Impacts of Different Tillage and Straw Management Systems on Herbicide Degradation and Human Health Risks in Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Incubation of Soils
2.3. Pretreatment Method
2.4. Analysis Method
2.5. Human Health Risk Assessment Method
2.6. Degradation Kinetics
2.7. Data Analysis
3. Results
3.1. Pesticide Residues in Soil Samples
3.2. Soil Physicochemical Properties Under Different Tillage Practices
3.3. Correlation Analysis
3.4. Human Health Risk Assessment
3.5. Degradation of Soil Pesticides Under Different Tillage Methods
3.5.1. The Degradation of Pesticides Under the Same Tillage Treatment
3.5.2. Degradation Patterns of Individual Herbicides Under Different Tillage Treatment
3.5.3. The Degradation of Pesticides at the Mid-Incubation Period
4. Discussion
4.1. Implications of Pesticide Residue Variability
4.2. Impact of Different Tillage Practices on Soil Physicochemical Properties
4.3. Discussion on Correlation
4.4. Discussion on Human Health Risk Assessment
4.5. Influence of Tillage Practices on Herbicide Degradation
4.5.1. Comparative Degradation of Pesticides Under the Same Tillage Treatment
4.5.2. Degradation Responses of Individual Pesticides to Different Tillage Treatments
4.5.3. Cross-Pesticide Comparison of Degradation Efficiency at the Mid-Incubation Period
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOM | Soil organic matter |
| GC–MS | Gas chromatography–mass spectrometry |
| HPLC | High performance liquid chromatography |
| NaCl | Sodium chloride |
| EI | Electron impact |
| ST | Straw incorporation with traditional tillage |
| SS | Straw incorporation with strip tillage |
| CK | No-till without straw incorporation |
| USEPA | U.S. Environmental Protection Agency |
| HQ | Hazard quotient |
| HI | Hazard index |
| RfD | Reference dose |
| IRi | Ingestion rate |
| IRih | Inhalation rate |
| BW | Body weight |
| AT | Averaging lifetime |
| EF | Exposure frequency |
| ED | Exposure duration |
| DA | Exposed dermal area |
| DAF | Dermal adherence factor |
| AF | Dermal absorption factor |
| PEF | Particle emission factor |
| CF | Conversion factor |
| PCA | Principal Component Analysis |
| ND | Not detected |
| CV | Coefficient of variance |
References
- Wang, X.; Fan, W.; Zha, C.; Wang, Z.; Yu, J.; Wu, L.; Zhang, X.; Luo, F.; Chen, Z.; Zhou, L. Occurrence, fate and dietary risk assessment of pesticides in chrysanthemum from garden to cup. J. Hazard. Mater. 2025, 493, 138363. [Google Scholar] [CrossRef]
- Sultan, M.; Hamid, N.; Junaid, M.; Duan, J.; Pei, D. Organochlorine pesticides (OCPs) in freshwater resources of Pakistan: A review on occurrence, spatial distribution and associated human health and ecological risk assessment. Ecotoxicol. Environ. Saf. 2023, 249, 114362. [Google Scholar] [CrossRef]
- Cui, K.; Fang, L.; Ding, R.; Ni, R.; Liang, J.; Li, T.; Wang, J.; Liu, J.; Guan, S.; Dong, Z.; et al. Dissipation and metabolism of fluxapyroxad, oxathiapiprolin and penthiopyrad in grapes: A comprehensive risk assessment from field to raisins. Food. Chem. 2025, 485, 144510. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Liu, Q.; Lin, L.; Xie, Y.; Du, C. Distribution Characteristics and Risk Assessment of 57 Pesticides in Farmland Soil and the Surrounding Water. Toxics 2024, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Cara, I.G.; Filip, M.; Bulgariu, L.; Raus, L.; Topa, D.; Jitareanu, G. Environmental Remediation of Metribuzin Herbicide by Mesoporous Carbon—Rich from Wheat Straw. Appl. Sci. 2021, 11, 4935. [Google Scholar] [CrossRef]
- Onyando, Z.O.; Omukunda, E.; Okoth, P.; Khatiebi, S.; Omwoma, S.; Otieno, P.; Osano, O.; Lalah, J. Screening and Prioritization of Pesticide Application for Potential Human Health and Environmental Risks in Largescale Farms in Western Kenya. Agriculture 2023, 13, 1178. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef]
- Schwarz, E.; Khurana, S.; Chakrawal, A.; Chavez Rodriguez, L.; Wirsching, J.; Streck, T.; Manzoni, S.; Thullner, M.; Pagel, H. Spatial Control of Microbial Pesticide Degradation in Soil: A Model-Based Scenario Analysis. Environ. Sci. Technol. 2022, 56, 14427–14438. [Google Scholar] [CrossRef]
- Harmon O’Driscoll, J.; Siggins, A.; Healy, M.G.; McGinley, J.; Mellander, P.; Morrison, L.; Ryan, P.C. A risk ranking of pesticides in Irish drinking water considering chronic health effects. Sci. Total. Environ. 2022, 829, 154532. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, Z.; Lu, J.; Qiao, C.; Wang, C.; Pang, T.; Guo, L.; Li, J.; Pang, R.; Xie, H. Residual behaviors and health risk assessment of dinotefuran, flonicamid, and their metabolites during apple growth, storage, and processing. Food. Res. Int. 2025, 205, 115970. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Hu, B.; Li, T.; Tian, H. Adsorption Behavior and Residue Degradation of Triazine Herbicides in Soil Amended with Rice Straw Biochar. Agriculture 2023, 13, 1282. [Google Scholar] [CrossRef]
- Wang, H.; Ren, W.; Xu, Y.; Wang, X.; Ma, J.; Sun, Y.; Hu, W.; Chen, S.; Dai, S.; Song, J.; et al. Long-term herbicide residues affect soil multifunctionality and the soil microbial community. Ecotox. Environ. Safe 2024, 283, 116783. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M.; la Cecilia, D.; McBratney, A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef]
