The Thermal Properties of Gliadins and Glutenins Fortified with Flavonoids and Their Glycosides Studied via Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Dough—Flavonoid/Glycoside Sample Preparation
2.3. Extraction of Gliadins and Glutenins from Gluten Samples
2.4. Thermogravimetric Analysis of Gliadins and Glutenins (TGA)
2.5. Differential Scanning Calorimetry of Gliadins and Glutenins (DSC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
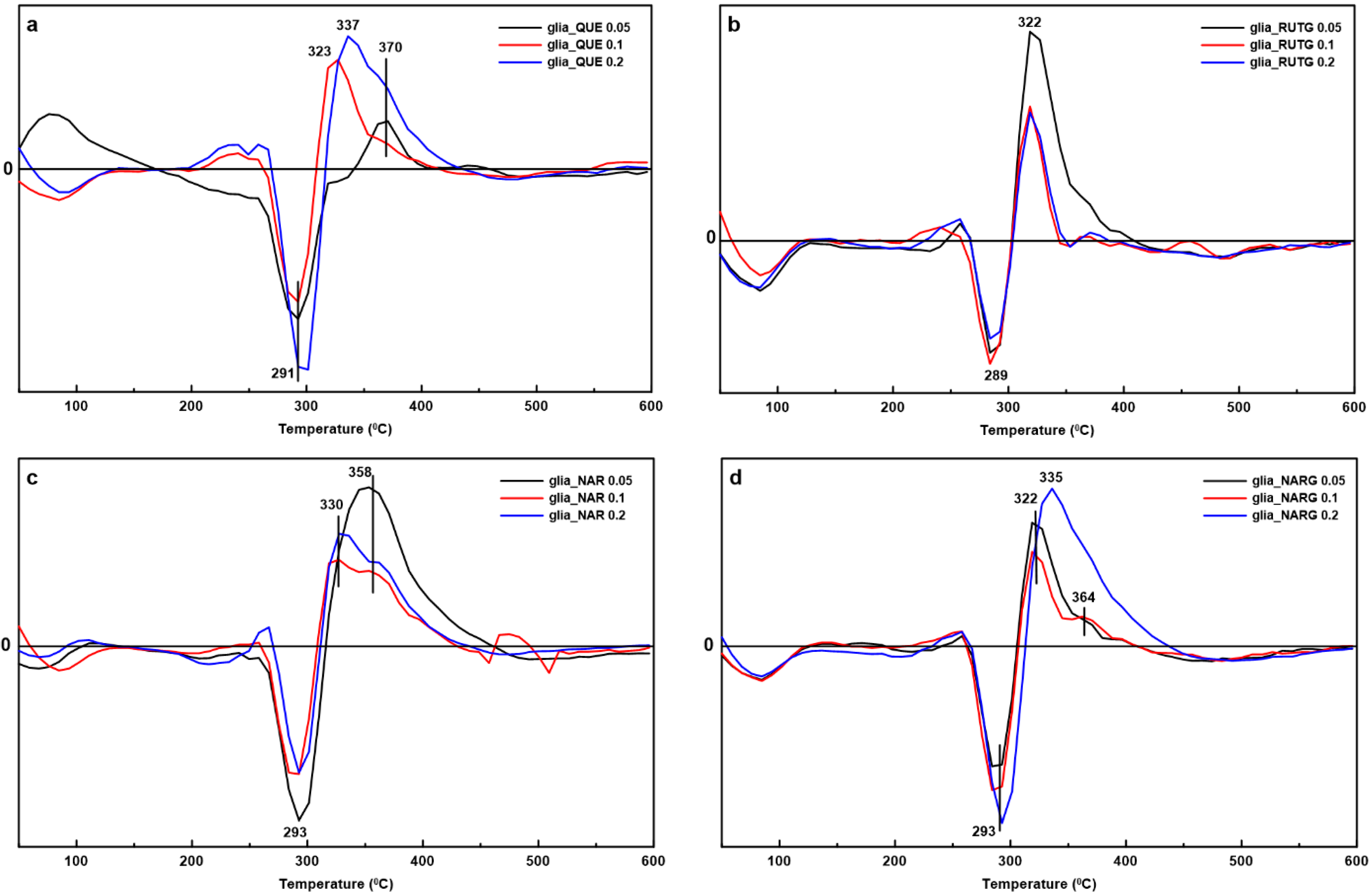
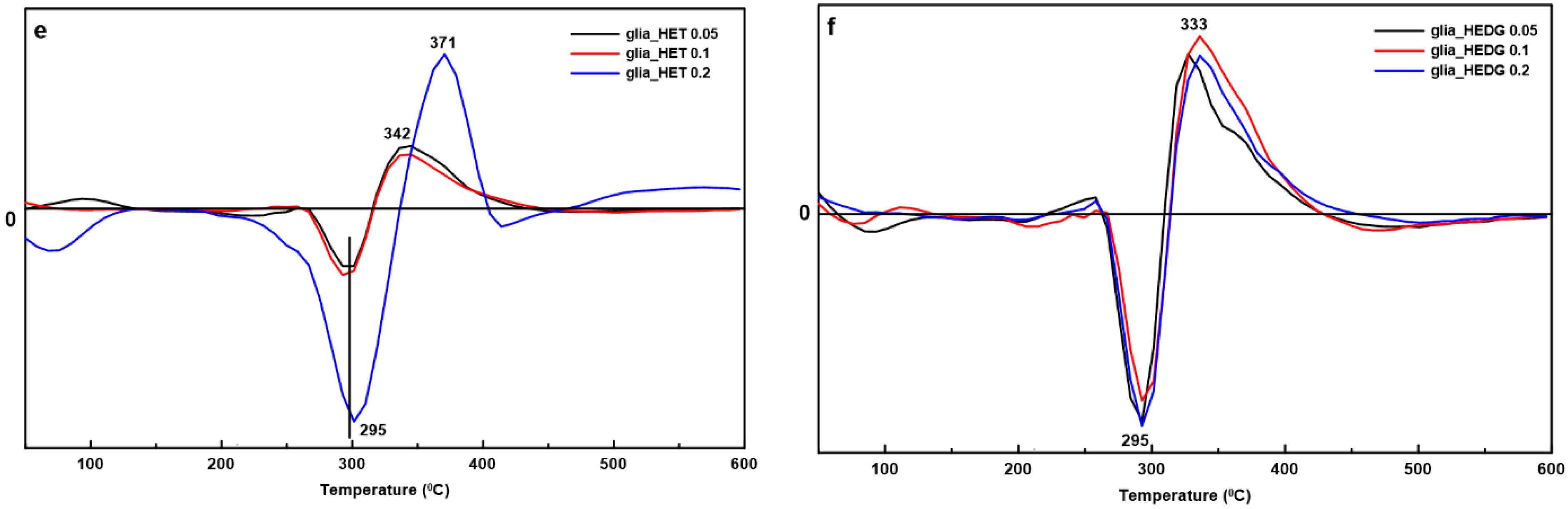
3.2. Differential Scanning Calorimetry (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2017, 10, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves—The influence of protein–phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-M.; Koh, B.-K. Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J. Sci. Food Agric. 2010, 91, 604–608. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Barak, S.; Mudgil, D. Effects of gliadin addition on the rheological, microscopic and thermal characteristics of wheat gluten. Int. J. Biol. Macromol. 2013, 53, 38–41. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Li, C.; Xue, F. Comparative studies on physicochemical properties of gluten- and glutenin-based films functionalized by polyphenols. Cereal Chem. 2022, 99, 439–703. [Google Scholar] [CrossRef]
- Ma, S.; Han, W.; Li, L.; Zheng, X.; Wang, X. The thermal stability, structural changeability, and aggregability of the glutenin and gliadin proteins induced by wheat bran dietary fiber. Food Funct. 2018, 10, 172–179. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Aggregation of gluten proteins in model dough after fibre polysaccharide addition. Food Chem. 2017, 231, 51–60. [Google Scholar] [CrossRef]
- Rumińska, W.; Markiewicz, K.H.; Wilczewska, A.Z.; Nawrocka, A. Effect of oil pomaces on thermal properties of model dough and gluten network studied by thermogravimetry and differential scanning calorimetry. Food Chem. 2021, 358, 129882. [Google Scholar] [CrossRef]
