Abstract
The sustainable and economically viable production of microalgae biomass for biofuels and high-value bioproducts is highly dependent on precise, multi-parametric monitoring of cultivation systems. This review provides a comprehensive overview of current approaches and technological advances in multi-sensor systems applied to photobioreactors, including flow cytometry, IR spectroscopy, RGB sensors, in situ microscopy, and software-based sensors. The integration of artificial intelligence (AI), the Internet of Things (IoT) and metaheuristic algorithms into monitoring systems is also discussed as a promising way to optimise key ecological, physicochemical, and biological parameters in real time. The report highlights critical factors that influence biomass growth and product yield, such as nutrient concentrations, light intensity, CO2 levels, pH and temperature. In addition, current technological limitations are highlighted, and future strategies for improving monitoring accuracy, automating cultivation, and improving the biosynthesis of metabolites are outlined. Through a synthesis of the literature and technological trends, this work contributes to the development of smart photobioreactor systems and provides actionable insights to improve large-scale, highly efficient microalgae cultivation in energy and environmental biotechnology.
1. Introduction
One of the promising directions in the development of industrial biotechnology is the production and application of microalgae biomass [1,2]. Compared to other types of plant biomass, microalgae exhibit very high photosynthetic efficiency, fast growth rates, and resistance to various pollutants in their culture environment [3]. In addition, microalgae can be cultivated on land that is unsuitable for traditional agriculture or for investment purposes [4,5]. The wide variety of microalgae strains available enables their use in various fields, including bioenergy production, food production, synthesis of omega-3 fatty acids, animal feed, organic fertilisers, biodegradable plastics, recombinant proteins, pigments, pharmaceuticals, and vaccines [6,7].
Most scientific publications focus on the use of microalgae biomass for the production of biofuels such as biodiesel, bioethanol, biogas, biohydrogen, and aviation fuels [8,9,10,11,12]. However, the realisation of efficient and economically viable technologies for the production of microalgae biomass and their application in the commercial sector remains a major challenge [13]. Current issues facing researchers, engineers and operators of microalgae-based energy systems include the development of efficient cultivation protocols, methods for concentrating and separating the biomass from the culture medium, dewatering and drying processes, and techniques for extracting valuable bioproducts [14].
A crucial aspect is the identification and selection of factors that influence the intensification of microalgal biomass growth and the increase in the yield of target substances, including the optimisation of the chemical and physical parameters of the cultivation environment [15]. Based on previous research, an important prerequisite for the further development of microalgae cultivation on an industrial scale is the development of solutions that are both economically and technologically feasible and at the same time ecologically sustainable [16]. A key factor in improving cultivation efficiency is the provision of optimal conditions in photobioreactors (PBRs) [17]. This goal can be achieved primarily through the development and implementation of advanced systems for monitoring, control, and automation of microalgae culture processes [18].
For industrial-scale systems, especially for large-scale operations, the use of online monitoring is recommended. This enables real-time process monitoring and allows a rapid response to fluctuations, directly improving cultivation performance [19]. Such monitoring also contributes to greater efficiency in the removal of contaminants and the production of high-quality end products. The implementation of effective monitoring and control systems requires the integration of reliable sensors capable of continuously measuring the environmental conditions, the physico-chemical parameters of the culture medium and the biological variables related to the processes within the PBRs [20]. The current focus is on the development of intelligent systems based on Internet of Things (IoT) technologies, artificial intelligence (AI) algorithms, and process automation using advanced sensors and predictive algorithms. There is also a growing interest in smart PBRs that are able to regulate culture conditions in real time. The dynamic development of these technologies and the lack of comprehensive studies integrating the latest monitoring solutions justify the need for this overview.
The aim of this study is to present the current state of knowledge, scope, approaches, and methods for system monitoring of intensive production of microalgae and the detection of target and valuable end products that accumulate in the biomass. In the following chapters, a biobiological analysis of the analysed topic, the application directions and economic use of microalgae are presented, and the parameters of the microalgae cultivation process and the environmental conditions that require close monitoring are described, as well as the tools used. In addition, current and future solutions in the field of monitoring and automated control methods are presented, which will allow an intensification of the metabolic processes specific to microalgae and consequently improve the overall technological and economic efficiency of the production system.
2. Bibliographical Analysis, Research Directions, and Scientific Potential
The optimisation of microalgal biomass production has received increasing attention in recent years. This trend is evidenced by the results of analyses of resources available in major scientific databases and scholarly publications. In the present study, an evaluation of existing research trends in this field was conducted based on statistics obtained from searches of selected terms. The analysis included the following keywords: “optimisation of microalgae production,” “monitoring of microalgae cultivation,” “on-line monitoring of microalgae cultivation,” and “automisation of microalgae production” over the period 2010–2024 (Figure 1).
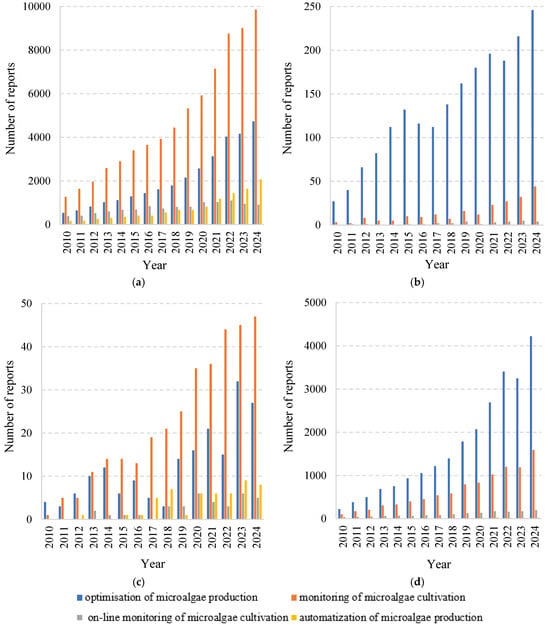
Figure 1.
Article search results in (a) Google Scholar, (b) Scopus, (c) Scilit, and (d) Science Direct between 2010 and 2024 for the keywords “optimisation of microalgae production”, “monitoring of microalgae cultivation”, “on-line monitoring of microalgae cultivation” and “automisation of microalgae production”. Accessed 6 April 2025.
Considering the number of references found in the databases Google Scholar, Scopus, Scilit, and Science Direct, it is evident that this topic remains relevant, and the growing number of reports on monitoring systems for microalgae cultivation demonstrates the sustained interest of researchers, academic institutions, and commercial entities worldwide.
Google Scholar contains the highest number of mentions of the term “monitoring of microalgae cultivation,” which increased from 1270 in 2010 to 9860 in 2024. Similarly, in the Scilit database, this keyword appears most frequently among the terms analysed, with mentions increasing from 1 in 2010 to 47 in 2024. In the remaining databases, the most significant changes were observed for the term “optimisation of microalgae production.” Between 2010 and 2024, the number of mentions increased from 27 to 246 in Scopus and from 222 to 4221 in Science Direct. Notably, no entries were found in Scopus for the term “automatization of microalgae production.”
3. Application Sectors and Valuable Products from Microalgae Biomass
Technologies related to the production and utilisation of microalgae biomass are increasingly seen as promising solutions that can be applied in various economic sectors as well as in the field of environmental protection [21]. Previous studies have shown that microalgae-based systems can be effectively used in wastewater and leachate treatment, waste and sewage sludge neutralisation, carbon dioxide biostorage, biogas upgrading, and flue gas cleaning [22,23,24,25,26,27].
Microalgae biomass is also a promising raw material for the production of renewable fuels, the use of which is associated with a significant reduction in pollutant emissions compared to conventional fossil fuels [28]. Figure 2 shows the generalised use of microalgae biomass. The main energy carriers obtained from microalgae include biodiesel produced from the lipids accumulated in their cells, hydrogen produced by photobiological processes, biomethane produced by fermentation processes, and biomass that can be used by pyrolysis, gasification, or direct combustion technologies [29,30,31]. The application of technologies based on microalgae biomass in the broader context of environmental protection is shown in Table 1.
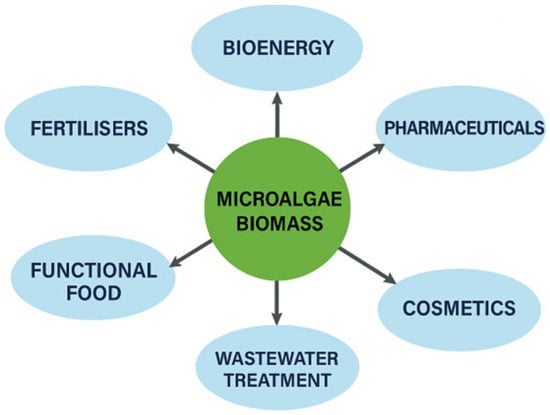
Figure 2.
Use of microalgae biomass.

Table 1.
Utilisation of microalgal biomass in environmental engineering and bioenergy.
An important direction in the utilisation of microalgae is their use as a substrate for the production of numerous economically valuable chemical compounds, which increases both the economic and environmental benefits of these technologies [73,74]. Microalgae are a rich source of carbohydrates, proteins, fatty acids, enzymes, and dietary fibre [75]. Its biomass provides almost all essential vitamins, including A, B1, B2, B6, B12, C, E, biotin, niacin, folic acid, and pantothenic acid, and it is also characterised by a balanced composition of micronutrients such as sodium, potassium, calcium, magnesium, iron, and zinc [76,77].
One of the most important products from microalgae is pigments, which are widely used in the cosmetics and food industries. The most commonly used pigments include phycocyanin, a blue pigment produced by cyanobacteria; phycoerythrin, a red pigment synthesised by red algae; and astaxanthin, produced by green algae [78,79]. In addition, certain microalgae have the ability to biosynthesise fatty acids, including γ-linolenic acid (18:3 ω6), arachidonic acid (20:4 ω6), eicosapentaenoic acid (EPA, 20:5 ω3), and docosahexaenoic acid (DHA, 22:6 ω3) [80]. Since long-chain polyunsaturated fatty acids are not synthesised by higher plants, animals, or humans, their presence in the diet is essential [81]. The main uses of the biomass of selected microalgae species grown under controlled conditions for industrial purposes are listed in Table 2.

Table 2.
Summary of selected economically valuable final products obtained from microalgae biomass.
Both the bibliometric data presented and the overview of the applications and products derived from microalgae biomass are crucial for a proper understanding of the need to further develop optimisation and monitoring methods in the cultivation technologies of these organisms. The analysis of publication trends and research activity in global databases clearly shows the growing interest of the scientific and industrial communities in issues related to the intensification of microalgae production, process automation, and the implementation of tools for monitoring physicochemical and biological parameters. At the same time, the diverse applications of microalgae in the energy, food, pharmaceutical, cosmetics, and environmental sectors, as well as the growing number of valuable substances that can be extracted from their biomass, emphasise the enormous economic potential of these organisms. However, for this potential to be effectively exploited on an industrial scale, advanced strategies for optimising cultivation conditions and precise, multi-parametric monitoring systems must be developed and implemented. In this context, the remaining parts of the thesis focus on discussing the key factors that influence the efficiency and composition of microalgae biomass, as well as advanced technologies that enable their control and regulation. The aim is to show how integrated monitoring and optimisation strategies can help to increase the efficiency, sustainability, and cost-effectiveness of microalgae-based biotechnologies.
4. Optimisation of the Microalgae Biomass Cultivation and Valuable Products Synthesis
In order to achieve the desired technological effect in the cultivation of microalgae and to obtain valuable end products, it is essential to create and maintain suitable incubation conditions for the biomass. This includes modifying the physical and chemical parameters of the culture medium as well as the operating conditions of the production process. These modifications include the regulation of temperature, oxygen and carbon dioxide concentration, illumination type, photoperiod, and radiation intensity [99]. Modern technologies also utilise genetic engineering methods that allow the DNA of microalgae to be modified in order to increase the production efficiency of certain compounds [100].
Induction methods for the accumulation phase of valuable products in microalgae cells usually involve limiting the availability of essential building blocks such as nitrogen, phosphorus, or silicon (in the case of diatoms), which leads to a halt in cell division and activation of biosynthetic pathways [101]. It is important to maintain a balance between the growth rate of biomass and the efficiency of the accumulation of storage substances, which can be achieved by two-stage cultivation strategies [102].
Another approach to increase autotrophic biomass growth is the appropriate design of PBRs. For example, automated systems equipped with sensors that orientate the PBR perpendicular to sunlight can increase the proportion of photosynthetically active radiation and improve photosynthetic efficiency [103]. Alternatively, chemical compounds can be added to the microalgae cultures to influence the metabolic pathways of biosynthesis in order to increase the accumulation of valuable products. For example, studies have shown that the addition of myo-inositol to Dunaliella sp. cultures increases cellular lipid content by 33% [104].
However, challenges to widespread application include the regulatory mechanisms of gene expression and the limited number of microalgae strains suitable for genetic modification. Research to increase the activity of enzymes involved in lipid biosynthesis, such as acetyl-CoA carboxylase, and to stimulate starch storage in mutants is an example of efforts to improve production efficiency [105]. Mathematical models for optimising the cultivation processes of microalgae, such as dynamic flux balance analysis (DFBA), allow the prediction of the effects of changes in illumination and other conditions on the composition of metabolites [106]. These models are validated against experimental data, which increases their accuracy in predicting production yields [107].
An equally important aspect is the understanding of the interactions between microalgae and other microorganisms, especially bacteria. The reciprocal relationships between microalgae and bacteria can improve the growth of microalgae and increase the production of metabolites, including carbohydrates, pigments, and lipids, which opens up new possibilities for optimising cultivation processes [108]. The methods used and described in the literature to optimise the production of microalgae biomass are summarised in Table 3.

Table 3.
Selected optimisation methods used in microalgae biomass cultivation technology.
5. Monitored and Controlled Indicators Critical to Microalgae Cultivation
5.1. The Concentration of Nutrients and Microelements
Nutrient elements such as nitrogen, phosphorus, and iron play a decisive role in the effective growth of microalgae biomass [117]. High nitrogen concentrations in the cultivation medium generally stimulate and accelerate the growth of microalgal biomass, although nitrogen can become a limiting factor under certain conditions, such as the presence of elevated ammonium ion concentrations [118]. Nitrogen deficiency has been shown to significantly reduce microalgal growth rates and overall cultivation efficiency [119]. Similarly, the availability of phosphorus has a significant effect on biomass productivity, and a lack of phosphorus can inhibit the process [120]. The results show that 1 kg of phosphorus can enable the production of about 50 kg of dry microalgae biomass [121].
In contrast to nitrogen and phosphorus, the availability of organic carbon is generally not a limiting factor for the autotrophic growth of microalgae. In heterotrophic or mixotrophic culture systems, however, it can be a decisive limiting factor [122]. In PBRs with high concentrations of organic carbon, the proliferation of competing microorganisms such as bacteria can lead to medium turbidity and reduced light penetration, which directly suppresses the growth of the microalgal biomass [123].
Many microalgae species require external sources of vitamins for intensive growth, including thiamine, biotin, vitamin B12, and riboflavin, as well as purines, pyrimidines, silicon, and other growth-promoting factors [124]. The nutrient ratio in cultivation media such as wastewater is usually C:N:P = 20:8:1, while the optimal ratio for the production of microalgae biomass should be about 106:15:1 [125]. Supplementation with carbon—mainly in inorganic form—is therefore required to ensure adequate quality of the cultivation medium [126]. This can be achieved by increasing CO2 saturation or by introducing leachate from fermentation chambers into the culture system.
5.2. Carbon Dioxide Concentration
The carbonate equilibrium system in natural water comprises three main forms: dissolved CO2 (both physically dissolved and in the form of carbonic acid, H2CO3), HCO3− (bicarbonate), and CO32− (carbonate). The distribution of these species in aqueous solution depends on pH, temperature, and CO2 partial pressure. HCO3− is usually the predominant form of dissolved inorganic carbon (DIC) at pH values between 6 and 9. The typical DIC concentration is between 1 and 5 mmol/L, although it can be significantly higher in hard waters (up to about 10 mmol/L). CO2 (aq), or dissolved carbon dioxide, in equilibrium with the atmosphere (pCO2~400 ppm), is generally found in concentrations of around 0.01–0.04 mmol/L. However, in waters that are rich in organic matter or have a low pH value (e.g., lakes containing humus, waters affected by fermentation processes), concentrations can reach 1–3 mmol/L or even higher in exceptional cases. A concentration of 10 mmol/L is occasionally observed in waters with intensive decomposition of organic substances (e.g., wastewater, anoxic waters), deep groundwater, or fermenting soil sediments. It has been shown that with intensive cultivation, almost 2.0 kg of CO2 must be supplied to the algae per 1 kg of dry biomass produced [127].
5.3. pH Value
The assimilation of carbon dioxide and increased oxygen production by microalgae during photosynthesis can lead to significant fluctuations in pH, with values rising to 10–11 in both open systems and closed PBRs [128]. Although increased pH values can be beneficial as they limit the proliferation of pathogenic organisms and harmful microbes in the cultivation medium, they can also lead to reduced growth rates of the microalgae. Therefore, it is important to maintain a controlled pH in the culture systems, with the recommended range being between pH 6 and pH 8 [129]. While certain eurybiontic strains tolerate a broader pH range, studies have shown that the metabolic activity of microalgae belonging to the genus Chlorella is significantly inhibited when the pH falls below 5 or rises above 9 [130].
5.4. Temperature
The optimal temperature range for most microalgae species is between 20 °C and 35 °C [131]. However, maintaining such conditions is often a challenge, especially in open cultivation systems in temperate climates. Climatic conditions may necessitate the operation of bioreactors at lower temperatures or the use of heating systems, which in turn affects the economic efficiency of biomass production [132]. Studies have shown that increasing the cultivation temperature from 25 °C to 30 °C can double the growth rate of biomass and improve the efficiency of pollutant removal from wastewater [133]. Conversely, other studies have shown that psychrophilic microalgae can effectively participate in the treatment process at temperatures as low as 15 °C [134].