- Sharipov, U.; Kočárek, M.; Jursík, M.; Nikodem, A.; Borůvka, L. Adsorption and degradation behavior of six herbicides in different agricultural soils. Environ. Earth Sci. 2021, 80, 702. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Peng, M.; Cheng, X. Contamination Levels and the Ecological and Human Health Risks of Potentially Toxic Elements (PTEs) in Soil of Baoshan Area, Southwest China. Appl. Sci. 2022, 12, 1693. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Sharmin, S.; Wang, Q.; Islam, M.R.; Wang, W.; Wang, Y.; Enyoh, C.E.; Rana, M.S. Assessment of Health Risks from Agricultural Soils Contaminated with Polycyclic Aromatic Hydrocarbons (PAHs) Across Different Land-Use Categories of Bangladesh. Appl. Sci. 2025, 15, 56. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Nascimento, O.R.; Martin-Neto, L. Hydrophobic Interactions between Spin-Label 5-SASL and Humic Acid As Revealed by ESR Spectroscopy. Environ. Sci. Technol. 2001, 35, 761–765. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Liu, J.; Wu, J.; Chen, W.; Wu, J.; Zhu, L.; Bian, X. Effects of ditch-buried straw return on soil organic carbon and rice yields in a rice–wheat rotation system. Catena 2015, 127, 56–63. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Cong, P.; Wang, J.; Guo, W.; Pang, H.; Zhang, L. Depth of straw incorporation significantly alters crop yield, soil organic carbon and total nitrogen in the North China Plain. Soil Till. Res. 2021, 205, 104772. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.C.; Shen, A.L. Long-term decomposed straw return positively affects the soil microbial community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Li, X.; Wang, H.; Su, Z.; Wang, X.; Zhang, H. Dynamic changes in microbial communities during the bioremediation of herbicide (chlorimuron-ethyl and atrazine) contaminated soils by combined degrading bacteria. PLoS ONE 2018, 13, e194753. [Google Scholar] [CrossRef]
- Lopes, P.R.M.; Cruz, V.H.; de Menezes, A.B.; Gadanhoto, B.P.; Moreira, B.R.D.A.; Mendes, C.R.; Mazzeo, D.E.C.; Dilarri, G.; Montagnolli, R.N. Microbial bioremediation of pesticides in agricultural soils: An integrative review on natural attenuation, bioaugmentation and biostimulation. Rev. Environ. Sci. Bio./Technol. 2022, 21, 851–876. [Google Scholar] [CrossRef]
- Jing, X.; Chai, X.; Long, S.; Liu, T.; Si, M.; Zheng, X.; Cai, X. Urea/sodium hydroxide pretreatments enhance decomposition of maize straw in soils and sorption of straw residues toward herbicides. J. Hazard. Mater. 2022, 431, 128467. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Liu, T.; Chai, X.; Wang, Y.; Zhang, X.; Cai, X. Persulfate pretreatment facilitates decomposition of maize straw in soils and accumulation of straw residues with high adsorption capacity. Chem. Eng. J. 2023, 475, 145956. [Google Scholar] [CrossRef]
- Minhas, W.A.; Mumtaz, N.; Ur-Rehman, H.; Farooq, S.; Farooq, M.; Ali, H.M.; Hussain, M. Weed infestation and productivity of wheat crop sown in various cropping systems under conventional and conservation tillage. Front. Plant Sci. 2023, 14, 1176738. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e192953. [Google Scholar] [CrossRef]
- Xin, X.L.; Yang, W.L.; Zhu, Q.G.; Zhang, X.F.; Zhu, A.N.; Zhang, J.B. Abundance and depth stratification of soil arthropods as influenced by tillage regimes in a sandy loam soil. Soil Use Manag. 2018, 34, 286–296. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhou, S.; Wang, Z. Meta-analysis of no-tillage effect on wheat and maize water use efficiency in China. Sci. Total. Environ. 2018, 635, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Integrated Risk Information System; Office of Emergency and Remedial Response; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2020. Available online: https://www.epa.gov/iris (accessed on 2 May 2024).
- Zeng, S.; Ma, J.; Yang, Y.; Zhang, S.; Liu, G.; Chen, F. Spatial assessment of farmland soil pollution and its potential human health risks in China. Sci. Total Environ. 2019, 687, 642–653. [Google Scholar] [CrossRef]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef] [PubMed]
- El-Kahawy, R.M.; Mabrouk, M.S. Benthic foraminifera as bioindicators for the heavy metals in the severely polluted Hurghada Bay, Red Sea coast, Egypt. Environ. Sci. Pollut. Res. Int. 2023, 30, 70437–70457. [Google Scholar] [CrossRef]
- Sharma, K.K.; Tripathy, V.; Mohapatra, S.; Matadha, N.Y.; Pathan, A.R.K.; Sharma, B.N.; Dubey, J.K.; Katna, S.; George, T.; Tayade, A.; et al. Dissipation kinetics and consumer risk assessment of novaluron + lambda-cyhalothrin co-formulation in cabbage. Ecotox. Environ. Safe 2021, 208, 111494. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.; Fontanals, N.; Borrull, F.; Marcé, R.M. Multiresidue analytical method for high production volume chemicals in dust samples, occurrence and human exposure assessment. Chemosphere 2022, 301, 134639. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Atrazine Draft Human Health Risk Assessment for Registration Review; Atrazine Human Health Risk Assessment; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2018. Available online: https://www.epa.gov/pesticides/atrazine-human-health-risk-assessment-now-available-public-comment (accessed on 15 June 2024).
