Labeling Peptides with Radioiodine: An Overview of Traditional and Emerging Techniques
Abstract
1. Introduction
2. Direct Radioiodination Methods
2.1. Electrophilic Iodination
2.2. Isotope/Halogen Exchange of Unnatural Amino Acids Bearing Halogen Substituents
3. Indirect Radioiodination Methods: The Conjugation of the Prosthetic Groups
3.1. Activated Esters and Maleimide Derivatives
3.2. Cycloadditions
4. Indirect Radioiodination Methods: The Labelling of the Prosthetic Groups
4.1. Iododestannylation
4.2. Iododesilylation
4.3. Diazotisation
4.4. Iodonium Salts
4.5. Boronic Acids
5. Purification and Other Considerations for Radioiodination
6. Modern Examples of Biological Applications of Radioiodinated Peptides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveira, M.C.; Correia, J.D. Biomedical applications of radioiodinated peptides. Eur. J. Med. Chem. 2019, 179, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Petrov, S.A.; Yusubov, M.S.; Beloglazkina, E.K.; Nenajdenko, V.G. Synthesis of radioiodinated compounds. Classical approaches and achievements of recent years. Int. J. Mol. Sci. 2022, 23, 13789. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.; Floresta, G.; Abbate, V. Peptide-based positron emission tomography probes: Current strategies for synthesis and radiolabelling. RSC Med. Chem. 2023, 14, 592–623. [Google Scholar] [CrossRef] [PubMed]
- Saccullo, E.; Patamia, V.; Tomarchio, E.G.; Zagni, C.; Floresta, G.; Rescifina, A. Unveiling the chemistry of antibody conjugation for enhanced PET imaging: Current trends and future directions. Bioorganic Chem. 2025, 155, 108115. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jung, J.-h.; Son, S.H.; Kim, C.-Y.; Hong, C.M.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Difference of clinical and radiological characteristics according to radioiodine avidity in pulmonary metastases of differentiated thyroid cancer. Nucl. Med. Mol. Imaging 2014, 48, 55–62. [Google Scholar] [CrossRef]
- Oh, J.-R.; Ahn, B.-C.; Jeong, S.Y.; Lee, S.-W.; Lee, J. Radioiodine scan index: A simplified, quantitative treatment response parameter for metastatic thyroid carcinoma. Nucl. Med. Mol. Imaging 2015, 49, 174–181. [Google Scholar] [CrossRef][Green Version]
- Van Nostrand, D. The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 2009, 19, 1381–1391. [Google Scholar] [CrossRef]
- Chen, M.-K.; Yasrebi, M.; Samii, J.; Staib, L.H.; Doddamane, I.; Cheng, D.W. The utility of I-123 pretherapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid 2012, 22, 304–309. [Google Scholar] [CrossRef]
- Yan, R.; El-Emir, E.; Rajkumar, V.; Robson, M.; Jathoul, A.P.; Pedley, R.B.; Årstad, E. One-pot synthesis of an 125I-labeled trifunctional reagent for multiscale imaging with optical and nuclear techniques. Angew. Chem. Int. Ed. 2011, 50, 6793–6795. [Google Scholar] [CrossRef]
- Blower, P.J. A nuclear chocolate box: The periodic table of nuclear medicine. Dalton Trans. 2015, 44, 4819–4844. [Google Scholar] [CrossRef]
