Plant-Based Biosorbents for Copper(II) Removal: A Comparative Study of Biomass and Essential Oil Residues
Abstract
1. Introduction
2. Materials and Methods
Adsorption Studies
3. Results and Discussion
3.1. Sample Characterization
3.1.1. XRD Analysis
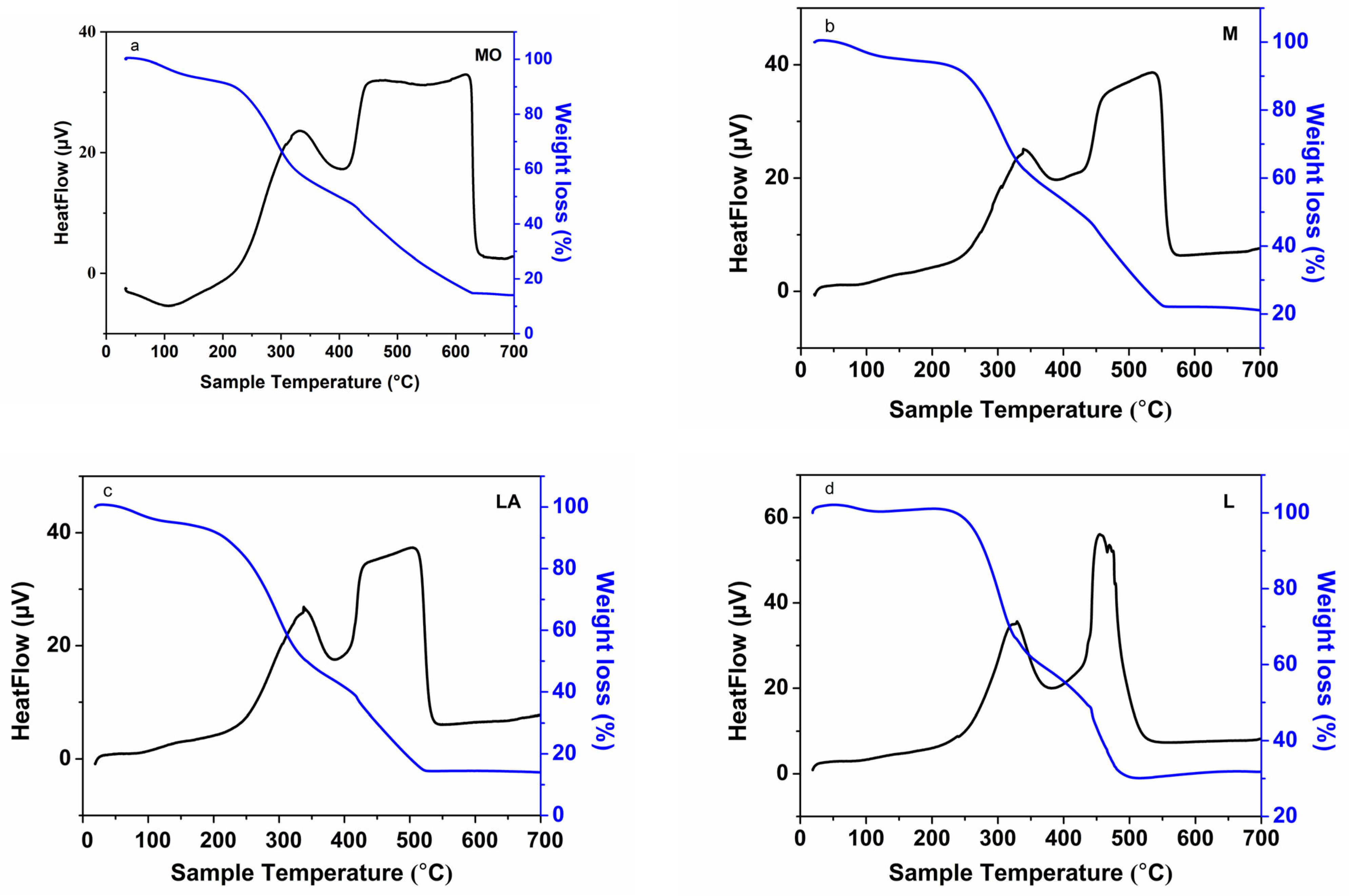
3.1.2. TG/DTA Analysis
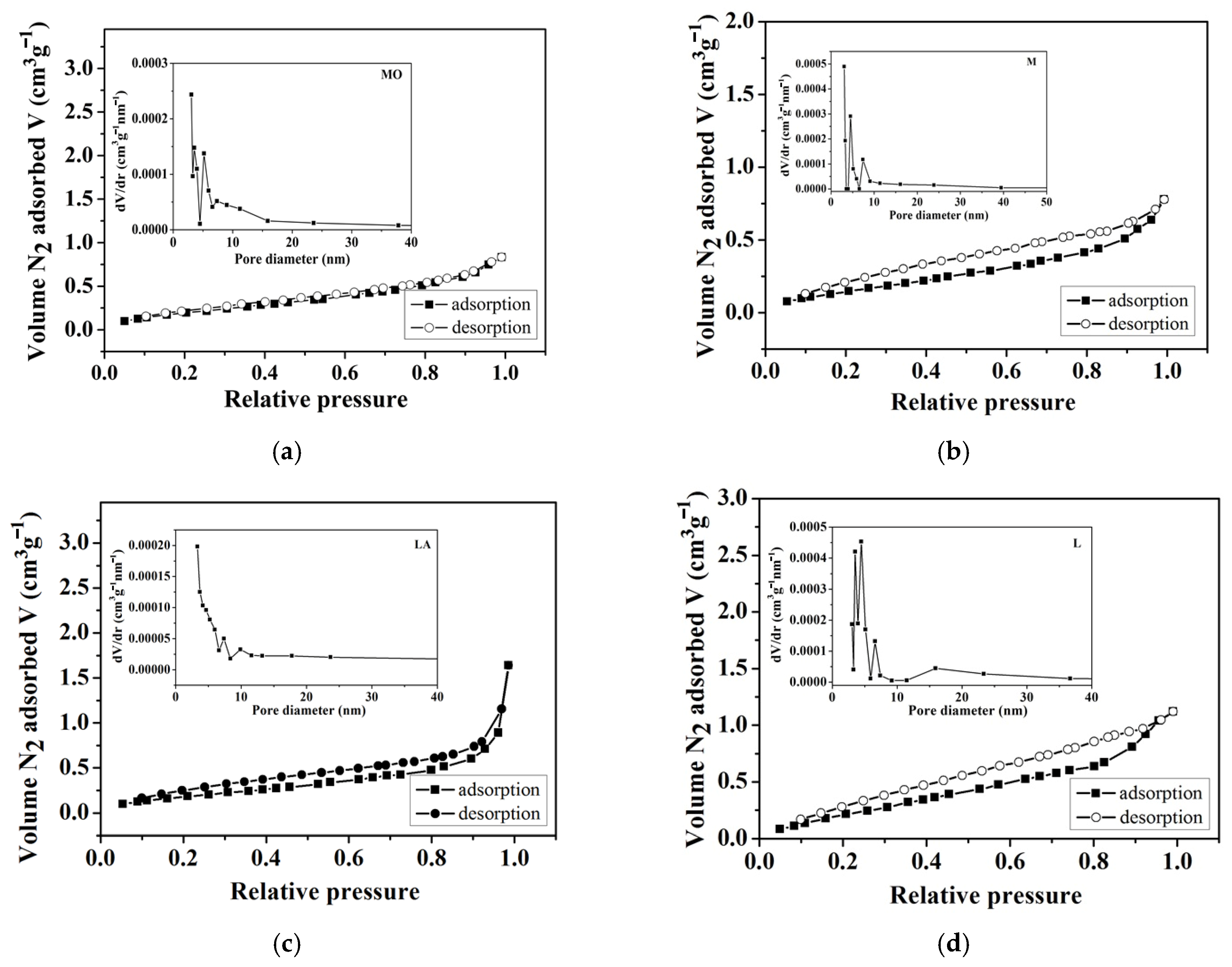
3.1.3. Texture Parameters
3.1.4. SEM Analysis
3.2. Adsorption Studies
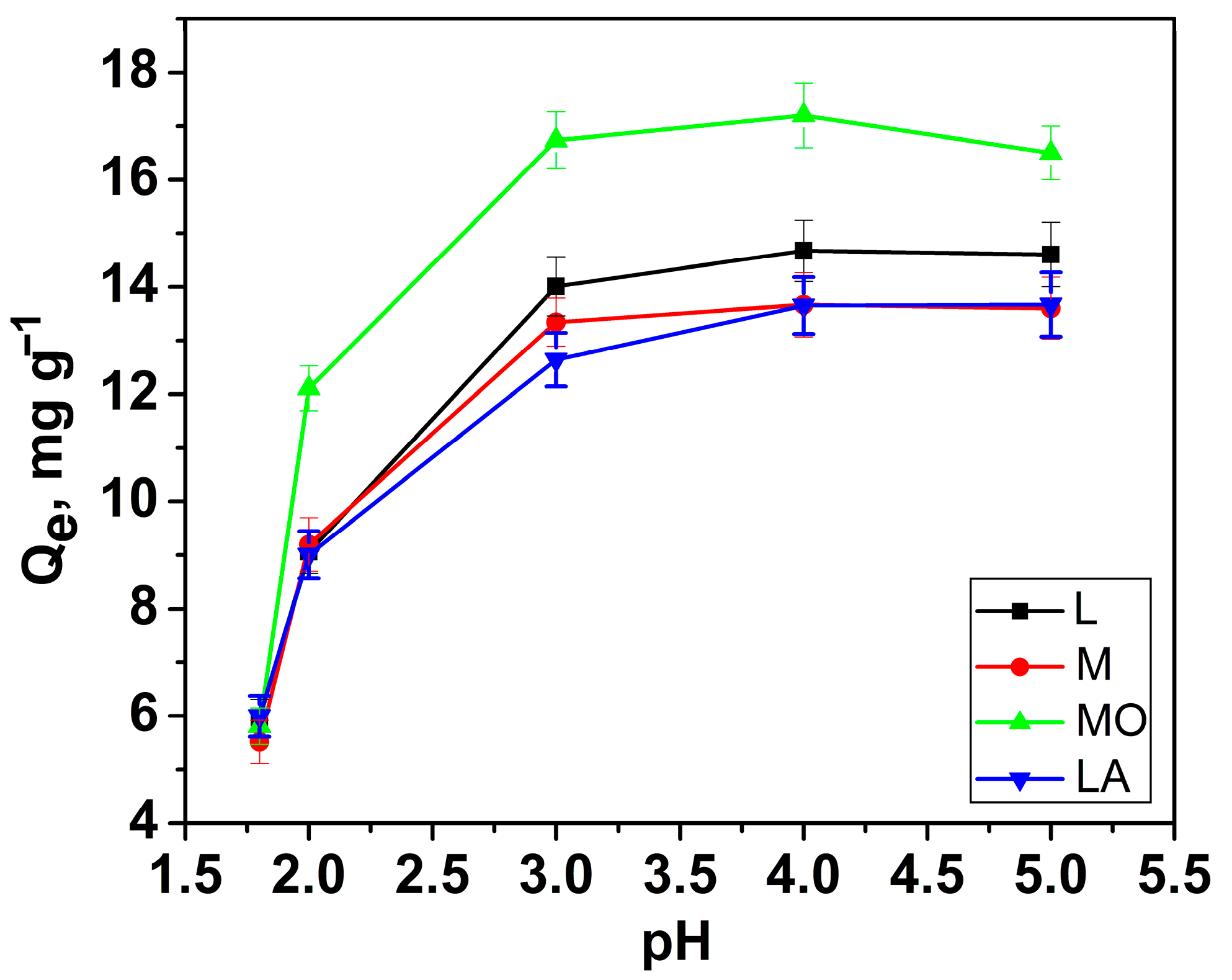
3.2.1. Effect of pH
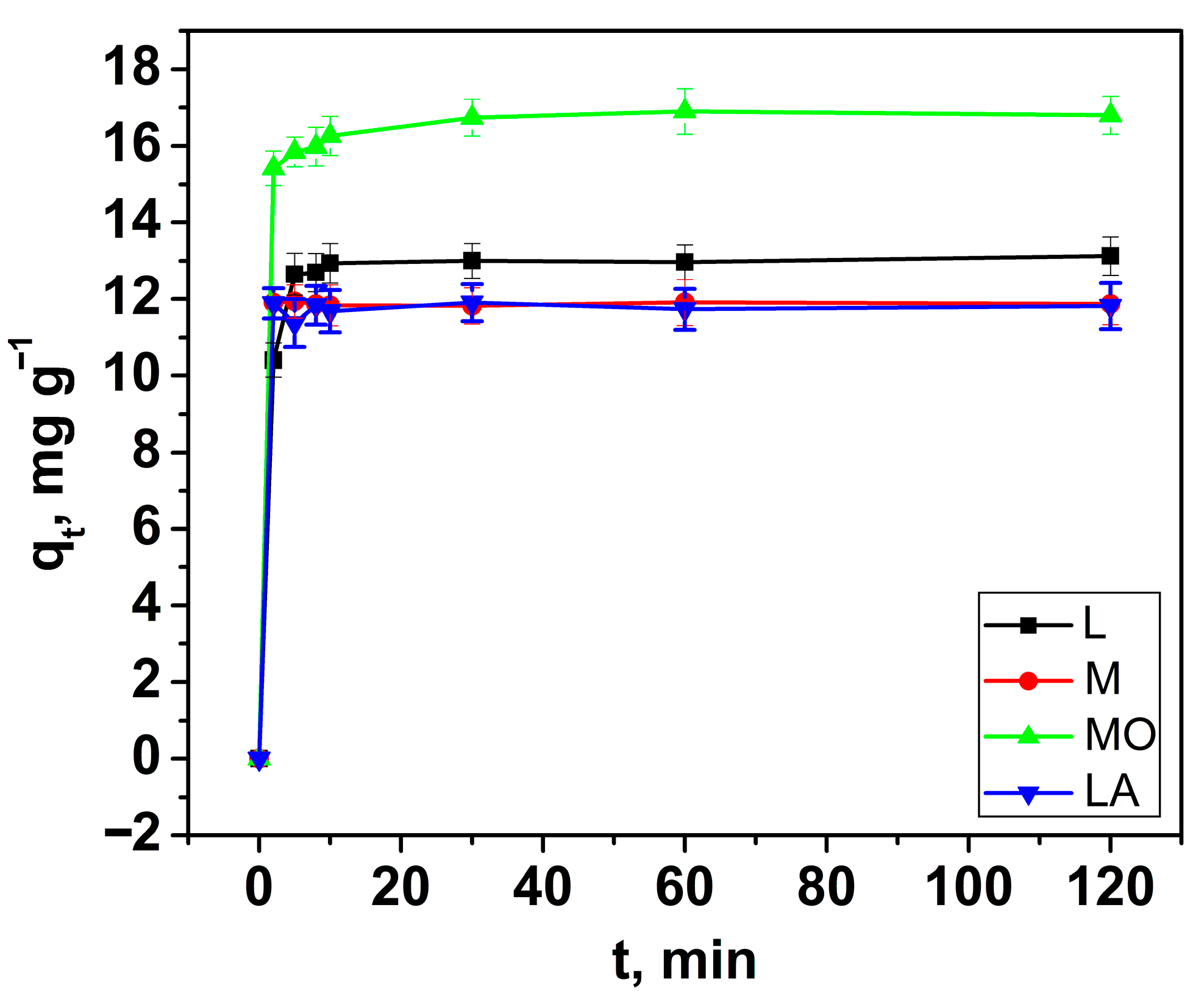
3.2.2. Effect of Contact Time
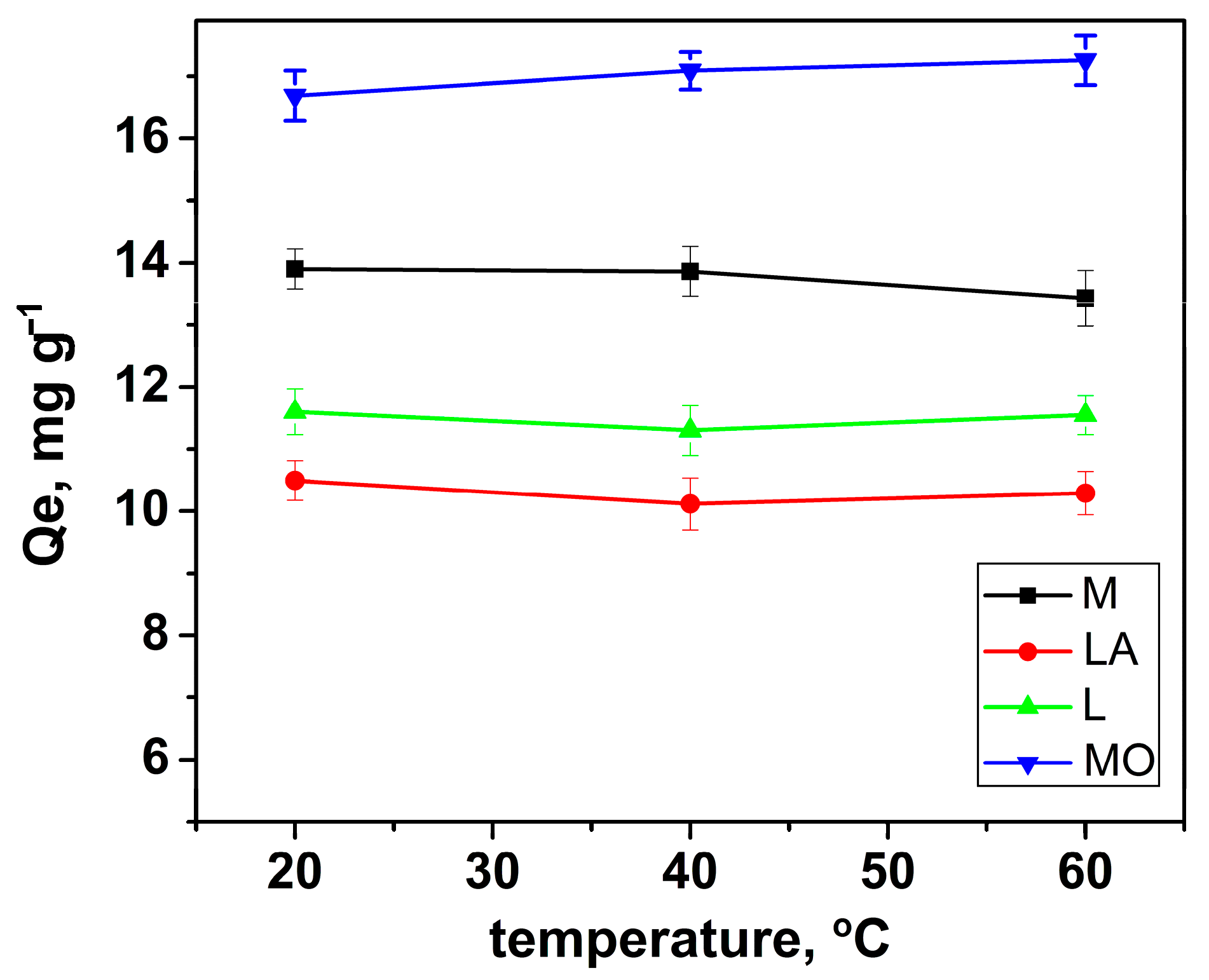
3.2.3. Effect of Temperature and Thermodynamic Studies
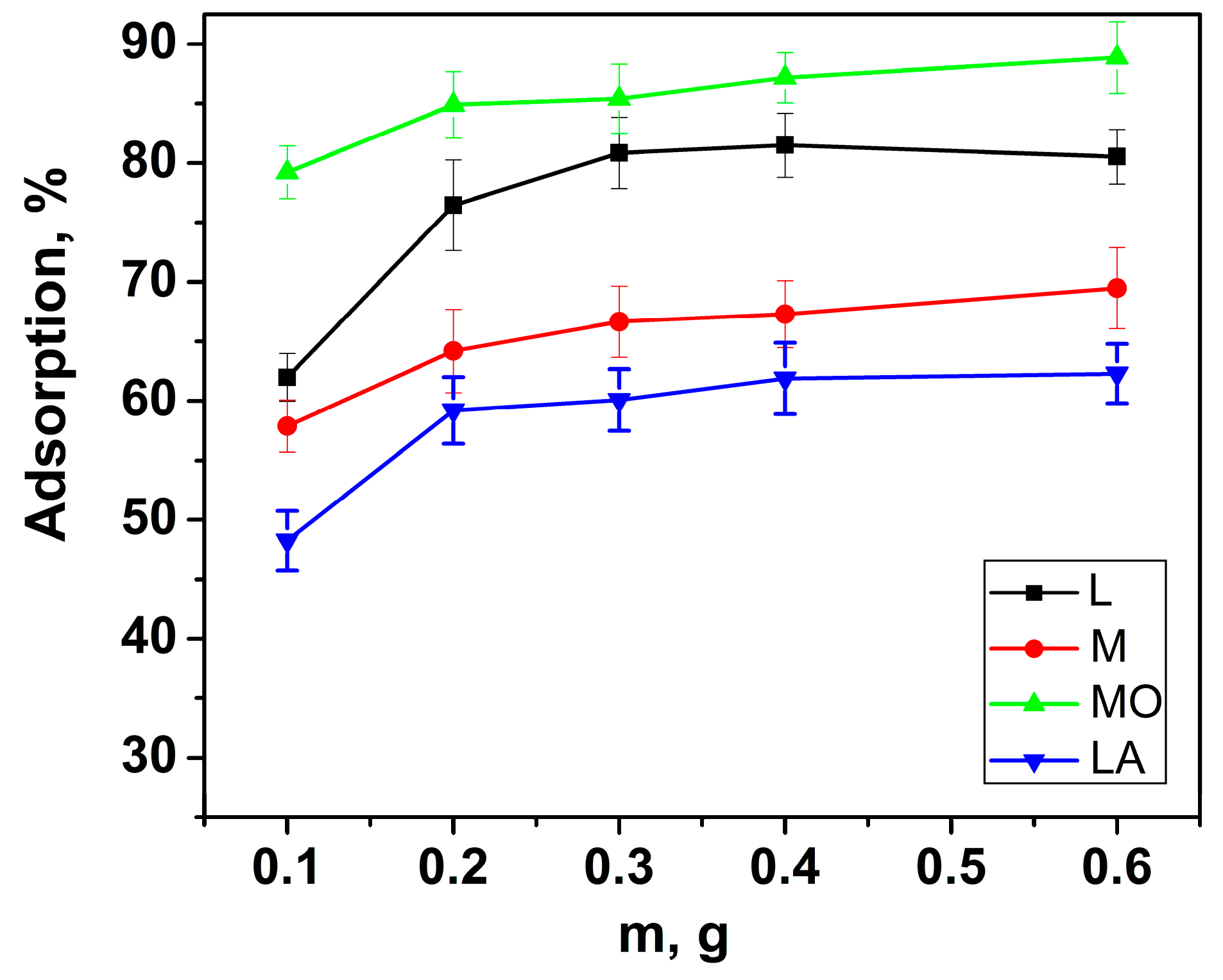
3.2.4. Effect of Sorbent Amount
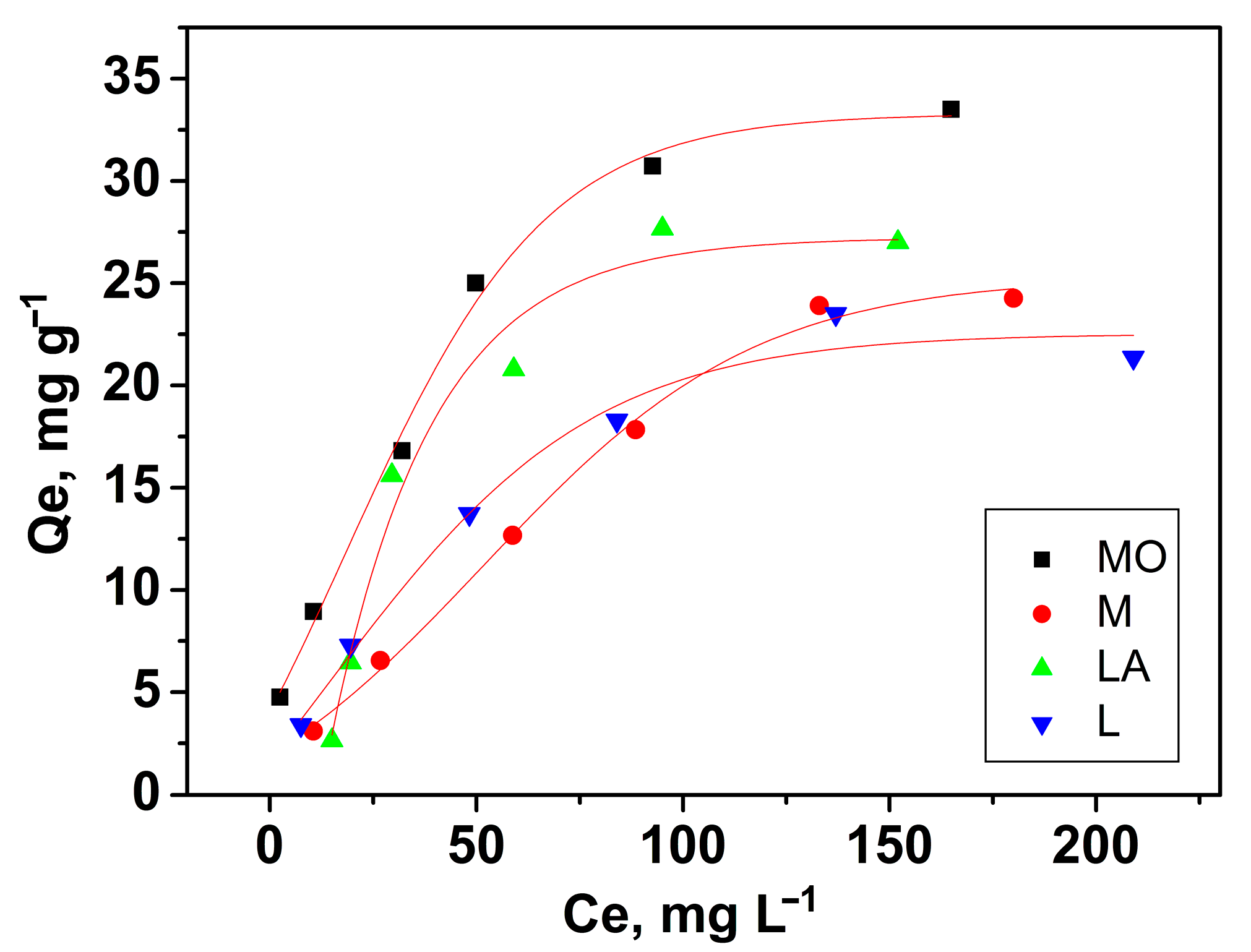
3.2.5. Effect of Initial Concentration
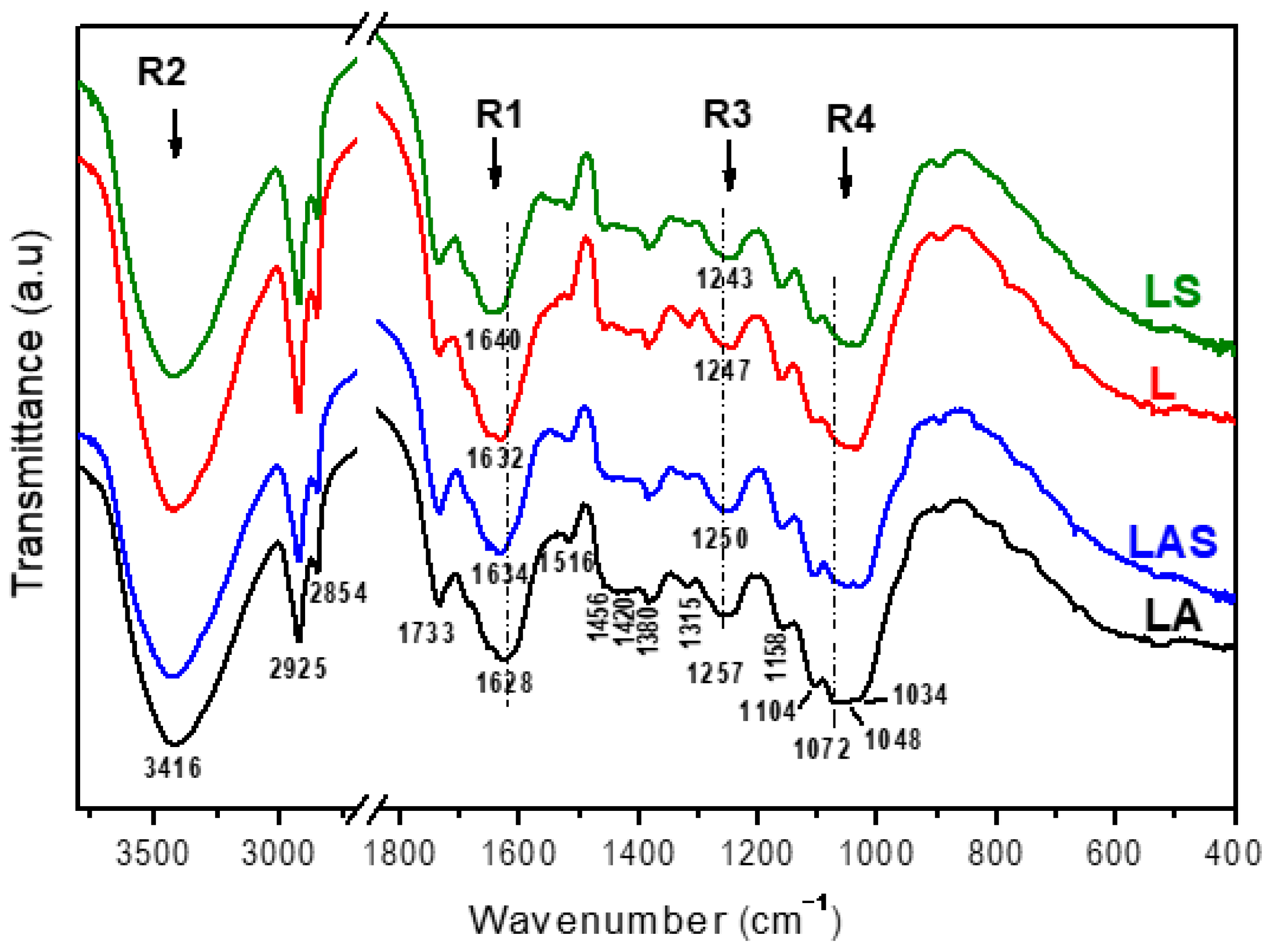
3.2.6. FTIR Analysis
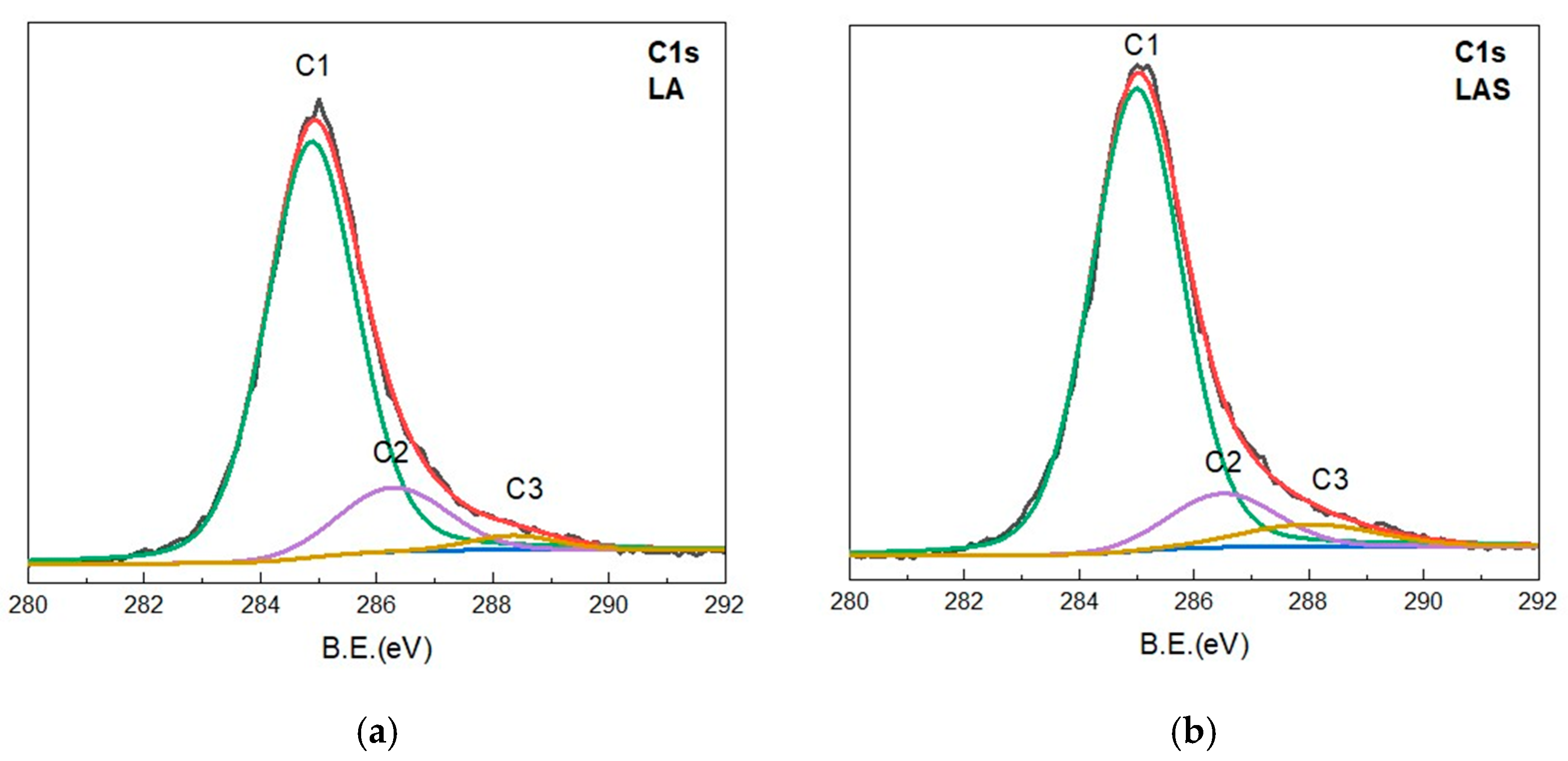
3.2.7. XPS—Analysis
- The major C1 peak, located at approximately ~285 eV, corresponds to the C–C bond.
- The second C2 peak, observed at ~286.5 eV, is associated with the C–OH, C–O–C, or C–N bonds.
- The C3 peak, situated at ~288.0 eV, corresponds to the O–C=O bond (Figure 13 for the samples LA and LAS as representative for all plant materials).
3.2.8. Experiments with Real Contaminated Water
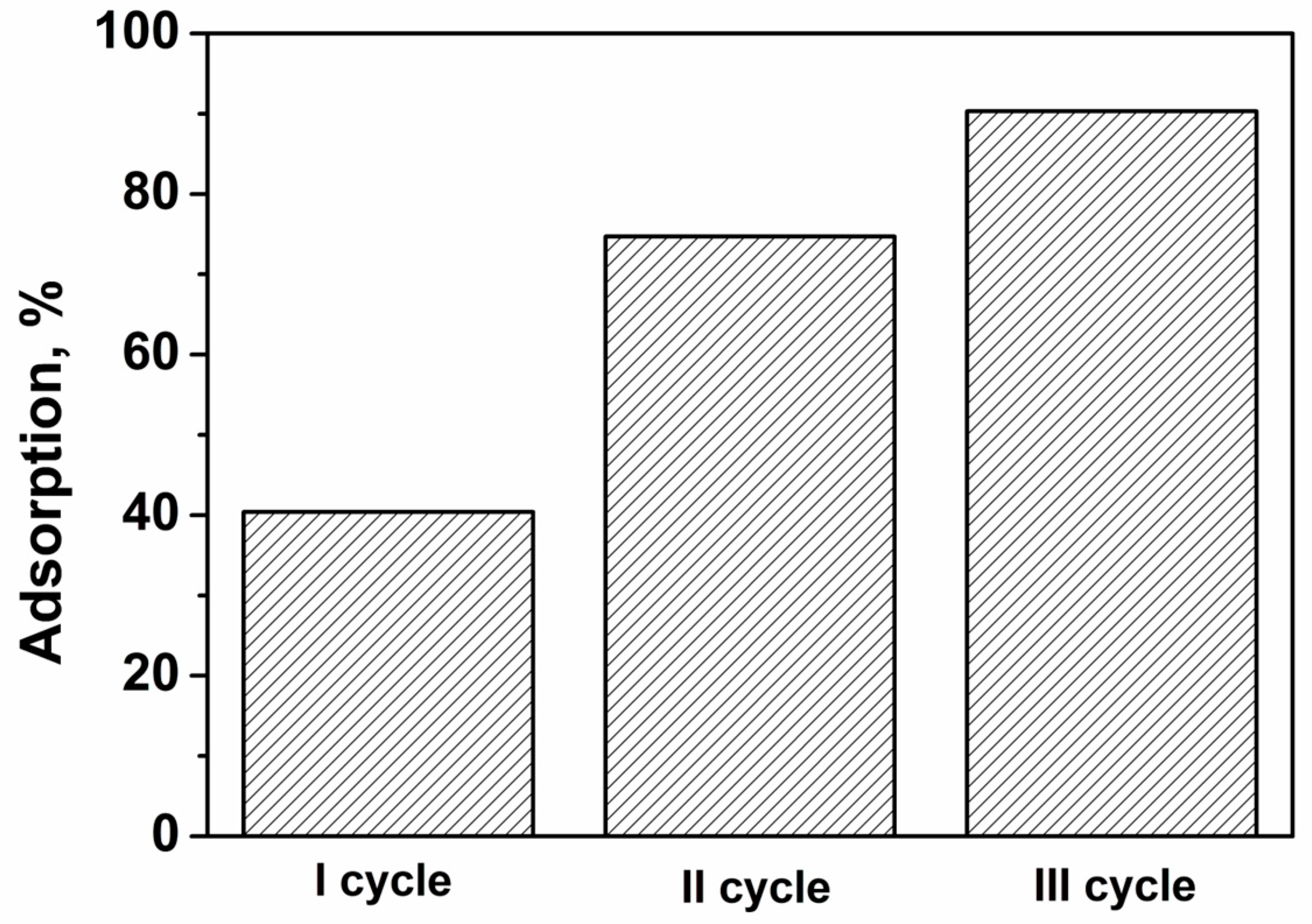
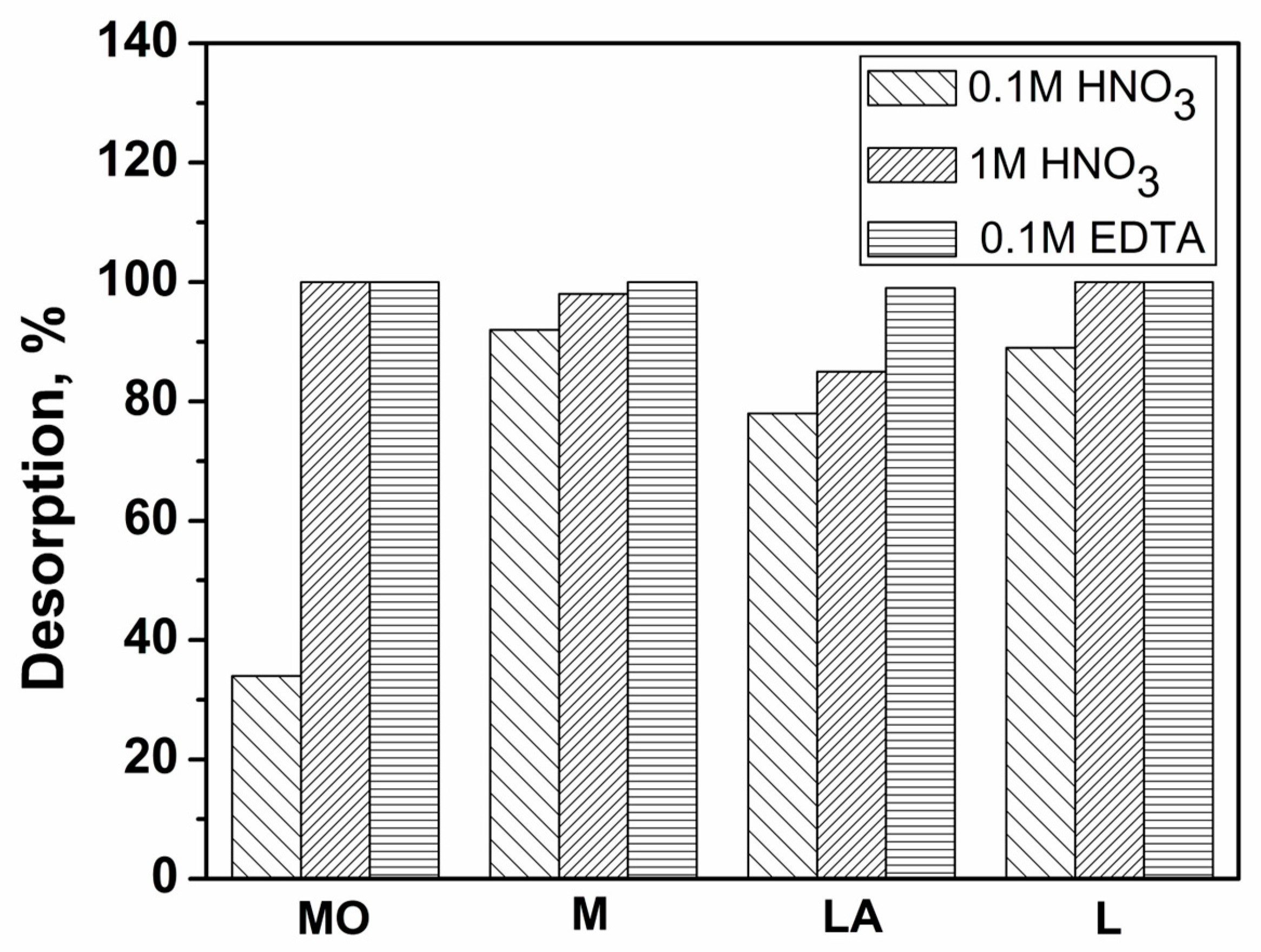
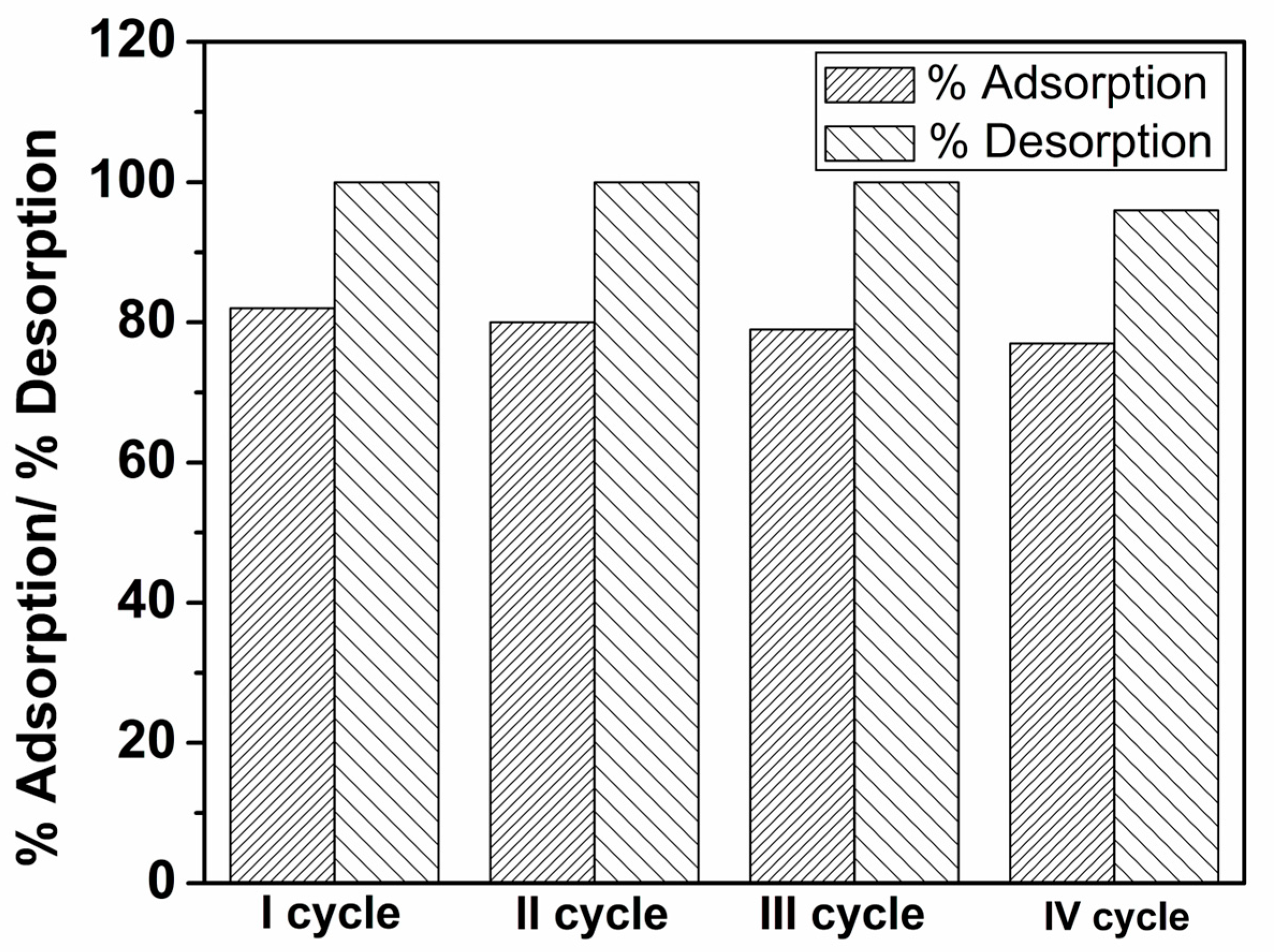
3.2.9. Desorption Experiments and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alizadeh Behbahani, B.; Shahidi, F. Melissa officinalis Essential Oil: Chemical Compositions, Antioxidant Potential, Total Phenolic Content and Antimicrobial Activity. Nutr. Food Sci. Res. 2019, 6, 17–25. [Google Scholar] [CrossRef]