Excessive temperature and light intensity can inhibit the growth of microalgae populations [135]. In many regions, lowering the temperature in biological systems represents a major technological challenge and is associated with considerable capital and operating costs. Various methods are available for temperature regulation, including heat exchangers and cooling systems with water spraying. However, these technologies are often expensive, especially for highly productive, large-scale culture systems [136]. An alternative approach could be the careful selection of microalgae species whose thermal optimum is adapted to the environmental conditions of a specific region [137].
5.5. Source and Intensity of Lighting
Light is a key factor influencing the efficiency of biomass production by microalgae [138]. Undoubtedly, sunlight is the most suitable energy source for the cultivation of microalgae [139]. However, solar energy is only available during the day, and its intensity varies considerably depending on the season and atmospheric conditions, such as cloud cover. In temperate climates, the intensity of natural sunlight also varies throughout the year [140]. In most microalgae species, photosynthetic activity increases at light intensities of 200 to 400 μmol E/m2-s, which corresponds to about 10% of the maximum direct sunlight they can receive [141]. Compared to land plants, microalgae generally require less light. Chlorella vulgaris, for example, grows well at an illumination of 50–100 W/m2, which corresponds to about 232–465 μmol E/m2-s in the Photosynthetically Active Radiation (PAR) range (13,000 to 26,000 lux) [142]. For many species, the optimal lighting conditions are in the range of 5000 lux (90 μmol/m2-s) to 13,000 lux (230 μmol/m2-s) and temperatures between 17–20 °C [143].
In recent years, daylight technology has developed considerably and includes modern optical fibre systems that enable their application in devices for intensive CO2 biosynthesis and wastewater treatment with microalgae [144]. As sunlight is only available during the day, hybrid systems that combine natural light with LED lighting have proven to be the optimal solution to ensure the continuous operation of CO2 capture and wastewater treatment systems.
Photoinhibition can occur during the midday hours, when light intensity can reach up to 4000 μmol E/m2-s. In diatoms, this phenomenon has been observed at a light intensity of about 10,000 lux (180 μmol E/m2-s), and similar results have been reported for cyanobacteria [145]. Planktothrix agardhii (formerly Oscillatoria), for example, exhibits a photoinhibition threshold above 180 μmol E/m2-s [146]. This effect is particularly pronounced in starter ponds, where the low concentration of microalgae biomass is directly exposed to intense solar radiation and the lack of self-shading of the cells promotes photoinhibition.
5.6. Oxygen Concentration
High concentrations of dissolved oxygen, especially in combination with intense lighting, can lead to photoinhibition, which significantly reduces photosynthetic efficiency and decreases the productivity of the microalgal biomass [147]. An increase in oxygen levels to 29 mg/L can lead to a significant decrease in photosynthetic activity—studies have shown that photosynthetic efficiency can decrease by up to 98% under such conditions [148]. These changes can trigger oxidative stress, which leads to cell damage in microalgae and has a negative effect on their growth.
In addition, increased oxygen levels favour the development of parasitic organisms such as Chytridium spp. fungi, which can infect microalgae and further impair cultivation performance [149]. In such cases, an effective strategy is to control the oxygen concentration in the culture system, which can be achieved by periodically reducing the oxygen content [150]. These measures can limit the growth of aerobic parasites and improve the overall efficiency of microalgae biomass production in PBRs. Regular monitoring and control of oxygen levels in combination with optimised cultivation conditions can help to minimise the risks associated with photoinhibition and infection. Table 4 shows a number of important environmental parameters that influence the growth efficiency of microalgae biomass and the production of economically valuable end products.

Table 4.
Range of applied technological and environmental parameters for biomass production of selected microalgae species.
6. Methods of Microalgae Cultivation Monitoring
Physicochemical variables monitored online in microalgae cultivation systems are summarised in Table 5. Figure 3 shows a schematic of the PBR system with multi-parameter monitoring.

Table 5.
Controlled variables in microalgae cultivation.
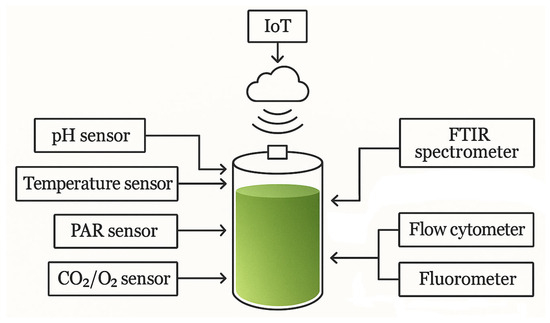
Figure 3.
PBR system with multiparameter monitoring.
6.1. Light Monitoring
The type of illumination used, the light intensity reaching the microalgae culture, the wavelength, and the photoperiod are decisive factors that largely determine the technological efficiency of the cultivation process for photosynthetic species [154]. The optimal light intensity for most microalgae is between 50 and 500 μmol photons/m2-s, which corresponds to photosynthetically active radiation (PAR) in the range of 400 to 700 nm. Direct sunlight can provide up to 2000–2500 μmol photons/m2-s on a clear day, which corresponds to 100–200 klux in equatorial regions [154]. On cloudy days, this value can drop to 20–50 klux, which is still optimal for efficient production of microalgae biomass. It should be noted that the conversion of illuminance expressed in lux (lx) to μmol photons/m2-s within the PAR range requires consideration of the type of light source and a conversion factor that depends, among other things, on the source. Typical conversion factors (lx/μmol photons/m2-s) are around 54–57 for sunlight, 74–80 for white fluorescent lamps, 60–75 for white LEDs, and 120–150 for sodium lamps. PAR accounts for about 40% (in W/m2) of the visible solar spectrum [154].
Among the sensors used to monitor microalgae culture under controlled conditions, PAR photometers play a key role [155]. The data obtained with this light detection method provides precise information about the wavelength range specifically used by microalgae and cyanobacteria for photosynthesis, which in turn affects biomass growth and the accumulation of target cellular metabolites [155]. Another way to describe the lighting system is to use the correlated colour temperature, expressed in Kelvin (K) [156]. This approach is considered simple, facilitates standardisation of the culture, saves energy, and can be used to control the chemical composition of the biomass. It is an effective tool for both research and industrial microalgae cultivation [156].
Common methods for measuring light intensity in microalgae biomass production systems are typical light sensors (photometers) [157]. Depending on the cultivation system, they can measure the light reaching the surface of an open pond or the transparent wall of a closed photobioreactor (PBR). In such cases, the photon flux density that penetrates the active volume of a PBR and reaches a selected point in the culture medium is modelled theoretically [158]. The models are often based on Lambert–Beer’s law with experimentally determined extinction coefficients. The photon flux density is usually measured in the PAR region using planar or spherical quantum sensors [159]. It is important to consider the relationship between the light intensity reaching the cell, the exposure time, and the complex interactions with other process parameters, including mixing, cell concentration, and incident light intensity [160].
However, a crucial variable affecting microalgae growth is the light intensity provided by the operator inside the culture, rather than the intensity falling on the surface of the culture substrate or the transparent wall of the PBR. The illumination intensity is attenuated before it reaches the microalgae cells due to absorption and self-shading effects caused by dense cultures inside the bioreactor [161]. Modern measurement systems allow the continuous monitoring of the light intensity reaching the cells of the growing microalgae population and the tracking of temporal changes in this parameter. Underwater light metres and photometers are used for this purpose. A typical sensor for underwater measurements is the Apogee SQ-520 (Apogee Instruments, Logan, UT) [155]. This digital PAR sensor is mainly used in PBR monitoring, where precise control of light intensity is crucial for optimising the photosynthesis of microalgae. Its application enables integration into automated cultivation systems, providing continuous real-time data recording and allowing the control system to regulate the activation of the light sources within the PBR. It has digital calibration and responds quickly to changes in light intensity, especially in PBR systems with variable illumination [155]. Another example is the LI-COR LI-190R sensor (Lincoln, NB, USA), which is equipped with a photodiode and high sensitivity and enables accurate measurements in research laboratories and when evaluating the effectiveness of different light sources [162]. The Delta-T Devices (Cambridge, UK) quantum sensor is designed for analysing light intensity in microalgae cultures in open ponds, where light is incident from different angles and is more heterogeneous [163]. Its linear measurement capability enables the recording of light intensity over larger areas, which is particularly useful in open systems with non-uniform illumination. In all cases where measurements are taken underwater, it is critical that the sensors are properly sealed or specifically designed for use in water.
Research is continuing to optimise both the lighting and the methods for its precise measurement. Scientists at Bialystok University of Technology have developed an innovative device for measuring the intensity parameters of optical radiation [164]. This device enables the precise measurement of radiation from modern LED sources, which have a significant proportion of blue light. Such solutions are essential to ensure appropriate light conditions in the cultivation of microalgae. Advanced bioreactors, such as the PhotoBionicCell system developed by Festo (Metamorfosi, Greece), incorporate quantum technology sensors from Q.ANT (Stuttgart, Germany) [165]. These high-precision sensors provide real-time data on the growth of the microorganisms and enable continuous adjustment of the cultivation parameters. A hybrid system has also been developed that combines open ponds with efficient light distribution systems used in PBRs [166]. This approach enables better utilisation of sunlight and thus increases the efficiency of microalgae biomass production. These sensors automatically switch the light sources on or off depending on the ambient brightness. When cultivating microalgae, they can simulate the natural light cycles and thus promote optimal culture growth.
6.2. Culture Temperature Monitoring
The optimum cultivation temperature for microalgae is species- or strain-specific. In general, the optimum range for most strains is between 20 °C and 30 °C. Certain species of cyanobacteria can grow effectively at higher temperatures, up to 35 °C. There are also strains, including genetically modified strains, for which temperatures in the range of 10 °C to 15 °C are sufficient to achieve satisfactory cultivation results [167]. Deviations from the optimum temperature significantly reduce the overall productivity of the biomass and inhibit the synthesis of the desired metabolites by the microalgae. Temperatures exceeding the optimal range should be avoided, as they cause a significant decrease in the quantum efficiency of photosynthesis. In larger cultivation systems, shading, immersion of the PBR in water tanks, or spraying with water are commonly used for temperature control [168]. However, shading reduces productivity. In open ponds and water channels, the temperature is rarely controlled. Low temperatures inhibit the rate of biochemical reactions, which has a direct impact on the technological outcome of the cultivation process.
Temperature is usually measured using a standard industrial Pt-100 sensor (Eppendorf AG, Hamburg, Germany), which is often integrated into control and automation systems in more advanced PBR systems [169]. When the temperature exceeds a threshold, the sensor signal triggers automation functions to stabilise the temperature, including various heating or cooling mechanisms. In lab-scale bioreactors or smaller units for cultivating microalgae, multiparameter sensors that combine temperature measurement with monitoring of other environmental parameters are often used [170]. Sensors for temperature, pH, and suspended solids concentration are often installed and functionally integrated with transducers with RS-485 communication interfaces [171]. This solution enables precise monitoring of the cultivation conditions in real time and allows the operator to react quickly.
Advanced thermostatic temperature measurement systems are less commonly used. Examples include Peltier modules, which transfer heat between two sides of the device in response to an electrical current flow. These modules allow precise temperature measurement and control in the range of 4 °C to 95 °C [172]. Such systems are primarily used in UV/VIS spectrophotometric analyses where temperature control is critical to measurement accuracy. Although they are more commonly used for laboratory analyses, their application in microalgae cultivation can improve environmental control. External thermostatting modules are sometimes used for temperature regulation in PBRs, and such solutions are increasingly being adapted for microalgae cultivation monitoring.
Thermostatting, i.e., the precise control and stabilisation of temperature, is a key factor in microalgae cultivation. Temperature affects the growth rate, photosynthetic efficiency, and biochemical composition of the microalgae biomass [173]. Therefore, the application of appropriate methods for temperature monitoring and regulation is essential for the optimisation of cultivation processes. In open systems, such as open ponds, continuous flow bioreactors, and other open tanks, temperature control is difficult due to direct exposure to ambient conditions. In such cases, the culture temperature depends on the local climatic conditions, which can lead to undesirable fluctuations [109]. In closed cultivation systems, such as various PBR designs, electric heaters are often used, especially in small units. More commonly, temperature-controlled heating and cooling jackets with circulating fluid are used [174]. This approach allows the precise maintenance of optimal thermal conditions inside the PBR regardless of the external conditions. Heat exchangers, both internal and external, are used in bioreactors for various purposes. Their use enables not only the heating of the cultivation vessels but also the rapid removal of excess heat generated by exothermic biochemical reactions during microalgae cultivation. This allows a stable temperature to be maintained, which is crucial for the efficiency of the biological process. In some cases, a hot or cold liquid is injected directly into the culture to achieve the desired temperature [175]. This method is usually used in emergency situations where drastic and rapid thermal adjustments tailored to the needs of a specific culture are required.
6.3. Monitoring of pH Changes in the Culture Medium and CO2 Content
The pH value has a direct effect on the growth rate of the biomass by characterising the environmental conditions specific to the microalgae species and strains. This parameter also influences the availability and utilisation efficiency of the nutrients present in the culture medium. The acceptable pH range for most microalgae species is between 7 and 9, with a typical optimum at 8.2–8.7. Some species are adapted to more acidic or alkaline environments [176].
In intensive photoautotrophic microalgae cultivation carried out at light intensities above the compensation point—where the photosynthetic rate equals the respiration rate—the pH of the culture tends to increase. This occurs because during active microalgae growth there is an efficient uptake of CO2 and HCO3− from the culture medium and an assimilation of nitrates. Conversely, CO2 introduced into the culture medium from outside dissolves easily and forms carbonic acid (H2CO3), which dissociates into bicarbonate (HCO3−) and hydrogen ions (H+). An increase in the CO2 concentration leads to a higher H+ ion concentration and lowers the pH value (acidification). Conversely, a decrease in the CO2 concentration reduces the H+ concentration and increases the pH value [176,177]. Therefore, in practice, the monitoring and stabilisation of the pH value in microalgae culture systems is often combined with the control of the CO2 concentration in the culture medium. Controlled CO2 dosing is often used to prevent excessive pH increase, maintain acid–base balance, and create optimal conditions for microalgae growth [178].
pH is measured with electrodes or optical pH probes and is usually controlled by injecting CO2 into the culture or inlet gas stream. The most commonly used method for pH measurement uses glass electrodes, which are characterised by high precision and fast response time [179]. In biotechnology, including microalgae cultivation, electrodes with pressurised liquid electrolytes and integrated temperature sensors are used to minimise measurement error and ensure stable readings [180]. An alternative to conventional electrodes are optical sensors that utilise the fluorescence properties of pH-sensitive materials [181]. Although they are less widely used, they offer advantages such as lower susceptibility to interference and no need for calibration. pH control with mineral acids can be advantageous in some cases [110]. More advanced pH control algorithms have been investigated to improve the efficiency of CO2 utilisation [182]. Improved pH control through control algorithms reduces the pH gradients within the culture, thereby increasing the average photosynthetic rate and biomass productivity.
Methods for measuring CO2 concentration in the culture medium are based on electrochemical sensors that perform direct CO2 measurements. These sensors recognise changes in the electrical potential in response to the presence of CO2 and thus make it possible to determine the concentration. Optical sensors, on the other hand, utilise the absorption of infrared radiation by CO2 molecules. Changes in absorption correspond to the CO2 concentration, which enables precise measurement. Effective regulation of the CO2 concentration in the culture medium is also possible based on the relationship between pH and carbonate hardness [183]. Monitoring the pH provides information about the CO2 content, while the carbonate hardness determines the buffering capacity of the medium to stabilise the pH in response to changes in CO2 concentration. Proper regulation of these parameters is critical to ensure optimal growth conditions for microalgae.
In cultivation systems with controlled exhaust or flue gas flow, analysing the gas mixture composition allows indirect determination of the CO2 concentration in the culture medium. Precise monitoring of the CO2 concentration in the gases supplying the PBR can be carried out using various measurement methods. Non-dispersive infrared (NDIR) gas analysers make use of the fact that CO2 molecules absorb infrared radiation at certain wavelengths [184]. Measuring the absorption intensity makes it possible to determine the CO2 concentration in a gas sample. This method is highly precise and is often used to monitor biotechnological processes. In gas chromatography, the components of a gas mixture are separated and analysed. The gas sample passes through a chromatographic column in which the individual components, including CO2, are separated. Detectors such as thermal conductivity detectors (TCD) or flame ionisation detectors (FID) with methanisers enable quantitative CO2 determination [185].
Electrochemical gas electrodes measure CO2 by detecting the changes in electrochemical potential caused by the presence of CO2. They are used in biotechnological monitoring but require regular calibration and are sensitive to variations in temperature and humidity [186]. Raman spectroscopy enables the analysis of gas composition by light scattering from molecules [187]. Although less common for routine analyses, it offers simultaneous monitoring of multiple gas components, including CO2. Classical chemical methods, such as titration, involve CO2 absorption in an alkaline solution and subsequent quantification of the absorbed gas [188]. Although they are less precise and more time-consuming than instrumental methods, they are still used in some laboratories.
In biotechnological practice, especially in the cultivation of microalgae, NDIR analysers are frequently used due to their speed and precision [189]. In studies on the utilisation of microalgae biomass for biogas upgrading, for example, monitoring the CO2 content in the exhaust gases is critical for evaluating process efficiency. In addition, controlling the CO2 concentration in hybrid PBR systems, where CO2-enriched air is added to the culture medium, enables the optimisation of growth conditions for the microorganisms [190].
6.4. Monitoring of O2 Concentration in the Culture Medium and Gaseous Metabolites
The analysis of the O2 content in the culture medium of PBRs is a decisive factor for the optimisation of biotechnological processes. Oxygen plays an important role in the metabolism of these organisms. In PBRs, oxygen is present in both the liquid and the gas phase. As a product of photosynthesis, it exerts an inhibitory effect on the growth rate of the culture when the oxygen concentration exceeds 200–500% of the saturation values, i.e., above 15–25 mg O2/L [160]. As a rule, oxygen is continuously removed from the culture medium by aerating the culture with CO2-enriched air or other gas mixtures. This process simultaneously integrates three interdependent functions: removal of O2, supply of CO2, and pH control in the culture system.