- Goddard, C.P.; Stead, A.H.; Mason, P.A.; Law, B.; Moffat, A.C.; McBrien, M.; Cosby, S. An iodine-125 radioimmunoassay for the direct detection of benzodiazepines in blood and urine. Analyst 1986, 111, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.J. Radioimmunoassay: Review of basic principles. Semin. Nucl. Med. 1975, 5, 125–152. [Google Scholar] [CrossRef] [PubMed]
- Cavina, L.; van der Born, D.; Klaren, P.H.; Feiters, M.C.; Boerman, O.C.; Rutjes, F.P. Design of radioiodinated pharmaceuticals: Structural features affecting metabolic stability towards in vivo deiodination. Eur. J. Org. Chem. 2017, 2017, 3387–3414. [Google Scholar] [CrossRef]
- Dubost, E.; McErlain, H.; Babin, V.; Sutherland, A.; Cailly, T. Recent Advances in Synthetic Methods for Radioiodination. J. Org. Chem. 2020, 85, 8300–8310. [Google Scholar] [CrossRef] [PubMed]
- Patamia, V.; Zagni, C.; Brullo, I.; Saccullo, E.; Coco, A.; Floresta, G.; Rescifina, A. Computer-Assisted Design of Peptide-Based Radiotracers. Int. J. Mol. Sci. 2023, 24, 6856. [Google Scholar] [CrossRef]
- Bapat, K.; Chintalwar, G.; Pandey, U.; Thakur, V.; Sarma, H.; Samuel, G.; Pillai, M.; Chattopadhyay, S.; Venkatesh, M. Preparation and in vitro evaluation of radioiodinated bakuchiol as an anti tumor agent. Appl. Radiat. Isot. 2005, 62, 389–393. [Google Scholar] [CrossRef]
- Sadri, K.; Gandomkar, M.; Babaei, M.; Najafi, R.; Zakavi, S.; Sadat Ebrahimi, S. Synthesis and biodistribution studies of iodine-131 D-amino acid YYK peptide as a potential therapeutic agent for labeling an anti-CD20 antibody. J. Label. Compd. Radiopharm. 2009, 52, 289–294. [Google Scholar] [CrossRef]
- Mangner, T.J.; Wu, J.L.; Wieland, D.M. Solid-phase exchange radioiodination of aryl iodides. Facilitation by ammonium sulfate. J. Org. Chem. 1982, 47, 1484–1488. [Google Scholar] [CrossRef]
- Chezal, J.-M.; Papon, J.; Labarre, P.; Lartigue, C.; Galmier, M.-J.; Decombat, C.; Chavignon, O.; Maublant, J.; Teulade, J.-C.; Madelmont, J.-C. Evaluation of radiolabeled (hetero) aromatic analogues of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and targeted radionuclide therapy of melanoma. J. Med. Chem. 2008, 51, 3133–3144. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. Preparation of N-succinimidyl 3-[*I] iodobenzoate: An agent for the indirect radioiodination of proteins. Nat. Protoc. 2006, 1, 707–713. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, J.; Quinn, T.P.; Jurisson, S.S. Radioiodination of rhenium cyclized α-melanocyte-stimulating hormone resulting in enhanced radioactivity localization and retention in melanoma. Cancer Res. 2004, 64, 1411–1418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adam, M.J.; Wilbur, D.S. Radiohalogens for imaging and therapy. Chem. Soc. Rev. 2005, 34, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pimlott, S.L.; Sutherland, A. Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef]
