Effects of Different Titanium Anodized Surfaces on Peri-Implant Soft Tissue Healing Around Dental Abutments: In Vitro and Proteomic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Morphological Characterization
2.3. In Vitro Experimentation
2.3.1. Cell Cultures
2.3.2. Cell Adhesion
2.3.3. Collagen Synthesis
2.3.4. Inflammatory Potential: Cytokine Secretion
2.4. Proteomics
2.4.1. Protein Layer Elution
2.4.2. nLC-MS/MS Measurements and Bioinformatics Analyses
2.5. Statistical Analysis
3. Results
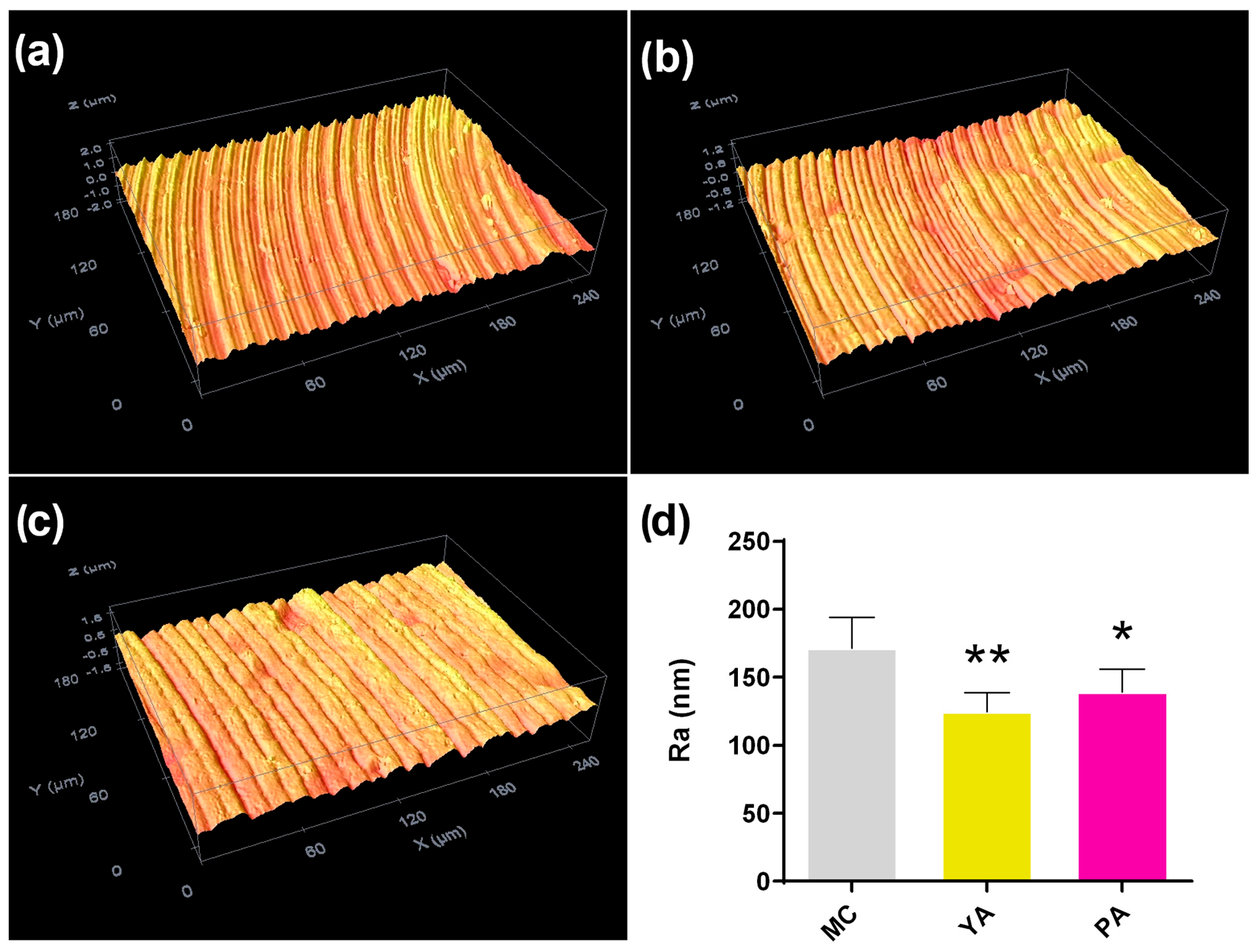
3.1. Morphological Characterization
3.2. In Vitro Experimentation
3.2.1. Cytoskeletal Arrangement and Collagen Secretion
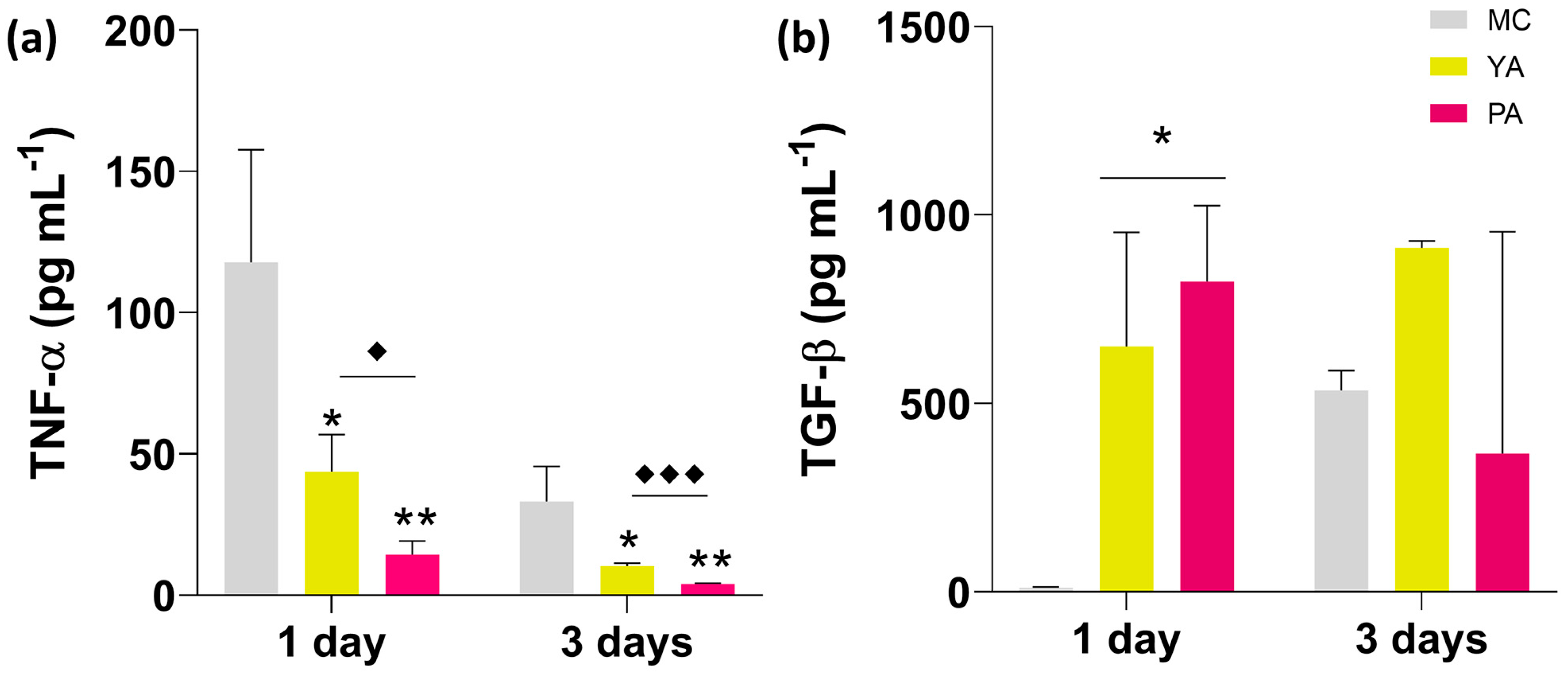
3.2.2. Inflammatory Potential: Cytokine Quantification by ELISA
3.3. Proteomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of Modified Titanium Abutment Surface on Peri-implant Soft Tissue Behavior: A Systematic Review of In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Annunziata, M.; Pesce, P.; Tommasato, G.; Nastri, L.; Guida, L. Influence of abutment material and modifications on peri-implant soft-tissue attachment: A systematic review and meta- analysis of histological animal studies. J. Prosthet. Dent. 2021, 125, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Titanium for Medical Applications. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin, Germany, 2001; pp. 12–24. [Google Scholar]
- Kordbacheh Changi, K.; Finkelstein, J.; Papapanou, P.N. Peri-implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clin. Oral Implant. Res. 2019, 30, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen. Dent. 2018, 66, 18–25. [Google Scholar]
- Amberg, R.; Elad, A.; Rothamel, D.; Fienitz, T.; Szakacs, G.; Heilmann, S.; Witte, F. Design of a migration assay for human gingival fibroblasts on biodegradable magnesium surfaces. Acta Biomater. 2018, 79, 158–167. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania Nanotubes for Local Drug Delivery from Implant Surfaces. In Electrochemically Engineered Nanoporous Materials: Methods, Properties and Applications; Losic, D., Santos, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 307–355. [Google Scholar]