- Lakušic, B.; Lakušic, D.; Ristic, M.; Marčetic, M.; Slavkovska, V. Seasonal Variations in the Composition of the Essential Oils of Lavandula angustifolia (Lamiaceae). Nat. Prod. Commun. 2014, 9, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, M.; Jianu, C.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Şoica, C.; Stana, L.G. Chemical Composition, In Vitro and In Silico Antioxidant Potential of Melissa officinalis subsp. officinalis Essential Oil. Antioxidants 2021, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, Ö.; Fidan, H.; Iliev, I.; Petkova, N.; Dincheva, I.; Gandova, V.; Stankov, S.; Stoyanova, A. Chemical composition, biological activities, and surface tension properties of Melissa officinalis L. essential oil. Turk. J. Agric. For. 2023, 47, 67–78. [Google Scholar] [CrossRef]
- Kıvrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Liquid and Vapour Phase of Lavandin (Lavandula × intermedia) Essential Oil: Chemical Composition and Antimicrobial Activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef]
- Ye, R.; Tian, K.; Hu, H.; Li, P.; Tian, X. Extraction process optimization of essential oil from Mellissa officinalis L. using a new ultrasound-microwave hybrid-assisted Clevenger hydrodistillation. Ind. Crops Prod. 2023, 203, 117165. [Google Scholar] [CrossRef]
- Souihi, M.; Amri, I.; Souissi, A.; Hosni, K.; Ben Brahim, N.; Annabi, M. Essential oil and fatty acid composition of Melissa officinalis L. Prog. Nutr. 2020, 22, 253–258. [Google Scholar] [CrossRef]