- Zhu, L.; Ploessl, K.; Kung, H.F. PET/SPECT imaging agents for neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6683–6691. [Google Scholar] [CrossRef]
- Sloan, N.L.; Luthra, S.K.; McRobbie, G.; Pimlott, S.L.; Sutherland, A. A one-pot radioiodination of aryl amines via stable diazonium salts: Preparation of 125I-imaging agents. Chem. Commun. 2017, 53, 11008–11011. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, A.; Beiki, D.; Rafiee, H.; Najafi, R. A new and simple synthesis of N-succinimidyl-4-[127/125I]iodobenzoate involving a microwave—Accelerated iodination step. J. Label. Compd. Radiopharm. 2001, 44, 235–240. [Google Scholar] [CrossRef]
- Vivier, M.; Rapp, M.; Papon, J.; Labarre, P.; Galmier, M.-J.; Sauzière, J.; Madelmont, J.-C. Synthesis, Radiosynthesis, and Biological Evaluation of New Proteasome Inhibitors in a Tumor Targeting Approach. J. Med. Chem. 2008, 51, 1043–1047. [Google Scholar] [CrossRef]
- Marek, A.; Brož, B.; Kriegelstein, M.; Nováková, G.; Hojcsková, J.; Blechová, M.; Žáková, L.; Jiráček, J.; Maletínská, L. Late-stage labeling of diverse peptides and proteins with iodine-125. J. Pharm. Anal. 2025, 101198. [Google Scholar] [CrossRef]
- Yamada, A.; Traboulsi, A.; Dittert, L.W.; Hussain, A.A. Chloramine-T in radiolabeling techniques: III. Radioiodination of biomolecules containing thioether groups. Anal. Biochem. 2000, 277, 232–235. [Google Scholar] [CrossRef]
- Shechter, Y.; Burstein, Y.; Patchornik, A. Selective oxidation of methionine residues in proteins. Biochemistry 1975, 14, 4497–4503. [Google Scholar] [CrossRef]
- Hussien, H.; Goud, A.; Amin, A.; El-Sheikh, R.; Seddik, U. Comparative study between chloramine-T and iodogen to prepare radioiodinated etodolac for inflammation imaging. J. Radioanal. Nucl. Chem. 2011, 288, 9–15. [Google Scholar] [CrossRef]
- Li, C.H. Kinetics of reactions between iodine and histidine. J. Am. Chem. Soc. 1944, 66, 225–227. [Google Scholar] [CrossRef]
- Young, T.S.; Schultz, P.G. Beyond the Canonical 20 Amino Acids: Expanding the Genetic Lexicon. J. Biol. Chem. 2010, 285, 11039–11044. [Google Scholar] [CrossRef]
- Kil, K.-E.; Zhu, A.; Zhang, Z.; Choi, J.-K.; Kura, S.; Gong, C.; Brownell, A.-L. Development of [123I] IPEB and [123I] IMPEB as SPECT radioligands for metabotropic glutamate receptor subtype 5. ACS Med. Chem. Lett. 2014, 5, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Cant, A.A.; Champion, S.; Bhalla, R.; Pimlott, S.L.; Sutherland, A. Nickel-mediated radioiodination of aryl and heteroaryl bromides: Rapid synthesis of tracers for SPECT imaging. Angew. Chem. Int. Ed. 2013, 52, 7829–7832. [Google Scholar] [CrossRef]
- Hasnowo, L.A.; Larkina, M.S.; Plotnikov, E.; Bodenko, V.; Yuldasheva, F.; Stasyuk, E.; Petrov, S.A.; Zyk, N.Y.; Machulkin, A.E.; Vorozhtsov, N.I.; et al. Synthesis, 123I-Radiolabeling Optimization, and Initial Preclinical Evaluation of Novel Urea-Based PSMA Inhibitors with a Tributylstannyl Prosthetic Group in Their Structures. Int. J. Mol. Sci. 2023, 24, 12206. [Google Scholar] [CrossRef]
