Bio-Agronomic Assessment and Quality Evaluation of Sugarcane with Optimized Juice Fermentation in View of Producing Sicilian “Rum Agricole”
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Cultivation Practices
2.2. Meteorological Data
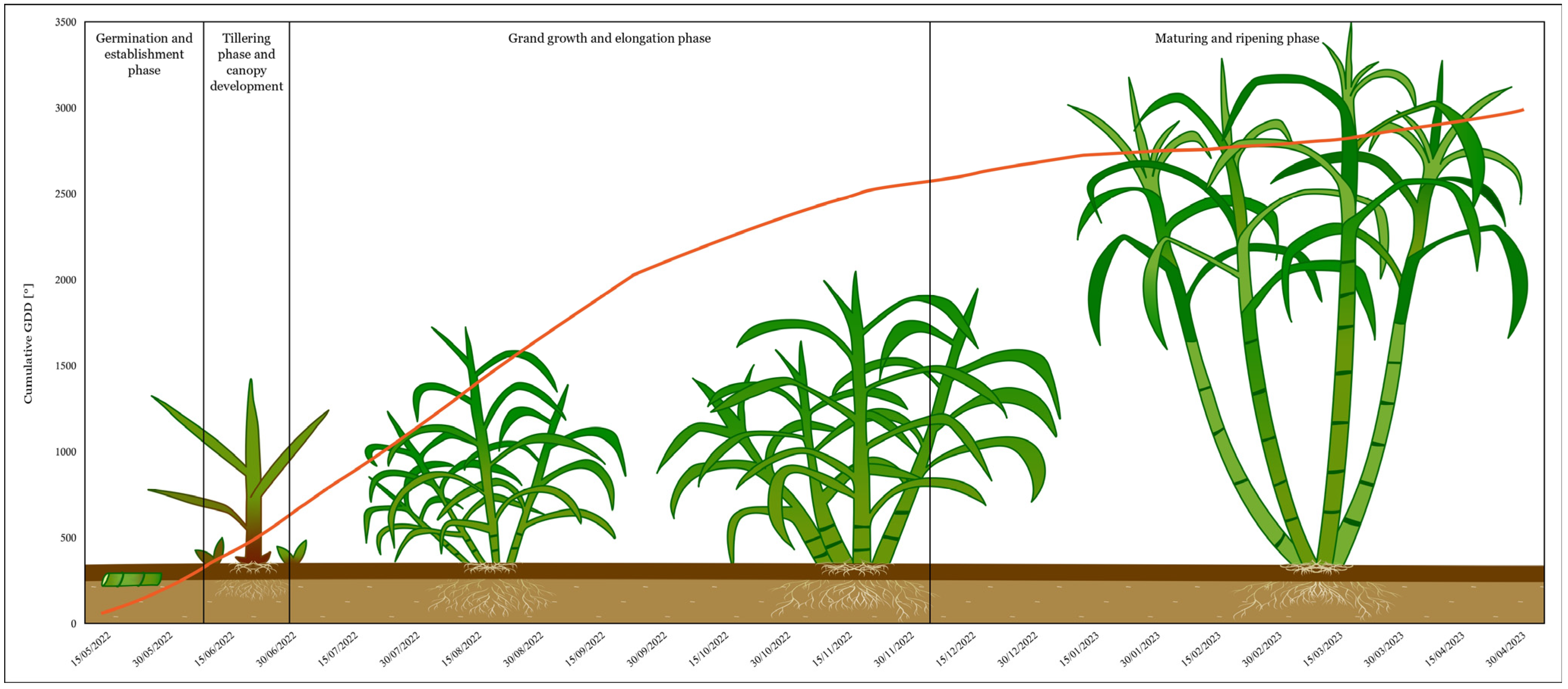
2.3. Growing Degree Days
2.4. Characteristics of Sugarcane Juice and Production Process
2.5. Yeast Strains and Media
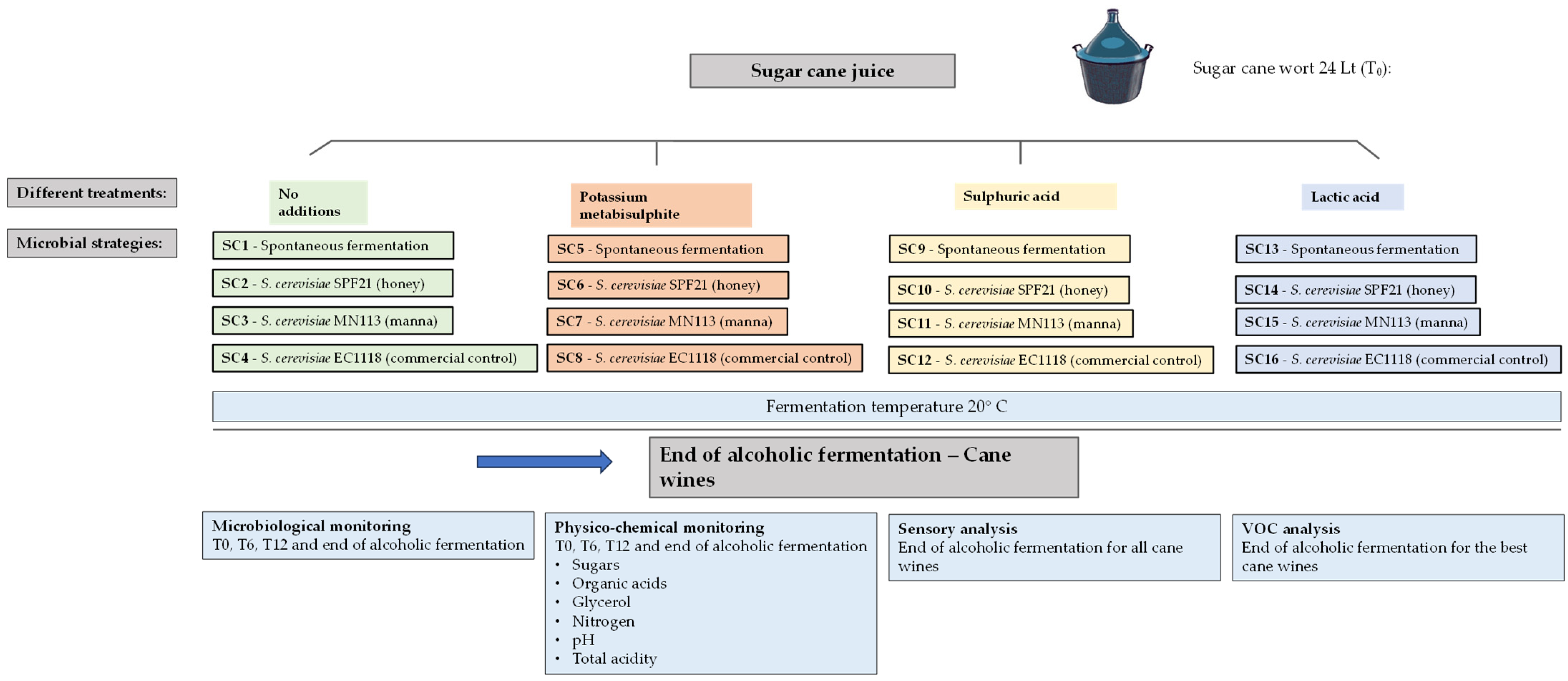
2.6. Experimental Plan and Sample Collection for Sugarcane Fermentation
2.7. Monitoring Sugarcane Juice Fermentation
2.8. Yeast Isolation, Molecular Identification, and Strain Typing
2.9. Determination of Physicochemical Parameters
2.10. Sensory Analysis
2.11. Analysis of VOCs of Fermented Sugarcane Samples
2.12. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Rainfall and Air Temperature Trends at the Experimental Site
3.2. Plant Growth Phases and Cumulative Growing Degree Days
3.3. Field Evaluation of Sugarcane Genotypes
3.4. Juice Quality and Yield
3.5. Wort Fermentation of Sugarcane Samples