- Marcelino, S.; Gaspar, P.D.; Paço, A. Sustainable Waste Management in the Production of Medicinal and Aromatic Plants—A Systematic Review. Sustainability 2023, 15, 13333. [Google Scholar] [CrossRef]
- Arabian, S.; Ziarati, P.; Sawicka, B. Waste Herbal and Black Tea as a Novel Adsorbent for Detoxification of Pharmaceutical Effluent. J. Med. Discov. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Ivanova, L.P.; Detcheva, A.K.; Vassileva, P.S. Characterization of Two Bulgarian Herbs for Use as Biosorbents for Copper(II). Anal. Lett. 2019, 52, 2650–2662. [Google Scholar] [CrossRef]
- Tanasa, A.; Puitel, A.C.; Zaharia, C.; Suteu, D. Sorption of reactive dyes from aqueous media using the lavender waste as biosorbent. Desalin Water Treat. 2021, 236, 348–358. [Google Scholar] [CrossRef]
- Hanif, A.; Bhatti, H.N.; Hanif, M.A. Removal and recovery of Cu(II) and Zn(II) using immobilized Mentha arvensis distillation waste biomass. Ecol. Eng. 2009, 35, 1427–1434. [Google Scholar] [CrossRef]
- Riaz, B.; Ansari, T.M.; Hanif, M.A.; Riaz, S.; Khan, M.A.; Riaz, M.; Jilani, M.I. Utilization of Extensively Available Environmental Waste Mentha spicata for Uptake of Pb(II) from Aqueous Solutions. Asian J. Chem. 2013, 25, 4551–4555. [Google Scholar] [CrossRef]
- Iftikhar, A.R.; Bhatti, H.N.; Hanif, M.A.; Nadeem, R. Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. J. Hazard. Mater. 2009, 161, 941–947. [Google Scholar] [CrossRef]
- Nasir, M.H.; Nadeem, R.; Akhtar, K.; Hanif, M.A.; Khalid, A.M. Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for lead(II) and zinc(II) removal from aqueous solutions. J. Hazard. Mater. 