- Billaud, E.M.; Vidal, A.l.; Vincenot, A.l.; Besse, S.; Bouchon, B.; Debiton, E.; Miot-Noirault, E.; Miladi, I.; Rbah-Vidal, L.; Auzeloux, P. Development and preliminary evaluation of TFIB, a new bimodal prosthetic group for bioactive molecule labeling. ACS Med. Chem. Lett. 2015, 6, 168–172. [Google Scholar] [CrossRef]
- Lin, R.; Liu, N.; Yang, Y.; Li, B.; Liao, J.; Jin, J. Radioiodination of protein using 2,3,5,6-tetrafluorophenyl 3-(nido-carboranyl) propionate (TCP) as a potential bi-functional linker: Synthesis and biodistribution in mice. Appl. Radiat. Isot. 2009, 67, 83–87. [Google Scholar] [CrossRef]
- Janabi, M.; Pollock, C.M.; Chacko, A.-M.; Hunter, D.H. Resin-supported arylstannanes as precursors for radiolabeling with iodine: Benzaldehydes, benzoic acids, benzamides, and NHS esters. Can. J. Chem. 2015, 93, 207–217. [Google Scholar] [CrossRef]
- Bolton, A.; Hunter, W. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Application to the radioimmunoassay. Biochem. J. 1973, 133, 529–538. [Google Scholar] [CrossRef]
- Russell, J.; O’Donoghue, J.A.; Finn, R.; Koziorowski, J.; Ruan, S.; Humm, J.L.; Ling, C.C. Iodination of annexin V for imaging apoptosis. J. Nucl. Med. 2002, 43, 671–677. [Google Scholar] [PubMed]
- Patamia, V.; Zagni, C.; Fiorenza, R.; Fuochi, V.; Dattilo, S.; Riccobene, P.M.; Furneri, P.M.; Floresta, G.; Rescifina, A. Total Bio-Based Material for Drug Delivery and Iron Chelation to Fight Cancer through Antimicrobial Activity. Nanomaterials 2023, 13, 2036. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, M.S.; Ranga, R.; Luker, G.D.; Rehemtulla, A.; Ross, B.D.; Van Dort, M.E. Synthesis and investigation of a radioiodinated F3 peptide analog as a SPECT tumor imaging radioligand. PLoS ONE 2011, 6, e22418. [Google Scholar] [CrossRef]
- Khawli, L.A.; Van Den Abbeele, A.D.; Kassis, A.I. N-(m-[125I] iodophenyl) maleimide: An agent for high yield radiolabeling of antibodies. Int. J. Radiat. Appl. Instrum. B 1992, 19, 289–295. [Google Scholar] [CrossRef]
- Lahnsteiner, M.; Kastner, A.; Mayr, J.; Roller, A.; Keppler, B.K.; Kowol, C.R. Improving the Stability of Maleimide-Thiol Conjugation for Drug Targeting. Chemistry 2020, 26, 15867–15870. [Google Scholar] [CrossRef]
- Szijj, P.A.; Bahou, C.; Chudasama, V. Minireview: Addressing the retro-Michael instability of maleimide bioconjugates. Drug Discov. Today 2018, 30, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bibi, I.; Mushtaq, S.; Lee, K.C.; Park, J.A.; Kim, J.Y. From molecules to medicine: Thiol selective bioconjugation in synthesis of diagnostic and therapeutic radiopharmaceuticals. Theranostics 2024, 14, 2396–2426. [Google Scholar] [CrossRef]
- Mushtaq, S.; Nam, Y.R.; Kang, J.A.; Choi, D.S.; Park, S.H. Efficient and Site-Specific 125I-Radioiodination of Bioactive Molecules Using Oxidative Condensation Reaction. ACS Omega 2018, 3, 6903–6911. [Google Scholar] [CrossRef]