The measurement of dissolved oxygen concentration is considered a reliable and sensitive indicator of the physiological state of microalgae in terms of biomass growth rate and productivity of target metabolites [117]. The most commonly used methods for measuring oxygen in the culture medium are oxygen electrodes, such as the Clark polarographic electrode and galvanic electrodes [191]. These work on the principle of oxygen reduction at the cathode and generate an electric current that is proportional to the oxygen concentration in the sample. These methods offer high sensitivity and precision but require regular calibration and can be affected by temperature fluctuations and the presence of other gases.
An alternative to electrodes are optical sensors that utilise oxygen-induced luminescence quenching [192]. This method uses luminescent dyes whose light emission is quenched in the presence of oxygen. The change in luminescence intensity is proportional to the oxygen concentration in the medium. Optical methods are less invasive and do not consume oxygen during the measurement, which is a considerable advantage.
The polarographic analyser (also known as an anodic oxygen analyser) is based on the electrochemical reduction of oxygen at the electrode surface [193]. The oxygen dissolved in the liquid (e.g., water) is reduced at the cathode and generates an electric current that is proportional to the oxygen concentration in the sample. Changes in the electrode voltage or current allow the oxygen to be quantified. In polarography, the voltage fluctuations due to the interaction of the oxygen with the electrolyte are measured, and the current fluctuations are used to determine the oxygen content. Polarographic oxygen analysers such as the YSI ProDSS (YSI, Yellow Spring, OH, USA), the Orion 9830/9835 (Boston, MA, USA), and the AquaSensors O2 Sensor (Thermo Scientific, Beverly, MA, USA) are widely used in applications that require precise measurement of dissolved oxygen, especially in microalgae cultivation, aquaculture, the food industry, and environmental monitoring. They are valued for their accuracy, ease of use, and ability to continuously monitor water quality.
Classical chemical methods, such as Winkler titration, involve the chemical binding of oxygen and subsequent titration of the reaction products [194]. Although these methods are less practical and more time-consuming than instrumental methods, they are still used in some laboratory studies. Sensor and analyser measurements are subsequently used to regulate the air flow or other gas supply to the culture.
The oxygen content in the exhaust gas can also be measured using a paramagnetic gas analyser [195]. The paramagnetic analyser makes use of the unique magnetic properties of oxygen. Oxygen is a paramagnetic gas, i.e., its molecules have unpaired electrons that react to magnetic fields. Paramagnetic oxygen (O2) interacts with an external magnetic field, which attracts the oxygen molecules. The analyser uses a magnet and a detector to measure changes in the attraction or repulsion of oxygen molecules. The movement of the oxygen molecules in the magnetic field depends on their concentration in the gas sample. Changes in the magnetic force are proportional to the amount of oxygen and enable precise measurement [195].
When cultivating microalgae, especially in closed systems, monitoring the oxygen concentration is critical to ensure optimal growth conditions. An excess of oxygen can lead to photooxidation of the chlorophyll, which has a negative effect on the efficiency of photosynthesis. Conversely, a lack of oxygen can limit the metabolic processes of microalgae [196]. Therefore, the choice of oxygen measurement method depends on the specifics of the cultivation and the availability of equipment.
The choice of a method for analysing oxygen in microalgae culture media depends on factors such as the required precision, the speed of measurement, and the accessibility of the equipment. Electrochemical and optical methods are most commonly used due to their accuracy and ability to continuously monitor the process. Chemical methods are more time-consuming but still serve as valuable tools in certain laboratory applications.
6.5. Monitoring of Nutrient Content in the Culture Medium
The determination of nutrient concentrations in microalgae culture media is a key element of quality control of the culture environment, as it directly affects the efficiency of microalgae growth and biomass production. Nutrient analysis methods include a variety of techniques, including spectrophotometry, chromatography, flame photometry, and electrochemical methods. Each method is used depending on the type of substance being analysed [197].
Nitrogen compounds such as ammonium nitrogen (NH4+), nitrate nitrogen (NO3−), and nitrite nitrogen (NO2−) are of crucial importance in the cultivation of microalgae, as these microorganisms intensively utilise nitrogen in their metabolic processes [198]. Ammonium nitrogen can be determined by a colourimetric method, in which ammonia reacts with reagents such as phenol, or by an electrochemical method using ion-selective electrodes. Nitrate and nitrite nitrogen are usually measured spectrophotometrically using the reaction with the Griess reagent, which produces a characteristic coloured compound. Ion-selective electrodes are also used for these types of nitrogen. To determine the total nitrogen, the Kjeldahl method is used, in which the sample is digested with sulphuric acid and the ammonium nitrogen is then quantified, or the persulphate method, in which the nitrogen in the sample is oxidised to nitrate and then measured spectrophotometrically.
Phosphorus is another important nutrient in microalgae culture media, which is usually determined as orthophosphate (PO43−). The standard method for phosphorus determination is the molybdenum blue method, in which orthophosphates react with ammonium molybdate under acidic conditions to form a blue-coloured complex. The colour intensity is measured spectrophotometrically, normally at 880 nm. Other spectrophotometric reagents can be used to increase selectivity in the presence of interfering substances. Total phosphorus can also be measured spectrophotometrically after the sample has been mineralised with sulphuric acid [199].
Iron, an essential micronutrient, must also be monitored in microalgae cultures. Iron in the forms Fe2+ and Fe3+ is usually determined spectrophotometrically, where Fe2+ reacts with 1,10-phenanthroline to form a red complex. The absorbance of this complex is measured at 510 nm. Alternatively, a colourimetric method using 2,2′-bipyridyl reagent, which forms a characteristic colour with iron, is also used [200].
The chemical oxygen demand (COD) is a measure of the oxygen demand of water due to the oxidation of organic and inorganic compounds. The manganese method is most commonly used for COD determination, in which organic compounds are oxidised with potassium permanganate and the reagent consumption is measured spectrophotometrically. This is a standard method for analysing COD in water. Alternative methods include rate-based approaches that monitor the changes in oxygen concentration during the oxidation reaction [201].
Biochemical oxygen demand over 5 days (BOD5) quantifies the oxygen consumption by microorganisms during a 5-day incubation at 20 °C. The standard method involves incubating the sample for 5 days and measuring the oxygen consumption before and after incubation. This method is time-consuming but accurate. A faster approach is the respirometric method, which allows real-time monitoring of oxygen consumption [202].
Potassium, an important macronutrient in microalgae cultivation, is often determined using flame photometry. The sample is exposed to a flame, which excites potassium atoms that emit light at a characteristic wavelength. The emission intensity is proportional to the potassium concentration in the sample [203]. Another method is inductively coupled plasma optical emission spectroscopy (ICP-OES), which enables the simultaneous determination of potassium and other micronutrients.
Micronutrients such as copper (Cu), zinc (Zn), manganese (Mn), molybdenum (Mo), and others are mainly analysed using spectroscopic techniques. The most commonly used method is ICP-OES (inductively coupled plasma optical emission spectroscopy), which enables the simultaneous analysis of several elements in water or culture media. Other popular methods include atomic absorption spectroscopy (AAS), which measures the absorption of light by gaseous metal atoms, and high-performance liquid chromatography (HPLC), which is used to analyse inorganic micronutrients.
All the above methods of nutrient analysis for microalgae culture media are supported by modern analytical models such as the Thermo Scientific iCAP 7400 ICP-OES (Beverly, MA, USA), PerkinElmer Optima 8300 ICP-OES (Waltham, MA, USA), Shimadzu AA-7000 AAS (Tokyo, Japan), and Hach DR 5000 UV-VIS spectrophotometer (Düsseldorf, Germany) [204]. These instruments provide high accuracy and precision in nutrient analysis and enable effective monitoring of cultivation conditions and optimisation of microalgae growth processes.
6.6. Dynamics of Growth, Concentration, and Biomass Composition
There are numerous analytical methods for assessing these parameters, which provide precise information on the growth rate of microalgae and their biochemical composition. Monitoring these parameters is not only important in scientific research but also in industry, particularly in biotechnology, biofuel production, and water treatment.
One of the basic methods for monitoring the growth dynamics of microalgae biomass is the measurement of optical density using spectrophotometers. In this technique, the absorption of light by the microalgae sample is measured at a specific wavelength, typically 680 nm, which corresponds to the characteristic absorption of chlorophyll. Changes in absorbance over time allow an estimation of the growth rate of the microalgae, as higher biomass leads to higher absorbance. A commonly used instrument for this method is the Hach DR 6000 UV-Vis spectrophotometer (Düsseldorf, Germany), which allows fast and precise absorbance measurements in different spectral ranges. Another widely used method is dry weight measurement, where the microalgae biomass sample is dried in an oven to a constant weight. Although this method is more time-consuming, it is considered one of the most accurate methods for determining total biomass. It can be combined with chemical analyses to determine the content of specific components such as carbohydrates, proteins, lipids, and others. High-precision analytical balances such as the Mettler Toledo XPR6 (Greifensee, Switzerland) are used for this purpose [20].
Optical turbidimetric sensors that measure the turbidity of the culture medium are also used to monitor the biomass concentration in real time. Biomass growth correlates with increasing turbidity, as microalgae particles scatter the light and thus cause higher turbidity values. Devices such as the OTT Optic 410 (Kempten, Germany) or ABB Turbidity Analyser (Zurich, Switzerland) are used to continuously monitor turbidity in the culture medium [205]. Fluorometric methods for quantifying biomass based on chlorophyll fluorescence measurement are another technique for evaluating the growth dynamics of microalgae. Microalgae absorb light at specific wavelengths and emit fluorescence proportional to their biomass content. Fluorometric analysers such as the Trilogy Fluorometer from Turner Designs allow accurate monitoring of chlorophyll fluorescence and provide a rapid and non-destructive method for assessing the status of microalgae culture [206].
For more detailed analyses of the biochemical composition of the microalgae biomass, techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are used. These methods enable the precise determination of various biomass components, including lipids, proteins, carbohydrates, and pigments such as chlorophylls, carotenoids, and other bioactive compounds. Instruments such as the Agilent 1200 HPLC system enable precise separation and analysis of biochemical components, while mass spectrometers such as the Thermo Fisher LTQ Orbitrap XL (Cleveland, OH, USA) allow detailed identification and quantification of individual compounds [207].
Elemental analysers for carbon/hydrogen/nitrogen (C/H/N), such as the Elementar Vario EL Cube (Hanau, Germany), are used to determine the organic carbon content in the microalgae biomass. These devices allow accurate quantification of carbon in the samples, which is essential for the evaluation of photosynthetic efficiency and biomass production [208]. Regarding the composition of microelements in biomass, techniques such as inductively coupled plasma optical emission spectroscopy (ICP-OES) and atomic absorption spectroscopy (AAS) are used. These methods enable precise quantification of micronutrients such as copper, zinc, manganese, molybdenum, and iron. Instruments such as the PerkinElmer Optima 8300 ICP-OES (Waltham, MA, USA) and Analytik Jena AAS (Jena, Germany) enable reliable determination of these elements, which is particularly important in studies investigating the influence of trace elements on the growth and composition of microalgae [209].
To summarise, a variety of methods—including spectrophotometry, fluorometry, chromatography, mass spectrometry, and microelement analysis—are used to comprehensively monitor the biomass, growth rate, concentration, and biochemical composition of microalgae. Supported by modern analytical instruments, these technologies enable effective management of microalgae cultures, which is critical for industrial and research applications in biotechnology.
6.7. Photosynthetic Activity
Numerous devices are available on the market for analysing the photosynthetic activity of microalgae using various measurement techniques such as chlorophyll fluorescence, gas exchange, and electron transport measurement in photosystems. A popular instrument is the bbe Moldaenke Chlorophyll Fluorometer, which allows direct measurement of chlorophyll fluorescence and assessment of the photosynthetic performance of microalgae by analysing the emitted light signal [210]. This fluorometer works by exciting the chlorophyll at specific wavelengths and recording the resulting fluorescence emission. This enables the identification of different microalgae groups based on their characteristic fluorescence signatures. The device also enables the assessment of photosystem II (PSII) efficiency and parameters such as Fv/Fm (variable fluorescence relative to maximum fluorescence), which are important indicators of the photosynthetic state of microalgae and their response to changing environmental conditions. By using multiple excitation wavelengths, the bbe Moldaenke Chlorophyll Fluorometer can distinguish the major microalgae groups, including cyanobacteria, green algae, diatoms, and cryptomonads. This instrument is widely used in ecotoxicological studies, surface water monitoring, and controlled microalgae cultures, where rapid and non-destructive analysis of photosynthetic activity is essential for optimising growth conditions and assessing the health of the culture. It can be operated online, enabling continuous monitoring of cultures in real time, which is especially useful in industrial PBRs and in applications related to bioremediation and the production of biofuels from microalgae.
Similar opportunities are offered by pulse amplitude modulated (PAM) fluorometers such as the Walz WATER-PAM and the MINI-PAM-II, which measure the variability of chlorophyll fluorescence in response to light of different intensities, enabling the assessment of PSII efficiency and microalgae response to environmental stress [211]. Another solution is the Turner Designs Trilogy Fluorometer, which allows the measurement of chlorophyll fluorescence at different wavelengths and can be used to analyse the biomass and photosynthetic activity of phytoplankton [212]. More compact and portable devices include the AquaPen-C 100 from Photon Systems Instruments (PSI) (Drásov, Czech Republic), which enables rapid measurements of photosynthetic parameters under both field and laboratory conditions [213]. Similarly, the Handy PEA from Hansatech Instruments records chlorophyll fluorescence kinetics and allows detailed analysis of photosynthetic dynamics [214]. For more advanced applications, the LI-COR LI-6800 Portable Photosynthesis System (Lincoln, NB, USA) combines the measurement of gas exchange with chlorophyll fluorescence analysis, enabling the simultaneous monitoring of CO2 uptake, oxygen production, and photosynthetic efficiency of microalgae [215]. The choice of a suitable device depends on the specific research objectives, the required measurement accuracy and the experimental conditions.
7. New Approaches to Monitoring and Optimisation of Microalgae Cultivation Systems
Table 6 presents methods for monitoring biological variables in microalgae cultures.

Table 6.
Methods for monitoring microalgae cultivation variables.
7.1. Two-Dimensional and Infrared Spectroscopy
Two-dimensional spectroscopy and infrared (IR) spectroscopy are advanced analytical tools that enable precise monitoring of biotechnological processes, including the cultivation of microalgae. These technologies enable non-invasive analysis of the biochemical composition of biomass and the tracking of metabolic changes in real time. IR spectroscopy is an optical technique that can be used to detect a wide range of organic compounds and thus offers significant potential for online monitoring of bioprocesses [20]. Organic molecules have characteristic signatures in the IR region of the electromagnetic spectrum. The most useful parts of this region for monitoring purposes are the mid-infrared region (MIR; 200–4000 cm−1) and the near-infrared region (NIR; 4000–13,000 cm−1). In addition, NIR and MIR spectroscopic sensors can detect multiple compounds simultaneously [226]. Due to the complex nature of the spectral signals obtained with these techniques, the application of multivariate analysis methods such as principal component analysis (PCA) and partial least squares (PLS) regression is essential to obtain relevant information. NIR spectra or absorbance at specific wavelengths were used to analyse processed and dried microalgae samples.
Two-dimensional spectroscopy, including techniques such as two-dimensional correlation infrared spectroscopy (2D-IR) and two-dimensional optical spectroscopy, enables the analysis of protein, lipid, and carbohydrate structures in microalgae. The use of two-dimensional methods enables the unfolding of overlapping absorption bands and thus improves the differentiation of individual cellular components. Studies suggest that 2D-IR is particularly useful to assess the dynamics of structural changes of proteins and lipids under environmental stress conditions such as nutrient deprivation or elevated CO2 concentrations [227].
Infrared spectroscopy, in particular Fourier transform infrared spectroscopy (FTIR), is frequently used to analyse the biochemical composition of microalgae and facilitates the identification and quantitative assessment of key biomass components such as proteins, lipids, and polysaccharides. FTIR in ATR (attenuated total reflectance) mode enables rapid and non-invasive monitoring of changes in chemical composition during cultivation. Analysing FTIR spectra enables the determination of lipid accumulation in microalgae cells, which is crucial for biofuel production research [228]. Quantitative determination of biomass composition, including proteins, lipids, carbohydrates, or silicates, by FTIR in the range of 4000–700 cm−1 has been performed with microalgae biomass or isolated components [229]. Recently, devices have been developed that integrate spectrometers with bioprocessing units. Two classes of such devices are flow-through ATR cells, where the sample stream flows through the measurement chamber of the FTIR spectrometer [230], and autoclavable optical fibres with ATR crystals at the tip [231]. At least two companies, Mettler Toledo International Inc. and Remspec Corp., have introduced fibre-optic ATR probes to the market.
The integration of spectroscopic techniques into in situ monitoring systems enables the continuous control of cultivation parameters, such as metabolic changes induced by abiotic stress or the adjustment of environmental conditions to optimise the production of desired secondary metabolites. The use of FTIR spectroscopy in combination with multivariate analysis (e.g., PCA) enables the classification of microalgae based on their biochemical profiles, which is valuable in strain selection for improved production performance [232]. In recent years, advances in two-dimensional spectroscopy combined with multiparametric analysis have opened up new possibilities for advanced monitoring of microalgae cultivation processes. The ability to precisely assess biochemical changes at the molecular level while minimising the invasiveness of the analysis makes these techniques extremely valuable tools in microalgae biotechnology. Further research in this area could contribute to improved cultivation efficiency and higher yields of biofuels, pigments, and other industrially important compounds.
7.2. Multiparameter Flow Cytometry
Multiparameter flow cytometry (MFC) is an advanced analytical tool that enables rapid and precise monitoring of microalgae cultivation processes by simultaneously analysing multiple physiological and biochemical parameters of individual cells. The MFC provides information on cell size and cellular components by measuring the light scatter and, if desired, the fluorescence of individual cells stained with fluorescent dyes. Flow cytometry can be used as a rapid and reliable off-line method for monitoring lipid content [233]. This technique allows the assessment of population heterogeneity, the identification of metabolic changes, and the determination of microalgae responses to variable environmental conditions, making it extremely useful for microalgae biotechnology.