2007, 147, 1006–1014. [Google Scholar] [CrossRef]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of copper speciation and water quality factors that affect aqueous copper tasting response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Aleksandrov, L.I. Structural, thermal, optical and photo-luminescent properties of barium tellurite glasses doped with rare-earth ions. J. Non-Cryst. Solids 2017, 476, 67–74. [Google Scholar] [CrossRef]
- Ivanova, L.P.; Koleva, V.G.; Vassileva, P.S.; Avramova, I.A.; Detcheva, A.K. Study on the adsorption mechanism of copper (II) ions onto Anethum graveolens L. Comptes Rendus Académie Bulg. Sci. 2021, 74, 1305–1313. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose—Structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Hongzhang, C. Biotechnology of Lignocellulose: Theory and Pratice; Springer: London, UK, 2014. [Google Scholar]
- Yuvaraja, G.; Krishnaiah, N.; Subbaiah, M.V.; Krishnaiah, A. Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf. B Biointerfaces 2014, 114, 75–81. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Leon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moawed, E.A.; Eissa, M.S.; Al-Tantawy, S.A. Application of polyurethane foam/zinc oxide nanocomposite for antibacterial activity, detection, and removal of basic dyes from wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 7767–7774. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, H.-J. Persimmon leaf bio-waste for adsorptive removal of heavy metals from aqueous solution. J. Environ. Manag. 2018, 209, 382–392. [Google Scholar] [CrossRef]
- Redha, A.A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Ali, S.; La Nafie, N.; Taba, P. Biosorption of Cu (II) Metal Ions By Dragon Fruit Skin (Hylocereus polyrhizus). J. Akta Kim. Indones. 2018, 11, 21–31. [Google Scholar] [CrossRef]