- Cinquini, C.; Marchio, V.; Di Donna, E.; Alfonsi, F.; Derchi, G.; Nisi, M.; Barone, A. Histologic Evaluation of Soft Tissues around Dental Implant Abutments: A Narrative Review. Materials 2022, 15, 3811. [Google Scholar] [CrossRef]
- Lyu, Z.; Yu, Q.; Chen, H. Interactions of Biomaterials Surfaces with Proteins and Cells. In Polymeric Biomaterials for Tissue Regeneration: From Surface/Interface Design to 3D Constructs; Gao, C., Ed.; Springer: Singapore, 2016; pp. 103–122. [Google Scholar] [CrossRef]
- Othman, Z.; Cillero Pastor, B.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Gomes, N.C.; Ródenas, J.; Sánchez, A.; Azkargorta, M.; Iloro, I.; Elortza, F.; Arnáez, I.G.; Gurruchaga, M.; Goñi, I.; et al. Proteome analysis of human serum proteins adsorbed onto different titanium surfaces used in dental implants. Biofouling 2017, 33, 98–111. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Cerqueira, A.; Anitua, E.; Tejero, R.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Izquierdo, R.; Azkargorta, M.; Elortza, F.; et al. Protein adsorption/desorption dynamics on Ca-enriched titanium surfaces: Biological implications. J. Biol. Inorg. Chem. 2021, 26, 715–726. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Sanchez-Pérez, A.M.; Araújo-Gomes, N.; Azkargorta, M.; Iloro, I.; Elortza, F.; Gurruchaga, M.; Goñi, I.; Suay, J. Proteomic analysis of silica hybrid sol-gel coatings: A potential tool for predicting the biocompatibility of implants in vivo. Biofouling 2017, 33, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Izquierdo, R.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Suay, J.; et al. Characterization of magnesium doped sol-gel biomaterial for bone tissue regeneration: The effect of Mg ion in protein adsorption. Mater. Sci. Eng. Mater. Biol. Appl. C 2021, 125, 112114. [Google Scholar] [CrossRef] [PubMed]
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gavilán, F.; Araújo-Gomes, N.; Cerqueira, A.; García-Arnáez, I.; Martínez-Ramos, C.; Azkargorta, M.; Iloro, I.; Elortza, F.; Gurruchaga, M.; Suay, J.; et al. Proteomic analysis of calcium-enriched sol–gel biomaterials. J. Biol. Inorg. Chem. 2019, 24, 563–574. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Araújo-Gomes, N.; Romero-Gavilán, F.; Zhang, Y.; Martinez-Ramos, C.; Elortza, F.; Azkargorta, M.; de Llano, J.M.; Gurruchaga, M.; Goñi, I.; Beucken, J.v.D.; et al. Complement proteins regulating macrophage polarisation on biomaterials. Colloids Surf. B Biointerfaces 2019, 181, 125–133. [Google Scholar] [CrossRef]