A major application of MFC in the cultivation of microalgae is the analysis of cell viability and metabolic activity. The use of fluorophores, which selectively colour cellular structures, enables the simultaneous assessment of membrane integrity, mitochondrial potential, and enzymatic activity. Dyes such as SYTOX Green or propidium iodide allow differentiation between dead and living cells, while the JC-1 probe facilitates the assessment of mitochondrial activity, which is valuable in studies investigating the effects of environmental stress on microalgae [234].
Another important application of MFC in the cultivation of microalgae is the assessment of cell cycle dynamics. The analysis of DNA content with intercalating dyes such as Hoechst 33,342 or DAPI allows the determination of the proportion of cells in different cell cycle phases (G0/G1, S, G2/M). This method can be used to evaluate the proliferation rates of microalgae depending on the cultivation conditions, e.g., changes in nutrient availability or light intensity [235]. MFC is also used to analyse the accumulation of industrially relevant compounds such as lipids, pigments and polysaccharides. Fluorescent dyes such as Nile red and BODIPY 505/515 allow a quantitative assessment of lipid content in individual cells, which is critical when researching microalgae as a raw material source for biofuel production. In addition, the measurement of chlorophyll autofluorescence enables the assessment of photosynthetic efficiency and the overall health of the microalgae [236].
A notable advantage of MFC is the ability to analyse oxidative stress in microalgae cultures, which is important for both basic research and applied studies. Fluorogenic probes such as H2DCF-DA allow the detection of reactive oxygen species (ROS) and facilitate the assessment of microalgae responses to stress factors such as high light intensity, salinity, or the presence of environmental toxins [237].
Flow cytometry methods for monitoring lipid content, enzymatic activity, cell viability, and cell number in microbial biofuel production have been reviewed [238]. Total cellular lipid content was measured after staining with Nile red and calibration of fluorescence signals using standard methods. The cell size and shape distribution of the microalgae were determined by forward scatter measurements in flow cytometry [239]. Due to the staining required, the implementation of at-line and on-line monitoring has been challenging, and, until recently, off-line applications in microalgae cultures have been reported. The first commercial system capable of working on-line in microalgae bioprocessing, including automated sampling and dilution, was announced by FlowCam in January 2013 [231]. Other online flow cytometry monitoring systems, such as the Cytosense flow cytometer [240], are designed for monitoring low to very low cell densities in marine or freshwater ecosystems. In yeast fermentation monitoring, flow cytometry has been used in combination with an autosampler and a flow injection system for sample processing in at-line mode [241].
In recent years, growing interest in the integration of MFC with multivariate data analysis methods such as principal component analysis (PCA) and k-means clustering has enabled more advanced approaches to data interpretation and identification of metabolic patterns in microalgae cultures. The application of these methods in combination with automation and machine learning opens up new possibilities for the precise monitoring and optimisation of microalgae cultivation conditions, which can lead to higher biomass yields and improved production of industrially relevant secondary metabolites.
7.3. Metaheuristic-Based Predictions
Modern technologies for monitoring the cultivation of microalgae increasingly use predictive algorithms based on metaheuristic methods to optimise growth conditions and predict biomass dynamics and metabolite accumulation. Metaheuristics such as evolutionary algorithms, particle swarm optimisation (PSO), and simulated annealing (SA) enable the modelling of complex, non-linear biological processes under variable cultivation conditions.
One of the most important applications of metaheuristic methods in the cultivation of microalgae is the prediction of biomass growth based on multidimensional environmental data, including light intensity, temperature, pH, and nutrient availability. Models based on genetic algorithms (GA) enable the calibration of mathematical models describing microalgae growth and allow accurate prediction of cell proliferation rates under different cultivation conditions [242]. The optimisation of cultivation parameters using metaheuristics is also applied to increase the production yield of secondary metabolites such as lipids, pigments, and polysaccharides. PSO and SA algorithms are used to determine optimal environmental parameters that maximise the content of desired products in the microalgal biomass. Studies indicate that these methods significantly improve the efficiency of biodiesel production by precisely regulating the nitrogen and phosphorus content in the culture medium [243].
In the context of in situ monitoring, metaheuristic algorithms play a crucial role in analysing microscopic images and spectral data that enable automatic classification of microalgae cells based on their morphology and biochemical composition. The use of ant colony optimisation (ACO) algorithms and artificial neural networks optimised by genetic algorithms enables the effective detection of anomalies within microalgae populations and the prediction of environmental stress effects based on image analysis [244]. Another important application of metaheuristics in microalgae monitoring is the prediction of metabolic responses to stress factors. Hybrid models combining evolutionary algorithms with machine learning methods such as random forest (RF) and artificial neural networks (ANN) facilitate the development of accurate predictive models describing the effects of environmental changes on the expression of genes related to lipid and carbohydrate metabolism [245].
The application of metaheuristic methods in the monitoring of microalgae culture opens up new possibilities for the optimisation and automation of biotechnological processes. The integration of predictive algorithms into real-time cultivation control systems enables a significant improvement in the efficiency of biomass production and increases process stability. The further development of these methods, especially in combination with the increasing amount of data from spectroscopic and microscopic imaging methods, can contribute to even more precise bioprocess control and improved commercial microalgae production.
7.4. Internet of Things
The Internet of Things (IoT) is increasingly being used in biotechnology and enables the automation and precise control of cultivation processes. In the context of microalgae cultivation, IoT systems facilitate the monitoring of environmental parameters in real time, the optimisation of growth conditions, and the integration of advanced analytical algorithms to predict biomass growth and the efficiency of secondary metabolite production.
A fundamental advantage of IoT technology for microalgae cultivation is the ability to remotely collect and analyse key environmental data such as temperature, pH, light intensity, CO2 concentration, and nutrient levels. Wireless sensors integrated into IoT platforms enable automatic data transmission to cloud systems, where the data is processed to identify trends and detect potential problems in cultivation [246]. Advanced analytical algorithms and artificial intelligence (AI) used in IoT systems enable the modelling of microalgae growth dynamics and the prediction of optimal cultivation conditions. Machine learning (ML) algorithms in particular analyse historical data to predict changes in microalgae populations and their metabolism. This facilitates the implementation of adaptive bioreactor control strategies that automatically adjust environmental parameters in response to changing cultivation conditions [247].
The application of the Internet of Things (IoT) significantly improves the efficiency of cultivation monitoring through the integration of sensors with mobile devices and cloud platforms. Modern IoT-based control systems utilise communication protocols such as MQTT (message queuing telemetry transport) and LoRaWAN (long range wide area network), which enable reliable data transmission from bioreactors to central databases even with limited connectivity [248]. An important aspect of IoT utilisation in the monitoring of microalgae is the integration of spectroscopic and microscopic imaging technologies. Optical sensors that detect chlorophyll fluorescence intensity and the spectral properties of secondary metabolites enable non-destructive assessment of microalgae physiology. The combination of this data with IoT systems enables the automatic classification of growth phases and the early detection of environmental stress.
The future development of IoT technology in microalgae biotechnology is moving towards full integration with robotics and process automation systems, enabling autonomous bioreactors capable of self-regulating cultivation parameters. It is expected that future IoT systems will utilise blockchain technology to ensure data integrity and digital twin technologies to simulate and predict the cultivation processes in real time. The integration of IoT into microalgae culture monitoring represents a significant step towards smart biotechnological systems that can contribute to greater efficiency in the production of biomass and secondary metabolites and enable precise resource management under nutrient-limited conditions, such as in extreme environments or space applications.
7.5. RGB Sensors (Red–Green–Blue)
RGB (red–green–blue) sensors are increasingly used in microalgae culture monitoring and provide fast, non-destructive methods for assessing the growth dynamics and physiological changes of organisms. Their functionality is based on analysing the intensity of reflected and transmitted light within three primary wavelength bands and enables the determination of parameters such as biomass concentration, changes in pigment composition, and the metabolic state of microalgae cultures. Compared to conventional spectrophotometric methods, RGB sensors are characterised by lower costs, ease of implementation, and applicability in remote monitoring systems, including Internet of Things (IoT) technologies [249].
One of the main applications of RGB sensors in the cultivation of microalgae is the monitoring of growth based on changes in the colour of the culture medium. The growth of microalgae leads to increased light absorption in the red and blue range, combined with increased reflectance in the green range, reflecting the chlorophyll content. Devices such as the TCS3200 (ams AG, Premstätten, Austria) and its newer version, the TCS34725 (ams AG, Premstätten, Austria), are typically used for such analyses. These sensors are very sensitive to changes in colour intensity and allow calibration against reference wavelengths so that biomass growth can be accurately tracked in real time [250].
Another important application of RGB sensors (ams AG, Premstätten, Austria) in the cultivation of microalgae is the assessment of environmental stress and changes in pigment composition, particularly chlorophyll a and carotenoid content. The AS7341 device (ams AG, Premstätten, Austria), a more advanced spectral sensor covering eight visible light bands, enables a more precise determination of pigment changes in microalgae under factors such as nutrient deficiency, oxidative stress, or excessive light exposure [251]. In the context of cultivation automation, RGB sensors are increasingly being integrated into IoT systems and robotic bioreactor control units. One example of such an implementation is the MAX44009 sensor (Maxim Integrated Products, Inc, San Jose, CA, USA), which is used in systems for monitoring photosynthetic intensity and dynamic changes in large-scale cultures due to its high sensitivity to visible light intensity and low power consumption [252].
Thanks to integration with microcontrollers such as ESP32 (Espressif Systems Company, Shanghai, China) or Arduino (Ivrea, Italy), RGB sensors (ams AG, Premstätten, Austria) allow remote access to data and automatic adjustment of cultivation parameters, e.g., illumination intensity or nutrient medium composition [253]. RGB sensors are also used in image analysis of microalgae cultures, especially in systems that use industrial and microscope cameras. Devices such as the Raspberry Pi Camera Module V2 (Pencoed, UK), which is based on the Sony IMX219 sensor (Atsugi, Japan), enable high-resolution analysis of morphological and pigmentary changes in individual microalgae colonies [254]. The data from such systems can then be processed by artificial intelligence algorithms to classify cell condition and predict growth trends.
To summarise, the application of RGB sensors in the monitoring of microalgae cultures is an efficient and cost-effective alternative to conventional spectrophotometric and fluorescence-based methods. Their integration into IoT systems and image analysis algorithms enables the continuous tracking of biomass growth, the assessment of environmental stress, and the optimisation of cultivation conditions. The development of RGB sensor technologies, especially with regard to higher spectral sensitivity and automated data analysis, could contribute significantly to increasing the biotechnological production efficiency of microalgae in the future.
7.6. Smart Photobioreactors
Smart photobioreactors (SPBRs) are an advanced solution in microalgae biotechnology. They enable precise control of cultivation conditions through the integration of advanced sensor technologies, Internet of Things (IoT) systems, and artificial intelligence (AI) algorithms. Compared to conventional bioreactors, SPBR systems enable automatic adjustment of environmental parameters in real time, resulting in improved biomass production efficiency and secondary metabolite yields. A key component of smart photobioreactors is the integrated sensor system that monitors physico-chemical parameters of the culture medium, such as temperature, pH, CO2 concentration, light intensity, and dissolved oxygen content. An example of a modern photobioreactor equipped with a multi-parameter monitoring system is the ALGEM® Labscale Photobioreactor from Algenuity (Bedfordshire, UK). This device enables precise control of light intensity and wavelength as well as real-time monitoring of microalgae growth rates. The system is used both in laboratory research and in the optimisation of cultivation conditions for industrial applications [255].
Another example of an intelligent photobioreactor is the Phyco-Flow from Varicon Aqua (Worcester, UK), which was developed for the continuous cultivation of microalgae under controlled conditions. This system integrates optical and spectroscopic sensors that allow the biomass concentration to be assessed based on light transmission and absorption in the visible and near-infrared range. In addition, Phyco-Flow uses AI algorithms to predict crop growth and automatically regulate CO2 supply, which improves photosynthetic efficiency and biomass accumulation [256].
The application of IoT technology in smart photobioreactors enables the remote control of cultivation processes and the real-time analysis of large data sets. The FytoScope FS 130 system from Photon Systems Instruments (PSI) combines controlled LED illumination with precise monitoring of the photosynthetic performance of microalgae via chlorophyll fluorescence. By using communication protocols such as MQTT and LoRaWAN, the device enables data transfer to the cloud and remote management of cultivation conditions, making it an ideal solution for long-term biotechnological experiments [257].
In the context of scalable solutions for industrial cultivation, the Green Wall Panel II system developed by Subitec is noteworthy. This is an integrated photobioreactor system with automatic CO2 supply control and optical sensors for monitoring photosynthetic efficiency. This device is used for the production of high-quality microalgae biomass for pharmaceutical, cosmetic, and food applications [258].
Intelligent photobioreactors are increasingly using artificial intelligence technologies to optimise cultivation processes. One example of an advanced research platform is the microalgae high-throughput screening system (HTS-MAB) developed by Wageningen University, which integrates machine learning and real-time image analysis to automatically select the most productive microalgae strains under dynamically changing cultivation conditions [259].
SPBRs are revolutionising microalgae cultivation processes by automating the monitoring and control of growth conditions. The integration of sensors, IoT systems, and AI algorithms enables precise regulation of environmental parameters, increasing production efficiency and allowing long-term experiments to be performed more effectively than with conventional cultivation systems. The ongoing development of this technology focuses on increasing the autonomy of bioreactors and adapting them to extreme conditions such as space exploration and microalgae production in degraded terrestrial ecosystems.
8. Classification of Monitoring Techniques and Principles for Their Application
To support the development of smart and scalable monitoring strategies for the cultivation of microalgae, this review presents an original conceptual framework that integrates technological, functional, and practical perspectives. The diversity of monitoring methods currently used in microalgae biotechnology requires a structured classification that reflects not only their analytical focus but also the level of technological readiness, degree of invasiveness, and economic feasibility. Techniques range from low-cost, widely available sensors for measuring basic environmental parameters to sophisticated, AI-powered diagnostic systems capable of performing biofunctional analyses in real time and autonomous control. These systems vary considerably in their precision, complexity, and suitability for use at scales ranging from laboratory-scale studies to full industrial deployment.
A comprehensive classification of monitoring technologies used in microalgae cultivation, structured along four key dimensions—the type of parameters monitored, the technological readiness level (TRL), the cost and accessibility, and the degree of system integration—is presented in Table 7. Physico-chemical sensors, such as pH, temperature, and gas sensors, continue to form the backbone of cultivation monitoring due to their sophistication and simplicity. However, biological and optical instruments such as flow cytometry, spectroscopy (NIR, FTIR), and RGB image analysis offer more comprehensive data that provide insight into biomass dynamics and metabolite accumulation [260]. The table also highlights the discrepancy in technological readiness, where only basic sensors are fully operational (TRL 8–9), while AI-integrated systems and advanced analytical platforms are still at the experimental or pilot stage (TRL 3–6). In addition, economic feasibility is a major barrier to implementation: low-cost tools are suitable for routine use, while high-end systems are limited to well-funded laboratories or demonstration facilities. This classification facilitates strategic decisions about which technologies are suitable for specific scales, objectives, and resource constraints.

Table 7.
Classification framework for microalgae cultivation monitoring technologies.
Table 8 outlines the integrated monitoring model for microalgae management (IMMM), a five-layer conceptual structure that reflects the increasing sophistication and automation of monitoring systems. Each layer corresponds to a specific functional and technological advancement [261].

Table 8.
Proposed theoretical framework: integrated monitoring model for microalgae cultivation (IMMM).
At the base, basic sensing refers to the real-time measurement of essential environmental conditions, which is the minimum requirement for any controlled microalgae culture. Bio-functional monitoring builds on this by incorporating non-invasive assessments of physiological status—e.g., growth rates and intracellular compounds—using advanced techniques such as fluorometry and NIR. As cultivation systems evolve, predictive diagnostics introduce machine learning models that analyse time series data to predict critical events or shifts in biomass productivity. These diagnoses form the basis for the autonomous control layer, in which AI algorithms take over important operational decisions such as nutrient dosing or CO2 injection. At the top of the model is scalability and adaptive optimisation, which requires open, flexible architectures capable of integrating new sensors, databases, and control strategies as they become available [261]. This theoretical framework provides a practical vision for incrementally improving the intelligence and robustness of algae cultivation systems (Table 8).
The key research and technology priorities that have emerged from the review conducted and the model proposed are listed in Table 9. They aim to accelerate the implementation of advanced monitoring technologies under industrial conditions. One of the main challenges is the standardisation and validation of monitoring methods to enable comparability of results between different laboratories and systems. Equally important is the integration of sensors and ensuring their interoperability, which enables the creation of modular and scalable platforms. The use of artificial intelligence and adaptive prediction can enable precise management of the cultivation process, customised to a specific strain and environmental conditions [242]. Cost and scalability analyses are also necessary to assess the profitability of investments in intelligent monitoring systems. Finally, the development of open databases and IoT platforms promotes collaboration, transparency, and knowledge sharing between research teams and industry. The identified directions define a strategic action plan for the development of modern microalgae cultivation technologies.

Table 9.
Strategic research and development directions in microalgae cultivation monitoring.
9. Summary and Future Perspectives
With the growing interest in microalgae as a sustainable source of biomass for bioenergy, high-value bioproducts, and environmental applications, cultivation technologies are developing rapidly. A literature analysis has shown that the number of publications on the use of microalgae has increased significantly over the last ten years, and, as of 2021, over 3000 articles per year can be found in the Scopus database. The main research directions focus on improving biomass productivity, optimising cultivation conditions, and improving the synthesis of valuable secondary metabolites such as lipids, pigments, and proteins.
To meet the challenges of large-scale microalgae production, the introduction of advanced monitoring and optimisation systems is essential. These include optical sensors, near-infrared spectroscopy (NIR), flow cytometry, RGB imaging, and, increasingly, artificial intelligence (AI), predictive algorithms, and Internet of Things (IoT) platforms. For example, RGB sensors enable fast and non-destructive biomass assessment, while flow cytometry enables real-time analysis of lipid content in microalgae cells. The integration of these tools into smart photobioreactor systems enables higher production efficiency, cost reduction, and improved process stability. Intelligent control of parameters such as nutrient concentration, light intensity, CO2 content, pH, and temperature can increase biomass yield by 30–50% compared to non-optimised systems.
