Heat Capacities and Thermal Coefficients of Sodium’s and Eutectic Sodium–Potassium’s Coolants for Nuclear Reactors
Abstract
1. Introduction
2. Theory
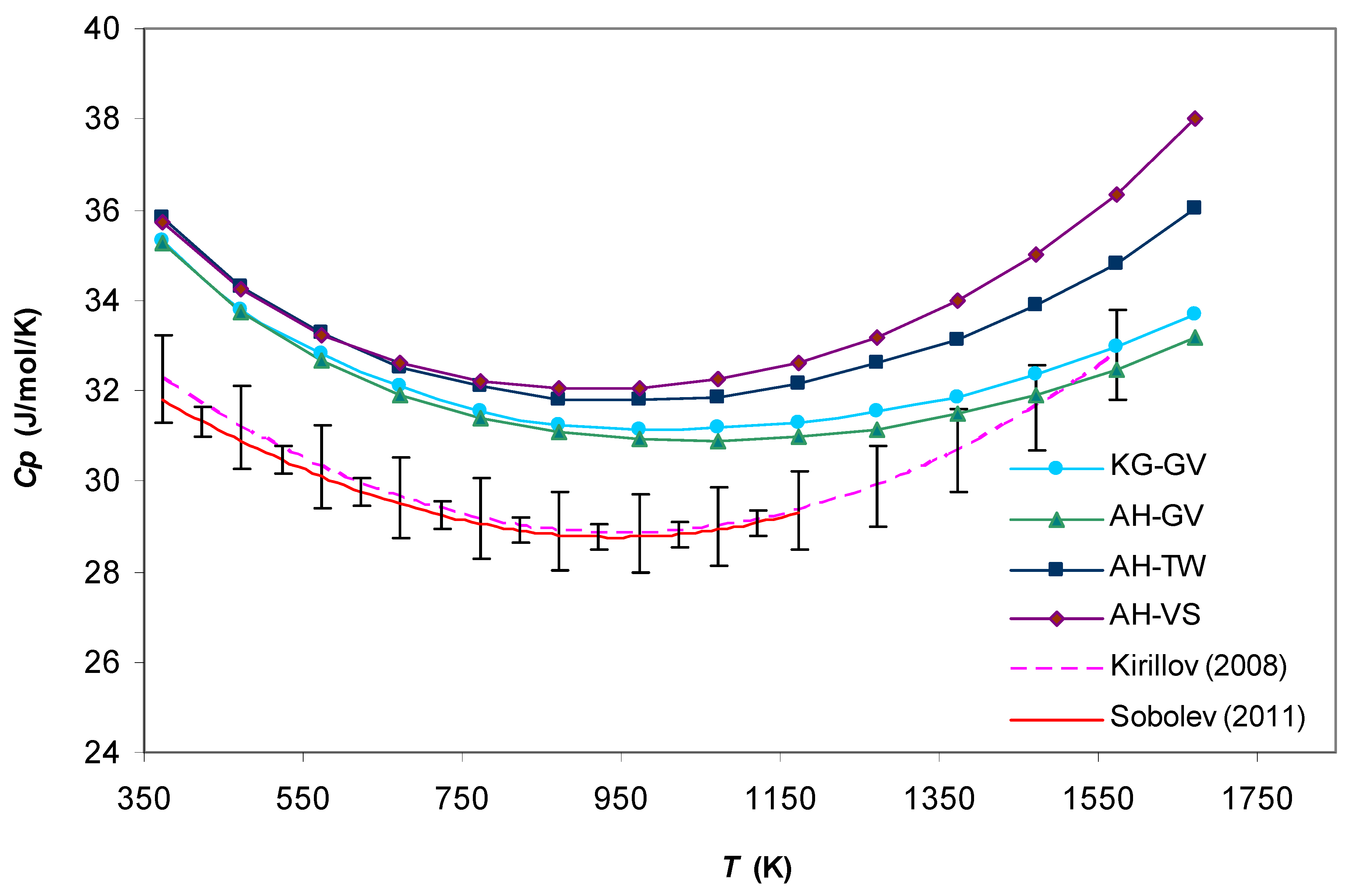
3. Results and Discussion
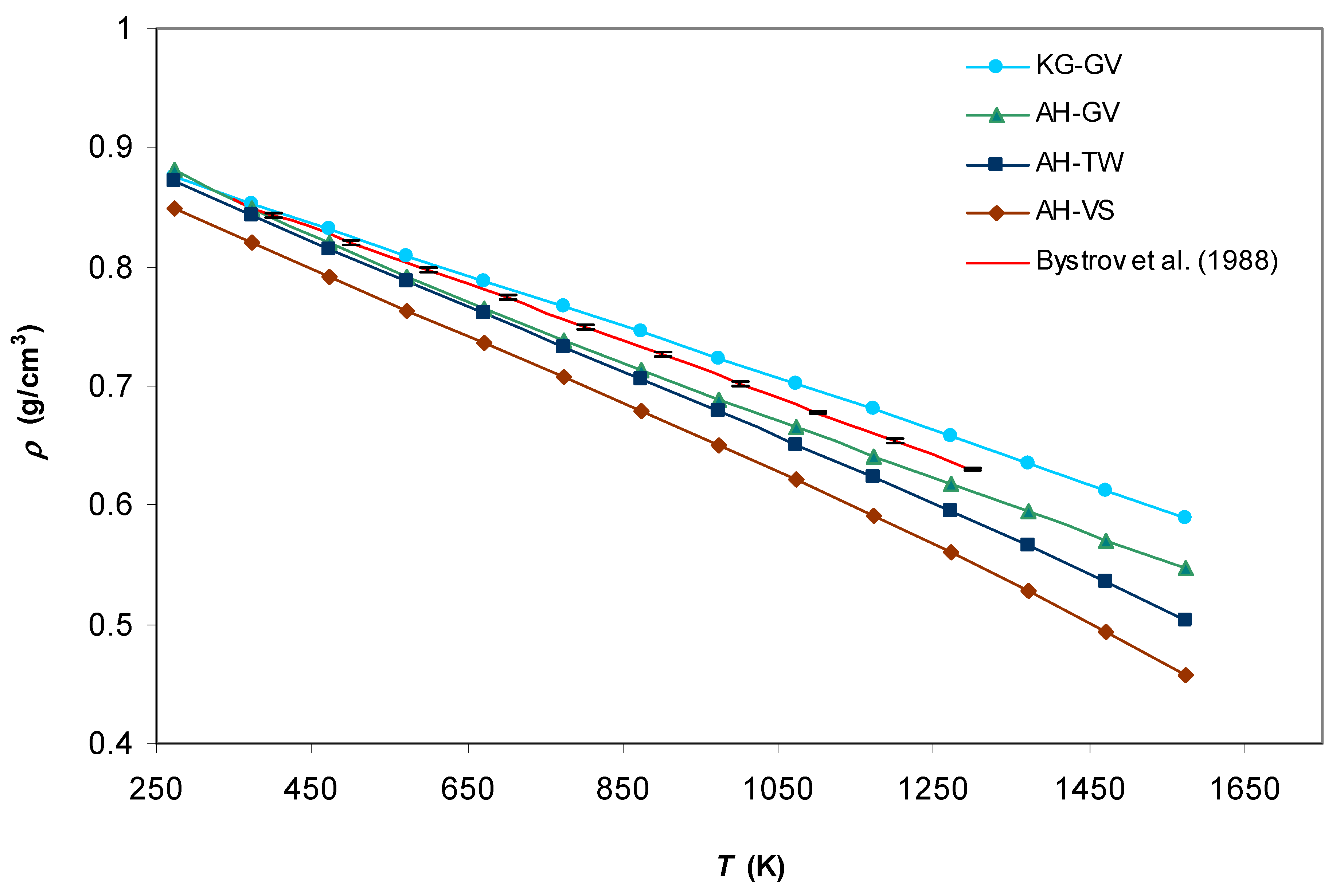
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subbotin, V.I.; Arnol’dov, M.N.; Kozlov, F.A.; Shimkevich, A.L. Liquid-metal coolants for nuclear power. At. Energy 2002, 92, 29–40. [Google Scholar] [CrossRef]
- Olivier, T.J.; Radel, R.F.; Nowlen, S.P.; Blanchat, T.K.; Hewson, J.C. Metal Fire Implications for Advanced Reactors, Part 1: Literature Review; Sandia Report, SAND2007-6332, Unlimited Release; OSTI: Oak Ridge, TN, USA, 2007. [Google Scholar]
- Kirillov, P.L. (Ed.) Thermophysical Properties of Materials for Nuclear Engineering: A Tutorial and Collection of Data; IAEA: Vienna, Austria, 2008. [Google Scholar]
- Sobolev, V. Database of Thermophysical Properties of Liquid Metal Coolants for GEN-IV. Sodium, Lead, Lead-Bismuth Eutectic (and Bismuth), Scientific Report of the Belgian Nuclear Research Centre, SCK-CEN-BLG-1069, November 2010. [rev. Dec. 2011]; Belgian Nuclear Research Centre: Mol, Belgium, 2011. [Google Scholar]
- Bale, C.W. The K-Na (potassium-sodium) system. Bull. Alloy Phase Diagr. 1982, 3, 313–318. [Google Scholar] [CrossRef]
- Loginov, N.I. Sodium-potassium eutectic alloy: Achievements and problems. Quest. At. Sci. Technol. Nucl.-React. Constants 2024, 2, 259–268. (In Russian) [Google Scholar]
- Ulyanov, V.V.; Koshelev, M.M.; Kremleva, V.S.; Bragin, D.S.; Prikazchikova, A.A. Features of purification and control of sodium-potassium eutectic alloy. Izv. Vyss. Uchebnykh Zaved. Yad. Ehnergetika 2024, 127–137. (In Russian) [Google Scholar] [CrossRef]
- Leonchuk, S.S.; Falchevskaya, A.S.; Nikolaev, V.; Vinogradov, V.V. NaK alloy: Underrated liquid metal. J. Mater. Chem. A 2022, 10, 22955–22976. [Google Scholar] [CrossRef]
- Leonchuk, S.S.; Falchevskaya, A.S.; Morozova, P.A.; Gromov, N.V.; Vinogradov, V.V. NaK alloy as a versatile reagent for template-free synthesis of porous metal- and metalloid-based nanostructures. Chem. Commun. 2024, 60, 4814–4817. [Google Scholar] [CrossRef] [PubMed]