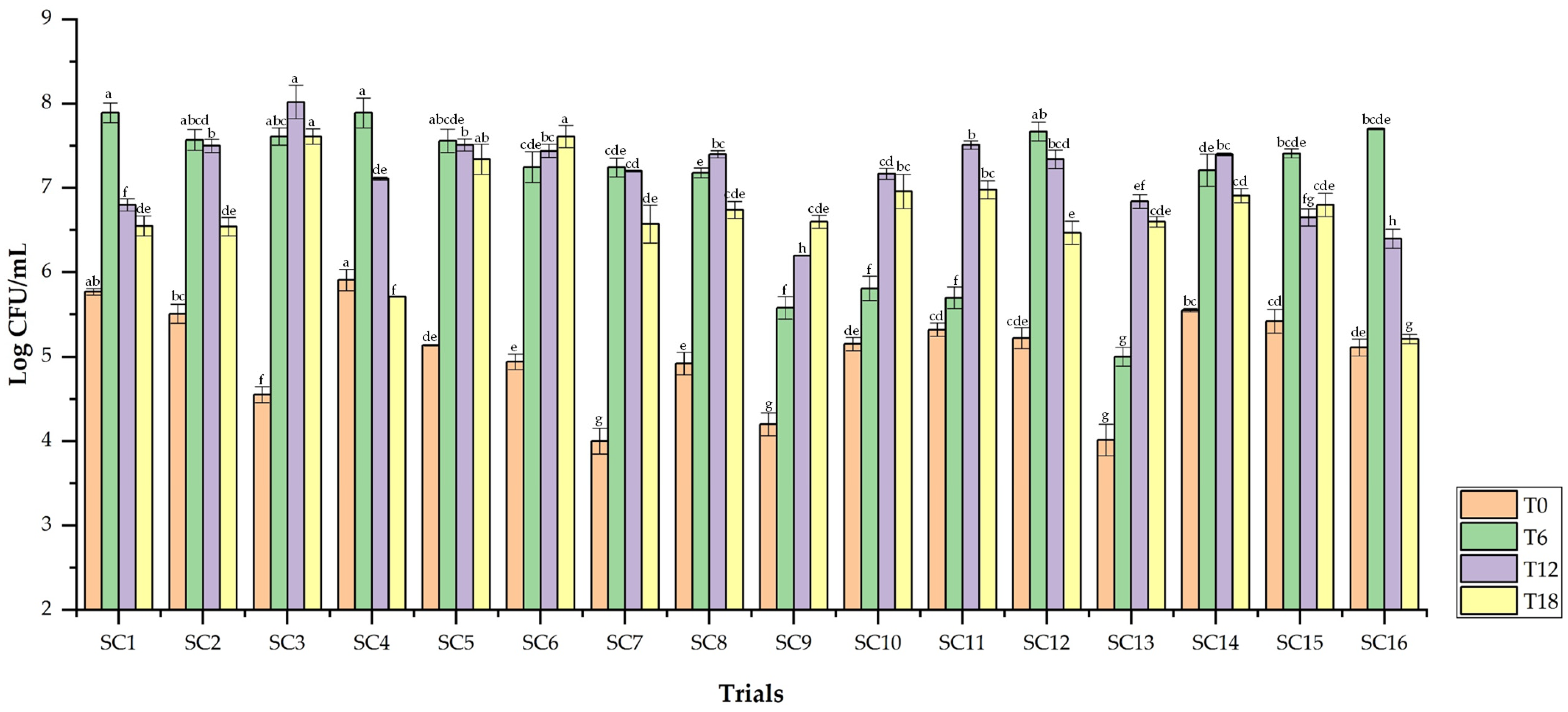
3.5.1. Yeasts
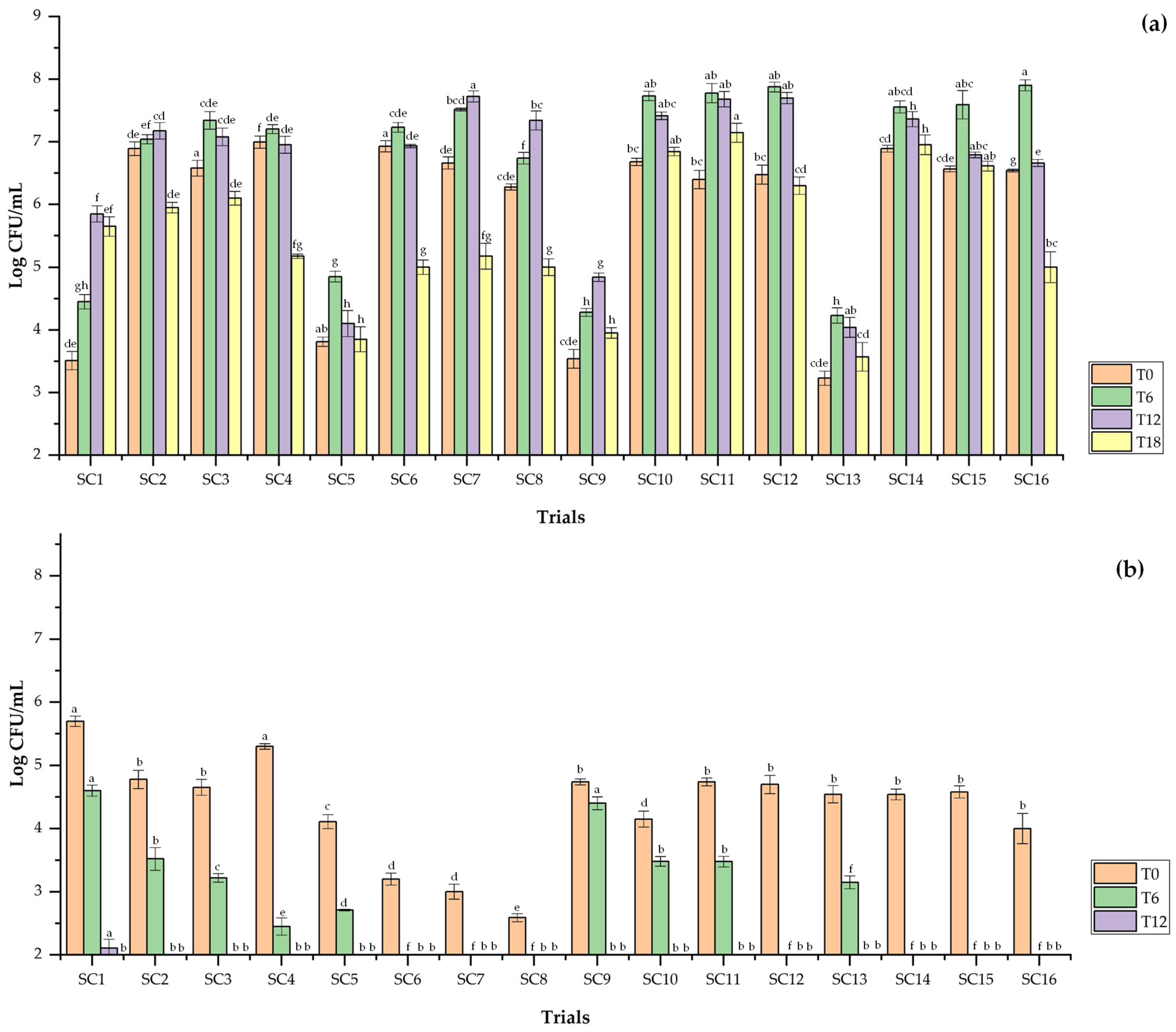
3.5.2. Lactic Acid Bacteria
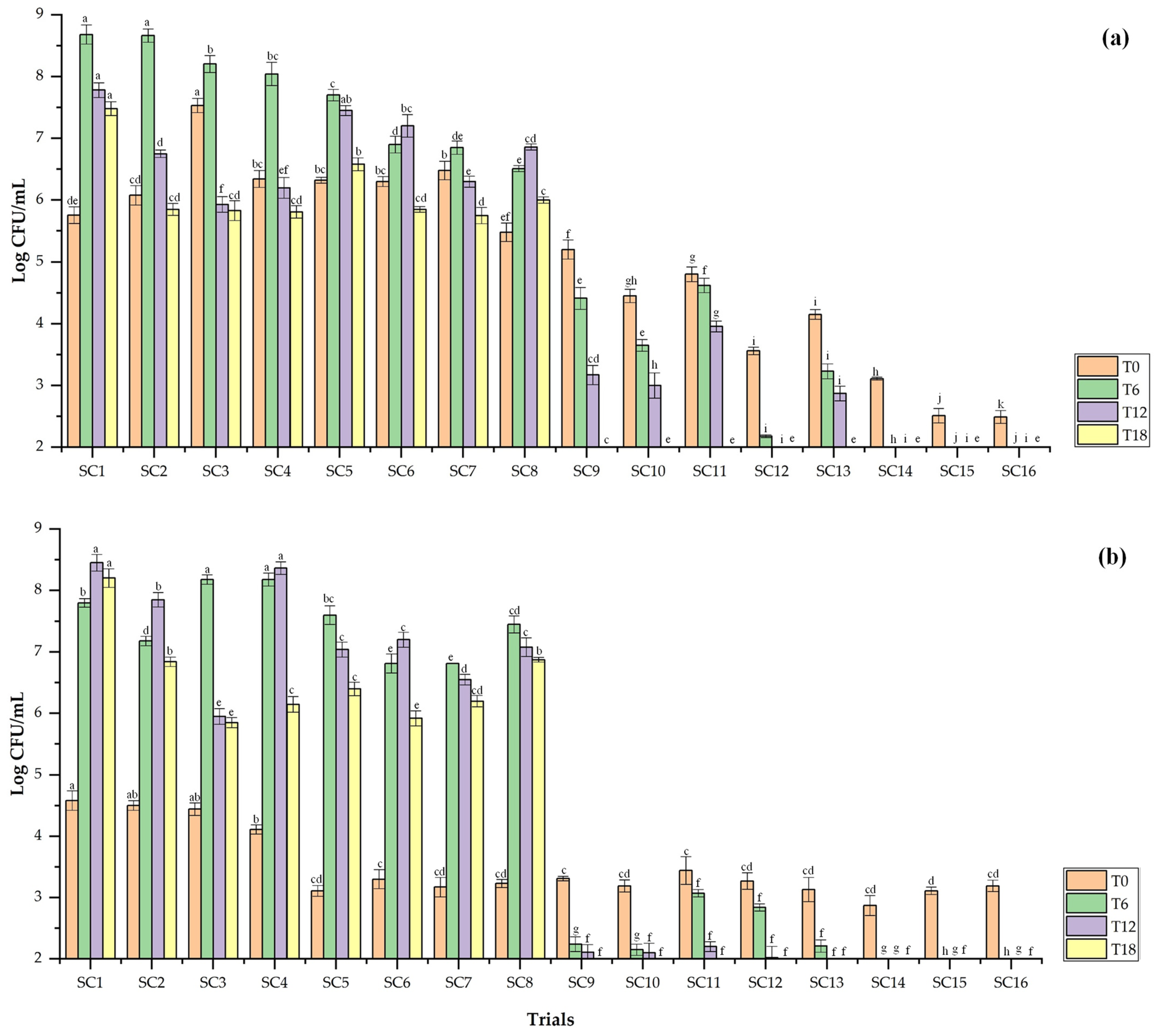

3.6. Dominance of Inoculated Yeasts
3.7. Physicochemical Parameters
3.8. Sensory Evaluation of Aroma
3.9. Volatile Organic Compounds of Cane Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 9 September 2024).
- Moraes, M.A.F.D.; Oliveira, F.C.R.; Diaz-Chavez, R.A. Socio-economic impacts of Brazilian sugarcane industry. Environ. Dev. 2015, 16, 31–43. [Google Scholar] [CrossRef]
- Li, Y.R.; Yang, L.T. Sugarcane agriculture and sugar industry in China. Sugar. Tech. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.R. Climate change and sugarcane production: Potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 547386. [Google Scholar] [CrossRef]
- Teruel, D.A.; Barbieri, V.; Ferraro, L.A., Jr. Sugarcane leaf area index modeling under different soil water conditions. Sci. Agric. 1997, 54, 39–44. [Google Scholar] [CrossRef]
- Cardozo, N.P.; Sentelhas, P.C. Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Sci. Agric. 2013, 70, 449–456. [Google Scholar] [CrossRef]
- Som-Ard, J.; Atzberger, C.; Izquierdo-Verdiguier, E.; Vuolo, F.; Immitzer, M. Remote sensing applications in sugarcane cultivation: A review. Remote Sens. 2021, 13, 4040. [Google Scholar] [CrossRef]
- Corbion, C.; Smith-Ravin, J.; Marcelin, O.; Bouajila, J. An overview of spirits made from sugarcane juice. Molecules 2023, 28, 6810. [Google Scholar] [CrossRef]
- Stewart, G.G. A short history of rum. In Whisky and Other Spirits, 3rd ed.; Stewart, G., Kellersshohn, J., Russel, I., Eds.; Academic Press: London, UK, 2022; pp. 457–462. [Google Scholar]
- Franitza, L.; Nicolotti, L.; Granvogl, M.; Schieberle, P. Differentiation of rums produced from sugar cane juice (Rhum Agricole) from rums manufactured from sugar cane molasses by a metabolomics approach. J. Agric. Food. Chem. 2018, 66, 3038–3045. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0787 (accessed on 29 January 2025).