Although this review provides a comprehensive summary of monitoring technologies and optimisation strategies for microalgae cultivation, it is important to note that these methods vary significantly in terms of efficiency, cost-effectiveness, and practicality for large-scale applications. Advanced techniques such as flow cytometry and Fourier transform infrared spectroscopy (FTIR) offer high analytical precision and enable multiparametric in situ assessment of microalgae biomass. However, their application is mainly limited to laboratory or pilot plants due to high operating costs and complexity. In contrast, less expensive devices such as RGB sensors, PAR metres, or turbidimetric sensors offer lower resolution but are easier to integrate into industrial photobioreactors. There is therefore a trade-off between analytical accuracy and scalability.
Many of the technologies discussed, including IoT-integrated sensors and machine learning-based optimisation algorithms, are currently at TRL 4–6—they have been proven in labs or pilot environments but are not yet commercially deployed on a large scale. Only the basic optical and environmental sensors (pH, temperature, light) can be considered mature (TRL 8–9) and ready for routine industrial use. This means that despite promising successes, many systems still need to be further developed and validated under real operating conditions. The high capital and operating costs of multi-sensor platforms remain a major challenge. Commercial flow cytometers, for example, can cost over USD 50,000, and modern FTIR probes require specialised calibration and maintenance. In contrast, RGB cameras or turbidity sensors can be implemented at a fraction of the cost (less than USD 500), albeit at the expense of limited specificity. This imbalance between cost and benefit limits the wide acceptance of advanced analytical systems.
The literature reviewed contains conflicting reports on the effects of elevated oxygen concentrations on photosynthetic inhibition or optimal light intensity for maximum lipid accumulation. In addition, studies with different strains under different environmental conditions often lead to contradictory results, making direct comparisons difficult. There is also a lack of standardised protocols for evaluating sensor performance, especially in mixed cultures or under wastewater conditions. Key bottlenecks in the practical implementation of monitoring technologies include sensor fouling and drift over time, the lack of robust, low-maintenance calibration procedures, interference from coexisting microorganisms (e.g., bacteria or fungi), the difficulty of integrating multiple sensors into a single control platform, and the limited availability of open-source bioprocess control software compatible with AI.
Addressing these gaps requires both interdisciplinary research and collaboration between universities, device manufacturers, and bioprocess engineers. Future work should focus on comparative testing of monitoring systems under standard test conditions, conducting life cycle and cost-benefit analyses, and developing modular, interoperable hardware platforms suitable for different photobioreactor configurations. In summary, effective optimisation and monitoring of microalgae cultivation processes are fundamental to the development of algae biotechnology. Future advances will depend on the convergence of sensor technologies, real-time data analysis, and predictive modelling, which will ultimately enable the full automation and scalability of photobioreactor systems. This review provides a comprehensive and up-to-date resource for researchers and engineers working on the development of novel cultivation systems that support the development of a sustainable bioeconomy based on microalgae.
Author Contributions
Conceptualisation, M.D.; methodology, M.D.; formal analysis, M.Z.; investigation, M.D., J.K. and M.Z.; resources, M.D., J.K. and M.Z.; writing—original draft preparation, M.D. and J.K.; writing—review and editing, M.D. and J.K.; visualisation, M.D. and J.K.; supervision, M.D.; project administration, M.Z.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by works No. 29.610.023-110 of the University of Warmia and Mazury in Olsztyn and WZ/WB-IIŚ/3/2025 of the Bialystok University of Technology, funded by the Ministry of Science and Higher Education.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Balamurugan, S.; Li, D.W.; Wang, X.; Li, H.Y. Unleashing the Potential of Biotechnological Strategies for the Sustainable Production of Microalgal Polysaccharides. Crit. Rev. Food Sci. Nutr. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Deamici, K.M.; Figueiredo, D.; Guerra, I.; Letras, P.; Pereira, H. Global Market and Future Trends of Microalgae-Based Products. Algal Bioreact. Sci. Eng. Technol. Upstream Process. 2025, 1, 11–25. [Google Scholar] [CrossRef]
- Yeheyo, H.A.; Ealias, A.M.; George, G.; Jagannathan, U. Bioremediation Potential of Microalgae for Sustainable Soil Treatment in India: A Comprehensive Review on Heavy Metal and Pesticide Contaminant Removal. J. Environ. Manag. 2024, 363, 121409. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y.; Zhang, Q. Assessing Global Carbon Sequestration and Bioenergy Potential from Microalgae Cultivation on Marginal Lands Leveraging Machine Learning. Sci. Total Environ. 2024, 948, 174462. [Google Scholar] [CrossRef]
- Samoraj, M.; Çalış, D.; Trzaska, K.; Mironiuk, M.; Chojnacka, K. Advancements in Algal Biorefineries for Sustainable Agriculture: Biofuels, High-Value Products, and Environmental Solutions. Biocatal. Agric. Biotechnol. 2024, 58, 103224. [Google Scholar] [CrossRef]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Musa Ardo, F.; Wei Lim, J.; Ramli, A.; Kee Lam, M.; Kiatkittipong, W.; Alaaeldin Abdelfattah, E.; Kashif Shahid, M.; Usman, A.; Wongsakulphasatch, S.; Tasnim Sahrin, N. A Review in Redressing Challenges to Produce Sustainable Hydrogen from Microalgae for Aviation Industry. Fuel 2022, 330, 125646. [Google Scholar] [CrossRef]
- Dębowski, M.; Świca, I.; Kazimierowicz, J.; Zieliński, M. Large Scale Microalgae Biofuel Technology–Development Perspectives in Light of the Barriers and Limitations. Energies 2022, 16, 81. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The Cultivation of Biohydrogen-Producing Tetraselmis Subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability 2022, 14, 12085. [Google Scholar] [CrossRef]
- Falfushynska, H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology 2024, 4, 548–575. [Google Scholar] [CrossRef]
- Mrinal; Jaiswal, K.S.; Singh, S.; Khalid, Z.; Alam, S.N.; Sundaramurthy, S.; Shukla, N.K.; Ramaswamy, A.P.; Jaiswal, K.K.; Singh, B.; et al. Microalgal Biofuels Production and Advances in Sustainable Applications. In Microalgal Biofuels; Woodhead Publishing: Cambridge, UK, 2025; pp. 395–413. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Halimatul Munawaroh, H.S.; Ho, S.H.; Rajesh Banu, J.; Show, P.L. Advancement of Green Technologies: A Comprehensive Review on the Potential Application of Microalgae Biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Patial, S.K.; Singh, S. Algae Biomass: Importance, Harvesting Techniques, Extraction Methods, and Associated Challenges. In Value Added Products from Bioalgae Based Biorefineries: Opportunities and Challenges; Springer Nature: Singapore, 2024; pp. 67–94. [Google Scholar] [CrossRef]
- Yadav, G.; Mathimani, T.; Sekar, M.; Sindhu, R.; Pugazhendhi, A. Strategic Evaluation of Limiting Factors Affecting Algal Growth—An Approach to Waste Mitigation and Carbon Dioxide Sequestration. Sci. Total Environ. 2021, 796, 149049. [Google Scholar] [CrossRef]
- Olabi, A.G.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef] [PubMed]
- Khor, W.H.; Kang, H.S.; Lim, J.W.; Iwamoto, K.; Tang, C.H.H.; Goh, P.S.; Quen, L.K.; Bin Shaharuddin, N.M.R.; Lai, N.Y.G. Microalgae Cultivation in Offshore Floating Photobioreactor: State-of-the-Art, Opportunities and Challenges. Aquac. Eng. 2022, 98, 102269. [Google Scholar] [CrossRef]
- Psachoulia, P.; Chatzidoukas, C. Illumination Policies for Stichococcus Sp. Cultures in an Optimally Operating Lab-Scale PBR toward the Directed Photosynthetic Production of Desired Products. Sustainability 2021, 13, 2489. [Google Scholar] [CrossRef]
- Porras Reyes, L.; Havlik, I.; Beutel, S. Software Sensors in the Monitoring of Microalgae Cultivations. Rev. Environ. Sci. Bio/Technol. 2024, 23, 67–92. [Google Scholar] [CrossRef]
- Havlik, I.; Beutel, S.; Scheper, T.; Reardon, K.F. On-Line Monitoring of Biological Parameters in Microalgal Bioprocesses Using Optical Methods. Energies 2022, 15, 875. [Google Scholar] [CrossRef]
- Yu, B.S.; Pyo, S.; Lee, J.; Han, K. Microalgae: A Multifaceted Catalyst for Sustainable Solutions in Renewable Energy, Food Security, and Environmental Management. Microb. Cell Factories 2024, 23, 308. [Google Scholar] [CrossRef]
- Chieti, M.G.; Petrucciani, A.; Mollo, L.; Gerotto, C.; Eusebi, A.L.; Fatone, F.; Norici, A.; González-Camejo, J. Acclimated Green Microalgae Consortium to Treat Sewage in an Alternative Urban WWTP in a Coastal Area of Central Italy. Sci. Total Environ. 2024, 945, 174056. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Vdovychenko, A.; Kazimierowicz, J. The Use of the Autotrophic Culture of Arthrospira Platensis for CO2 Fixation from Biogas Combustion. Processes 2024, 12, 396. [Google Scholar] [CrossRef]
- Emalya, N.; Munawar, E. Synergistic Removal of Organic and Nutrients from Landfill Leachate Using Photobiore-Actor-Cultivated Microalgae-Bacteria Consortium. Glob. J. Environ. Sci. Manag. 2024, 10, 683–698. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Bastos, L.P.; Nariyoshi, Y.N.; Borges, R.M.; Keller, R.; Silveira, D.D. Assessment of Selected Parameters in CO2 and CH4 Mass Transfer During Photosynthetic Biogas Upgrading Using Bubble Columns Filled with Wastewater-Derived Microalgae. Energy Fuels 2025, 39, 7314–7325. [Google Scholar] [CrossRef]
- Mallick, N.C.; Mozammil, S.; Koshta, E. Investigation on Chlorella Sp. HS2 Microalgae Growth Using CO2 from Engine Exhaust to Study Carbon Sequestration. Biofuels 2024, 15, 279–290. [Google Scholar] [CrossRef]
- Song, Y.; Xie, L.; Zhang, X.; Hu, Z.; Li, S.; Zhang, P.; Yang, X. Enhancement of Biomass, Lipid Accumulation, and Carbon Sequestration Potential in Microalgae via Cultivation with Aggregation-Induced Emission Light-Conversion Films. Chem. Eng. J. 2024, 483, 149148. [Google Scholar] [CrossRef]
- Sirohi, R.; Il Choi, H.; Sim, S.J. Microalgal Fuels: Promising Energy Reserves for the Future. Fuel 2022, 312, 122841. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Kazimierowicz, J.; Quattrocelli, P.; Rusanowska, P.; Barczak, Ł.; Nowicka, A.; Zieliński, M. Analysis of Multi-Biofuel Production during Cultivation of the Green Microalga Tetraselmis Subscordiformis. Energies 2024, 17, 3670. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from Microalgae Biomass: A Review of Conversion Processes and Procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal Biomass Valorization for Biofuel Production and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Ferreira Carraro, C.d.F.; Almeida Loures, C.C.; de Castro, J.A. Microalgae Bioremediation and CO2 Fixation of Industrial Wastewater. Clean. Eng. Technol. 2022, 8, 100466. [Google Scholar] [CrossRef]
- Sforza, E.; Kumkum, P.; Barbera, E.; Kumar, S. Bioremediation of Industrial Effluents: How a Biochar Pretreatment May Increase the Microalgal Growth in Tannery Wastewater. J. Water Process Eng. 2020, 37, 101431. [Google Scholar] [CrossRef]
- Dȩbowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella Vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of Microalgae on Liquid Anaerobic Digestate for Depollution, Biofuels and Cosmetics: A Review. Environ. Chem. Lett. 2022, 20, 3631–3656. [Google Scholar] [CrossRef]
- Kandimalla, P.; Vatte, P.; Bandaru, C.S.R. Phycoremediation of Automobile Exhaust Gases Using Green Microalgae. Environ. Dev. Sustain. 2021, 23, 6301–6322. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.F.; Sun, Z.L. Mass Transfer Characteristics and Effect of Flue Gas Used in Microalgae Culture. Appl. Microbiol. Biotechnol. 2022, 106, 7013–7025. [Google Scholar] [CrossRef]
- Dahai, H.; Zhihong, Y.; Lin, Q.; Yuhong, L.; Lei, T.; Jiang, L.; Liandong, Z. The Application of Magical Microalgae in Carbon Sequestration and Emission Reduction: Removal Mechanisms and Potential Analysis. Renew. Sustain. Energy Rev. 2024, 197, 114417. [Google Scholar] [CrossRef]
- Tamil Selvan, S.; Balasubaramaian, A.; Manickam Dakshinamoorthi, B.K.; Velramar, B.; Fareed, M. Green Integrative Large Scale Treatment of Tannery Effluent, CO2 Sequestration, and Biofuel Production Using Oleaginous Green Microalga Nannochloropsis Oculata TSD05: An Ecotechnological Approach. Biocatal. Agric. Biotechnol. 2024, 61, 103370. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Abdelaziz, N.I.M.; Nazir, M.A.; Jamshaid, M.; Hassouna, M.S.; El Nemr, A. Box–Behnken Modeling of Biodiesel Production from Botryococcus Braunii Microalgae. Biofuels Bioprod. Biorefining 2024, 18, 1321–1354. [Google Scholar] [CrossRef]
- Moradi, P.; Saidi, M. Biodiesel Production from Chlorella Vulgaris Microalgal-Derived Oil via Electrochemical and Thermal Processes. Fuel Process. Technol. 2022, 228, 107158. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, K.; Pan, X.; Liu, G.; Cai, Y.; Zaidi, A.A.; Zhang, K. Substrate Degradation, Biodiesel Production, and Microbial Community of Two Electro-Fermentation Systems on Treating Oleaginous Microalgae Nannochloropsis Sp. Bioresour. Technol. 2021, 329, 124932. [Google Scholar] [CrossRef]
- Acebu, P.I.G.; de Luna, M.D.G.; Chen, C.Y.; Abarca, R.R.M.; Chen, J.H.; Chang, J.S. Bioethanol Production from Chlorella Vulgaris ESP-31 Grown in Unsterilized Swine Wastewater. Bioresour. Technol. 2022, 352, 127086. [Google Scholar] [CrossRef] [PubMed]
- Ma’mun, S.; Prasetio, M.W.; Anugrah, A.R.; Ruliandi, A.P.; Pramuwardani, D. Bioethanol from Arthrospira Platensis Biomass Using a Combined Pretreatment. Chem. Eng. J. Adv. 2024, 19, 100616. [Google Scholar] [CrossRef]
- Silva, G.; Cerqueira, K.; Rodrigues, J.; Silva, K.; Coelho, D.; Souza, R. Cultivation of Microalgae Chlorella Vulgaris in Open Reactor for Bioethanol Production. Phycology 2023, 3, 325–336. [Google Scholar] [CrossRef]
- El-Moustaqim, K.; Rachiq, T.; Mabrouki, J.; Slaoui, M.; Hmouni, D. Application of Microalgae in Green Hydrogen Production: A Diverse Biomass for Multiple Applications. Environ. Sci. Eng. 2024, Part F3279, 231–240. [Google Scholar] [CrossRef]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current Strategies and Future Perspectives in Biological Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, Y.; Wang, X. Recent Advances on Using Functional Materials to Increase the Pollutant Removal Capabilities of Microalgae and Bacteria: Especially for Their Symbiotic Systems. Curr. Pollut. Rep. 2023, 9, 272–291. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Świca, I.; Zieliński, M. Ultrasonic Disintegration to Improve Anaerobic Digestion of Microalgae with Hard Cell Walls—Scenedesmus Sp. and Pinnularia Sp. Plants 2022, 12, 53. and Pinnularia Sp. Plants 2022, 12, 53. [Google Scholar] [CrossRef]
- Xie, T.; Herbert, C.; Zitomer, D.; Kimbell, L.; Stafford, M.; Venkiteshwaran, K. Biogas Conditioning and Digestate Recycling by Microalgae: Acclimation of Chlorella Vulgaris to H2S-Containing Biogas and High NH4-N Digestate and Effect of Biogas: Digestate Ratio. Chem. Eng. J. 2023, 453, 139788. [Google Scholar] [CrossRef]
- Torres, A.; Padrino, S.; Brito, A.; Díaz, L. Biogas Production from Anaerobic Digestion of Solid Microalgae Residues Generated on Different Processes of Microalgae-to-Biofuel Production. Biomass Convers. Biorefinery 2023, 13, 4659–4672. [Google Scholar] [CrossRef]