- Mon, K.K. Application of hard sphere perturbation theory for thermodynamics of model liquid metals. Phys. Rev. E 2001, 63, 061203. [Google Scholar] [CrossRef]
- Mizuno, A.; Masaki, T.; Itami, T. Theoretical prediction of atomic volume for liquid metals based on the hard sphere model combined with NFE theory. Chem. Phys. Lett. 2002, 363, 337–342. [Google Scholar] [CrossRef]
- Dubinin, N.E. Thermodynamics of alkali metals melts. J. Opt. Adv. Mater. 2003, 5, 1259–1262. [Google Scholar]
- Grosdidier, B.; Al-Busaidi, M.S.; Osman, S.M. Transferable individual local pseudopotential for expanded liquid metals. J. Non-Cryst. Solids 2007, 353, 3484–3487. [Google Scholar] [CrossRef]
- Dubinin, N.; Yuryev, A.; Vatolin, N. Gibbs–Bogoliubov variational procedure with the square-well reference system. J. Non-Equilibr. Thermodyn. 2010, 35, 289–300. [Google Scholar] [CrossRef]
- Dubinin, N.E. The variational calculation of bulk moduli for liquid binary alloys of alkali metals. Acta Phys. Polon. A 2017, 131, 237–239. [Google Scholar]
- Dubinin, N.E. The heat capacity at constant pressure in the nearly-free-electron approximation for binary liquid alloys of alkali metals. High Temp.-High Press. 2018, 47, 205–211. [Google Scholar]
- Uddin, M.S.; Gosh, R.C.; Bhuiyan, G.M. Investigation of surface tension, viscosity and diffusion coefficients for liquid simple metals. J. Non-Cryst. Solids 2018, 499, 426–433. [Google Scholar] [CrossRef]
- Bhuiyan, G.M.; Abbas, F.I. Local minimum in pair potentials of polyvalent metals: A limitation of pseudopotential theory. Int. J. Mod. Phys. B 2019, 33, 1950049. [Google Scholar] [CrossRef]
- Dubinin, N.E. Square-well self-diffusion coefficients in liquid binary alloys of alkali metals within the mean spherical approximation. J. Alloys Compd. 2019, 803, 1100–1104. [Google Scholar]
- Bryk, T.; Demchuk, T.; Wax, J.-F.; Jakse, N. Pressure-induced effects in the spectra of collective excitations in pure liquid metals. J. Phys. Condens. Matter 2020, 32, 184002. [Google Scholar]