- Fahrasmane, L.; Ganou-Parfait, B. De la Canne au Rhum; Inra-Quae: Paris, France, 1997. [Google Scholar]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Portugal, C.B.; Alcarde, A.R.; Bortoletto, A.M.; de Silva, A.P. The role of spontaneous fermentation for the production of cachaça: A study of case. Eur. Food Res. Technol. 2016, 242, 1587–1597. [Google Scholar] [CrossRef]
- Fahrasmane, L.; Ganou-Parfait, B. Microbial flora of rum fermentation media. J. Appl. Microbiol. 1998, 84, 921–928. [Google Scholar]
- Oliveira, V.A.; Vicente, M.A.; Fietto, L.G.; de Miranda Castro, I.; Coutrim, M.X.; Schüller, D.; Alves, H.; Casal, M.; Santos, J.O.; Araújo, L.D.; et al. Biochemical and molecular characterization of Saccharomyces cerevisiae strains obtained from sugar-cane juice fermentations and their impact in cachaca production. Appl. Environ. Microbiol. 2008, 74, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.H.; Yoon, K.S.; Shankar, S.; Rhim, J.W. Comparative antibacterial and antifungal activities of sulfur nanoparticles capped with chitosan. Microb. Pathogen. 2020, 144, 104178. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial activity of volatile sulfur compounds in foods. In Volatile Sulfur Compounds in Food; Quian, M.C., Fan, X., Mahattanatawee, K., Eds.; American Chemical Society: Washington, WA, USA, 2011; pp. 323–338. [Google Scholar]
- Gaglio, R.; Alfonzo, A.; Francesca, N.; Corona, O.; Di Gerlando, R.; Columba, P.; Moschetti, G. Production of the Sicilian distillate “Spiritu re fascitrari” from honey by-products: An interesting source of yeast diversity. Int. J. Food Microbiol. 2017, 261, 62–72. [Google Scholar] [CrossRef]
- Guarcello, R.; Gaglio, R.; Todaro, A.; Alfonzo, A.; Schicchi, R.; Cirlincione, F.; Moschetti, G.; Francesca, N. Insights into the cultivable microbial ecology of “Manna” ash products extracted from Fraxinus angustifolia (Oleaceae) trees in Sicily, Italy. Front. Microbiol. 2019, 10, 984. [Google Scholar] [CrossRef]
- Francesca, N.; Pirrone, A.; Gugino, I.; Prestianni, R.; Naselli, V.; Settanni, L.; Todaro, A.; Guzzon, R.; Maggio, A.; Porrello, A.; et al. A novel microbiological approach to impact the aromatic composition of sour loquat beer. Food Biosci. 2023, 55, 103011. [Google Scholar] [CrossRef]
- Bonnett, G.D. Developmental stages (phenology). In Sugarcane: Physiology, Biochemistry, and Functional Biology; Moore, P.H., Botha, F.C., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2013; pp. 35–53. [Google Scholar] [CrossRef]
- Chen, J.C.; Chou, C.C. Cane Sugar Handbook: A Manual for Cane Sugar Manufacturers and Their Chemists; John Wiley & Sons: Toronto, ON, Canada, 1993. [Google Scholar]
- Pirrone, A.; Prestianni, R.; Naselli, V.; Todaro, A.; Farina, V.; Tinebra, I.; Guzzon, R.; Badalamenti, N.; Maggio, A.; Gaglio, R.; et al. Influence of indigenous Hanseniaspora uvarum and Saccharomyces cerevisiae from sugar-rich substrates on the aromatic composition of loquat beer. Int. J. Food Microbiol. 2022, 379, 109868. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Sorrentino, E.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Letizia, F.; Albanese, G.; Karaulli, J.; Ruci, M.; Pistillo, M.; Germinara, G.S.; Messia, M.C.; Succi, M.; et al. Versatility of Saccharomyces cerevisiae 41CM in the Brewery Sector: Use as a Starter for “Ale” and “Lager” Craft Beer Production. Processes 2022, 10, 2495. [Google Scholar] [CrossRef]
- Iris, L.; Antonio, M.; Antonia, B.M.; Antonio, S.L.J. Isolation, selection, and identification techniques for non-Saccharomyces yeasts of oenological interest. In Biotechnological Progress and Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Kidlington, UK, 2020; pp. 467–508. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Francesca, N.; Sannino, C.; Settanni, L.; Corona, O.; Barone, E.; Moschetti, G. Microbiological and chemical monitoring of Marsala base wine obtained by spontaneous fermentation during large-scale production. Ann. Microbiol. 2014, 64, 1643–1657. [Google Scholar] [CrossRef]
- Cavazza, A.; Grando, M.S.; Zini, C. Rilevazione della flora microbica di mosti e vini. Vignevini 1992, 9, 17–20. [Google Scholar]
- Francesca, N.; Gaglio, R.; Matraxia, M.; Naselli, V.; Prestianni, R.; Settanni, L.; Badalamenti, N.; Columba, P.; Bruno, M.; Maggio, A.; et al. Technological screening and application of Saccharomyces cerevisiae strains isolated from fermented honey by-products for the sensory improvement of Spiritu re fascitrari, a typical Sicilian distilled beverage. Food Microbiol. 2022, 104, 103968. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Alfonzo, A.; Prestianni, R.; Gaglio, R.; Matraxia, M.; Maggio, A.; Naselli, V.; Craparo, V.; Badalamenti, N.; Bruno, M.; Vagnoli, P.; et al. Effects of different yeast strains, nutrients and glutathione-rich inactivated yeast addition on the aroma characteristics of Catarratto wines. Int. J. Food Microbiol. 2021, 360, 109325. [Google Scholar] [CrossRef]
- Compendium of International Methods of Analysis-OIV Total Acidity-Method OIV-MA-AS313-01. Available online: https://www.oiv.int/public/medias/3731/oiv-ma-as313-01.pdf (accessed on 19 May 2024).
- Matraxia, M.; Alfonzo, A.; Prestianni, R.; Francesca, N.; Gaglio, R.; Todaro, A.; Alfeo, V.; Perretti, G.; Columba, P.; Settanni, L.; et al. Non-conventional yeasts from fermented honey by-products: Focus on Hanseniaspora uvarum strains for craft beer production. Food Microbiol. 2021, 99, 103806. [Google Scholar] [CrossRef]
- Chawafambira, A. The effect of incorporating herbal (Lippia javanica) infusion on the phenolic, physicochemical, and sensorial properties of fruit wine. Food Sci. Nutr. 2021, 9, 4539–4549. [Google Scholar] [CrossRef]
- Freitas Schwan, R.; Mendonça, A.T.; da Silva, J.J.; Rodrigues, V.; Wheals, A.E. Microbiology and physiology of cachaça (aguardente) fermentations. Antonie Van Leeuwenhoek 2001, 79, 89–96. [Google Scholar] [CrossRef]
- Catania, P.; Vallone, M.; Farid, A.; De Pasquale, C. Effect of O2 control and monitoring on the nutraceutical properties of extra virgin olive oils. J. Food Eng. 2016, 169, 179–188. [Google Scholar] [CrossRef]
- dos Santos Luciano, A.C.; Picoli, M.C.A.; Rocha, J.V.; Franco, H.C.J.; Sanches, G.M.; Leal, M.R.L.V.; Le Maire, G. Generalized space-time classifiers for monitoring sugarcane areas in Brazil. Remote Sens. Environ. 2018, 215, 438–451. [Google Scholar] [CrossRef]
- Lofton, J.; Tubana, B.S.; Kanke, Y.; Teboh, J.; Viator, H.; Dalen, M. Estimating sugarcane yield potential using an in-season determination of normalized difference vegetative index. Sensors 2012, 12, 7529–7547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, J.; Wang, J.; Zhang, K.; Kuang, Z.; Zhong, S.; Song, X. Object-oriented classification of sugarcane using time-series middle-resolution remote sensing data based on AdaBoost. PLoS ONE 2015, 10, e0142069. [Google Scholar] [CrossRef]
- Baez-Gonzalez, A.D.; Kiniry, J.R.; Meki, M.N.; Williams, J.; Alvarez-Cilva, M.; Ramos-Gonzalez, J.L.; Magallanes-Estala, A.; Zapata-Buenfil, G. Crop parameters for modeling sugarcane under rainfed conditions in Mexico. Sustainability 2017, 9, 1337. [Google Scholar] [CrossRef]
- Cuadra, S.V.; Costa, M.H.; Kucharik, C.J.; Da Rocha, H.R.; Tatsch, J.D.; Inman-Bamber, G.; Da Rocha, R.; Leite, C.; Cabral, O.M.R. A biophysical model of sugarcane growth. Glob. Change Biol. Bioenergy 2012, 4, 36–48. [Google Scholar] [CrossRef]
- Scarpari, M.S.; Beauclair, E.G.F.D. Sugarcane maturity estimation through edaphic-climatic parameters. Sci. Agric. 2004, 61, 486–491. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Soares, R.A.B.; Landell, M.G.D.A.; Campana, M.P. Agronomic performance of sugarcane families in response to water stress. Bragantia 2008, 67, 655–661. [Google Scholar] [CrossRef][Green Version]