- Eusébio, A.; Santos, C.A.; Marques, I.P. Anaerobic Digestion of Microalga Chlorella Protothecoides and Metagenomic Analysis of Reddish-Colored Digestate. Appl. Sci. 2023, 13, 3325. [Google Scholar] [CrossRef]
- Markou, G.; Ilkiv, B.; Brulé, M.; Antonopoulos, D.; Chakalis, L.; Arapoglou, D.; Chatzipavlidis, I. Methane Production through Anaerobic Digestion of Residual Microalgal Biomass after the Extraction of Valuable Compounds. Biomass Convers. Biorefinery 2022, 12, 419–426. [Google Scholar] [CrossRef]
- Manthos, G.; Tsigkou, K.; Koutra, E.; Mingou, L.; Kornaros, M. Exploring the Impact of Microalgal Fatty Acid Content on Anaerobic Digestion and Methane Production: An Experimental and Mathematical Modeling Study. Renew. Energy 2024, 230, 120859. [Google Scholar] [CrossRef]
- Dębowski, M.; Dudek, M.; Nowicka, A.; Quattrocelli, P.; Kazimierowicz, J.; Zieliński, M. Suitability of Pre-Digested Dairy Effluent for Mixotrophic Cultivation of the Hydrogen-Producing Microalgae Tetraselmis Subcordiformis. Environ. Technol. 2024, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas Subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Sitthikitpanya, N.; Sittijunda, S.; Khamtib, S.; Reungsang, A. Co-Generation of Biohydrogen and Biochemicals from Co-Digestion of Chlorella Sp. Biomass Hydrolysate with Sugarcane Leaf Hydrolysate in an Integrated Circular Biorefinery Concept. Biotechnol. Biofuels 2021, 14, 197. [Google Scholar] [CrossRef]
- Raheem, A.; Abbasi, S.A.; Mangi, F.H.; Ahmed, S.; He, Q.; Ding, L.; Memon, A.A.; Zhao, M.; Yu, G. Gasification of Algal Residue for Synthesis Gas Production. Algal Res. 2021, 58, 102411. [Google Scholar] [CrossRef]
- Scopel, L.; Marcantonio, V. Gasification of Chlorella Vulgaris for Syngas Production and Energy Generation Through Gas Turbine. Energies 2024, 17, 6085. [Google Scholar] [CrossRef]
- Banihashemi, F.; Ibrahim, A.F.M.; Deng, S.; Lin, J.Y.S. Pyrolysis and Gasification Characteristics of Galdieria Sulphuraria Microalgae. Bioenergy Res. 2023, 16, 611–621. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.; Sharma, P.K.; Manna, S.; Pugazhendhi, A.; Matsakas, L.; Patel, A. Holistic Utilization of Chlorella Pyrenoidosa Microalgae for Extraction of Renewable Fuels and Value-Added Biochar through in Situ Transesterification and Pyrolysis Reaction Process. Biomass Convers. Biorefinery 2024, 14, 5261–5274. [Google Scholar] [CrossRef]
- Makowska, M.; Dziosa, K. Influence of Different Pyrolysis Temperatures on Chemical Composition and Graphite-like Structure of Biochar Produced from Biomass of Green Microalgae Chlorella Sp. Environ. Technol. Innov. 2024, 35, 103667. [Google Scholar] [CrossRef]
- Saiyud, N.; Deethayat, T.; Asanakham, A.; Duongbia, N.; Kamopas, W.; Kiatsiriroat, T. Biochar Production from Co-Pyrolysis of Coffee Ground and Native Microalgae Consortium. Biomass Convers. Biorefinery 2024, 14, 6855–6863. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Chen, Y.; Hu, J.; Yang, H.; Chen, H. Preparation of Low-Nitrogen and High-Quality Bio-Oil from Microalgae Catalytic Pyrolysis with Zeolites and Activated Carbon. J. Anal. Appl. Pyrolysis 2021, 159, 105182. [Google Scholar] [CrossRef]
- Mahfud, M.; Qadariyah, L.; Haqqyana, H.; Aswie, V. Optimization Bio-Oil Production from Chlorella Sp. through Microwave-Assisted Pyrolysis Using Response Surface Methodology. Green Energy Resour. 2024, 2, 100057. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Mohammed, U.A.; Rawat, I.; Bux, F.; Isa, Y.M. Production of High-Quality Pyrolytic Bio-Oils from Nutrient-Stressed Scenedesmus Obliquus Microalgae. Fuel 2023, 332, 126299. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wang, T.; Zhang, X.; Wang, L.; Hu, X.; Guo, Q. Co-Production of Upgraded Bio-Oils and H2-Rich Gas from Microalgae via Chemical Looping Pyrolysis. Int. J. Hydrogen Energy 2021, 46, 24942–24955. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Ma, J.; Kong, J.; Feng, S.; Wang, Z.; Xiong, L. Pyrolysis of Spirulina Platensis, Tetradesmus Obliquus and Chlorella Vulgaris by TG-FTIR and Py-GC/MS: Kinetic Analysis and Pyrolysis Behaviour. Energy 2022, 244, 123165. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Humaid, L.; Al-Dahmash, N.D.; Aldawsari, M. Assessing the Benefits of Chlorella Vulgaris Microalgal Biodiesel for Internal Combustion Engines: Energy and Exergy Analyses. Fuel 2023, 344, 128055. [Google Scholar] [CrossRef]
- Romadinullah, R.A.; Sukarni, S.; Zakaria, Y.; Aminullah, A.Y.; Permanasari, A.A.; Anis, S.; Johari, A. Thermal Behavior and Combustion Kinetics of Arthrospira Platensis-Activated Carbon Mixture at Mass Ratio of 10:3. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2024; Volume 3124. [Google Scholar] [CrossRef]
- Dessì, F.; Mureddu, M.; Ferrara, F.; Fermoso, J.; Orsini, A.; Sanna, A.; Pettinau, A. Thermogravimetric Characterisation and Kinetic Analysis of Nannochloropsis sp. and Tetraselmis sp. Microalgae for Pyrolysis, Combustion and Oxy-Combustion. Energy 2021, 217, 119394. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The Potential and Challenge of Microalgae as Promising Future Food Sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Loke Show, P. Global Market and Economic Analysis of Microalgae Technology: Status and Perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a Sustainable Source of Edible Proteins and Bioactive Peptides—Current Trends and Future Prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Reyes, J.A.; González-Hernández, J.C. Vitamins from Microalgae. In Handbook of Food and Feed from Microalgae; Academic Press: Cambridge, MA, USA, 2023; pp. 111–115. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Mutaf-Kılıc, T.; Oncel, S.S. Algal and Cyanobacterial Colorants. From Chlorophyll to Phycocyanin. In Microbial Colorants: Chemistry, Biosynthesis and Applications; Wiley: Hoboken, NJ, USA, 2025; pp. 81–105. [Google Scholar] [CrossRef]
- Nair, A.; Rao, A.S.; Ayesha, B.; Salu, H.A.; Ashraf, Q.; Hebbar, A.S.; Yuktha, K.; Khadyal, P.; Poulamee, S.; Veena, S.M.; et al. Phycobiliproteins. Algal and Cyanobacterial Pigments Radiating Vivid Colors. In Microbial Colorants: Chemistry, Biosynthesis and Applications; Wiley: Hoboken, NJ, USA, 2025; pp. 241–259. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K. Fatty Acids of Microalgae: Diversity and Applications. Rev. Environ. Sci. Bio/Technol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Kaamoush, M.; El-Agawany, N.; El Salhin, H.; El-Zeiny, A. Monitoring Effect of Nickel, Copper, and Zinc on Growth and Photosynthetic Pigments of Spirulina Platensis with Suitability Investigation in Idku Lake. Environ. Sci. Pollut. Res. 2022, 29, 78942–78959. [Google Scholar] [CrossRef]
- Reshma, R.; Chitra Devi, K.; Dinesh Kumar, S.; Santhanam, P.; Perumal, P.; Krishnaveni, N.; Begum, A.; Pragnya, M.; Arthikha, R.; Dhanalakshmi, B.; et al. Enhancement of Pigments Production in the Green Microalga Dunaliella Salina (PSBDU05) under Optimized Culture Condition. Bioresour. Technol. Rep. 2021, 14, 100672. [Google Scholar] [CrossRef]
- Grujić, V.J.; Todorović, B.; Ambrožič-Dolinšek, J.; Kranvogl, R.; Ciringer, T. Diversity and Content of Carotenoids and Other Pigments in the Transition from the Green to the Red Stage of Haematococcus Pluvialis Microalgae Identified by HPLC-DAD and LC-QTOF-MS. Plants 2022, 11, 1026. [Google Scholar] [CrossRef]
- Ma, W.; Liu, M.; Zhang, Z.; Xu, Y.; Huang, P.; Guo, D.; Sun, X.; Huang, H. Efficient Co-Production of EPA and DHA by Schizochytrium Sp. via Regulation of the Polyketide Synthase Pathway. Commun. Biol. 2022, 5, 1356. [Google Scholar] [CrossRef]
- Navarro López, E.; Jiménez Callejón, M.J.; Macías Sánchez, M.D.; González Moreno, P.A.; Robles Medina, A. Obtaining Eicosapentaenoic Acid-Enriched Polar Lipids from Microalga Nannochloropsis Sp. by Lipase-Catalysed Hydrolysis. Algal Res. 2023, 71, 103073. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Cao, J.; Wang, Y.; Zhang, L.; Yan, X.; Li, Y.; Xu, J. Enhancement of Docosahexaenoic Acid and Eicosapentaenoic Acid Biosynthesis in Isochrysis Galbana by Bacillus Jeotgali. Mar. Biotechnol. 2024, 26, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; El Latif, A.A.; Elkaw, E.M.; Alotaibi, A.S.; Alenzi, A.M.; Hamza, H.A. Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella Vulgaris and Its Blend with Different Vitamins. Molecules 2022, 27, 1602. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; El-Boraey, N.G.; El Bialy, B.E.; Alrdahe, S.S.; Darwish, D.B.E. Vitamin Supplements Enhance Spirulina Platensis Biomass and Phytochemical Contents. Green Process. Synth. 2022, 11, 266–274. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella Vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qi, F.; Yang, F.; Khan, Q.U.; Faizan; Fu, Q.; Li, J.; Wang, X.; Wang, X.; Wang, J.; et al. Nutritional Composition and Functional Properties of A. Platensis-Derived Peptides: A Green and Sustainable Protein-Rich Supplement. Processes 2024, 12, 2608. [Google Scholar] [CrossRef]
- Lopes, D.; Rey, F.; Gomes, A.; Duarte, L.; Pereira, J.; Pinho, M.; Melo, T.; Domingues, R. Tracing the Impact of Domestic Storage Conditions on Antioxidant Activity and Lipid Profiles in the Edible Microalgae Chlorella Vulgaris and Tetraselmis Chui. Mar. Drugs 2024, 22, 254. [Google Scholar] [CrossRef]
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I.; et al. Potential of Exopolysaccharide from Porphyridium Marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Mar. Drugs 2021, 19, 66. [Google Scholar] [CrossRef]
- Wang, T.; Li, D.; Tian, X.; Huang, G.; He, M.; Wang, C.; Kumbhar, A.N.; Woldemicael, A.G. Mitigating Salinity Stress through Interactions between Microalgae and Different Forms (Free-Living & Alginate Gel-Encapsulated) of Bacteria Isolated from Estuarine Environments. Sci. Total Environ. 2024, 926, 171909. [Google Scholar] [CrossRef]
- Sarkar, P.; Bandyopadhyay, T.K.; Gopikrishna, K.; Nath Tiwari, O.; Bhunia, B.; Muthuraj, M. Algal Carbohydrates: Sources, Biosynthetic Pathway, Production, and Applications. Bioresour. Technol. 2024, 413, 131489. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Chairi, H.; Baltemimi, A.; Essafi, A.; Abrini, J.; Senhaji, N.S. Evaluation of the Antioxidant Properties of Microalgae Naturally Isolated from Mediterranean Morocco. Egypt. J. Aquat. Biol. Fish. 2022, 26, 311. [Google Scholar] [CrossRef]
- Basha, A.N.; Akhir, F.N.M.; Othman, N.; Hara, H. Antioxidant and Anticancer Potential of Bioactive Compounds from Locally Isolated Microalgae. J. Health Qual. Life 2024, 3, 40–54. [Google Scholar] [CrossRef]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the Bio-Prospective of Microalgae by Different Light Systems and Photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Sproles, A.E.; Fields, F.J.; Smalley, T.N.; Le, C.H.; Badary, A.; Mayfield, S.P. Recent Advancements in the Genetic Engineering of Microalgae. Algal Res. 2021, 53, 102158. [Google Scholar] [CrossRef]
- Mourya, M.; Khan, M.J.; Ahirwar, A.; Schoefs, B.; Marchand, J.; Rai, A.; Varjani, S.; Rajendran, K.; Banu, J.R.; Vinayak, V. Latest Trends and Developments in Microalgae as Potential Source for Biofuels: The Case of Diatoms. Fuel 2022, 314, 122738. [Google Scholar] [CrossRef]
- Villanova, V.; Roques, J.A.C.; Forghani, B.; Shaikh, K.M.; Undeland, I.; Spetea, C. Two-Phase Microalgae Cultivation for RAS Water Remediation and High-Value Biomass Production. Front. Plant Sci. 2023, 14, 1186537. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, N.; Ahmad, M.D.; Koji, I.; Yuzir, A. Harnessing Solar Radiation for Potential Algal Biomass Production. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 421–449. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tohidfar, M.; Mousavi Derazmahalleh, S.M.; Sulaiman, A.; Baharuddin, A.S.; Tabatabaei, M. Biochemical Modulation of Lipid Pathway in Microalgae Dunaliella Sp. for Biodiesel Production. Biomed Res. Int. 2015, 2015, 597198. [Google Scholar] [CrossRef]
- Rengel, R.; Smith, R.T.; Haslam, R.P.; Sayanova, O.; Vila, M.; León, R. Overexpression of Acetyl-CoA Synthetase (ACS) Enhances the Biosynthesis of Neutral Lipids and Starch in the Green Microalga Chlamydomonas Reinhardtii. Algal Res. 2018, 31, 183–193. [Google Scholar] [CrossRef]
- Tibocha-Bonilla, J.D.; Zuñiga, C.; Godoy-Silva, R.D.; Zengler, K. Advances in Metabolic Modeling of Oleaginous Microalgae Mike Himmel. Biotechnol. Biofuels 2018, 11, 241. [Google Scholar] [CrossRef]
- Daly, G.; Ghini, V.; Adessi, A.; Fondi, M.; Buchan, A.; Viti, C. Towards a Mechanistic Understanding of Microalgae–Bacteria Interactions: Integration of Metabolomic Analysis and Computational Models. FEMS Microbiol. Rev. 2022, 46, fuac020. [Google Scholar] [CrossRef]
- Maria González-González, L.; De-Bashan, L.E.; Khozin-Goldberg, I.; Zilberg, D.; Solovchenko, A. Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in Microalgae Engineering and Synthetic Biology Applications for Biofuel Production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for High-Value Compounds and Biofuels Production: A Review with Focus on Cultivation under Stress Conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Sharma, K.K.; Garg, S.; Li, Y.; Malekizadeh, A.; Schenk, P.M. Critical Analysis of Current Microalgae Dewatering Techniques. Biofuels 2013, 4, 397–407. [Google Scholar] [CrossRef]
- Vonshak, A. (Ed.) Spirulina Platensis Arthrospira: Physiology, Cell-Biology And Biotechnology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Ravishankar, G.A.; Ambati, R.R. Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Huang, Y.; Lou, C.; Luo, L.; Wang, X.C. Insight into Nitrogen and Phosphorus Coupling Effects on Mixotrophic Chlorella Vulgaris Growth under Stably Controlled Nutrient Conditions. Sci. Total Environ. 2021, 752, 141747. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella Vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef]
- Plouviez, M.; Bolot, P.; Shilton, A.; Guieysse, B. Phosphorus Uptake and Accumulation in Chlamydomonas Reinhardtii: Influence of Biomass Concentration, Phosphate Concentration, Phosphorus Depletion Time, and Light Supply. Algal Res. 2023, 71, 103085. [Google Scholar] [CrossRef]
- Medeiros, D.L.; Moreira, Í.T.A. Microalgae Biomass Production from Cultivation in Availability and Limitation of Nutrients: The Technical, Environmental and Economic Performance. J. Clean. Prod. 2022, 370, 133538. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Dragone, G. Challenges and Opportunities to Increase Economic Feasibility and Sustainability of Mixotrophic Cultivation of Green Microalgae of the Genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Sun, Y.; Yan, B.; Chen, W.; Fan, D. The Potential Use of Microalgae for Nutrient Supply and Health Enhancement in Isolated and Confined Environments. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13418. [Google Scholar] [CrossRef]
- da Gama, R.C.N.; Assemany, P.P.; de Assis, L.R.; Oliveira, L.V.; Cecon, P.R.; Calijuri, M.L. Influence of C/N Ratio on Microalgae-Bacteria Joint Culture: Treatment Performance and Phytoplankton Dynamics in Mixed Wastewaters. Bioresour. Technol. Rep. 2023, 23, 101516. [Google Scholar] [CrossRef]
- Roy, U.K.; Wagner, J.; Radu, T. Production of Metabolites in Microalgae Under Alkali Halophilic Growth Medium Using a Dissolved Inorganic Carbon Source. Waste Biomass Valorization 2023, 14, 3339–3354. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J. Mass Cultivation and Harvesting of Microalgal Biomass: Current Trends and Future Perspectives. Bioresour. Technol. 2022, 344, 126406. [Google Scholar] [CrossRef]
- Filali, R.; Tian, H.; Micheils, E.; Taidi, B. Evaluation of the Growth Performance of Microalgae Based on Fine PH Changes. Austin J. Biotechnol. Bioeng. 2021, 8, 1109. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. The Effects of CO2 Addition along a PH Gradient on Wastewater Microalgal Photo-Physiology, Biomass Production and Nutrient Removal. Water Res. 2015, 70, 9–26. [Google Scholar] [CrossRef]