- Le Saux, L.; Haddad, F.; Gestin, J.-F.; Eychenne, R.; Guérard, F. Sydnone-based prosthetic groups for radioiodination. Bioorganic Med. Chem. 2024, 113, 117904. [Google Scholar] [CrossRef]
- Albu, S.A.; Al-Karmi, S.A.; Vito, A.; Dzandzi, J.P.; Zlitni, A.; Beckford-Vera, D.; Blacker, M.; Janzen, N.; Patel, R.M.; Capretta, A. 125I-Tetrazines and inverse-electron-demand Diels–Alder chemistry: A convenient radioiodination strategy for biomolecule labeling, screening, and biodistribution studies. Bioconjugate Chem. 2016, 27, 207–216. [Google Scholar] [CrossRef]
- Choi, M.H.; Shim, H.E.; Yun, S.-J.; Kim, H.R.; Mushtaq, S.; Lee, C.H.; Park, S.H.; Choi, D.S.; Lee, D.-E.; Byun, E.-B. Highly efficient method for 125I-radiolabeling of biomolecules using inverse-electron-demand Diels–Alder reaction. Bioorganic Med. Chem. 2016, 24, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- McIntee, J.W.; Sundararajan, C.; Donovan, A.C.; Kovacs, M.S.; Capretta, A.; Valliant, J.F. A convenient method for the preparation of fluorous tin derivatives for the fluorous labeling strategy. J. Org. Chem. 2008, 73, 8236–8243. [Google Scholar] [CrossRef] [PubMed]
- Rajerison, H.; Faye, D.; Roumesy, A.; Louaisil, N.; Boeda, F.; Faivre-Chauvet, A.; Gestin, J.-F.; Legoupy, S. Ionic liquid supported organotin reagents to prepare molecular imaging and therapy agents. Org. Biomol. Chem. 2016, 14, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Zalutsky, M.R. No-carrier-added synthesis of meta-[131I]iodobenzylguanidine. Appl. Radiat. Isot. 1993, 44, 621–628. [Google Scholar] [CrossRef]
- Nakagawa, C.; Toyama, M.; Takeuchi, R.; Takahashi, T.; Tanaka, H. Synthesis of [123I]-iodometomidate from a polymer-supported precursor with a large excluded volume. RSC Adv. 2016, 6, 12215–12218. [Google Scholar] [CrossRef]
- Navarro, L.; Berdal, M.; Chérel, M.; Pecorari, F.; Gestin, J.-F.; Guérard, F. Prosthetic groups for radioiodination and astatination of peptides and proteins: A comparative study of five potential bioorthogonal labeling strategies. Bioorganic Med. Chem. 2019, 27, 167–174. [Google Scholar] [CrossRef]
- Guérard, F.; Lee, Y.S.; Baidoo, K.; Gestin, J.F.; Brechbiel, M.W. Unexpected behavior of the heaviest halogen astatine in the nucleophilic substitution of aryliodonium salts. Chemistry 2016, 22, 12332–12339. [Google Scholar] [CrossRef]
- Guérard, F.; Navarro, L.; Lee, Y.-S.; Roumesy, A.; Alliot, C.; Chérel, M.; Brechbiel, M.; Gestin, J.-F. Bifunctional aryliodonium salts for highly efficient radioiodination and astatination of antibodies. Bioorganic Med. Chem. 2017, 25, 5975–5980. [Google Scholar] [CrossRef]
- Kondo, Y.; Kimura, H.; Sasaki, M.; Koike, S.; Yagi, Y.; Hattori, Y.; Kawashima, H.; Yasui, H. Effect of Water on Direct Radioiodination of Small Molecules/Peptides Using Copper-Mediated Iododeboronation in Water–Alcohol Solvent. ACS Omega 2023, 8, 24418–24425. [Google Scholar] [CrossRef]
- King, A.E.; Brunold, T.C.; Stahl, S.S. Mechanistic Study of Copper-Catalyzed Aerobic Oxidative Coupling of Arylboronic Esters and Methanol: Insights into an Organometallic Oxidase Reaction. J. Am. Chem. Soc. 2009, 131, 5044–5045. [Google Scholar] [CrossRef]