- Singh, M.P.; Comstock, J.C.; Davidson, W.; Gordon, V.; Sandhu, H.S.; McCord, P.; Zhao, D.; Sood, S.; Baltazar, M.; McCorkle, K. Registration of ‘CP 06-2425’,‘CP 06-2495’,‘CP 06-2964’,‘CP 06-3103’, and ‘CP 07-1313’Sugarcane for Sand Soils in Florida. J. Plant Regist. 2017, 11, 143–151. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Gilbert, R.A.; Perdomo, R.E.; Shine, J.M., Jr.; Powell, G.; Montes, G. Sugarcane leaf area development under field conditions in Florida, USA. Field Crops Res. 2004, 88, 171–178. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Qiang, H.; Zhou, B.; Xu, G.; Wang, Z. Sugarcane nodes identification algorithm based on sum of local pixel of minimum points of vertical projection function. Comput. Electron. Agric. 2021, 182, 105994. [Google Scholar] [CrossRef]
- Batista, L.M.T.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Bufon, V.B.; Sousa, R.Z.; Vinson, C.C.; Deuner, S. Effect of irrigation on sugarcane morphophysiology in the Brazilian cerrado. Plants 2024, 13, 937. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.W.; Gordon, V.S.; Sandhu, H.S.; McCord, P.; Zhao, D.; Comstock, J.C.; Zhao, D.; Comstock, J.C.; Singh, M.P.; Sood, S.; et al. Registration of ‘CP 09-1952’sugarcane. J. Plant Regist. 2018, 12, 340–346. [Google Scholar] [CrossRef]
- Sood, N.; Gupta, P.K.; Srivastava, R.K.; Gosal, S.S. Comparative studies on field performance of micropropagated and conventionally propagated sugarcane plants. Plant Tissue Cult. Biotechnol. 2006, 16, 25–29. [Google Scholar] [CrossRef]
- Ghosh, A.M.; Balakrishnan, M. Pilot demonstration of sugarcane juice ultrafiltration in an Indian sugar factory. J. Food Eng. 2003, 58, 143–150. [Google Scholar] [CrossRef]
- Bomdespacho, L.D.Q.; Silva, B.T.R.D.; Lapa-Guimaraes, J.; Ditchfield, C.; Petrus, R.R. Cultivar affects the color change kinetics of sugarcane juice. Food Sci. Technol. 2018, 38, 96–102. [Google Scholar] [CrossRef]
- Pimenta, F.C.; Moraes, T.C.K.; Dacanal, G.C.; Oliveira, A.L.D.; Petrus, R.R. The potential use of supercritical carbon dioxide in sugarcane juice processing. npj Sci. Food 2024, 8, 6. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Rai, M.K. Sugarcane production: Impact of climate change and its mitigation. Biodiversitas 2012, 13, 214–227. [Google Scholar] [CrossRef]
- Bonnett, G.D.; Hewitt, M.L.; Glassop, D. Effects of high temperature on the growth and composition of sugarcane internodes. Aust. J. Agric. Res. 2006, 57, 1087–1095. [Google Scholar] [CrossRef]
- Wagih, M.E.; Ala, A.; Musa, Y. Evaluation of sugarcane varieties for maturity earliness and selection for efficient sugar accumulation. Sugar Tech. 2004, 6, 297–304. [Google Scholar] [CrossRef]
- Martini, C.; Verruma-Bernardi, M.R.; Borges, M.T.M.R.; Margarido, L.A.C.; Ceccato-Antonini, S.R. Yeast composition of sugar cane juice in relation to plant varieties and seasonality. Biosci. J. 2011, 27, 710–717. [Google Scholar]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef]
- Tzeng, D.I.; Chia, Y.C.; Tai, C.Y.; Ou, A.S.M. Investigation of chemical quality of sugarcane (Saccharum officinarum L.) wine during fermentation by Saccharomyces cerevisiae. J. Food Qual. 2010, 33, 248–267. [Google Scholar] [CrossRef]
- Laluce, C.; Leite, G.R.; Zavitoski, B.Z.; Zamai, T.T.; Ventura, R. Fermentation of sugarcane juice and molasses for ethanol production. In Sugarcane-Based Biofuels and Bioproducts; O’Hara, I.M., Mundree, S.G., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 53–86. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef]
- Bortoletto, A.M.; Silvello, G.C.; Alcarde, A.R. Chemical and microbiological quality of sugar cane juice influences the concentration of ethyl carbamate and volatile congeners in cachaça. J. Inst. Brew. 2015, 121, 251–256. [Google Scholar] [CrossRef]
- Lino, F.S.D.O.; Misiakou, M.A.; Kang, K.; Li, S.S.; da Costa, B.L.V.; Basso, T.O.; Panagiotou, G.; Sommer, M.O.A. Strain dynamics of specific contaminant bacteria modulate the performance of ethanol biorefineries. bioRxiv 2021. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8 S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Evol. Microbiol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Guillamón, J.M.; Sabaté, J.; Barrio, E.; Cano, J.; Querol, A. Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch. Microbiol. 1998, 169, 387–392. [Google Scholar] [CrossRef]
- Kaavya, R.; Pandiselvam, R.; Kothakota, A.; Banuu Priya, E.P.; Arun Prasath, V. Sugarcane juice preservation: A critical review of the state of the art and way forward. Sugar Tech. 2019, 21, 9–19. [Google Scholar] [CrossRef]
- Appellations of Origin and Local Customs: The Need for Innovation “Le Rhum De La Martinique”: An Example of AOC Recognition of a Product in an Industrialised Context. Available online: https://ideas.repec.org/p/ags/eaae67/241745.html (accessed on 31 January 2025).
- de Ullivarri, M.F.; Mendoza, L.M.; Raya, R.R. Killer activity of Saccharomyces cerevisiae strains: Partial characterization and strategies to improve the biocontrol efficacy in winemaking. Antonie Van Leeuwenhoek 2014, 106, 865–878. [Google Scholar] [CrossRef]
- Senne de Oliveira Lino, F.; Bajic, D.; Vila, J.C.C.; Sánchez, A.; Sommer, M.O.A. Complex yeast–bacteria interactions affect the yield of industrial ethanol fermentation. Nat. Commun. 2021, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Remize, F.; Sablayrolles, J.M.; Dequin, S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 2000, 88, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.T.; Zhang, Y.S.; Wen, X.; Song, X.W.; Meng, D.; Li, B.J.; Wang, M.Y.; Tao, Y.Q.; Zhao, H.; Guan, W.Q.; et al. The glycerol and ethanol production kinetics in low-temperature wine fermentation using Saccharomyces cerevisiae yeast strains. Int. J. Food Sci. Technol. 2019, 54, 102–110. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Characterization of the key aroma compounds in two commercial rums by means of the sensomics approach. J. Agric. Food Chem. 2016, 64, 637–645. [Google Scholar] [CrossRef]
- Ickes, C.M.; Cadwallader, K.R. Characterization of sensory differences in mixing and premium rums through the use of descriptive sensory analysis. J. Food Sci. 2017, 82, 2679–2689. [Google Scholar] [CrossRef]
- De Souza, M.D.; Vásquez, P.; Del Mastro, N.L.; Acree, T.E.; Lavin, E.H. Characterization of cachaca and rum aroma. J. Agric. Food Chem. 2006, 54, 485–488. [Google Scholar] [CrossRef]
- Ickes, C.M.; Lee, S.Y.; Cadwallader, K.R. Novel creation of a rum flavor lexicon through the use of web-based material. J. Food Sci. 2017, 82, 1216–1223. [Google Scholar] [CrossRef]
- Sampaio, O.M.; Reche, R.V.; Franco, D.W. Chemical profile of rums as a function of their origin. The use of chemometric techniques for their identification. J. Agric. Food Chem. 2008, 56, 1661–1668. [Google Scholar] [CrossRef]
- Belmonte-Sánchez, J.R.; Gherghel, S.; Arrebola-Liébanas, J.; González, R.R.; Vidal, J.L.M.; Parkin, I.; Frenich, A.G. Rum classification using fingerprinting analysis of volatile fraction by headspace solid phase microextraction coupled to gas chromatography-mass spectrometry. Talanta 2018, 187, 348–356. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Evaluation of volatile compounds and antioxidant capacity of some commercial rums from Dominican Republic. Int. J. Food Sci. Technol. 2011, 46, 988–993. [Google Scholar] [CrossRef]
- The Effect of Distillation Conditions and Molasses Concentration on the Volatile Compounds of Unaged Rum. Available online: https://openrepository.aut.ac.nz/server/api/core/bitstreams/680ae4cc-dd56-4874-a6d5-af12c2ca187d/content (accessed on 31 January 2025).