- Rajanren, J.R.; Ismail, H.M. Investigation of Chlorella Vulgaris Microalgae as a Source for Renewable Fuel. Biofuels 2017, 8, 37–47. [Google Scholar] [CrossRef]
- Saputri, D.A.E.; Prabaningtyas, S.; Aridowi, D. Effect of Temperature Differences on Cell Growth Chlorella Vulgaris Culture by Fixing Bacteria Nitrogen and Producing IAA (Indole Acetic Acid). In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2025; Volume 3186. [Google Scholar] [CrossRef]
- Talaei, M.; Mahdavinejad, M.; Azari, R.; Haghighi, H.M.; Atashdast, A. Thermal and Energy Performance of a User-Responsive Microalgae Bioreactive Façade for Climate Adaptability. Sustain. Energy Technol. Assess. 2022, 52, 101894. [Google Scholar] [CrossRef]
- Soares, L.T.; Vilas-Bôas, R.N.; Mendes, M.F.; de Mendonça, H.V. Assessment of Biofuel Production Yield Using Microalgae Biomass in Cattle Wastewater. Sustain. Water Resour. Manag. 2024, 10, 135. [Google Scholar] [CrossRef]
- Iakovidou, G.; Itziou, A.; Tsiotsias, A.; Lakioti, E.; Samaras, P.; Tsanaktsidis, C.; Karayannis, V. Application of Microalgae to Wastewater Bioremediation, with CO2 Biomitigation, Health Product and Biofuel Development, and Environmental Biomonitoring. Appl. Sci. 2024, 14, 6727. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Benavides, A.M.S.; Torzillo, G. Variables Governing Photosynthesis and Growth in Microalgae Mass Cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Rasheed, R.; Schipper, K.; Gifuni, I.; Al-Jabri, H.; Barbosa, M.J.; Gonçalves, O.; Pruvost, J. Thermal Regulation of Algae Cultures in Raceway Ponds Utilizing Ground Heat: Improving Techno-Economic Feasibility and Process Sustainability of Large-Scale Algae Production in Qatar. Sustain. Energy Technol. Assess. 2023, 60, 103497. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, E.; Sánchez-Zurano, A.; Guzmán, J.L.; Acién, G.; Visioli, A. A Seasonal Simulation Approach for Culture Depth Influence on the Temperature for Different Characterized Microalgae Strains. Biotechnol. J. 2022, 17, 2100489. [Google Scholar] [CrossRef]
- Kwan, P.P.; Banerjee, S.; Shariff, M.; Md. Yusoff, F. Influence of Light on Biomass and Lipid Production in Microalgae Cultivation. Aquac. Res. 2021, 52, 1337–1347. [Google Scholar] [CrossRef]
- Rehman, M.; Kesharvani, S.; Dwivedi, G.; Gidwani Suneja, K. Impact of Cultivation Conditions on Microalgae Biomass Productivity and Lipid Content. Mater. Today Proc. 2022, 56, 282–290. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. A Critical Review on the Status and Progress of Microalgae Cultivation in Outdoor Photobioreactors Conducted over 35 Years (1986–2021). Energies 2023, 16, 3105. [Google Scholar] [CrossRef]
- Köktürk, G.; Ünal, A.; Tokuç, A. Of an Innovative Building-Integrated Photobioreactor. In Exergetic, Energetic and Environmental Dimensions; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Zieliński, M. Influence of the Light Source on the Chlorella Vulgaris Biomass Growth in the Culture Medium Supplemented with Anaerobic Digestate. Annu. Set Environ. Prot. 2020, 22, 605–621. [Google Scholar]
- De Bhowmick, G.; Sen, R.; Sarmah, A.K. Consolidated Bioprocessing of Wastewater Cocktail in an Algal Biorefinery for Enhanced Biomass, Lipid and Lutein Production Coupled with Efficient CO2 Capture: An Advanced Optimization Approach. J. Environ. Manag. 2019, 252, 109696. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Kexin, R.; Jingmiao, Z.; Yun, H.; Xianqing, Z.; Xun, Z.; Qiang, L.; Ao, X.; Kexin, R.; Jingmiao, Z.; et al. Promotion of Carbon Fixation and Emission Reduction by Microalgae with Optical Fiber/Light Guide Tubes. COAL Sci. Technol. 2024, 52, 329–337. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, W.W.; Cui, K.H.; Lau, B.P.Y.; Lee, F.W.F.; Xu, S.J.L. Feasibility and Efficiency of Microalgae Cultivation for Nutrient Recycling and Energy Recovery from Food Waste Filtrate. PLoS ONE 2025, 20, e0315801. [Google Scholar] [CrossRef]
- Contreras-Ropero, J.E.; Lidueñez-Ballesteros, V.S.; Rodríguez-Bohórquez, A.D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A.; Contreras-Ropero, J.E.; et al. The Effect of LEDs on Biomass and Phycobiliproteins Production in Thermotolerant Oscillatoria Sp. Appl. Sci. 2022, 12, 11664. [Google Scholar] [CrossRef]
- Serôdio, J.; Campbell, D.A. Photoinhibition: Fundamentals and Implications for Primary Productivity. In Life Below Water; Springer: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Gao, S.; Edmundson, S.; Huesemann, M. Oxygen Stress Mitigation for Microalgal Biomass Productivity Improvement in Outdoor Raceway Ponds. Algal Res. 2022, 68, 102901. [Google Scholar] [CrossRef]
- Liebscher, L.-J.; Höger, A.-L.; Kleinert, C.; Matthes, S.; Griehl, C.; Ecke, M. Prevention and Control of Parasitic Contamination in Industrial Microalgae Cultures. J. Appl. Phycol. 2024, 37, 1–13. [Google Scholar] [CrossRef]
- Nordio, R.; Belachqer-El Attar, S.; Clagnan, E.; Sánchez-Zurano, A.; Pichel, N.; Viviano, E.; Adani, F.; Guzmán, J.L.; Acién, G. Exploring Microbial Growth Dynamics in a Pilot-Scale Microalgae Raceway Fed with Urban Wastewater: Insights into the Effect of Operational Variables. J. Environ. Manag. 2024, 369, 122385. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hong-ying, H.; Ke, G.; Ying-xue, S. Effects of Different Nitrogen and Phosphorus Concentrations on the Growth, Nutrient Uptake, and Lipid Accumulation of a Freshwater Microalga Scenedesmus Sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Stevens, D.; Murray, J.; Diepeveen, D.; Toohey, D.; Stevens, J.D.; Murray, D.; Diepeveen, D.; Toohey, D. Adaptalight: An Inexpensive PAR Sensor System for Daylight Harvesting in a Micro Indoor Smart Hydroponic System. Horticulturae 2022, 8, 105. [Google Scholar] [CrossRef]
- Amaral, R.; Duci, D.; Cotta, F.C.; Bacellar, F.L.; Oliveira, S.; Verret, F.; Asadi, K.; Vandamme, L.K.J.; Reis, N.M.; Bryant, L.D.; et al. Ion-Driven Communication and Acclimation Strategies in Microalgae. Chem. Eng. J. 2023, 473, 144985. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Vlaskin, M.S.; Ryndin, K.G.; Butyrin, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Effects of Light Intensity on the Growth and Biochemical Composition in Various Microalgae Grown at High CO2 Concentrations. Plants 2023, 12, 3876. [Google Scholar] [CrossRef]
- Rögner, M. Photosynthesis: Biotechnological Applications with Microalgae; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2021; pp. 1–310. [Google Scholar] [CrossRef]
- Larochelle, J.; Klueppel, J.; McCormick, R.; Biegert, K.; Comella, L.M. A Low-Power Optical Sensor With Dynamically Adjustable Field of View for Photosynthetically Active Radiation (PAR) Measurement. IEEE Sens. J. 2024, 24, 7711–7728. [Google Scholar] [CrossRef]
- Muñoz, I.L.; Bernard, O. Modeling the Influence of Temperature, Light Intensity and Oxygen Concentration on Microalgal Growth Rate. Processes 2021, 9, 496. [Google Scholar] [CrossRef]
- Saccardo, A.; Bezzo, F.; Sforza, E. Microalgae Growth in Ultra-Thin Steady-State Continuous Photobioreactors: Assessing Self-Shading Effects. Front. Bioeng. Biotechnol. 2022, 10, 977429. [Google Scholar] [CrossRef]
- Ifrim, G.A.; Titica, M.; Deppe, S.; Frahm, B.; Barbu, M.; Caraman, S. Multivariable Control Strategy for the Photosynthetic Cultures of Microalgae. In Proceedings of the 2019 23rd International Conference on System Theory, Control and Computing (ICSTCC), Sinaia, Romania, 9–11 October 2019; pp. 218–223. [Google Scholar] [CrossRef]
- Wolf, J.; Stephens, E.; Steinbusch, S.; Yarnold, J.; Ross, I.L.; Steinweg, C.; Doebbe, A.; Krolovitsch, C.; Müller, S.; Jakob, G.; et al. Multifactorial Comparison of Photobioreactor Geometries in Parallel Microalgae Cultivations. Algal Res. 2016, 15, 187–201. [Google Scholar] [CrossRef]
- Jakubowski, P.; Gryc, I. PL243889B1 System for Measuring Intensity Parameters of Optical Radiation. 2020. Available online: https://worldwide.espacenet.com/patent/search/family/076547986/publication/PL243889B1?q=PL243889B1 (accessed on 12 April 2025).
- Festo GR. PhotoBionicCell. Available online: https://www.festo.com/gr/en/e/about-festo/research-and-development/bionic-learning-network/future-concepts-for-biologisation/photobioniccell-id_1330847/ (accessed on 27 April 2025).
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light Management Technologies for Increasing Algal Photobioreactor Efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Rossi, S.; Carecci, D.; Ficara, E. Thermal Response Analysis and Compilation of Cardinal Temperatures for 424 Strains of Microalgae, Cyanobacteria, Diatoms and Other Species. Sci. Total Environ. 2023, 873, 162275. [Google Scholar] [CrossRef]
- Legrand, J.; Artu, A.; Pruvost, J. A Review on Photobioreactor Design and Modelling for Microalgae Production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Agrawal, R. Fermentor. In Textbook of Industrial Microbiology; Springer: Singapore, 2024; pp. 97–150. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Scale-up of Photo-Bioreactors for Microalgae Cultivation by π-Theorem. Biochem. Eng. J. 2020, 153, 107398. [Google Scholar] [CrossRef]
- Ng, H.; Tan, C.H.; Nomanbhay, S.; Show, P.L. Sustainability and Development of Microalgae 4.0. In Microalgae for Environmental Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 397–420. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabrielyan, A.K.; Bobrovnikova, L.A.; Bedbenov, V.S.; Starikov, A.Y.; Zorina, A.A.; Gabel, B.V.; Los, D.A. Laboratory System for Intensive Cultivation of Microalgae and Cyanobacteria. Russ. J. Plant Physiol. 2023, 70, 20. [Google Scholar] [CrossRef]
- Carneiro, M.; Cicchi, B.; Maia, I.B.; Pereira, H.; Zittelli, G.C.; Varela, J.; Malcata, F.X.; Torzillo, G. Effect of Temperature on Growth, Photosynthesis and Biochemical Composition of Nannochloropsis Oceanica, Grown Outdoors in Tubular Photobioreactors. Algal Res. 2020, 49, 101923. [Google Scholar] [CrossRef]
- Shekh, A.; Sharma, A.; Schenk, P.M.; Kumar, G.; Mudliar, S. Microalgae Cultivation: Photobioreactors, CO2 Utilization, and Value-Added Products of Industrial Importance. J. Chem. Technol. Biotechnol. 2022, 97, 1064–1085. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Y.; Wang, X.; Yu, Z.; Mao, W.; Cheng, C.; Che, G.; Zhao, L.; Li, T.; Yang, W.; et al. Flue Gas Waste Heat Affects Algal Liquid Temperature for Microalgal Production in Column Photobioreactors. Heat Transf. 2024, 53, 2173–2190. [Google Scholar] [CrossRef]
- Meier, L.; Vilchez, C.; Cuaresma, M.; Torres-Aravena, Á.; Jeison, D. Effect of PH Change on the Microalgae-Based Biogas Upgrading Process. Appl. Sci. 2022, 12, 12194. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Gao, K. Approaches and Involved Principles to Control PH/PCO2 Stability in Algal Cultures. J. Appl. Phycol. 2021, 33, 3497–3505. [Google Scholar] [CrossRef]
- Savio, S.; Di Natale, C.; Paolesse, R.; Lvova, L.; Congestri, R. Keeping Track of Phaeodactylum Tricornutum (Bacillariophyta) Culture Contamination by Potentiometric E-Tongue. Sensors 2021, 21, 4052. [Google Scholar] [CrossRef]
- Kim, G.Y.; Heo, J.; Kim, K.; Chung, J.; Han, J.I. Electrochemical PH Control and Carbon Supply for Microalgae Cultivation. Chem. Eng. J. 2021, 426, 131796. [Google Scholar] [CrossRef]
- Sandaruwan, H.H.P.B.; Manatunga, D.C.; N. Liyanage, R.; Costha, N.P.; Dassanayake, R.S.; Wijesinghe, R.E.; Zhou, Y.; Liu, Y. Next-Generation Methods for Precise PH Detection in Ocular Chemical Burns: A Review of Recent Analytical Advancements. Anal. Methods 2025, 17, 408–431. [Google Scholar] [CrossRef]
- Caparroz, M.; Guzmán, J.L.; Berenguel, M.; Acién, F.G. A Novel Data-Driven Model for Prediction and Adaptive Control of PH in Raceway Reactor for Microalgae Cultivation. N. Biotechnol. 2024, 82, 1–13. [Google Scholar] [CrossRef]
- Idam, E.I.; Qadeer, A.; Oli, I.C.; Fagorite, V.I.; Baxter, T.; Grischek, T.; Nwobi, N.O.; Onyeagoro, R.U.; Adama, I.O.; Abass, G.F. Optimizing Wastewater Treatment: Algae-Mediated Calcite Formation and Carbon Sequestration through Bicarbonate Control. Algal Res. 2024, 82, 103631. [Google Scholar] [CrossRef]
- Cogne, G.; Ballestas, F.F.; Titica, M.; Legrand, J. A Tool for On-Line Monitoring Microalgal Bioprocesses Based on Gas Balance Analysis. Biotechnol. Bioeng. 2025, 122, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Severo, I.A.; Deprá, M.C.; Barin, J.S.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Bio-Combustion of Petroleum Coke: The Process Integration with Photobioreactors. Chem. Eng. Sci. 2018, 177, 422–430. [Google Scholar] [CrossRef]
- Yang, Y.W.; Li, M.J.; Hung, T.C. The Study on Coupled CO2 Fixation and Power Generation in Microalgae-Microbial Fuel Cells Embedded with Oxygen-Consuming Biofilms. Fuel 2024, 367, 131410. [Google Scholar] [CrossRef]
- Ansari, A.; Verma, N.K.; Saravanan, M.; Verma, N. Selective Conversion of Aqueous CO2 to Formate Using the Wastewater-Isolated Microalage in a Packed-Bed Photobioreactor Under Flow Condition. Available at SSRN 2025. [Google Scholar] [CrossRef]
- Du, N.; Gholami, P.; Kline, D.I.; DuPont, C.L.; Dickson, A.G.; Mendola, D.; Martz, T.; Allen, A.E.; Greg Mitchell, B. Simultaneous Quantum Yield Measurements of Carbon Uptake and Oxygen Evolution in Microalgal Cultures. PLoS ONE 2018, 13, e0199125. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nurun Nabi, M.; Brown, R.J. Investigation of Diesel Engine Performance and Exhaust Emissions of Microalgae Fuel Components in a Turbocharged Diesel Engine. Energy Convers. Manag. 2019, 186, 220–228. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Borodin, V.B. Specifics of the Clark-Type O2-Electrodes Application in Biological Studies. Russ. J. Plant Physiol. 2021, 68, 774–782. [Google Scholar] [CrossRef]
- Fallahi Chegeni, N.; Ijadi Maghsoodi, P.; Habibi, M.; Zare-Behtash, H.; Majles Ara, M.H.; Heydari, E. Hybrid Dissolved-Oxygen and Temperature Sensing: A Nanophotonic Probe for Real-Time Monitoring of Chlorella Algae. Sensors 2021, 21, 6553. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Li, X.; Yan, M.; Ma, H.; Leung, P.T.Y. Polarimetric Learning: A Siamese Approach to Learning Distance Metrics of Algal Mueller Matrix Images. Appl. Opt. 2018, 57, 3829–3837. [Google Scholar] [CrossRef]
- Rehman, R.; Kazmi, S.F.; Irshad, M.; Bilal, M.; Hafeez, F.; Ahmed, J.; Shaheedi, S.; Nazir, R. Microalgae-Assisted Treatment of Wastewater Originating from Varied Sources, Particularly in the Context of Heavy Metals and Antibiotic-Resistant Bacteria. Water 2024, 16, 3305. [Google Scholar] [CrossRef]
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Oxygen Balanced Mixotrophy under Day-Night Cycles. ACS Sustain. Chem. Eng. 2020, 8, 11682–11691. [Google Scholar] [CrossRef]
- Song, X.; Kong, F.; Liu, B.F.; Song, Q.; Ren, N.Q.; Ren, H.Y. Antioxidants Alleviated Low-Temperature Stress in Microalgae by Modulating Reactive Oxygen Species to Improve Lipid Production and Antioxidant Defense. Bioresour. Technol. 2024, 413, 131451. [Google Scholar] [CrossRef]
- Skok, A.; Bazel, Y.; Vishnikin, A. New Analytical Methods for the Determination of Sulfur Species with Microextraction Techniques: A Review. J. Sulfur Chem. 2022, 43, 443–471. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Alves, M.; Leitão, F.; Tacão, M.; Henriques, I.; Castro, P.M.L.; Amorim, C.L. Bioremediation of Coastal Aquaculture Effluents Spiked with Florfenicol Using Microalgae-Based Granular Sludge—A Promising Solution for Recirculating Aquaculture Systems. Water Res. 2023, 233, 119733. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Pachés, M.; Borrás, L.; Seco, A. Comprehensive Assessment of the Microalgae-Nitrifying Bacteria Competition in Microalgae-Based Wastewater Treatment Systems: Relevant Factors, Evaluation Methods and Control Strategies. Algal Res. 2022, 61, 102563. [Google Scholar] [CrossRef]