- Renier, G.; Clément, I.; Desfaits, A.C.; Lambert, A. Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-α production. Endocrinology 1996, 137, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Johari, V.; Loke, C. Brief Overview of the Coagulation Cascade. Dis. Mon. 2012, 58, 421–423. [Google Scholar] [CrossRef]
- Brummel-Ziedins, K.; Mann, K.G. Molecular Basis of Blood Coagulation. In Hematology, 7th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1885–1905.e8. [Google Scholar]
- Blanc-Brude, O.P.; Archer, F.; Leoni, P.; Derian, C.; Bolsover, S.; Laurent, G.J.; Chambers, R. Factor Xa stimulates fibroblast procollagen production, proliferation, and calcium signaling via PAR1 activation. Exp. Cell Res. 2005, 304, 16–27. [Google Scholar] [CrossRef]
- Schmaier, A.H.; McCrae, K.R. The plasma kallikrein-kinin system: Its evolution from contact activation. J. Thromb. Haemost. 2007, 5, 2323–2329. [Google Scholar] [CrossRef]
- Tsuchida-Straeten, N.; Ensslen, S.; Schäfer, C.; Wöltje, M.; Denecke, B.; Moser, M.; Gräber, S.; Wakabayashi, S.; Koide, T.; Jahnen-Dechent, W. Enhanced blood coagulation and fibrinolysis in mice lacking histidine-rich glycoprotein (HRG). J. Thromb. Haemost. 2007, 3, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Swanson, R.; Kroh, H.K.; Bock, P.E. Protein Z-dependent protease inhibitor (ZPI) is a physiologically significant inhibitor of prothrombinase function. J. Biol. Chem. 2019, 294, 7644–7657. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Ny, L.; Wilczynska, M.; Shen, Y.; Ny, T.; Parmer, R.J. Plasminogen receptors and fibrinolysis. Int. J. Mol. Sci. 2021, 22, 1712. [Google Scholar] [CrossRef]
- Iba, K.; Hatakeyama, N.; Kojima, T.; Murata, M.; Matsumura, T.; Wewer, U.M.; Wada, T.; Sawada, N.; Yamashita, T. Impaired cutaneous wound healing in mice lacking tetranectin. Wound Repair Regen. 2009, 17, 108–112. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2009, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef]
- Pilling, D.; Gomer, R.H. Persistent lung inflammation and fibrosis in serum amyloid P component (Apcs-/-) knockout mice. PLoS ONE 2014, 9, 29–33. [Google Scholar] [CrossRef]