- Todorciuc, T.; Bulgariu, L.; Popa, V.I. Adsorption of Cu(II) from aqueous solution on wheat straw lignin: Equilibrium and kinetic studies. Cellul. Chem. Technol. 2015, 49, 439–447. [Google Scholar]
- Krstić, V.; Urošević, T.; Pešovski, B. A review on adsorbents for treatment of water and wastewaters containing copper ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Shuhaimen, M.S.; Abdulah, E.N.; Salim, R.M.; Samah, M.A.A.; Omar, M.N.; Ahmad, M.N. Adsorption study on the removal of copper ions from aqueous solution using sodium hydroxide-modified Carica papaya peels. Malays. J. Anal. Sci. 2019, 23, 926–937. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Robalds, A.; Tran, H.N.; Mitrogiannis, D.; Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Dotto, G.L. Removal of heavy metals by leaves-derived biosorbents. Environ. Chem. Lett. 2018, 17, 755–766. [Google Scholar] [CrossRef]
- Chubar, N.; Carvalho, J.R.; Correia, M.J.N. Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II). Colloids Surf. A 2004, 230, 57–65. [Google Scholar] [CrossRef]
- Vassileva, P.S.; Detcheva, A.K.; Ivanova, L.P.; Evtimova, S.K. Biosorption of Cd2+ ions using Mentha spicata L. and Ruta graveolens L. Comptes Rendus Académie Bulg. Sci. 2017, 70, 497–504. [Google Scholar]
- Vassileva, P.; Detcheva, A.; Uzunov, I.; Uzunova, S. Removal of Metal Ions from Aqueous Solutions Using Pyrolyzed Rice Husks: Adsorption Kinetics and Equilibria. Chem. Eng. Commun. 2013, 200, 1578. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.M.; Ismail, K. Adsorptive removal of meth-ylene blue by chemically treated cellulosic waste banana (Musa sapientum) peels. J. Taibah Univ. Sci. 2018, 12, 809–819. [Google Scholar] [CrossRef]