- United States Environmental Protection Agency (US EPA). Acetochlor. In Pesticide Tolerances; A Rule by the Environmental Protection Agency; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2014; Available online: https://regulations.justia.com/regulations/fedreg/2013/02/27/2013-04532.html (accessed on 16 June 2024).
- United States Environmental Protection Agency (US EPA). Acetochlor. In Pesticide Tolerances; A Rule by the Environmental Protection Agency; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2007. Available online: https://www.govinfo.gov/content/pkg/FR-2007-09-26/pdf/E7-18967.pdf (accessed on 20 July 2024).
- United States Environmental Protection Agency (US EPA). Nicosulfuron. In Pesticide Tolerances; A Rule by the Environmental Protection Agency; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2015. Available online: https://www.govinfo.gov/content/pkg/FR-2015-11-04/pdf/2015-27887.pdf (accessed on 21 July 2024).
- United States Environmental Protection Agency (US EPA). Pesticide Fact Sheet, Mesotrione; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2001. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-122990_04-Jun-01.pdf (accessed on 10 August 2024).
- Cui, Y.; Bai, L.; Li, C.; He, Z.; Liu, X. Assessment of heavy metal contamination levels and health risks in environmental media in the northeast region. Sustain. Cities Soc. 2022, 80, 103796. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Pan, D.; Zhang, J.; Zhang, Y.; Lu, Z. Source and health risk assessment of soil polycyclic aromatic hydrocarbons under straw burning condition in Changchun City, China. Sci. Total. Environ. 2023, 894, 165057. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Bubak, A. Mobility, bioaccumulation in plants, and risk assessment of metals in soils. Sci. Total. Environ. 2023, 882, 163574. [Google Scholar] [CrossRef]
- Mei, L.; Xia, X.; Cao, J.; Zhao, Y.; Huang, H.; Li, Y.; Zhang, Z. Degradation of Three Herbicides and Effect on Bacterial Communities under Combined Pollution. Toxics 2024, 12, 562. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q. Spatial and Temporal Distribution Characteristics of Triazine Herbicides in Typical Agricultural Regions of Liaoning, China. Bull. Environ. Contam. Toxicol. 2020, 105, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Hu, Z.; Shi, Y. Effects of Acetochlor on Wheat Growth Characteristics and Soil Residue in Dryland. Gesunde. Pflanz. 2021, 73, 307–315. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil. Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Wang, L.; Kaur, M.; Zhang, P.; Li, J.; Xu, M. Effect of Different Agricultural Farming Practices on Microbial Biomass and Enzyme Activities of Celery Growing Field Soil. Int. J. Environ. Res. Public Health 2021, 18, 2862. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sultana, N.; Hoque, M.A.; Azam, M.G.; Islam, M.R.; Hossain, M.A. Conservation tillage (CT) for climate-smart sustainable intensification: Benchmarking CT to improve soil properties, water footprint and bulb yield productivity in onion cultivation. Heliyon 2024, 10, e39749. [Google Scholar] [CrossRef]
- Saharan, B.S.; Dhanda, D.; Mandal, N.K.; Kumar, R.; Sharma, D.; Sadh, P.K.; Jabborova, D.; Duhan, J.S. Microbial contributions to sustainable paddy straw utilization for economic gain and environmental conservation. Curr. Res. Microb. Sci. 2024, 7, 100264. [Google Scholar] [CrossRef]
- Yang, H.; Ma, J.; Rong, Z.; Zeng, D.; Wang, Y.; Hu, S.; Ye, W.; Zheng, X. Wheat Straw Return Influences Nitrogen-Cycling and Pathogen Associated Soil Microbiota in a Wheat-Soybean Rotation System. Front. Microbiol. 2019, 10, 1811. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Paul Voroney, R. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef]
- Badiane, A.; Faye, B.A.; Sambou, A.; Ba, I.; Diop, K.; Diallo, M.; Gueye, S.; Bamba, B.; Fall, S. Cultural mode and organo-mineral amendment effect on growth and yield of rice (Oryza sativa L.) and soil chemical properties in sulfated acid soils of Basse-Casamance. Heliyon 2023, 9, e18830. [Google Scholar] [CrossRef]
- Pratibha, G.; Manjunath, M.; Raju, B.M.K.; Srinivas, I.; Rao, K.V.; Shanker, A.K.; Prasad, J.V.N.S.; Rao, M.S.; Kundu, S.; Indoria, A.K.; et al. Soil bacterial community structure and functioning in a long-term conservation agriculture experiment under semi-arid rainfed production system. Front. Microbiol. 2023, 14, 1102682. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Yuan, S.; Wang, W.; Feng, Y.; Qiao, B. Effects of Long-term Conservation Tillage on Soil Nutrients in Sloping Fields in Regions Characterized by Water and Wind Erosion. Sci. Rep. 2015, 5, 17592. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Skoufogianni, E.; Lolas, A.; Skordas, K. The Impact of Different Cultivation Practices on Surface Runoff, Soil and Nutrient Losses in a Rotational System of Legume-Cereal and Sunflower. Plants 2022, 11, 3513. [Google Scholar] [CrossRef]