- Becker, S.; Meyer, N.; Xu, H.; Wax, J.-F. Viscosity of liquid Na–K alloys from molecular dynamics simulations. J. Phys. Condens. Matter 2020, 32, 194005. [Google Scholar]
- Wax, J.-F.; Mocchetti, E. Simulation study of the collective excitations in liquid sodium under high pressure. J. Phys. Condens. Matter 2023, 35, 304003. [Google Scholar]
- Kovneristyi, Y.K.; Vatolin, N.A.; Gurskaya, E.G.; Landa, A.I.; Romankevitch, M.V.; Yuryev, A.A. Ab initio calculation of the thermodynamic properties of liquid alloys with the application to Ni-Al. A nonlocal resonant pseudopotential approach. J. Non-Cryst. Solids 1990, 117–119, 589–592. [Google Scholar] [CrossRef]
- Landa, A.I.; Yuryev, A.A.; Ruban, A.V.; Gurskaya, E.G.; Kovneristyi, Y.K.; Vatolin, N.A. Pseudopotential calculation of thermodynamic properties and glass transition temperatures of binary Ni-Al alloys. J. Phys. Condens. Matter 1991, 3, 9229–9243. [Google Scholar] [CrossRef]
- Dubinin, N.E.; Yuryev, A.A.; Vatolin, N.A. Thermodynamic properties of ternary liquid metal alloys. High Temp. Mater. Process. 1995, 14, 285–290. [Google Scholar] [CrossRef]
- Kitamura, H. Equation of state for expanded fluid mercury: Variational theory with many body interaction. J. Chem. Phys. 2007, 126, 134509. [Google Scholar] [CrossRef] [PubMed]
- Greeff, C.W. Tests of Monte Carlo perturbation theory for the free energy of liquid copper. J. Chem. Phys. 2008, 128, 184104. [Google Scholar] [CrossRef]
- Tsai, K.H.; Wu, T.-M. Entropy of a model for liquid Ga: Contribution due to Friedel oscillations. Comput. Phys. Commun. 2011, 182, 62–64. [Google Scholar] [CrossRef]
- Dubinin, N.E.; Vatolin, N.A.; Filippov, V.V. Thermodynamic perturbation theory in studies of metal melts. Russ. Chem. Rev. 2014, 83, 987–1002. [Google Scholar] [CrossRef]
- Ueda, S.; Morita, K. Theoretical calculation of the free energy of mixing of liquid transition-metal alloys using a bond-order potential and thermodynamic perturbation theory. J. Non-Cryst. Solids 2020, 528, 119743. [Google Scholar] [CrossRef]