- Su, Y.Y.; Nishimoto, T.; Hoffman, S.; Nguyen, X.X.; Pilewski, J.M.; Feghali-Bostwick, C. Insulin-like growth factor binding protein-4 exerts antifibrotic activity by reducing levels of connective tissue growth factor and the C-X-C chemokine receptor 4. FASEB Bioadv. 2019, 1, 167–179. [Google Scholar] [CrossRef]
- Smith, Y.E.; Toomey, S.; Napoletano, S.; Kirwan, G.; Schadow, C.; Chubb, A.J.; Mikkelsen, J.H.; Oxvig, C.; Harmey, J.H. Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer. BMC Cancer 2018, 18, 1016. [Google Scholar] [CrossRef]



| YA/MC | PA/MC | ||||||
|---|---|---|---|---|---|---|---|
| Accession | Description | Protein Name | Unique Peptides | p Value | Ratio | p Value | Ratio |
| P00742 | FA10 | Coagulation factor X | 9 | 7.7 × 10−4 | 4.8 | 1.4 × 10−3 | 7.7 |
| P22692 | IBP4 | Insulin-like growth factor- binding protein 4 | 2 | 6.7 × 10−2 | 3.9 | 2.5 × 10−2 | 5.4 |
| Q9UK55 | ZPI | Protein Z-dependent protease inhibitor | 7 | 9.6 × 10−3 | 4.2 | 6.7 × 10−3 | 5.1 |
| P01766 | HV313 | Immunoglobulin heavy variable 3-13 | 1 | 2.4 × 10−2 | 5.1 | 2.8 × 10−2 | 4.5 |
| P05452 | TETN | Tetranectin | 9 | 1.2 × 10−4 | 2.3 | 7.1 × 10−6 | 2.8 |
| P08697 | A2AP | Alpha-2-antiplasmin | 3 | 6.6 × 10−3 | 2.6 | 1.4 × 10−2 | 2.2 |
| P04196 | HRG | Histidine-rich glycoprotein | 15 | 1.8 × 10−3 | 1.9 | 1.2 × 10−3 | 1.9 |
| P03952 | KLKB1 | Plasma kallikrein | 16 | 6.0 × 10−5 | 1.8 | 5.6 × 10−4 | 1.8 |
| P00747 | PLMN | Plasminogen | 36 | 9.4 × 10−6 | 1.9 | 3.9 × 10−5 | 1.7 |
| P20851 | C4BPB | C4b-binding protein beta chain | 5 | 1.5 × 10−2 | 1.5 | 1.6 × 10−3 | 1.7 |
| P02743 | SAMP | Serum amyloid P-component | 10 | 4.6 × 10−4 | 1.5 | 7.3 × 10−5 | 1.7 |
| O14791 | APOL1 | Apolipoprotein L1 | 6 | 5.9 × 10−4 | 1.7 | 1.0 × 10−3 | 1.6 |
| P05155 | IC1 | Plasma protease C1 inhibitor | 12 | 4.6 × 10−3 | 1.3 | 2.6 × 10−3 | 1.5 |
| Q13790 | APOF | Apolipoprotein F | 3 | 1.4 × 10−2 | 1.6 | 9.8 × 10−1 | 1.0 |
| O95445 | APOM | Apolipoprotein M | 4 | 8.3 × 10−3 | 0.6 | 7.8 × 10−1 | 0.9 |
| P55056 | APOC4 | Apolipoprotein C-IV | 4 | 1.2 × 10−2 | 0.6 | 2.2 × 10−1 | 0.8 |
| P03951 | FA11 | Coagulation factor XI | 31 | 9.5 × 10−1 | 1.0 | 1.2 × 10−2 | 0.7 |
| Q9BXR6 | FHR5 | Complement factor H-related protein 5 | 5 | 1.7 × 10−2 | 0.5 | 9.0 × 10−2 | 0.6 |
| P81605 | DCD | Dermcidin | 5 | 6.3 × 10−3 | 0.5 | 3.1 × 10−2 | 0.5 |