- Vassileva, P.S.; Ivanova, L.P.; Detcheva, A.K. Adsorption studies of a material based on the medicinal plant yarrow for the removal of Cu2+ ions from aqueous solutions. Bulg. Chem. Commun. 2022, 54, 019–024. [Google Scholar]
- Gentscheva, G.; Vassileva, P.; Marinkov, N.; Tzvetkova, C.; Kovacheva, D. Investi-gation of the possibility for removal of hexavalent chromium using manganese ferrite na-noparticles. Comptes Rendus Académie Bulg. Sci. 2020, 73, 1229–1238. [Google Scholar] [CrossRef]
- Zheng, J.-C.; Feng, H.-M.; Lam, M.H.-W.; Lam, P.K.-S.; Ding, Y.-W.; Yu, H.-Q. Re-moval of Cu(II) in aqueous media by biosorption using water hyacinth roots as a bio-sorbent material. J. Hazard. Mater. 2009, 171, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Calvo, M.; Sonia Freire, M.; Gonzalez-Alvarez, J.; Antorrena, G. Chestnut shell as heavy metal adsorbent: Optimization study of lead, copper and zinc cat-ions removal. J. Hazard. Mater. 2009, 172, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, X.; Lu, H. Ni(II) and Cu(II) removal from aqueous solution by a heavy metal-resistance bacterium: Kinetic, isotherm and mechanism studies. Water Sci. Technol. 2017, 76, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Ngu-yen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of plant based biosorbents in the removal of heavy metals: A review. Environ. Process 2016, 3, 495–523. [Google Scholar] [CrossRef]




| Biosorbent | BET Surface Area m2 g−1 | Pore Volume cm3 g−1 | Average Pore Diameter, nm | pH |
|---|---|---|---|---|