- Bhuiyan, G.M. Microscopic origin of immiscibility and segregation in liquid metallic binary alloys. Bangladesh J. Phys. 2020, 27, 1–25. [Google Scholar] [CrossRef]
- Abbas, F.I.; Bhuiyan, G.M.; Kasem, R. Critical properties of segregation for Al1−xBix liquid binary alloys. J. Phys. Soc. Jpn. 2020, 89, 114004. [Google Scholar]
- Bogdanova, Y.A.; Gubin, S.A.; Maklashova, I.V. Calculation of thermodynamic properties of metals and their binary alloys by the perturbation theory. Metals 2021, 11, 1548. [Google Scholar] [CrossRef]
- Abbas, F.I.; Bhuiyan, G.M. A study of thermodynamics of mixing for Al1−xZnx liquid binary alloy. Phys. B 2022, 647, 414365. [Google Scholar] [CrossRef]
- Lukes, T.L.; Jones, R. Inequalities and variational methods in classical statistical mechanics. J. Phys. A Proc. Phys. Soc. 1968, 1, 29–33. [Google Scholar] [CrossRef]
- Isihara, A. The Gibbs–Bogoliubov inequality. J. Phys. A Proc. Phys. Soc. 1968, 1, 539–548. [Google Scholar]
- Mansoori, G.A.; Canfield, F.B. Variational approach to the equilibrium thermodynamic properties of simple liquids. I. J. Chem. Phys. 1969, 51, 4958–4967. [Google Scholar] [CrossRef]
- Umar, I.H.; Meyer, A.; Watabe, M.; Young, W.H. Thermodynamic calculations for liquid alloys with an application to sodium-potassium. J. Phys. F Met. Phys. 1974, 4, 1691–1706. [Google Scholar] [CrossRef]
- Nozieres, P.; Pines, D. Correlation energy of a free electron gas. Phys. Rev. 1958, 111, 442–454. [Google Scholar] [CrossRef]
- Krasco, G.L.; Gurskii, Z.A. Concerning one model pseudopotential. Lett. ZhETF 1969, 9, 596–601. [Google Scholar]
- Animalu, A.O.E.; Heine, V. The screened model potential for 25 elements. Phil. Mag. 1965, 12, 1249–1270. [Google Scholar] [CrossRef]
- Vaks, V.G.; Trefilov, A.V. On the theory of the atomic properties of liquid metals. Solid State Phys. 1977, 19, 244–258. [Google Scholar]
- Ashcroft, N.W.; Langreth, D.C. Structure of binary liquid mixtures. I. Phys. Rev. 1967, 156, 685–692. [Google Scholar] [CrossRef]
- Ashcroft, N.W.; Lekner, J. Structure and resistivity of liquid metals. Phys. Rev. 1966, 145, 83–90. [Google Scholar] [CrossRef]
