of the Companion Proceedings of the The Web Conference 2018, Lyon, France, 23–27 April 2018; pp. 945–952.
- Knežević, D.; Babić, J.; Savić, M.; Radovanović, M. Evaluation of LID-aware graph embedding methods for node clustering. In Proceedings of the International Conference on Similarity Search and Applications, Bologna, Italy, 5–7 October 2022; Springer International Publishing: Cham, Switzerland, 2022; pp. 222–233. [Google Scholar]
- Gan, A.; Gong, A.; Ding, P.; Yuan, X.; Chen, M.; Fu, Y.; Cheng, Y. Computer-aided diagnosis of schizophrenia based on node2vec and Transformer. J. Neurosci. Methods 2023, 389, 109824. [Google Scholar] [CrossRef]
- Rosenthal, G.; Váša, F.; Griffa, A.; Hagmann, P.; Amico, E.; Goñi, J.; Avidan, G.; Sporns, O. Mapping higher-order relations between brain structure and function with embedded vector representations of connectomes. Nat. Commun. 2018, 9, 2178. [Google Scholar] [CrossRef]
- Cattai, T.; Caporali, C.; Corsi, M.-C.; Colonnese, S. Introducing the modularity graph: An application to brain functional networks. In Proceedings of the 2024 32nd European Signal Processing Conference (EUSIPCO), Lyon, France, 26–30 August 2024; IEEE: New York, NY, USA, 2024; pp. 1611–1615. [Google Scholar]
- Kim, J.-G.; Kim, H.; Hwang, J.; Kang, S.H.; Lee, C.-N.; Woo, J.; Kim, C.; Han, K.; Kim, J.B.; Park, K.-W. Differentiating amnestic from non-amnestic mild cognitive impairment subtypes using graph theoretical measures of electroencephalography. Sci. Rep. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Paap, K.R.; Anders-Jefferson, R.T.; Balakrishnan, N.; Majoubi, J.B. The many foibles of Likert scales challenge claims that self-report measures of self-control are better than performance-based measures. Behav. Res. Methods 2024, 56, 908–933. [Google Scholar] [CrossRef]
- McBride, C.; Atkinson, L.; Quilty, L.C.; Bagby, R.M. Attachment as moderator of treatment outcome in major depression: A randomized control trial of interpersonal psychotherapy versus cognitive behavior therapy. J. Consult. Clin. Psychol. 2006, 74, 1041. [Google Scholar] [CrossRef]
- Fraley, R.C.; Waller, N.G. Adult attachment patterns: A test of the typological model. In Attachment Theory and Close Relationships; The Guilford Press: New York, NY, USA, 1998; pp. 77–114. [Google Scholar]
- Gillath, O.; Karantzas, G. Attachment security priming: A systematic review. Curr. Opin. Psychol. 2019, 25, 86–98. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar]
- Van Veen, B.; van Drongelen, W.; Yuchtman, M.; Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997, 44, 867–880. [Google Scholar] [CrossRef]
- Decker, S.; Fillmore, P.T.; Roberts, A. Coherence: The measurement and application of brain connectivity. NeuroRegulation 2017, 4, 3. [Google Scholar] [CrossRef]
- Suresh, G.V.; Reddy, S. Uncertain data analysis with regularized XGBoost. Webology 2022, 19, 3722–3740. [Google Scholar] [CrossRef]
- Dark-Freudeman, A.; Pond, R.S.J.; Paschall, R.E.; Greskovich, L. Attachment style in adulthood: Attachment style moderates the impact of social support on depressive symptoms. J. Soc. Pers. Relatsh. 2020, 37, 2871–2889. [Google Scholar] [CrossRef]
- Rasool, A.; Aslam, S.; Xu, Y.; Wang, Y.; Pan, Y.; Chen, W. Deep neurocomputational fusion for ASD diagnosis using multi-domain EEG analysis. Neurocomputing 2025, 641, 130353. [Google Scholar] [CrossRef]
- Bunterngchit, C.; Wang, J.; Su, J.; Wang, Y.; Liu, S.; Hou, Z.-G. Temporal attention fusion network with custom loss function for EEG–fNIRS classification. J. Neural Eng. 2024, 21, 066016. [Google Scholar] [CrossRef]
- Liu, R.; Hirn, M.; Krishnan, A. Accurately modeling biased random walks on weighted networks using node2vec+. Bioinformatics 2023, 39, btad047. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, D.; Qiu, X.; Jiang, W. A pipeline for fair comparison of graph neural networks in node classification tasks. arXiv 2020, arXiv:2012.10619. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, M. Exact recovery of community structures using DeepWalk and node2vec. arXiv 2021, arXiv:2101.07354. [Google Scholar]


| Predicted | ||||||
|---|---|---|---|---|---|---|
| Secure | Avoidant | Anxious | Fearful Avoidant | Recall | ||
| Real | Secure | 29 | 3 | 4 | 0 | 80.55% |
| Avoidant | 2 | 28 | 11 | 1 | 53.70% | |
| Anxious | 5 | 16 | 31 | 2 | 57.41% | |
| Fearful avoidant | 0 | 2 | 1 | 22 | 88.00% | |
| Precision | 80.55% | 57.14% | 65.95% | 88.00% | Accuracy 68% | |
| Predicted | |||||
|---|---|---|---|---|---|
| Secure | Insecure | Extreme Insecure | Recall | ||
| Real | Secure | 32 | 4 | 0 | 88.88% |
| Insecure | 5 | 89 | 2 | 92.71% | |
| Extreme Insecure | 0 | 3 | 22 | 88.00% | |
| Precision | 86.49% | 92.71% | 91.66% | Accuracy 88.27% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizrahi, D.; Laufer, I.; Zuckerman, I. Predicting Attachment Class Using Coherence Graphs: Insights from EEG Studies on the Secretary Problem. Appl. Sci. 2025, 15, 9009. https://doi.org/10.3390/app15169009
Mizrahi D, Laufer I, Zuckerman I. Predicting Attachment Class Using Coherence Graphs: Insights from EEG Studies on the Secretary Problem. Applied Sciences. 2025; 15(16):9009. https://doi.org/10.3390/app15169009
Chicago/Turabian StyleMizrahi, Dor, Ilan Laufer, and Inon Zuckerman. 2025. "Predicting Attachment Class Using Coherence Graphs: Insights from EEG Studies on the Secretary Problem" Applied Sciences 15, no. 16: 9009. https://doi.org/10.3390/app15169009
APA StyleMizrahi, D., Laufer, I., & Zuckerman, I. (2025). Predicting Attachment Class Using Coherence Graphs: Insights from EEG Studies on the Secretary Problem. Applied Sciences, 15(16), 9009. https://doi.org/10.3390/app15169009