| MO | 0.8 | 0.001 | 6 | 5.8 |
| M | 0.6 | 0.001 | 8 | 6.0 |
| LA | 0.8 | 0.003 | 14 | 5.8 |
| L | 1.0 | 0.002 | 6 | 6.3 |
| Biosor- bent | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intraparticle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (min−1) | r2 | Qe (mg g−1) | k2 (g mg−1 min−1) | r2 | kid (mg g−1 min−1/2) | C (mg g−1) | r2 | |
| MO | 1.50 | 0.012 | 0.9353 | 13.55 | 1.720 | 0.9999 | 0.449 0.009 | 14.79 16.73 | 0.9531 0.8213 |
| Biosorbent | ΔH0 (kJ/mol) | ΔS0 (J/mol·K) | ΔG0 293 K (kJ/mol) | ΔG0 313 K (kJ/mol) | ΔG0 333 K (kJ/mol) |
|---|---|---|---|---|---|
| MO | 4.25 | 27.18 | −3.70 | −4.32 | −4.78 |
| LA | −0.96 | −0.55 | −0.85 | −0.68 | −0.84 |
| M | 4.80 | 19.53 | −0.89 | −1.40 | −1.66 |
| L | −4.75 | −5.49 | −3.03 | −3.22 | −2.82 |
| Biosorbent | Langmuir Parameters | Freundlich Parameters | Dubinin–Radushkevich Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 (mg g−1) | K1 (L mg−1) | r2 | χ2 | kF (mg1−nLn g−1) | n (L mg−1) | r2 | χ2 | Qm(o) (mg g−1) | E (kJ mol−1) | r2 | χ2 | |
| MO | 59.95 | 0.031 | 0.9709 | 0.0051 | 1.08 | 1.58 | 0.9525 | 0.3074 | 27.42 | 0.146 | 0.7970 | 0.0362 |
| M | 47.66 | 0.006 | 0.9352 | 0.0063 | 0.54 | 1.31 | 0.9855 | 0.0112 | 17.04 | 0.117 | 0.7139 | 0.1216 |
| LA | 40.52 | 0.016 | 0.8469 | 0.0252 | 0.44 | 1.12 | 0.7344 | 0.0809 | 27.60 | 0.074 | 0.9922 | 0.0052 |
| L | 28.08 | 0.020 | 0.9646 | 0.0074 | 1.21 | 1.71 | 0.9390 | 0.0298 | 16.99 | 0.166 | 0.7613 | 0.0798 |
| Sorbent | C1 C-C, % | C2 C-O-C, C-OH, C-N, % | C3 O-C=O, % | O1 C=O, % | O2 C-O, % |
|---|---|---|---|---|---|
| MO | 87.1 | 10.5 | 2.4 | 34.6 | 65.4 |
| MOS | 84.2 | 10.8 | 5.0 | 62.3 | 37.7 |