- Habib-ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; Issues, challenges, and opportunities in Asia. Front. Plant. Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Yuan, J.; Yan, L.; Li, G.; Sadiq, M.; Rahim, N.; Wu, J.; Ma, W.; Xu, G.; Du, M. Effects of conservation tillage strategies on soil physicochemical indicators and N(2)O emission under spring wheat monocropping system conditions. Sci. Rep. 2022, 12, 7066. [Google Scholar] [CrossRef]
- Szafranek-Nakonieczna, A.; Wolinska, A.; Zielenkiewicz, U.; Kowalczyk, A.; Stepniewska, Z.; Blaszczyk, M. Activity and Identification of Methanotrophic Bacteria in Arable and No-Tillage Soils from Lublin Region (Poland). Microbial. Ecol. 2019, 77, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, M.; Liu, Y.; Zhu, Z.; Fahad, S.; Qayyum, A.; Zhu, G. Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake. Antioxidants 2022, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.R.; Chotana, G.A.; Shafiq, M.; Hseu, Z. Distribution of Heavy Metals in the Soils Associated with the Commonly Used Pesticides in Cotton Fields. Scientifica 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Bhatt, P.; Verma, A.; Gangola, S.; Bhandari, G.; Chen, S. Microbial glycoconjugates in organic pollutant bioremediation: Recent advances and applications. Microb. Cell Fact 2021, 20, 18–72. [Google Scholar] [CrossRef]
- Kjellenberg, L.; Johansson, E.; Gustavsson, K.; Granstedt, A.; Olsson, M.E. Correlations between Polyacetylene Concentrations in Carrot (Daucus carota L.) and Various Soil Parameters. Foods 2016, 5, 60. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, S.; Cui, Y.; Rong, Y.; Song, J.; Lv, D. Study on environmental behaviour of fluopyram in different banana planting soil. Sci. Rep. 2021, 11, 15346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Gou, Q.; Zhang, Y.; Liu, J.; Gao, M. Succession of a natural desert vegetation community after long-term fencing at the edge of a desert oasis in northwest China. Front. Plant Sci. 2023, 14, 1091446. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotox. Environ. Safe 2024, 272, 116019. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Salman, H.M.S.; Li, Y.; Wang, M. Elucidating enantioselective fate and sensitive biomarkers in zebrafish of chiral pesticide fenpropidin: Insights into metabolic pathways and hazard assessment. J. Hazard. Mater. 2024, 480, 136293. [Google Scholar] [CrossRef]
- Iida, Y.; Sun, I.; Price, C.A.; Chen, C.; Chen, Z.; Chiang, J.; Huang, C.; Swenson, N.G. Linking leaf veins to growth and mortality rates: An example from a subtropical tree community. Ecol. Evol. 2016, 6, 6085–6096. [Google Scholar] [CrossRef]