- Chiellini, C.; Guglielminetti, L.; Pistelli, L.; Ciurli, A. Screening of Trace Metal Elements for Pollution Tolerance of Freshwater and Marine Microalgal Strains: Overview and Perspectives. Algal Res. 2020, 45, 101751. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, J.; Yin, Z.; Liu, Z.; Chen, J.; Xu, J.; Gao, Q.; Liu, J. A Method for Quantifying the Contribution of Algal Sources to CODMn in Water Bodies Based on Ecological Chemometrics and Its Potential Applications. J. Environ. Chem. Eng. 2024, 12, 111943. [Google Scholar] [CrossRef]
- Kumar, D.; Sahoo, D. Assessment of Physiochemical Parameters and Bioremediation of Complex Contaminated Yamuna River, India: An Algal-Based Approach. Water. Air. Soil Pollut. 2024, 235, 90. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of Microalgal Extracts as Biostimulants through Seed Treatment and Foliar Spray for Tomato Cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Martinez, A. Minerals and Trace Elements in Microalgal Biomass. In Handbook of Food and Feed from Microalgae; Academic Press: Cambridge, MA, USA, 2023; pp. 103–109. [Google Scholar] [CrossRef]
- Gorbunova, S.Y.; Avsiyan, A.L. Real-Time Automation and Monitoring of the Batch Growth of Microalga Tetraselmis Viridis and Cyanobacterium Limnospira Platensis. Syst. Microbiol. Biomanufacturing 2025, 5, 795–804. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Vasilieva, S.; Lobakova, E. Chlorophyll Fluorescence as a Valuable Multitool for Microalgal Biotechnology. Biophys. Rev. 2022, 14, 973–983. [Google Scholar] [CrossRef]
- Van Wychen, S.; Rowland, S.M.; Lesco, K.C.; Shanta, P.V.; Dong, T.; Laurens, L.M.L. Advanced Mass Balance Characterization and Fractionation of Algal Biomass Composition. J. Appl. Phycol. 2021, 33, 2695–2708. [Google Scholar] [CrossRef]
- Paula, S.F.A.; Chagas, B.M.E.; Pereira, M.; Rangel, A.H.N.; Sassi, C.F.C.; Borba, L.H.F.; Santos, E.S.; Asevedo, E.A.; Câmara, F.R.A.; Araújo, R.M. Pyrolysis-GCMS of Spirulina Platensis: Evaluation of Biomasses Cultivated under Autotrophic and Mixotrophic Conditions. PLoS ONE 2022, 17, e0276317. [Google Scholar] [CrossRef]
- Michalak, I.; Baśladyńska, S.; Mularczyk, M.; Marycz, K. Investigation on the Potential Sorbents—Aluminosilicate, Microalga and Grass Hay as Feed Additives. Environ. Technol. Innov. 2021, 24, 101816. [Google Scholar] [CrossRef]
- Ni, Z.; Tan, L.; Wang, J.; Chen, Y.; Zhang, N.; Meng, F.; Wang, J. Toxic Effects of Pristine and Aged Polystyrene and Their Leachate on Marine Microalgae Skeletonema Costatum. Sci. Total Environ. 2023, 857, 159614. [Google Scholar] [CrossRef]
- Williamson, C.J.; Anesio, A.M.B.; Benning, L.G.; Tranter, M. New Method Provides First Evidence of Fine-Scale in Situ Heterogeneity in Glacier Algal Photophysiology. Eur. J. Phycol. 2025, 60, 35–42. [Google Scholar] [CrossRef]
- Stablein, M.J.; Baracho, D.H.; Watson, J.T.; Silva, J.C.; Zhang, Y.; Lombardi, A.T. Microalgal Photosynthetic Inhibition and Mixotrophic Growth in Post Hydrothermal Liquefaction Wastewater (PHW). Algal Res. 2021, 60, 102548. [Google Scholar] [CrossRef]
- des Aulnois, M.G.; Roux, P.; Caruana, A.; Réveillon, D.; Briand, E.; Hervé, F.; Savar, V.; Bormans, M.; Amzil, Z. Physiological and Metabolic Responses of Freshwater and Brackish-Water Strains of Microcystis Aeruginosa Acclimated to a Salinity Gradient: Insight into Salt Tolerance. Appl. Environ. Microbiol. 2019, 85, e01614-19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Pang, T.; Liu, J. Comparative Transcriptome Profiling of Kappaphycus Alvarezii (Rhodophyta, Gigartinales) in Response to Two Extreme Temperature Treatments: An RNA-Seq-Based Resource for Photosynthesis Research. Eur. J. Phycol. 2019, 54, 162–174. [Google Scholar] [CrossRef]
- Hupp, J.; McCoy, J.I.E.; Millgan, A.J.; Peers, G. Simultaneously Measuring Carbon Uptake Capacity and Chlorophyll a Fluorescence Dynamics in Algae. Algal Res. 2021, 58, 102399. [Google Scholar] [CrossRef]
- Derksen, G.C.H.; Blommaert, L.; Bastiaens, L.; Hasşerbetçi, C.; Fremouw, R.; van Groenigen, J.; Twijnstra, R.H.; Timmermans, K.R. ATR-FTIR Spectroscopy Combined with Multivariate Analysis as a Rapid Tool to Infer the Biochemical Composition of Ulva Laetevirens (Chlorophyta). Front. Mar. Sci. 2023, 10, 1154461. [Google Scholar] [CrossRef]
- Ferro, L.; Gojkovic, Z.; Gorzsás, A.; Funk, C. Statistical Methods for Rapid Quantification of Proteins, Lipids, and Carbohydrates in Nordic Microalgal Species Using ATR–FTIR Spectroscopy. Molecules 2019, 24, 3237. [Google Scholar] [CrossRef]
- Dammak, M.; Ben Hlima, H.; Elleuch, F.; Pichon, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Flow Cytometry Assay to Evaluate Lipid Production by the Marine Microalga Tetraselmis Sp. Using a Two Stage Process. Renew. Energy 2021, 177, 280–289. [Google Scholar] [CrossRef]
- Satpati, G.G.; Pal, R. Rapid Detection of Neutral Lipid in Green Microalgae by Flow Cytometry in Combination with Nile Red Staining—An Improved Technique. Ann. Microbiol. 2015, 65, 937–949. [Google Scholar] [CrossRef]
- Havlik, I.; Reardon, K.F.; Ünal, M.; Lindner, P.; Prediger, A.; Babitzky, A.; Beutel, S.; Scheper, T. Monitoring of Microalgal Cultivations with On-Line, Flow-through Microscopy. Algal Res. 2013, 2, 253–257. [Google Scholar] [CrossRef]
- Connan, S. Spectrophotometric Assays of Major Compounds Extracted from Algae. Methods Mol. Biol. 2015, 1308, 75–101. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Ebbing, T.; Frick, K.; Schmid-Staiger, U.; Haasdonk, B.; Tovar, G.E.M. Improving Determination of Pigment Contents in Microalgae Suspension with Absorption Spectroscopy: Light Scattering Effect and Bouguer–Lambert–Beer Law. Mar. Drugs 2023, 21, 619. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, J.L.; Pérez, L.; Sanchez, Á.; Cancela, Á.; Míguez, C. Microalgal Biomass Quantification from the Non-Invasive Technique of Image Processing through Red–Green–Blue (RGB) Analysis. J. Appl. Phycol. 2022, 34, 871–881. [Google Scholar] [CrossRef]
- Sarrafzadeh, M.H.; La, H.J.; Lee, J.Y.; Cho, D.H.; Shin, S.Y.; Kim, W.J.; Oh, H.M. Microalgae Biomass Quantification by Digital Image Processing and RGB Color Analysis. J. Appl. Phycol. 2015, 27, 205–209. [Google Scholar] [CrossRef]
- White, S.; Anandraj, A.; Bux, F. PAM Fluorometry as a Tool to Assess Microalgal Nutrient Stress and Monitor Cellular Neutral Lipids. Bioresour. Technol. 2011, 102, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Thiviyanathan, V.A.; Ker, P.J.; Amin, E.P.P.; Tang, S.G.H.; Yee, W.; Jamaludin, M.Z. Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband. Molecules 2023, 28, 1318. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Hołaj-Krzak, J.T.; Palani, B.K.; Bolewski, T.; Dąbrowski, J.; Bartyzel, B.J.; Gruczyńska-Sękowska, E. The Analytical Possibilities of FT-IR Spectroscopy Powered by Vibrating Molecules. Int. J. Mol. Sci. 2023, 24, 1013. [Google Scholar] [CrossRef]
- Esther Elizabeth Grace, C.; Briget Mary, M.; Vaidyanathan, S.; Srisudha, S. Response to Nutrient Variation on Lipid Productivity in Green Microalgae Captured Using Second Derivative FTIR and Raman Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120830. [Google Scholar] [CrossRef]
- Zamanileha, E.F.; Mitantsoa, J.T.; Vaonalamihanta, P.F.; Razafintsalama, A.R.; Andrianony, F.A.; Ravelonandro, P.H.; Zamanileha, E.F.; Mitantsoa, J.T.; Vaonalamihanta, P.F.; Razafintsalama, A.R.; et al. Various Approaches to Fourier-Transform Infrared Spectroscopy (FTIR) for Bioanalytical and Biotechnological Applications in Marine Algae. In Recent Advances in Infrared Spectroscopy and Its Applications in Biotechnology; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Samokhvalov, A.; McCombs, S. In Situ Time-Dependent Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy of a Powdered Specimen in a Controlled Atmosphere: Monitoring Sorption and Desorption of Water Vapor. Appl. Spectrosc. 2023, 77, 308–319. [Google Scholar] [CrossRef]
- Havlik, I.; Lindner, P.; Scheper, T.; Reardon, K.F. On-Line Monitoring of Large Cultivations of Microalgae and Cyanobacteria. Trends Biotechnol. 2013, 31, 406–414. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zeng, L.H.; Ren, Z.H. Recent Application of Spectroscopy for the Detection of Microalgae Life Information: A Review. Appl. Spectrosc. Rev. 2020, 55, 26–59. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Karpiński, T.M. Live-Cell Systems in Real-Time Biomonitoring of Water Pollution: Practical Considerations and Future Perspectives. Sensors 2021, 21, 7028. [Google Scholar] [CrossRef] [PubMed]
- Harshkova, D.; Zielińska, E.; Aksmann, A. Optimization of a Microplate Reader Method for the Analysis of Changes in Mitochondrial Membrane Potential in Chlamydomonas Reinhardtii Cells Using the Fluorochrome JC-1. J. Appl. Phycol. 2019, 31, 3691–3697. [Google Scholar] [CrossRef]
- Howell, J.; Omwenga, S.; Jimenez, M.; Hammarton, T.C. Analysis of the Leishmania Mexicana Promastigote Cell Cycle Using Imaging Flow Cytometry Provides New Insights into Cell Cycle Flexibility and Events of Short Duration. PLoS ONE 2024, 19, e0311367. [Google Scholar] [CrossRef] [PubMed]
- Ozdalgic, B.; Ustun, M.; Dabbagh, S.R.; Haznedaroglu, B.Z.; Kiraz, A.; Tasoglu, S. Microfluidics for Microalgal Biotechnology. Biotechnol. Bioeng. 2021, 118, 1716–1734. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I. Photosynthesis: New Approaches to the Molecular, Cellular, and Organismal Levels; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–386. [Google Scholar] [CrossRef]
- Taborda, T.; Moniz, P.; Reis, A.; da Silva, T.L. Evaluating Low-Cost Substrates for Crypthecodinium Cohnii Lipids and DHA Production, by Flow Cytometry. J. Appl. Phycol. 2021, 33, 263–274. [Google Scholar] [CrossRef]
- Shan Ahamed, T.; Brindhadevi, K.; Krishnan, R.; Phuong, T.N.; Ali Alharbi, S.; Chinnathambi, A.; Mathimani, T. Invivo Detection of Triacylglycerols through Nile Red Staining and Quantification of Fatty Acids in Hyper Lipid Producer Nannochloropsis sp. Cultured under Adequate Nitrogen and Deficient Nitrogen Condition. Fuel 2022, 322, 124179. [Google Scholar] [CrossRef]
- Du, X.; Chen, X.; Gao, C.; Wang, J.; Huo, X.; Chen, J. Recent Developments (After 2020) in Flow Cytometry Worldwide and Within China. Biosensors 2025, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Broger, T.; Odermatt, R.P.; Huber, P.; Sonnleitner, B. Real-Time on-Line Flow Cytometry for Bioprocess Monitoring. J. Biotechnol. 2011, 154, 240–247. [Google Scholar] [CrossRef]
- Tamayol, A.; Arab-Tehrany, E.; Desobry, S.; Imamoglu, E. Artificial Intelligence and/or Machine Learning Algorithms in Microalgae Bioprocesses. Bioeng. 2024, 11, 1143. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Biji, A.P.; Lanuyanger, T.; Show, P.L.; Sriariyanun, M.; Upadhyayula, V.K.K.; Gadhamshetty, V.; Bhattacharyya, D. Artificial Intelligence and Machine Learning Tools for High-Performance Microalgal Wastewater Treatment and Algal Biorefinery: A Critical Review. Sci. Total Environ. 2023, 876, 162797. [Google Scholar] [CrossRef]
- Bilal, A.; Imran, A.; Baig, T.I.; Liu, X.; Abouel Nasr, E.; Long, H. Breast Cancer Diagnosis Using Support Vector Machine Optimized by Improved Quantum Inspired Grey Wolf Optimization. Sci. Rep. 2024, 14, 10714. [Google Scholar] [CrossRef] [PubMed]
- Mafat, I.H.; Palla, S.; Surya, D.V. Machine Learning and Artificial Intelligence for Algal Cultivation, Harvesting Techniques, Wastewater Treatment, Nutrient Recovery, and Biofuel Production and Optimization. In Value Added Products From Bioalgae Based Biorefineries: Opportunities and Challenges; Springer: Singapore, 2024; pp. 463–487. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Ho, S.H.; Show, P.L. Smart Microalgae Farming with Internet-of-Things for Sustainable Agriculture. Biotechnol. Adv. 2022, 57, 107931. [Google Scholar] [CrossRef]
- Abdullah, M.; Malik, H.A.; Ali, A.; Boopathy, R.; Vo, P.H.N.; Danaee, S.; Ralph, P.; Malik, S. AI-Driven Algae Biorefineries: A New Era for Sustainable Bioeconomy. Curr. Pollut. Reports 2025, 11, 1–24. [Google Scholar] [CrossRef]
- Maraveas, C.; Bartzanas, T. Application of Internet of Things (IoT) for Optimized Greenhouse Environments. AgriEngineering 2021, 3, 954–970. [Google Scholar] [CrossRef]
- Borah, D.; Eldiehy, K.S.H.; Hatiboruah, D.; Mandal, M.; Deka, D. An Integrated Approach for Simultaneous Monitoring and Data Acquisition on the Culture of Green Microalga Chlorella Homosphaera Using Different LED Illumination. Bioenergy Res. 2023, 16, 601–610. [Google Scholar] [CrossRef]
- Strachan, S.; Chakraborty, M.; Sallam, M.; Bhuiyan, S.A.; Ford, R.; Nguyen, N.T. Maximising Affordability of Real-Time Colorimetric LAMP Assays. Micromachines 2023, 14, 2101. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, A.; Moallem, M. Measuring Photosynthetic Photon Flux Density in the Blue and Red Spectrum for Horticultural Lighting Using Machine Learning Methods. IEEE Trans. Instrum. Meas. 2024, 73, 2501410. [Google Scholar] [CrossRef]
- Behzadipour, F.; Ghasemi Nezhad Raeini, M.; Abdanan Mehdizadeh, S.; Taki, M.; Khalil Moghadam, B.; Zare Bavani, M.R.; Lloret, J. A Smart IoT-Based Irrigation System Design Using AI and Prediction Model. Neural Comput. Appl. 2023, 35, 24843–24857. [Google Scholar] [CrossRef]
- Anggraini, L.E.K.; Kumi, J.A.; Karolina, L.B.; Akansah, E.; Sulyman, H.A.; Mendonça, I.; Aritsugi, M.; Elikem, E.; Senoo, K.; Anggraini, L.; et al. IoT Solutions with Artificial Intelligence Technologies for Precision Agriculture: Definitions, Applications, Challenges, and Opportunities. Electronics 2020, 13, 1894. [Google Scholar] [CrossRef]
- Gincley, B.; Khan, F.; Hartnett, E.; Fisher, A.; Pinto, A.J. Introducing ARTiMiS: A Low-Cost Flow Imaging Microscope for Phytoplankton Monitoring in Engineered and Natural Environments. bioRxiv 2024. [Google Scholar] [CrossRef]
- D’Adamo, S.; Schiano di Visconte, G.; Lowe, G.; Szaub-Newton, J.; Beacham, T.; Landels, A.; Allen, M.J.; Spicer, A.; Matthijs, M. Engineering the Unicellular Alga Phaeodactylum Tricornutum for High-Value Plant Triterpenoid Production. Plant Biotechnol. J. 2019, 17, 75–87. [Google Scholar] [CrossRef]
- Faulkner, M.; Andrews, F.; Scrutton, N. Improving Productivity of Citramalate from CO2 by Synechocystis sp. PCC 6803 through Design of Experiment. Biotechnol. Biofuels Bioprod. 2024, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Karlický, V.; Kmecová Materová, Z.; Kurasová, I.; Nezval, J.; Štroch, M.; Garab, G.; Špunda, V. Accumulation of Geranylgeranylated Chlorophylls in the Pigment-Protein Complexes of Arabidopsis Thaliana Acclimated to Green Light: Effects on the Organization of Light-Harvesting Complex II and Photosystem II Functions. Photosynth. Res. 2021, 149, 233–252. [Google Scholar] [CrossRef]
- Bradley, T.; Rajaeifar, M.A.; Kenny, A.; Hainsworth, C.; del Pino, V.; del Valle Inclán, Y.; Povoa, I.; Mendonça, P.; Brown, L.; Smallbone, A.; et al. Life Cycle Assessment of Microalgae-Derived Biodiesel. Int. J. Life Cycle Assess. 2023, 28, 590–609. [Google Scholar] [CrossRef]
- Carnovale, G.; Rosa, F.; Shapaval, V.; Dzurendova, S.; Kohler, A.; Wicklund, T.; Horn, S.J.; Barbosa, M.J.; Skjånes, K. Starch Rich Chlorella Vulgaris: High-Throughput Screening and Up-Scale for Tailored Biomass Production. Appl. Sci. 2021, 11, 9025. [Google Scholar] [CrossRef]
- Uguz, S.; Sahin, Y.S.; Kumar, P.; Yang, X.; Anderson, G. Real-Time Algal Monitoring Using Novel Machine Learning Approaches. Big Data Cogn. Comput. 2025, 9, 153. [Google Scholar] [CrossRef]
- Szymoniak, S.; Kuczy’nski, Ł.; Kuczy’nski, K. Overview of Modern Technologies for Acquiring and Analysing Acoustic Information Based on AI and IoT. Appl. Sci. 2025, 15, 6690. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).