- Jones, H. Method for finding the equation of state of liquid metals. J. Chem. Phys. 1971, 55, 2640–2642. [Google Scholar] [CrossRef]
- Geldart, D.J.W.; Vosko, S.H. The screening function of an interacting electron gas. Can. J. Phys. 1966, 44, 2137–2171. [Google Scholar] [CrossRef]
- Vashishta, P.; Singwi, K. Electron correlation at metallic densities. Phys. Rev. B 1972, 6, 875–887. [Google Scholar] [CrossRef]
- Toigo, F.; Woodruff, T.O. Calculation of the dielectric function for a degenerate electron gas with interactions. I. Phys. Rev. B 1970, 2, 3958–3966. [Google Scholar] [CrossRef]
- Ohse, R.W. (Ed.) Handbook of Thermodynamic and Transport Properties of Alkali Metals; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- Bystrov, P.M.; Kagan, D.N.; Krechetova, G.A.; Shpilrain, E.E. Liquid Metal Coolants of Heat Pipes and Power Plants; Nauka: Moscow, Russia, 1988. [Google Scholar]




| Metal | KG–GV | AH–GV | AH–TW | AH–VS | ||||
|---|---|---|---|---|---|---|---|---|
| (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | |
| Na | 3.242 | 0.4898 | −0.1752 | 2.0730 | −0.1958 | 2.1148 | −0.2136 | 2.174 |
| K | 2.965 | 0.6800 | −0.1813 | 2.9700 | −0.1853 | 2.9990 | −0.1949 | 3.082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubinin, N.E. Heat Capacities and Thermal Coefficients of Sodium’s and Eutectic Sodium–Potassium’s Coolants for Nuclear Reactors. Appl. Sci. 2025, 15, 7566. https://doi.org/10.3390/app15137566
Dubinin NE. Heat Capacities and Thermal Coefficients of Sodium’s and Eutectic Sodium–Potassium’s Coolants for Nuclear Reactors. Applied Sciences. 2025; 15(13):7566. https://doi.org/10.3390/app15137566
Chicago/Turabian StyleDubinin, Nikolay E. 2025. "Heat Capacities and Thermal Coefficients of Sodium’s and Eutectic Sodium–Potassium’s Coolants for Nuclear Reactors" Applied Sciences 15, no. 13: 7566. https://doi.org/10.3390/app15137566
APA StyleDubinin, N. E. (2025). Heat Capacities and Thermal Coefficients of Sodium’s and Eutectic Sodium–Potassium’s Coolants for Nuclear Reactors. Applied Sciences, 15(13), 7566. https://doi.org/10.3390/app15137566