| Q02413 | DSG1 | Desmoglein-1 | 14 | 3.8 × 10−2 | 0.2 | 3.4 × 10−1 | 0.5 |
| P02649 | APOE | Apolipoprotein E | 27 | 1.9 × 10−3 | 0.5 | 2.1 × 10−4 | 0.4 |
| Q14520 | HABP2 | Hyaluronan-binding protein 2 | 16 | 1.8 × 10−4 | 0.4 | 7.7 × 10−4 | 0.4 |
| P35542 | SAA4 | Serum amyloid A-4 protein | 4 | 9.4 × 10−5 | 0.4 | 2.4 × 10−5 | 0.4 |
| P12259 | FA5 | Coagulation factor V | 6 | 2.7 × 10−2 | 0.3 | 8.5 × 10−5 | 0.3 |
| P06702 | S10A9 | Protein S100-A9 | 5 | 9.8 × 10−2 | 0.2 | 2.4 × 10−2 | 0.2 |
| P04406 | G3P | Glyceraldehyde-3-phosphate dehydrogenase | 5 | 4.0 × 10−3 | 0.3 | 9.6 × 10−4 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Gavilán, F.; Cerqueira, A.; Arias-Mainer, C.; Peñarrocha-Oltra, D.; Salavert-Martínez, C.; Bernabeu-Mira, J.C.; García-Arnáez, I.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Effects of Different Titanium Anodized Surfaces on Peri-Implant Soft Tissue Healing Around Dental Abutments: In Vitro and Proteomic Study. Appl. Sci. 2025, 15, 7349. https://doi.org/10.3390/app15137349
Romero-Gavilán F, Cerqueira A, Arias-Mainer C, Peñarrocha-Oltra D, Salavert-Martínez C, Bernabeu-Mira JC, García-Arnáez I, Elortza F, Gurruchaga M, Goñi I, et al. Effects of Different Titanium Anodized Surfaces on Peri-Implant Soft Tissue Healing Around Dental Abutments: In Vitro and Proteomic Study. Applied Sciences. 2025; 15(13):7349. https://doi.org/10.3390/app15137349
Chicago/Turabian StyleRomero-Gavilán, Francisco, Andreia Cerqueira, Carlos Arias-Mainer, David Peñarrocha-Oltra, Claudia Salavert-Martínez, Juan Carlos Bernabeu-Mira, Iñaki García-Arnáez, Félix Elortza, Mariló Gurruchaga, Isabel Goñi, and et al. 2025. "Effects of Different Titanium Anodized Surfaces on Peri-Implant Soft Tissue Healing Around Dental Abutments: In Vitro and Proteomic Study" Applied Sciences 15, no. 13: 7349. https://doi.org/10.3390/app15137349
APA StyleRomero-Gavilán, F., Cerqueira, A., Arias-Mainer, C., Peñarrocha-Oltra, D., Salavert-Martínez, C., Bernabeu-Mira, J. C., García-Arnáez, I., Elortza, F., Gurruchaga, M., Goñi, I., & Suay, J. (2025). Effects of Different Titanium Anodized Surfaces on Peri-Implant Soft Tissue Healing Around Dental Abutments: In Vitro and Proteomic Study. Applied Sciences, 15(13), 7349. https://doi.org/10.3390/app15137349