- King, A.E.; Ryland, B.L.; Brunold, T.C.; Stahl, S.S. Kinetic and Spectroscopic Studies of Aerobic Copper(II)-Catalyzed Methoxylation of Arylboronic Esters and Insights into Aryl Transmetalation to Copper(II). Organometallics 2012, 31, 7948–7957. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, J.C.; Miras, H.N.; Isidro-Llobet, A.; Sproules, S.; Watson, A.J.B. Spectroscopic Studies of the Chan–Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity. J. Am. Chem. Soc. 2017, 139, 4769–4779. [Google Scholar] [CrossRef]
- Sauer, B.; Xiao, Y.H.; Zoontjes, M.; Kroll, C. Application of X-ray fluorescence spectrometry for screening pharmaceutical products for Elemental Impurities according to ICH guideline Q3D. J. Pharm. Biomed. Anal. 2020, 179, 113005. [Google Scholar] [CrossRef]
- Conlon, J.M. Purification of naturally occurring peptides by reversed-phase HPLC. Nat. Protoc. 2007, 2, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.B.; Kennel, S.J.; Richey, T.; Wooliver, C.; Osborne, D.; Williams, A.; Stuckey, A.; Wall, J.S. Dynamic PET and SPECT imaging with radioiodinated, amyloid-reactive peptide p5 in mice: A positive role for peptide dehalogenation. Peptides 2014, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Eberle, A.; Hübscher, W. α-Melanotropin Labelled at its Tyrosine2 Residue: Synthesis and Biological Activities of 3′-Iodotyrosine2-,3′-125Iodotyrosine2-,3′,5′-Diiodotyrosine2-, and (3′,5′-3H2)tyrosine2-α-Melanotropin, and of Related Peptides. Helv. Chim. Acta 1979, 62, 2460–2483. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Open letter to journal editors on: International Consensus Radiochemistry Nomenclature Guidelines. Ann. Nucl. Med. 2018, 32, 236–238. [Google Scholar] [CrossRef]
- Gillings, N.; Todde, S.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Hjelstuen, O.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. EANM guideline on the validation of analytical methods for radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2020, 5, 7. [Google Scholar] [CrossRef]
- Tago, T.; Toyohara, J. Step-by-step optimisation of the radiosynthesis of the brain HDAC6 radioligand [(18)F]FSW-100 for clinical applications. EJNMMI Radiopharm. Chem. 2024, 9, 45. [Google Scholar] [CrossRef]
- Fonseca, A.I.; Carmo, S.J.C.D.; Ivanna, H.; Alves, V.; Francisco, A.; Abrunhosa, A.J. Purification of Copper Radioisotopes for Medical Applications: Chromatographic Methods and Challenges. Sep. Purif. Rev. 2024, 53, 289–310. [Google Scholar] [CrossRef]
- Laferriere-Holloway, T.S.; Rios, A.; Carlucci, G.; van Dam, R.M. Rapid Purification and Formulation of Radiopharmaceuticals via Thin-Layer Chromatography. Molecules 2022, 27, 8178. [Google Scholar] [CrossRef] [PubMed]