- Pino, J.A.; Tolle, S.; Gök, R.; Winterhalter, P. Characterisation of odour-active compounds in aged rum. Food Chem. 2012, 132, 1436–1441. [Google Scholar] [CrossRef]
- Nascimento, E.S.P.; Cardoso, D.R.; Franco, D.W. Quantitative ester analysis in cachaça and distilled spirits by gas chromatography−mass spectrometry (GC−MS). J. Agric. Food Chem. 2008, 56, 5488–5493. [Google Scholar] [CrossRef]

| Variety/Accession | Origin | Centre |
|---|---|---|
| PSR 07-334 | Filippine | Philsurin |
| FR 87-83 | Guadeloupe | Visacane |
| KN 07-0037 | Sudan | Kenana |
| CPCL 02-1295 | USA—Florida | USDA |
| CP 06-2495 | USA—Florida | USDA |
| CP 09-1952 | USA—Florida | USDA |
| Mex 69-290 | Messico | Visacane |
| Ananas | Caribbean | - |
| Baltasià | Caribbean | - |
| Chemical Parameters | Wort |
|---|---|
| D-fructose (g/L) | 13.19 ± 0.12 |
| D-glucose (g/L) | 23.01 ± 0.22 |
| D-sucrose (g/L) | 168.50 ± 1.35 |
| Glycerol (g/L) | 0.32 ± 0.05 |
| Acetic acid (g/L) | 0.09 ± 0.06 |
| Lactic acid (g/L) | 0.06 ± 0.05 |
| Malic acid (g/L) | 0.02 ± 0.02 |
| Tartaric acid (g/L) | 0.03 ± 0.01 |
| Ammoniacal nitrogen (mg/L) | 0.00 ± 0.00 |
| Alpha-amino nitrogen (mg/L) | 74.08 ± 0.26 |
| pH | 5.11 ± 0.12 |
| Total acidity (TA; g/L tartaric acid) | 2.85 ± 0.10 |
| Variety/Accession | Plant Height (cm) | Millable Canes Length (cm) | Number of Nodes (n) | Average Node Diameter (mm) | Number of Tillers (n ha−1) | Number of Millable Canes (n ha−1) |
|---|---|---|---|---|---|---|
| PSR 07-334 | 190.93 ± 33.60 ab | 155.04 ± 3.24 abc | 14.91 ± 0.58 a | 28.08 ± 1.76 ab | 247,000.00 ± 1414.00 ab | 189,333.00 ± 3771.00 b |
| FR 87-83 | 212.16 ± 4.48 a | 164.47 ± 1.03 ab | 12.60 ± 0.56 bc | 28.31 ± 2.15 ab | 237,500.00 ± 3536.00 b | 160,000.00 ± 0.00 c |
| KN 07-0037 | 173.25 ± 4.60 bc | 143.50 ± 2.12 bc | 10.80 ± 0.28 c | 28.79 ± 2.14 ab | 188,350.00 ± 1180.00 d | 180,833.00 ± 8250.00 b |
| CPCL 02-1295 | 130.73 ± 2.92 d | 98.04 ± 6.66 d | 11.50 ± 0.35 c | 25.06 ± 1.14 ab | 118,000.00 ± 2828.00 f | 117,333.00 ± 1508.00 e |
| CP 06-2495 | 170.62 ± 17.10 bc | 167.95 ± 18.50 a | 13.90 ± 0.35 ab | 22.70 ± 0.73 b | 175,000.00 ± 0.00 e | 157,500.00 ± 1550.00 c |
| CP 09-1952 | 177.50 ± 0.70 bc | 134.57 ± 0.24 c | 11.45 ± 1.34 c | 20.96 ± 4.20 b | 183,333.00 ± 4714.00 de | 152,333.00 ± 6128.00 cd |
| Mex 69-290 | 196.83 ± 1.18 ab | 157.00 ± 3.77 abc | 11.50 ± 0.23 c | 26.42 ± 2.72 ab | 253,333.00 ± 9428.00 a | 206,667.00 ± 9428.00 a |
| Ananas | 142.57 ± 6.60 cd | 139.00 ±11.64 bc | 12.23 ± 0.22 bc | 27.90 ± 2.28 ab | 180,000.00 ± 1733.00 de | 138,333.00 ± 2357.00 d |
| Baltasià | 189.60 ± 39.00 ab | 156.50 ± 27.90 abc | 12.50 ± 0.35 bc | 31.98 ± 0.06 a | 217,000.00 ± 4243.00 c | 138,667.00 ± 7542.00 d |
| p-values | *** | *** | *** | * | *** | *** |
| Variety/Accession. | Juice Yield (L ha−1) | °Brix of Juice (°) | pH of Juice | Sucrose (g/cm3) | CCS (%) | Sugar Yield (t ha−1) |
|---|---|---|---|---|---|---|
| PSR 07-334 | 11,995.00 ± 1351.00 abc | 22.90 ± 1.17 a | 5.28 ± 0.08 d | 19.76 ± 0.04 b | 13.51 ± 0.39 b | 4.11 ± 0.04 ab |
| FR 87-83 | 11,292.50 ± 293.00 abc | 19.82 ± 0.00 b | 5.24 ± 0.05 d | 15.35 ± 1.55 d | 9.02 ± 2.82 c | 3.04 ± 0.01 ab |
| KN 07-0037 | 12,623.30 ± 3003.00 ab | 14.74 ± 1.24 c | 5.40 ± 0.03 ab | 12.33 ± 2.61 e | 8.30 ± 2.31 c | 2.31 ± 0.03 b |
| CPCL 02-1295 | 5850.00 ± 1344.00 d | 24.10 ± 0.60 a | 5.42 ± 0.04 a | 16.73 ± 1.17 cd | 10.06 ± 1.02 c | 1.84 ± 0.03 b |
| CP 06-2495 | 7871.50 ± 1419.00 c | 23.87 ± 0.24 a | 5.30 ± 0.02 bc | 18.98 ± 2.73 bc | 13.00 ± 2.00 b | 2.78 ± 0.05 ab |
| CP 09-1952 | 5932.50 ± 470.00 d | 23.82 ± 0.81 a | 5.44 ± 0.00 a | 23.11 ± 0.34 a | 16.66 ± 0.11 a | 3.03 ± 0.03 ab |
| Mex 69-290 | 9876.70 ± 2814.00 bc | 20.69 ± 0.28 b | 5.29 ± 0.08 cd | 19.61 ± 0.48 bc | 14.00 ± 0.57 ab | 3.55 ± 0.06 ab |
| Ananas | 9586.70 ± 2480.00 bc | 20.65 ± 1.39 b | 5.36 ± 0.09 bc | 19.54 ± 1.57 bc | 13.94 ± 1.19 ab | 3.86 ± 0.10 ab |
| Baltasià | 14,312.50 ± 3624.00 a | 20.24 ± 0.21 b | 5.42 ± 0.04 a | 19.56 ± 0.33 bc | 14.08 ± 0.40 ab | 5.33 ± 0.07 a |
| p-values | *** | *** | *** | *** | *** | * |
| Trials | D-Sucrose 1 | D-Glucose 1 | D-Fructose 1 | Glycerol 1 | L-Malic Acid 1 | Lactic Acid 1 | Acetic Acid 1 | Tartatic Acid 1 | pH | TA |