- León-Sobrino, C.; Ramond, J.; Maggs-Kölling, G.; Cowan, D.A. Nutrient acquisition, rather than stress response over diel cycles, drives microbial transcription in a hyper-arid Namib desert soil. Front. Microbiol. 2019, 10, 1054. [Google Scholar] [CrossRef]
- Yu, G.; Chen, F.; Zhang, H.; Wang, Z. Pollution and health risk assessment of heavy metals in soils of Guizhou, China. Ecosyst. Health Sust. 2021, 7, 1859948. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, H.; Chen, K.; Fei, X.; Li, M.; Ma, J.; He, W. Health Risk Assessment for Potential Toxic Elements in the Soil and Rice of Typical Paddy Fields in Henan Province. Toxics 2024, 12, 771. [Google Scholar] [CrossRef]
- Ene, A.; Sion, A.; Stihi, C.; Gheboianu, A.I.; Basliu, V.; Ceoromila, A.M.; Gosav, S. Metal Contamination and Human Health Risk Assessment of Soils from Parks of Industrialized Town (Galati, Romania). Appl. Sci. 2024, 14, 10379. [Google Scholar] [CrossRef]
- Feng, J.; Sun, J.; Xu, J.; Wang, H. Degradation of acetochlor in soil by adding organic fertilizers with different conditioners. Soil Till. Res. 2023, 228, 105651. [Google Scholar] [CrossRef]
- Potter, T.L.; Truman, C.C.; Bosch, D.D.; Bednarz, C. Fluometuron and pendimethalin runoff from strip and conventionally tilled cotton in the southern atlantic coastal plain. J. Environ. Qual. 2004, 33, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Armenova, N.; Tsigoriyna, L.; Arsov, A.; Petrov, K.; Petrova, P. Microbial Detoxification of Residual Pesticides in Fermented Foods: Current Status and Prospects. Foods 2023, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Song, X.; Fu, H.; Liu, C.; Yang, F. Effects of the decomposition agent application on the physicochemical properties and microbial community structure of wheat straw-returning soil. Environ. Technol. Innov. 2024, 35, 103668. [Google Scholar] [CrossRef]
- Wei, S.; Fang, J.; Zhang, T.; Wang, J.; Cheng, Y.; Ma, J.; Xie, R.; Liu, Z.; Su, E.; Ren, Y.; et al. Dynamic changes of soil microorganisms in rotation farmland at the western foot of the Greater Khingan range. Front. Bioeng. Biotechnol. 2023, 11, 1191240. [Google Scholar] [CrossRef]
- Alrumman, S.A.; Standing, D.B.; Paton, G.I. Effects of hydrocarbon contamination on soil microbial community and enzyme activity. J. King Saud. Univ. Sci. 2015, 27, 31–41. [Google Scholar] [CrossRef]
- Jing, X.; Li, Q.; Qiao, X.; Chen, J.; Cai, X. Effects of accumulated straw residues on sorption of pesticides and antibiotics in soils with maize straw return. J. Hazard. Mater. 2021, 418, 126213. [Google Scholar] [CrossRef]