- Molavipordanjani, S.; Tolmachev, V.; Hosseinimehr, S.J. Basic and practical concepts of radiopharmaceutical purification methods. Drug Discov. Today 2019, 24, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.S.; Balachandran, M.; Hancock, T.J.; Martin, E.B.; Macy, S.; Wooliver, C.; Richey, T.; Stuckey, A.; Williams, A.D.; Jackson, J.W.; et al. Development and characterization of a prototypic pan-amyloid clearing agent—A novel murine peptide-immunoglobulin fusion. Front. Immunol. 2023, 14, 1275372. [Google Scholar] [CrossRef]
- Zhou, Z.; Zalutsky, M.R.; Chitneni, S.K. Stapled peptides as scaffolds for developing radiotracers for intracellular targets: Preliminary evaluation of a radioiodinated MDM2-binding stapled peptide in the SJSA-1 osteosarcoma model. Bioorganic Med. Chem. Lett. 2022, 66, 128725. [Google Scholar] [CrossRef]
- Weber, M.; Hadaschik, B.; Ferdinandus, J.; Rahbar, K.; Bögemann, M.; Herrmann, K.; Fendler, W.P.; Kesch, C. Prostate-specific Membrane Antigen–based Imaging of Castration-resistant Prostate Cancer. Eur. Urol. Focus 2021, 7, 279–287. [Google Scholar] [CrossRef]
- Barrio, M.; Fendler, W.P.; Czernin, J.; Herrmann, K. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev. Mol. Diagn. 2016, 16, 1177–1188. [Google Scholar] [CrossRef]
- Kondo, Y.; Kimura, H.; Sasaki, I.; Watanabe, S.; Ohshima, Y.; Yagi, Y.; Hattori, Y.; Koda, M.; Kawashima, H.; Yasui, H.; et al. Copper-mediated radioiodination and radiobromination via aryl boronic precursor and its application to (125)I/(77)Br-labeled prostate-specific membrane antigen imaging probes. Bioorganic Med. Chem. 2022, 69, 116915. [Google Scholar] [CrossRef]
- Li, D.; Ding, J.; Liu, T.-l.; Wang, F.; Meng, X.-x.; Liu, S.; Yang, Z.; Zhu, H. SARS-CoV-2 receptor binding domain radio-probe: A non-invasive approach for angiotensin-converting enzyme 2 mapping in mice. Acta Pharmacol. Sin. 2022, 43, 1749–1757. [Google Scholar] [CrossRef]
- Floresta, G.; Memdouh, S.; Pham, T.; Ma, M.T.; Blower, P.J.; Hider, R.C.; Abbate, V.; Cilibrizzi, A. Targeting integrin αvβ6 with gallium-68 tris (hydroxypyridinone) based PET probes. Dalton Trans. 2022, 51, 12796–12803. [Google Scholar] [CrossRef]
- Kondo, N.; Kato, M.; Oshima, A.; Hirano, F.; Miyazaki, A.; Temma, T. Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation. Pharmaceuticals 2025, 18, 549. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Watanabe, H.; Ikehata, Y.; Ding, N.; Yoshimura, M.; Sano, K.; Saji, H. Radioiodination of BODIPY and its application to a nuclear and optical dual functional labeling agent for proteins and peptides. Sci. Rep. 2017, 7, 3337. [Google Scholar] [CrossRef]
- Karageorgou, M.-A.; Bouziotis, P.; Stiliaris, E.; Stamopoulos, D. Radiolabeled Iron Oxide Nanoparticles as Dual Modality Contrast Agents in SPECT/MRI and PET/MRI. Nanomaterials 2023, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Krönke, T.; Kopka, K.; Mamat, C. Enhancing the radionuclide theranostic concept through the radiohybrid approach. RSC Med. Chem. 2025, 16, 1856–1864. [Google Scholar] [CrossRef]