|---|---|---|---|---|---|---|---|---|---|---|
| SC1 | 109.00 ± 0.94 a | 5.75 ± 0.15 c | 12.20 ± 0.14 a | 2.14 ± 0.07 h | 0.07 ± 0.00 f | 2.86 ± 0.15 c | 4.01 ± 0.08 c | 0.03 ± 0.00 h | 3.32 ± 0.10 fgh | 16.10 ± 0.22 a |
| SC2 | 1.85 ± 0.10 d | 0.34 ± 0.04 ef | 1.20 ± 0.08 e | 9.80 ± 0.24 a | 0.08 ± 0.01 h | 1.20 ± 0.00 e | 4.73 ± 0.12 a | 0.23 ± 0.01 de | 3.76 ± 0.09 bc | 13.87 ± 0.09 b |
| SC3 | 1.74 ± 0.05 d | 1.55 ± 0.03 d | 0.87 ± 0.07 f | 9.14 ± 0.16 b | 0.09 ± 0.01 def | 0.90 ± 0.00 f | 4.28 ± 0.11 b | 0.35 ± 0.01 b | 3.92 ± 0.10 b | 11.25 ± 0.07 d |
| SC4 | 0.88 ± 0.07 d | 1.24 ± 0.07 de | 1.22 ± 0.06 e | 9.80 ± 0.24 a | 0.08 ± 0.00 ef | 0.70 ± 0.00 f | 4.62 ± 0.10 a | 0.26 ± 0.01 d | 3.79 ± 0.08 bc | 12.37 ± 0.08 c |
| SC5 | 78.40 ± 1.20 c | 1.21 ± 0.06 de | 11.50 ± 0.24 b | 3.84 ± 0.12 g | 0.09 ± 0.02 def | 2.09 ± 0.06 d | 0.53 ± 0.02 ef | 0.06 ± 0.00 gh | 3.36 ± 0.07 efgh | 13.50 ± 0.17 b |
| SC6 | 0.75 ± 0.08 d | 0.22 ± 0.04 f | 0.55 ± 0.11 gh | 5.17 ± 0.09 f | 0.07 ± 0.01 f | 1.17 ± 0.10 e | 0.55 ± 0.03 e | 0.14 ± 0.00 f | 3.65 ± 0.06 cd | 10.12 ± 0.18 e |
| SC7 | 0.85 ± 0.06 d | 0.55 ± 0.03 ef | 1.20 ± 0.07 e | 5.96 ± 0.09 e | 0.07 ± 0.01 f | 0.71 ± 0.02 f | 0.55 ± 0.04 e | 0.26 ± 0.02 d | 3.65 ± 0.10 cd | 6.00 ±0.07 h |
| SC8 | 1.10 ± 0.07 d | 1.67 ± 0.05 d | 0.78 ± 0.04 fg | 7.40 ± 0.12 c | 0.07 ± 0.01 f | 0.83 ± 0.01 f | 0.73 ± 0.03 ef | 0.26 ± 0.01 d | 3.98 ± 0.08 a | 7.65 ± 0.13 f |
| SC9 | 97.60 ± 1.32 b | 20.75 ± 0.84 a | 4.71 ± 0.15 d | 1.12 ± 0.05 i | 0.09 ± 0.02 def | 0.15 ± 0.09 g | 0.44 ± 0.02 ef | 0.39 ± 0.01 a | 3.13 ± 0.09 h | 10.87 ± 0.20 d |
| SC10 | 1.95 ± 0.09 d | 1.14 ± 0.12 def | 0.25 ± 0.11 h | 6.51 ± 0.13 d | 0.11 ± 0.00 cde | 0.07 ± 0.00 g | 0.46 ± 0.01 ef | 0.26 ± 0.01 d | 3.37 ± 0.07 efgh | 7.12 ± 0.17 g |
| SC11 | 0.85 ± 0.11 d | 0.97 ±0.04 def | 0.30 ± 0.02 h | 6.52 ± 0.11 d | 0.19 ± 0.02 a | 0.06 ± 0.00 g | 0.45 ± 0.01 ef | 0.31 ± 0.02 c | 3.39 ± 0.08 efg | 7.14 ± 0.16 g |
| SC12 | 0.65 ± 0.13 d | 1.15 ± 0.07 def | 0.63 ± 0.03 fg | 7.33 ± 0.12 c | 0.15 ± 0.01 b | 0.05 ± 0.01 g | 0.26 ± 0.01 gh | 0.20 ± 0.01 e | 3.21 ± 0.10 gh | 6.90 ± 0.09 g |
| SC13 | 99.20 ± 1.28 b | 19.37 ± 0.92 b | 5.39 ± 0.12 c | 4.24 ± 0.09 g | 0.15 ± 0.01 b | 5.37 ± 0.11 a | 0.42 ± 0.03 efg | 0.13 ± 0.01 f | 3.46 ± 0.05 def | 10.87 ± 0.21 d |
| SC14 | 0.88 ± 0.10 d | 0.77 ± 0.07 def | 0.55 ± 0.07 gh | 5.95 ± 0.15 e | 0.12 ± 0.01 bcd | 5.18 ± 0.10 ab | 0.37 ± 0.02 fg | 0.16 ± 0.01 f | 3.47 ± 0.09 def | 9.75 ± 0.08 e |
| SC15 | 1.79 ± 0.10 d | 0.55 ± 0.08 def | 0.68 ± 0.04 gh | 6.08 ± 0.12 e | 0.20 ± 0.01 a | 5.10 ± 0.09 b | 0.40 ± 0.01 efg | 0.08 ± 0.01 g | 3.57 ± 0.06 cde | 9.75 ± 0.12 e |
| SC16 | 1.52 ± 0.15 d | 1.10 ± 0.12 ef | 1.67 ± 0.05 fg | 7.44 ± 0.20 c | 0.14 ± 0.00 bc | 5.21 ± 0.07 ab | 0.19 ± 0.00 h | 0.07 ± 0.00 g | 3.42 ± 0.04 defg | 9.97 ± 0.08 e |
| p-values | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| RT 1 | KI 2 | Compounds 3 | CAS | SC9 4 | SC11 4 | SC12 4 | SC13 4 | SC15 4 | SC16 4 | p-Values |
|---|---|---|---|---|---|---|---|---|---|---|
| 9.123 | 858 | 1-Butanol, 3-methyl-, acetate | 000123-92-2 | 27.15 ± 1.05 a | 6.25 ± 0.45 b | 1.53 ± 0.09 de | 4.33 ± 0.25 c | 0.88 ± 0.08 e | 2.40 ± 0.12 d | *** |
| 10.831 | 899 | Oxime-, methoxy-phenyl-_ | 1000222-86-6 | 5.92 ± 0.25 b | 3.27 ± 0.24 d | 1.26 ± 0.11 e | 6.93 ± 0.33 a | 4.30 ± 0.18 c | 1.74 ± 0.11 e | *** |
| 14.481 | 989 | Hexanoic acid, ethyl ester | 000123-66-0 | n.d. | 3.88 ± 0.31 a | 1.61 ± 0.08 b | n.d. | 0.41 ± 0.03 c | 1.61 ± 0.12 c | *** |
| 18.559 | 1060 | Phenylethyl Alcohol | 000060-12-8 | 2.81 ± 0.15 a | n.d. | n.d. | n.d. | n.d. | n.d. | *** |
| 20.407 | 1179 | Octanoic acid | 000124-07-2 | n.d. | 6.34 ± 0.18 a | 3.70 ± 0.17 b | n.d. | 1.31 ± 0.15 d | 2.88 ± 0.21 c | *** |
| 21.222 | 1195 | Octanoic acid, ethyl ester | 000106-32-1 | 2.83 ± 0.12 c | 22.79 ± 0.74 a | 10.32 ± 0.37 b | 1.05 ± 0.09 d | 3.37 ± 0.25c | 9.81 ± 0.15 b | *** |
| 22.972 | 1248 | Propanoic acid, 2-methyl-, 2-phenylethyl ester | 000103-48-0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.04 ± 0.01 a | *** |
| 22.986 | 1256 | Acetic acid, 2-phenylethyl ester | 000103-45-7 | 26.74 ± 0.80 a | n.d. | n.d. | 8.71 ± 0.41 b | n.d. | n.d. | *** |