- Gao, Z.; Gu, C.; Fan, X.; Shen, L.; Jin, Z.; Wang, F.; Jiang, X. Biochemical insights into the biodegradation mechanism of typical sulfonylureas herbicides and association with active enzymes and physiological response of fungal microbes: A multi-omics approach. Environ. Int. 2024, 190, 108906. [Google Scholar] [CrossRef]
- Arunrat, N.; Sansupa, C.; Sereenonchai, S.; Hatano, R. Stability of soil bacteria in undisturbed soil and continuous maize cultivation in Northern Thailand. Front. Microbiol. 2023, 14, 1285445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Ge, S.; Peng, P.; Tang, H.; Wang, J. Sugarcane/soybean intercropping with reduced nitrogen addition enhances residue-derived labile soil organic carbon and microbial network complexity in the soil during straw decomposition. J. Integr. Agric. 2024, 23, 4216–4236. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Koskinen, W.C.; Graff, C.D.; Anderson, J.L.; Mulla, D.J.; Nater, E.A.; Alonso, D.G. Acetochlor Persistence in Surface and Subsurface Soil Samples. Water. Air. Soil. Pollut. 2013, 224, 1747. [Google Scholar] [CrossRef]
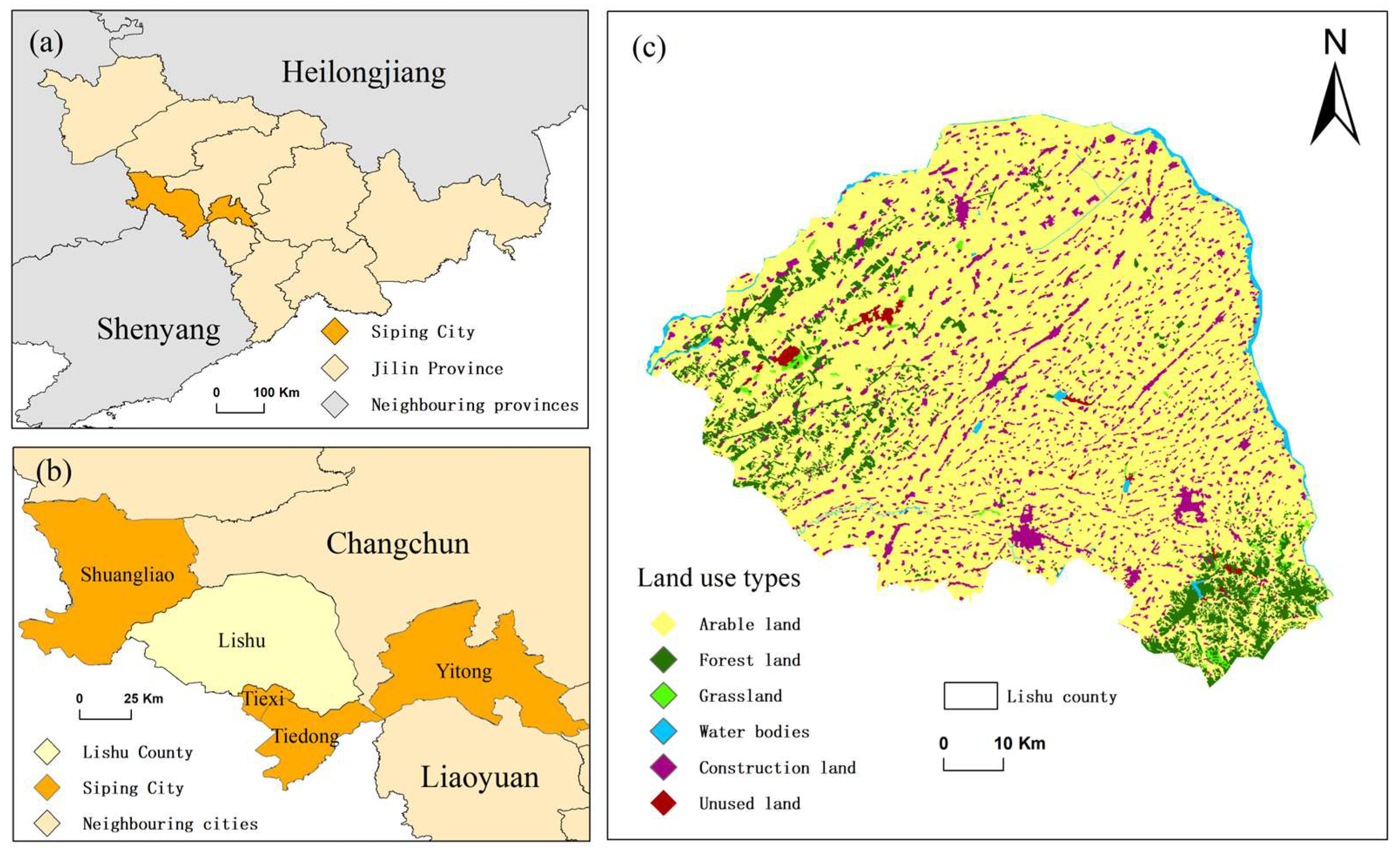
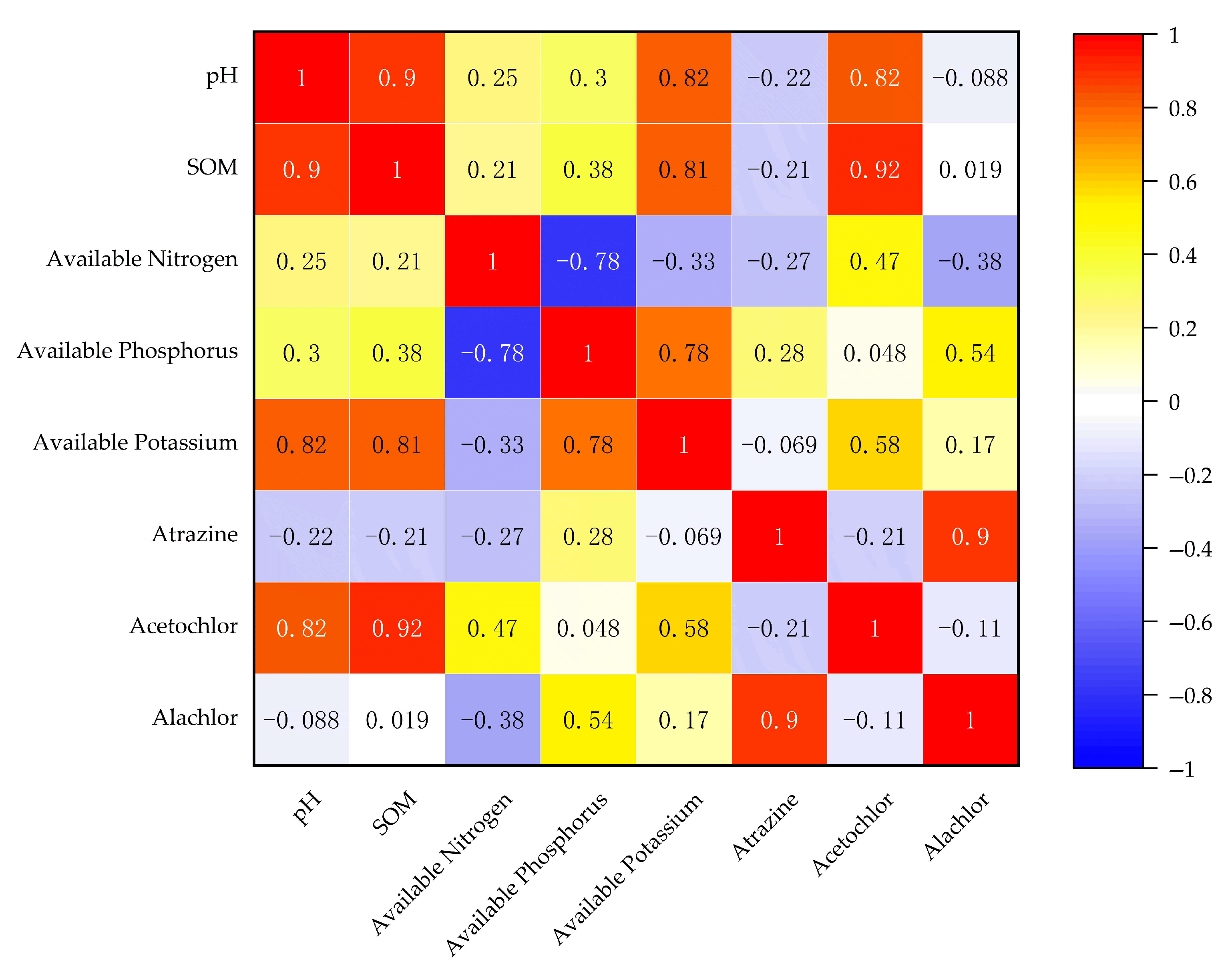
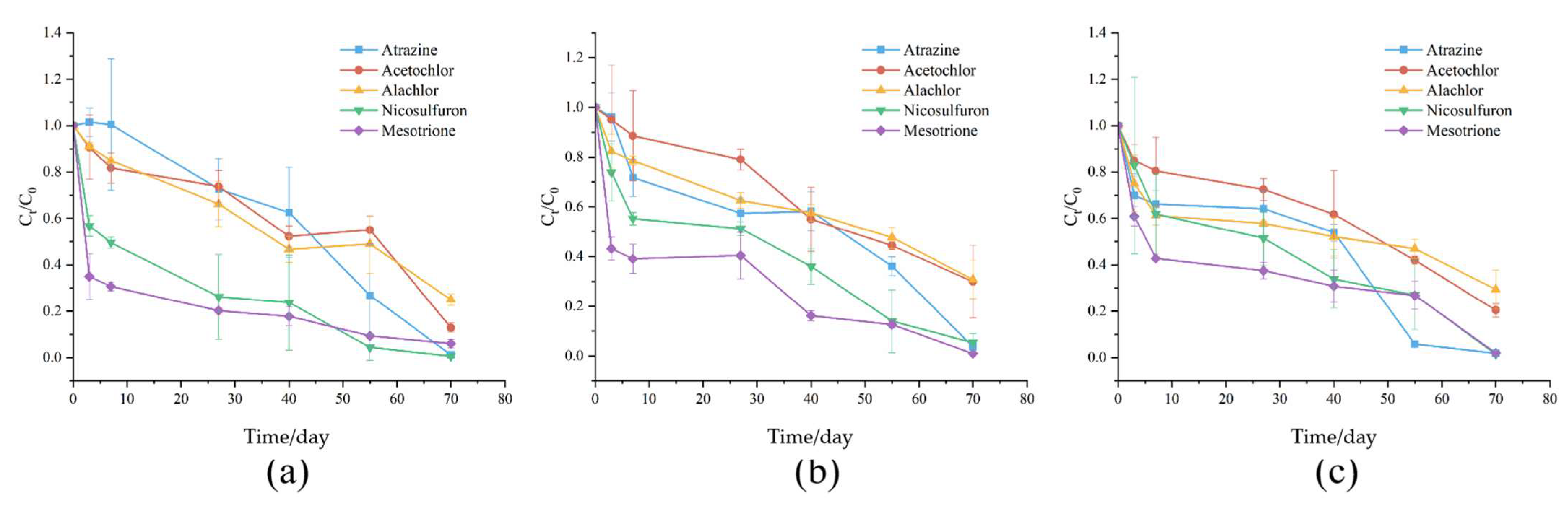
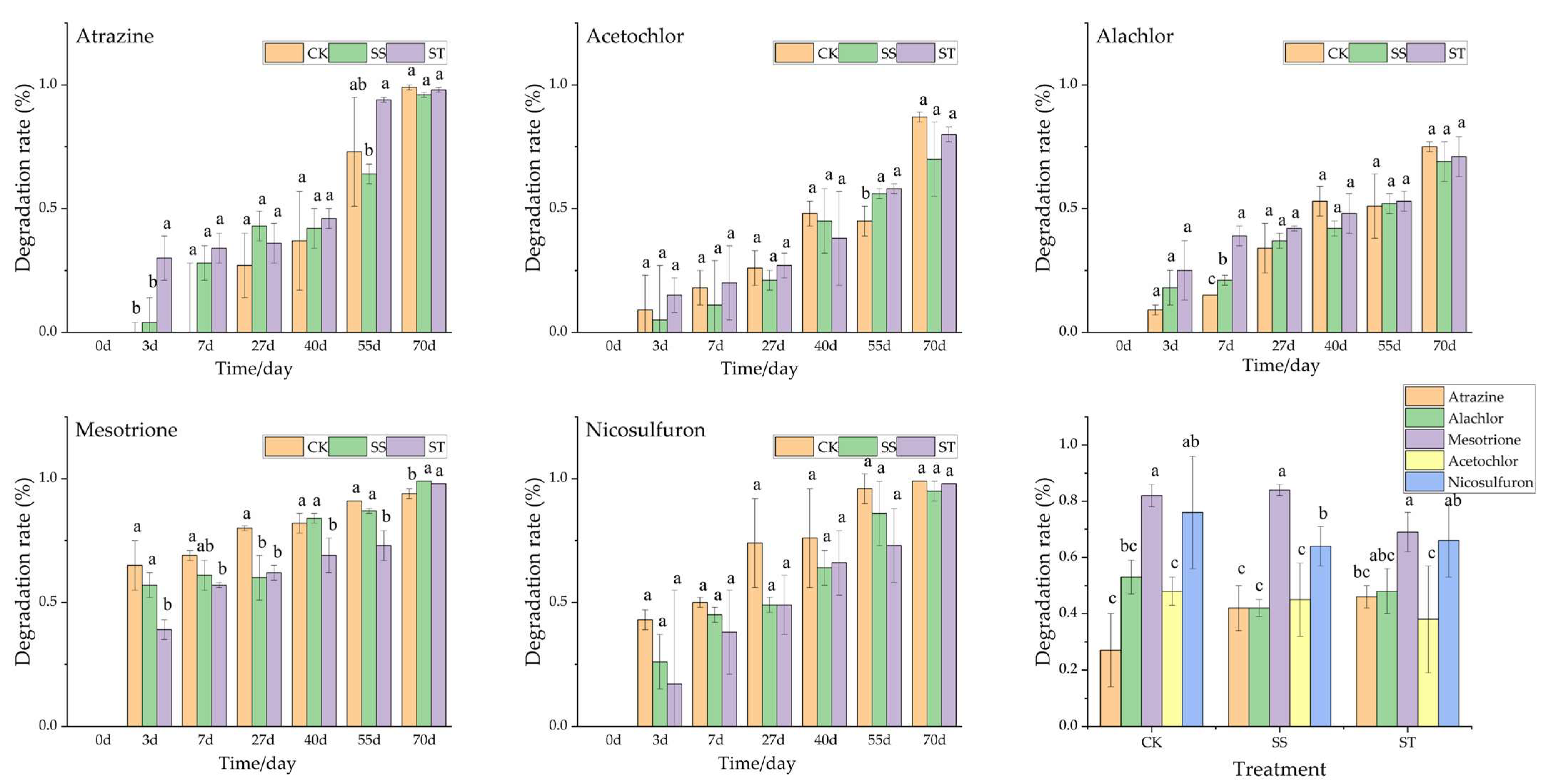
| Tillage Method | Pesticide (ug/kg) | Min | Max | Mean | SD | CV |
|---|---|---|---|---|---|---|
| CK | Atrazine | ND | 11.62 | 5.06 | 5.70 | 1.13 |
| Acetochlor | 19.35 | 30.62 | 23.78 | 4.47 | 0.19 | |
| Alachlor | ND | ND | ND | ND | ND | |
| Nicosulfuron | ND | ND | ND | ND | ND | |
| Mesotrione | ND | ND | ND | ND | ND | |
| SS | Atrazine | ND | 12.31 | 3.94 | 5.12 | 1.30 |
| Acetochlor | 2.29 | 48.79 | 23.66 | 18.34 | 0.78 | |
| Alachlor | ND | 5.71 | 1.02 | 2.30 | 2.25 | |
| Nicosulfuron | ND | ND | ND | ND | ND | |
| Mesotrione | ND | ND | ND | ND | ND | |
| ST | Atrazine | ND | 21.10 | 6.83 | 8.25 | 1.21 |
| Acetochlor | 4.38 | 120.61 | 28.34 | 45.46 | 1.60 | |
| Alachlor | ND | ND | ND | ND | ND | |
| Nicosulfuron | ND | ND | ND | ND | ND | |
| Mesotrione | ND | ND | ND | ND | ND | |
| Total | Atrazine | ND | 21.10 | 5.28 | 6.23 | 1.18 |
| Acetochlor | 2.29 | 120.61 | 25.26 | 26.79 | 1.06 | |
| Alachlor | ND | 5.71 | 0.34 | 1.34 | 3.95 | |
| Nicosulfuron | ND | ND | ND | ND | ND | |
| Mesotrione | ND | ND | ND | ND | ND |
| Tillage Method | pH | SOM (g/kg) | Available Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) |
|---|---|---|---|---|---|
| CK | 7.1 | 20.89 | 84.02 | 23.46 | 208 |