- Floresta, G. Leading designs of peptide-based chemical probes for medical imaging– the dawn of precision diagnostics. Future Med. Chem. 2025, 17, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Floresta, G.; Keeling, G.P.; Memdouh, S.; Meszaros, L.K.; de Rosales, R.T.M.; Abbate, V. NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines 2021, 9, 367. [Google Scholar] [CrossRef]
- Webb, E.W.; Scott, P.J.H. Potential Applications of Artificial Intelligence and Machine Learning in Radiochemistry and Radiochemical Engineering. PET Clin. 2021, 16, 525–532. [Google Scholar] [CrossRef]
- Georgiou, M.F.; Nielsen, J.A.; Chiriboga, R.; Kuker, R.A. An Artificial Intelligence System for Optimizing Radioactive Iodine Therapy Dosimetry. J. Clin. Med. 2023, 13, 117. [Google Scholar] [CrossRef]
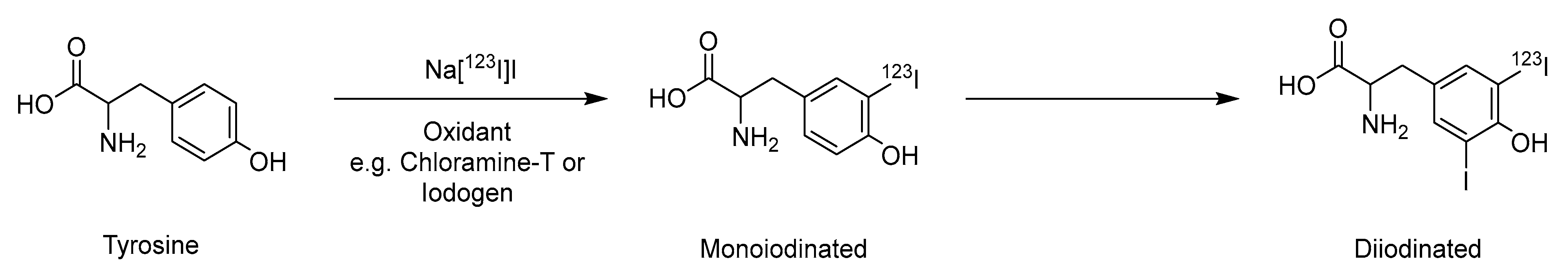
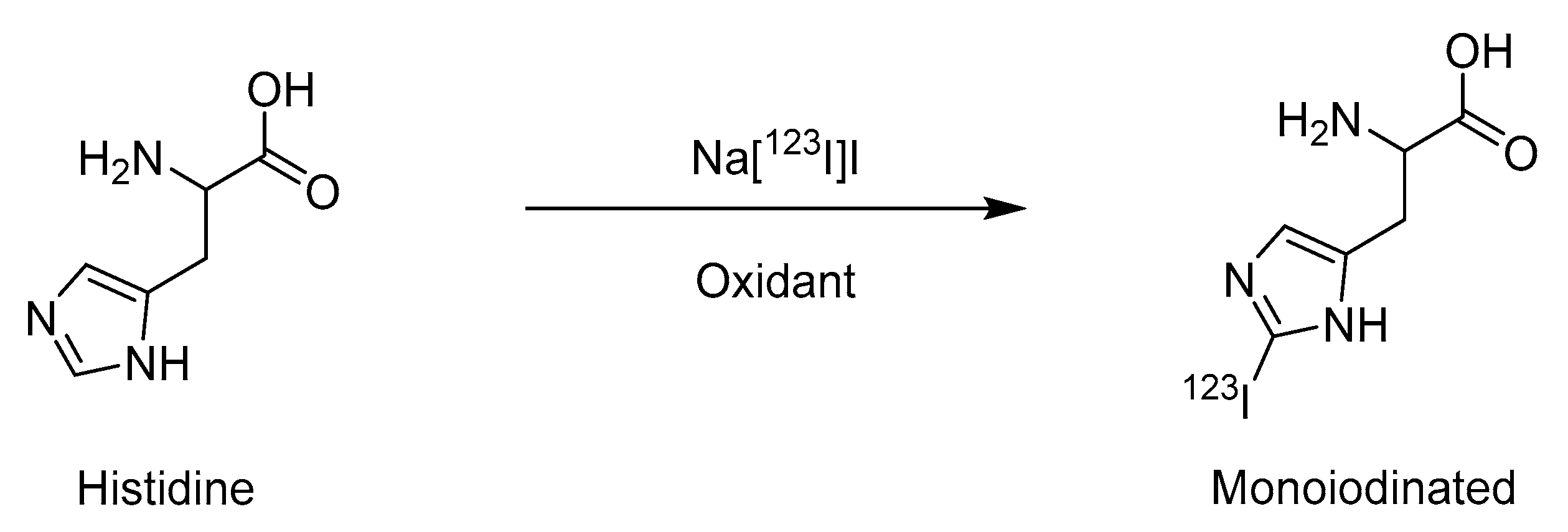
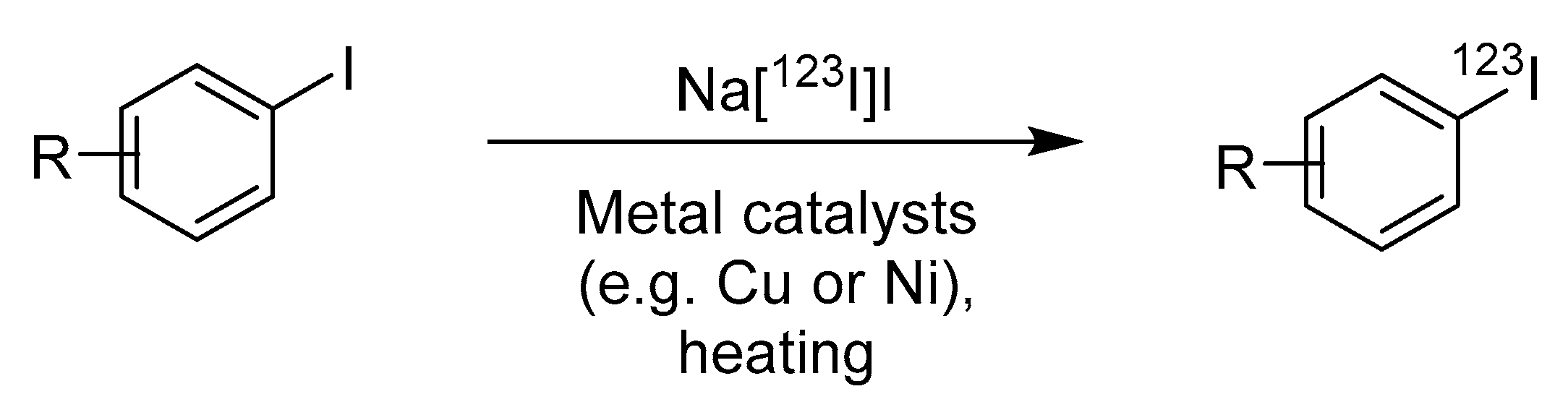
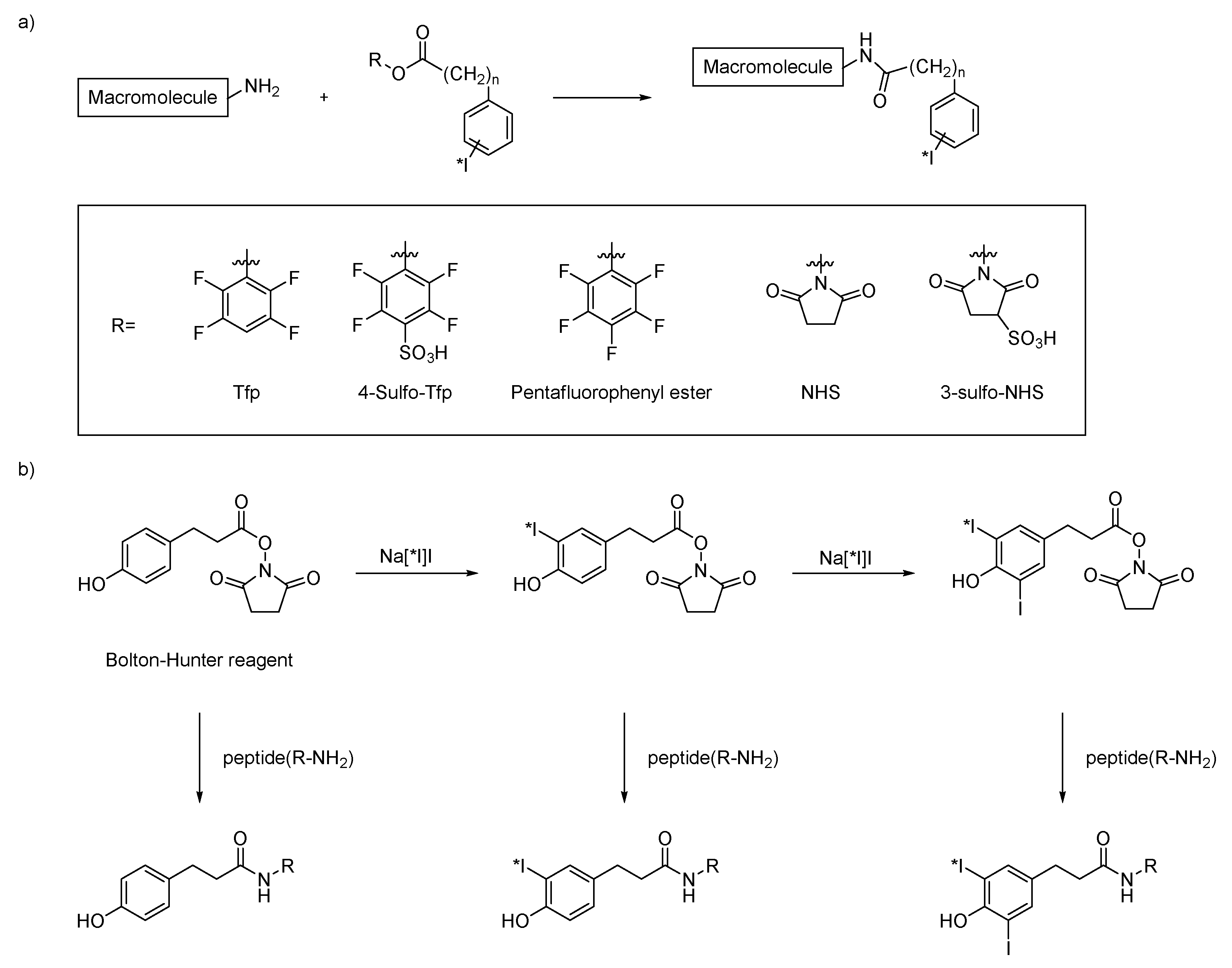
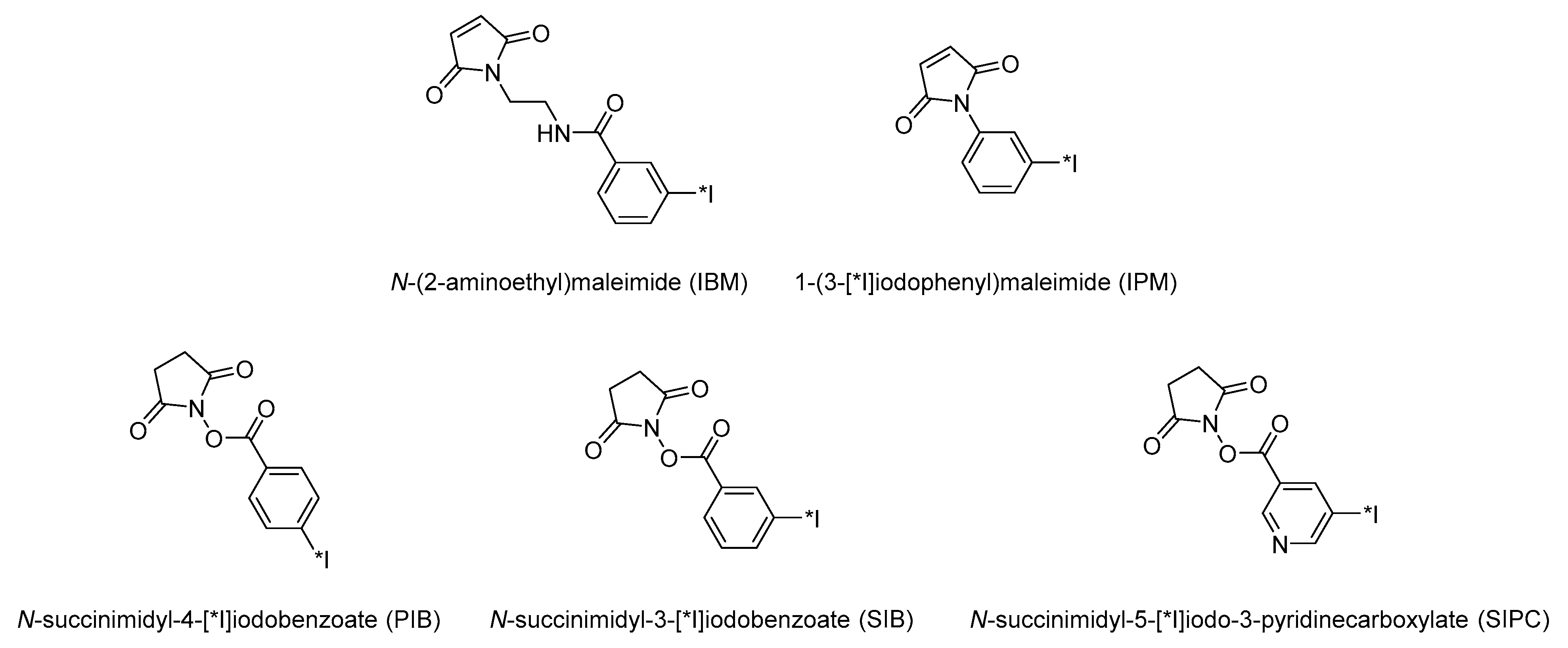

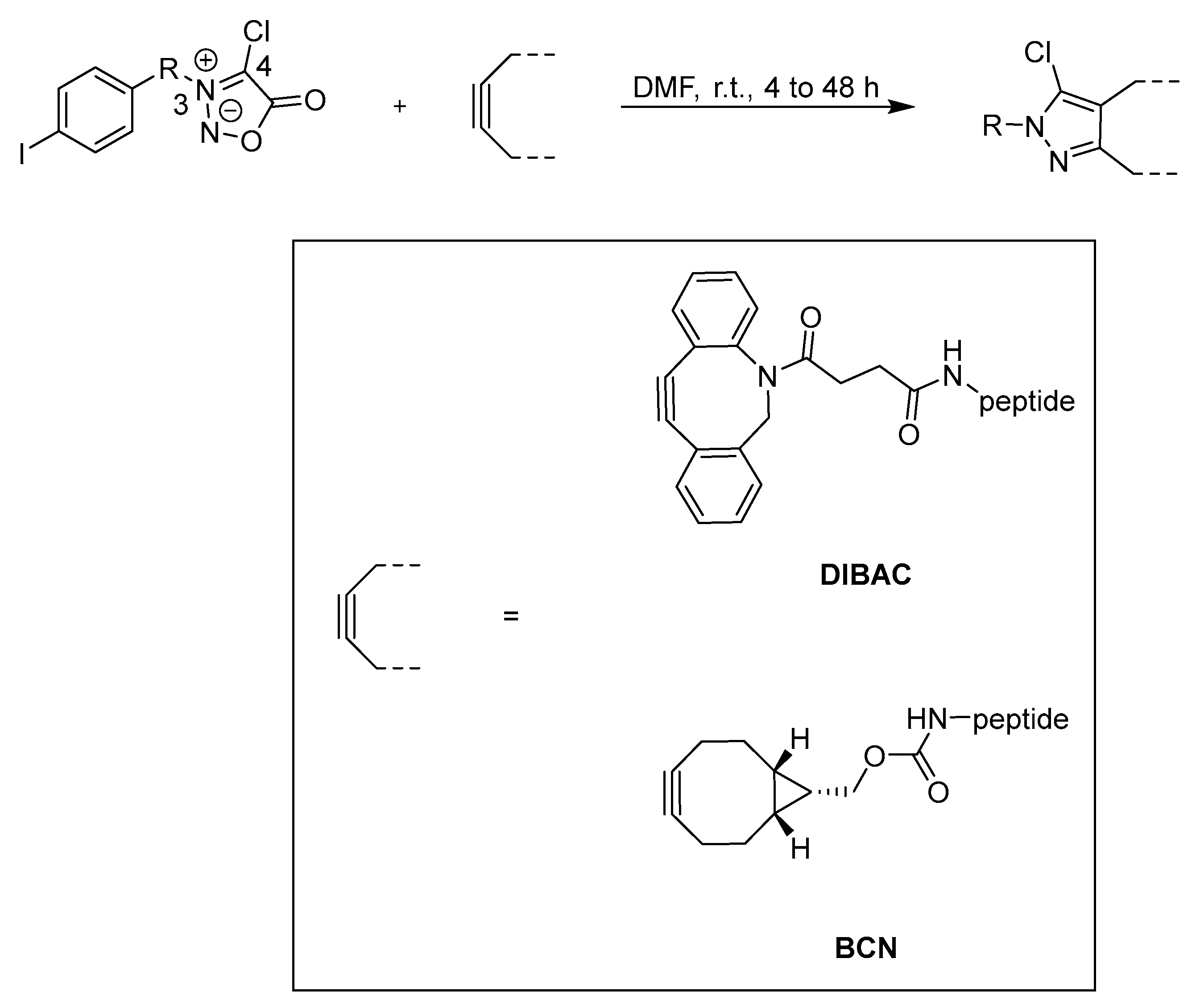

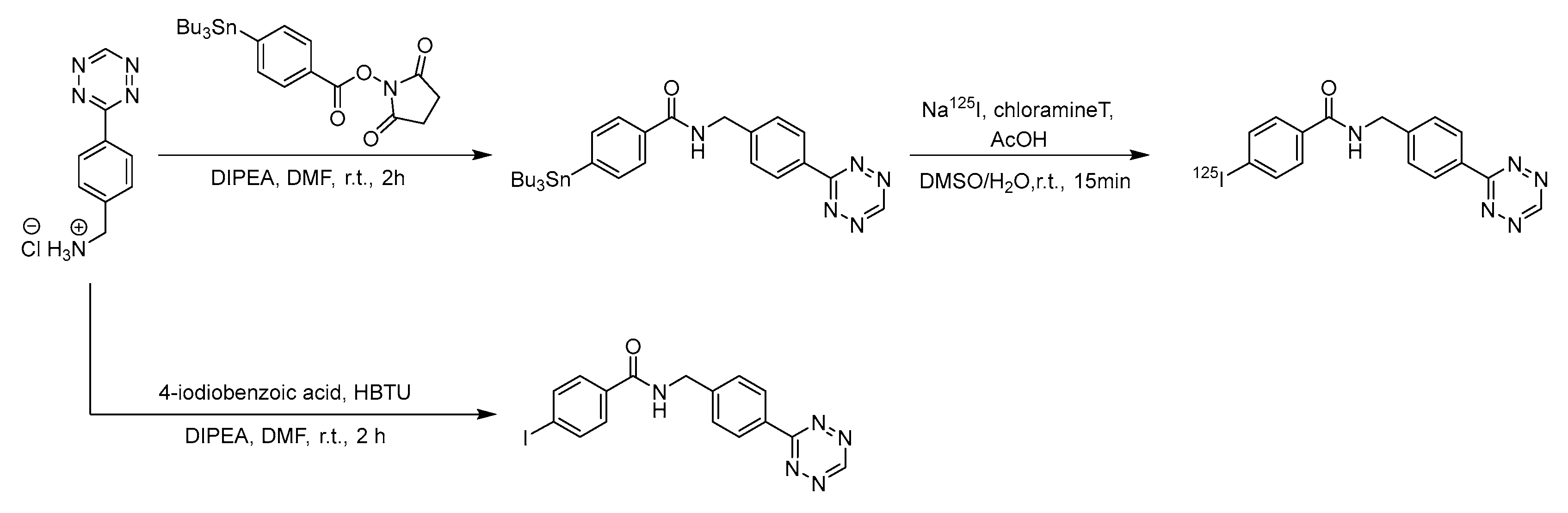
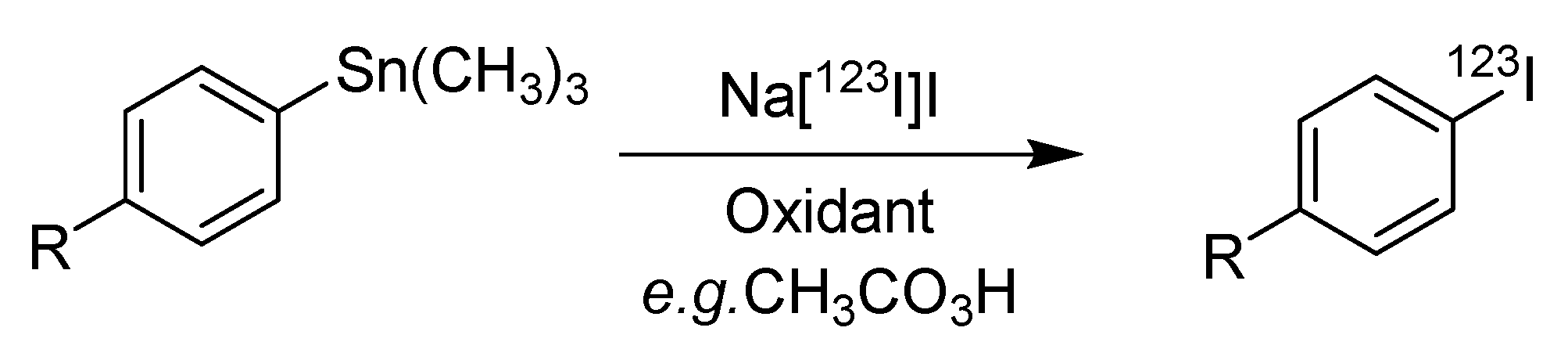
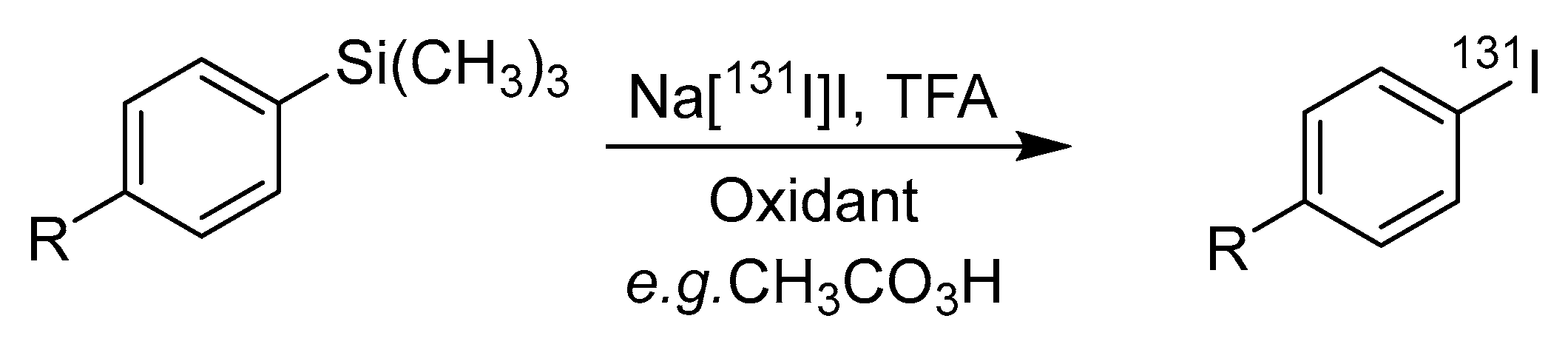



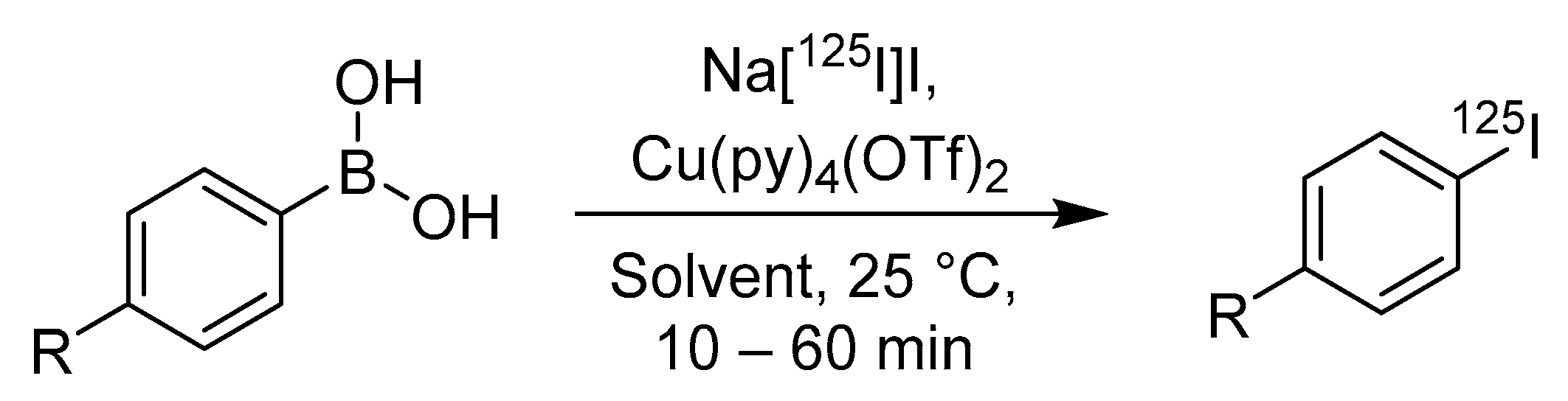

| Method | Key Features | Use Case | Key References |
|---|---|---|---|
| Electrophilic iodination | Mild conditions, wide substrate scope | Small molecules, tyrosine/histidine-containing peptides | [16,17] |
| Isotope/halogen exchange | Simple, no precursor synthesis | Molecules with existing halogen groups | [18,19] |
| Nucleophilic substitution | Requires good leaving groups | Prosthetic groups | [13,20,21] |
| Iododestannylation | One-step, highly efficient | Aryl-containing molecules, pre-functionalized systems | [22,23,24] |
| Iododesilylation | Lower radiochemical yields than Iododestannylation | Aryl-containing molecules, pre-functionalized systems | [2,14] |
| Diazotisation | High reactivity of intermediate | Aryl-containing molecules, pre-functionalized systems | [25,26,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patamia, V.; Saccullo, E.; Crocetti, L.; Procopio, A.; Floresta, G. Labeling Peptides with Radioiodine: An Overview of Traditional and Emerging Techniques. Appl. Sci. 2025, 15, 7803. https://doi.org/10.3390/app15147803
Patamia V, Saccullo E, Crocetti L, Procopio A, Floresta G. Labeling Peptides with Radioiodine: An Overview of Traditional and Emerging Techniques. Applied Sciences. 2025; 15(14):7803. https://doi.org/10.3390/app15147803
Chicago/Turabian StylePatamia, Vincenzo, Erika Saccullo, Letizia Crocetti, Antonio Procopio, and Giuseppe Floresta. 2025. "Labeling Peptides with Radioiodine: An Overview of Traditional and Emerging Techniques" Applied Sciences 15, no. 14: 7803. https://doi.org/10.3390/app15147803
APA StylePatamia, V., Saccullo, E., Crocetti, L., Procopio, A., & Floresta, G. (2025). Labeling Peptides with Radioiodine: An Overview of Traditional and Emerging Techniques. Applied Sciences, 15(14), 7803. https://doi.org/10.3390/app15147803