| 25.668 | 1311 | 1H-Indene, 2,3-dihydro-1,1,5,6-tetramethyl | 000942-43-8 | n.d. | n.d. | n.d. | 1.01 ± 0.05 a | n.d. | n.d. | *** |
| 26.082 | 1387 | n-Decanoic acid | 000334-48-5 | 1.55 ± 0.08 e | 6.06 ± 0.31 a | 3.79 ± 0.15 c | 4.69 ± 0.41 b | 2.53 ± 0.11 d | 4.04 ± 0.34 bc | *** |
| 26.52 | 1388 | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- | 023696-85-7 | 1.20 ± 0.09 a | n.d. | n.d. | 0.85 ± 0.06 b | n.d. | n.d. | *** |
| 26.655 | 1389 | Ethyl 9-decenoate | 067233-91-4 | n.d. | 4.04 ± 0.15 a | 0.49 ± 0.03 d | n.d. | 2.03 ± 0.10 e | 1.07 ± 0.09c | *** |
| 26.892 | 1391 | Decanoic acid, ethyl ester | 000110-38-3 | 13.16 ± 0.75 d | 29.38 ± 0.64 a | 27.46 ± 0.61b | 13.01 ± 0.65 d | 15.78 ± 0.55 c | 28.79 ± 0.64 ab | *** |
| 28.242 | 1471 | 3-Methylbutyl 2-ethylhexanoate | 1000099-99-3 | n.d. | n.d. | 0.47 ± 0.08 a | n.d. | n.d. | n.d. | *** |
| 28.247 | 1450 | Octanoic acid, 3-methylbutyl ester | 002035-99-6 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.41 ± 0.02 a | *** |
| 31.524 | 1580 | Dodecanoic acid | 000143-07-7 | n.d. | n.d. | 0.85 ± 0.05 ab | n.d. | 0.94 ± 0.05 a | 0.79 ± 0.04 b | *** |
| 32.088 | 1581 | Dodecanoic acid, ethyl ester | 000106-33-2 | 18.64 ± 0.95 c | 9.89 ± 0.35 d | 21.54 ± 0.55 b | 52.31 ± 1.05 a | 7.47 ± 0.31 e | 21.22 ± 0.71 b | *** |
| 33.298 | 1615 | Pentadecanoic acid, 3-methylbutylester | 002306-91-4 | n.d. | n.d. | 1.14 ± 0.10 b | n.d. | 0.97 ± 0.06 b | 1.57 ± 0.15 a | *** |
| 37.185 | 1687 | Ethyl tridecanoate | 028267-29-0 | n.d. | n.d. | 1.74 ± 0.11 b | 2.34 ± 0.12 a | n.d. | n.d. | *** |
| 37.195 | Octadecanoic acid, ethyl ester | 000111-61-5 | n.d. | n.d. | n.d. | n.d. | 1.09 ± 0.07 b | 1.65 ± 0.11 a | *** | |
| 37.204 | 1880 | Pentadecanoic acid, ethyl ester | 041114-00-5 | n.d. | n.d. | n.d. | 1.89 ± 0.24 a | n.d. | n.d. | *** |
| 43.893 | 1978 | Hexadecanoic acid, ethyl ester | 000628-97-7 | n.d. | n.d. | 18.32 ± 0.47 b | 1.44 ± 0.10 e | 27.54 ± 0.84 a | 15.67 ± 0.31 c | *** |
| 43.973 | 1980 | Ethyl 9-hexadecenoate | 054546-22-4 | n.d. | 8.1 ± 0.41 b | 5.78 ± 0.34 c | n.d. | 29.78 ± 0.67 a | 6.31 ± 0.34 c | *** |
| 50.206 | 2144 | Linoleic acid ethyl ester | 000544-35-4 | n.d. | n.d. | n.d. | n.d. | 0.39 ± 0.02 a | n.d. | *** |
| 50.383 | 2153 | 9,12-Octadecadienoic acid, ethyl ester | 007619-08-1 | n.d. | n.d. | n.d. | n.d. | 0.39 ± 0.03 a | n.d. | *** |
| 50.96 | 2162 | cis-Vaccenic acid | 000506-17-2 | n.d. | n.d. | n.d. | n.d. | 0.24 ± 0.01 a | n.d. | *** |
| 51.081 | 2173 | 9,12-Octadecadien-1-ol, (Z,Z)- | 000506-43-4 | n.d. | n.d. | n.d. | n.d. | 0.48 ± 0.02 a | n.d. | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirrone, A.; Iacuzzi, N.; Alfonzo, A.; Monte, M.; Naselli, V.; Alaimo, F.; Tortorici, N.; Busetta, G.; Garofalo, G.; Gaglio, R.; et al. Bio-Agronomic Assessment and Quality Evaluation of Sugarcane with Optimized Juice Fermentation in View of Producing Sicilian “Rum Agricole”. Appl. Sci. 2025, 15, 7696. https://doi.org/10.3390/app15147696
Pirrone A, Iacuzzi N, Alfonzo A, Monte M, Naselli V, Alaimo F, Tortorici N, Busetta G, Garofalo G, Gaglio R, et al. Bio-Agronomic Assessment and Quality Evaluation of Sugarcane with Optimized Juice Fermentation in View of Producing Sicilian “Rum Agricole”. Applied Sciences. 2025; 15(14):7696. https://doi.org/10.3390/app15147696
Chicago/Turabian StylePirrone, Antonino, Nicolò Iacuzzi, Antonio Alfonzo, Morgana Monte, Vincenzo Naselli, Federica Alaimo, Noemi Tortorici, Gabriele Busetta, Giuliana Garofalo, Raimondo Gaglio, and et al. 2025. "Bio-Agronomic Assessment and Quality Evaluation of Sugarcane with Optimized Juice Fermentation in View of Producing Sicilian “Rum Agricole”" Applied Sciences 15, no. 14: 7696. https://doi.org/10.3390/app15147696
APA StylePirrone, A., Iacuzzi, N., Alfonzo, A., Monte, M., Naselli, V., Alaimo, F., Tortorici, N., Busetta, G., Garofalo, G., Gaglio, R., De Pasquale, C., Francesca, N., Settanni, L., Tuttolomondo, T., & Moschetti, G. (2025). Bio-Agronomic Assessment and Quality Evaluation of Sugarcane with Optimized Juice Fermentation in View of Producing Sicilian “Rum Agricole”. Applied Sciences, 15(14), 7696. https://doi.org/10.3390/app15147696