| M | 84.3 | 9.9 | 5.8 | 63.3 | 36.7 |
| MS | 81.6 | 12.3 | 6.1 | 46.7 | 53.3 |
| LA | 83.6 | 13.9 | 2.5 | 48.2 | 51.8 |
| LAS | 83.9 | 10.6 | 5.5 | 51.3 | 48.7 |
| L | 87.9 | 8.9 | 3.2 | 61.4 | 38.6 |
| LS | 84.4 | 10.9 | 4.7 | 61.7 | 38.3 |
| Sorbent | C, at.% | O, at.% | N, at.% | Cu, at.% |
|---|---|---|---|---|
| MO | 79.21 | 17.93 | 2.86 | - |
| MOS | 83.24 | 14.59 | 1.66 | 0.51 |
| M | 79.46 | 18.35 | 2.20 | - |
| MS | 78.40 | 19.35 | 1.76 | 0.49 |
| LA | 84.71 | 13.14 | 2.15 | - |
| LAS | 83.80 | 14.47 | 1.42 | 0.31 |
| L | 86.72 | 11.73 | 1.54 | - |
| LS | 81.35 | 15.70 | 2.46 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, L.; Vassileva, P.; Detcheva, A.; Koleva, V.; Avramova, I. Plant-Based Biosorbents for Copper(II) Removal: A Comparative Study of Biomass and Essential Oil Residues. Appl. Sci. 2025, 15, 7695. https://doi.org/10.3390/app15147695
Ivanova L, Vassileva P, Detcheva A, Koleva V, Avramova I. Plant-Based Biosorbents for Copper(II) Removal: A Comparative Study of Biomass and Essential Oil Residues. Applied Sciences. 2025; 15(14):7695. https://doi.org/10.3390/app15147695
Chicago/Turabian StyleIvanova, Lidia, Paunka Vassileva, Albena Detcheva, Violeta Koleva, and Ivalina Avramova. 2025. "Plant-Based Biosorbents for Copper(II) Removal: A Comparative Study of Biomass and Essential Oil Residues" Applied Sciences 15, no. 14: 7695. https://doi.org/10.3390/app15147695
APA StyleIvanova, L., Vassileva, P., Detcheva, A., Koleva, V., & Avramova, I. (2025). Plant-Based Biosorbents for Copper(II) Removal: A Comparative Study of Biomass and Essential Oil Residues. Applied Sciences, 15(14), 7695. https://doi.org/10.3390/app15147695