| SS | 6.59 | 18.9 | 64 | 26.27 | 203 |
| ST | 6.34 | 17.6 | 82.6 | 20.7 | 172 |
| Pesticide | Tillage Method | Adults | Children | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HQi | HQd | HQih | HI | HQi | HQd | HQih | HI | ||
| CK | 3.36 × 10−8 | 1.74 × 10−8 | 4.32 × 10−12 | 5.10 × 10−8 | 1.73 × 10−8 | 4.73 × 10−9 | 2.23 × 10−12 | 2.21 × 10−8 | |
| Atrazine | SS | 2.61 × 10−8 | 1.35 × 10−8 | 3.36 × 10−12 | 3.97 × 10−8 | 1.35 × 10−8 | 3.68 × 10−9 | 1.74 × 10−12 | 1.72 × 10−8 |
| ST | 4.52 × 10−8 | 2.35 × 10−8 | 5.82 × 10−12 | 6.87 × 10−8 | 2.34 × 10−8 | 6.38 × 10−9 | 3.01 × 10−12 | 2.98 × 10−8 | |
| CK | 7.89 × 10−7 | 4.09 × 10−7 | 1.01 × 10−10 | 1.20 × 10−6 | 4.07 × 10−7 | 1.11 × 10−7 | 5.24 × 10−11 | 5.18 × 10−7 | |
| Acetochlor | SS | 7.84 × 10−7 | 4.07 × 10−7 | 1.01 × 10−10 | 1.19 × 10−6 | 4.05 × 10−7 | 1.11 × 10−7 | 5.21 × 10−11 | 5.16 × 10−7 |
| ST | 9.39 × 10−7 | 4.87 × 10−7 | 1.21 × 10−10 | 1.43 × 10−6 | 4.85 × 10−7 | 1.33 × 10−7 | 6.25 × 10−11 | 6.18 × 10−7 | |
| CK | ND | ND | ND | ND | ND | ND | ND | ND | |
| Alachlor | SS | 3.38 × 10−7 | 1.76 × 10−7 | 4.35 × 10−11 | 5.14 × 10−7 | 1.75 × 10−7 | 4.77 × 10−8 | 2.25 × 10−11 | 2.23 × 10−7 |
| ST | ND | ND | ND | ND | ND | ND | ND | ND | |
| CK | ND | ND | ND | ND | ND | ND | ND | ND | |
| Nicosulfuron | SS | ND | ND | ND | ND | ND | ND | ND | ND |
| ST | ND | ND | ND | ND | ND | ND | ND | ND | |
| CK | ND | ND | ND | ND | ND | ND | ND | ND | |
| Mesotrione | SS | ND | ND | ND | ND | ND | ND | ND | ND |
| ST | ND | ND | ND | ND | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, F.; Gao, Q.; Ma, Q. Impacts of Different Tillage and Straw Management Systems on Herbicide Degradation and Human Health Risks in Agricultural Soils. Appl. Sci. 2025, 15, 7840. https://doi.org/10.3390/app15147840
Chen Y, Zhang F, Gao Q, Ma Q. Impacts of Different Tillage and Straw Management Systems on Herbicide Degradation and Human Health Risks in Agricultural Soils. Applied Sciences. 2025; 15(14):7840. https://doi.org/10.3390/app15147840
Chicago/Turabian StyleChen, Yanan, Feng Zhang, Qiang Gao, and Qing Ma. 2025. "Impacts of Different Tillage and Straw Management Systems on Herbicide Degradation and Human Health Risks in Agricultural Soils" Applied Sciences 15, no. 14: 7840. https://doi.org/10.3390/app15147840
APA StyleChen, Y., Zhang, F., Gao, Q., & Ma, Q. (2025). Impacts of Different Tillage and Straw Management Systems on Herbicide Degradation and Human Health Risks in Agricultural Soils. Applied Sciences, 15(14), 7840. https://doi.org/10.3390/app15147840