- Krekora, M.; Markiewicz, K.H.; Wilczewska, A.Z.; Nawrocka, A. Raman and thermal (TGA and DSC) studies of gluten proteins supplemented with flavonoids and their glycosides. J. Cereal Sci. 2023, 111, 103672. [Google Scholar] [CrossRef]
- Krekora, M.; Nawrocka, A. Interactions of gliadins with flavonoids and their glycosides studied with application of FT-Raman spectroscopy. J. Cereal Sci. 2024, 117, 103915. [Google Scholar] [CrossRef]
- Krekora, M.; Szymańska-Chargot, M.; Niewiadomski, Z.; Miś, A.; Nawrocka, A. Effect of cinnamic acid and its derivatives on structure of gluten proteins—A study on model dough with application of FT-Raman spectroscopy. Food Hydrocoll. 2020, 107, 105935. [Google Scholar] [CrossRef]
- Taddei, P.; Zanna, N.; Tozzi, S. Raman characterization of the interactions between gliadins and anthocyanins. J. Raman Spec. 2013, 44, 1435–1439. [Google Scholar] [CrossRef]
- Leon, A.; Rosell, C.M.; Benedito de Barber, C. A differential scanning calorimetry study of wheat proteins. Eur. Food Res. Technol. 2003, 217, 13–16. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.-T.; Niakousari, M.; Hosseini, S.; Ghorani, B.; Lopez-Rubio, A. Active Food Packaging Coatings Based on Hybrid Electrospun Gliadin Nanofibers Containing Ferulic Acid/Hydroxypropyl-Beta-Cyclodextrin Inclusion Complexes. Nanomaterials 2018, 8, 919. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, W.; Su, G.; Cui, C.; Zhao, M. Comparison of the conformational and nutritional changes of deamidated wheat gliadin by citric acid and hydrochloric acid. J. Cereal Sci. 2014, 60, 143–150. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effect of physical and chemical factors on the structure of gluten, gliadins and glutenins as studied with spectroscopic methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef]
- Wang, P.; Xu, L.; Nikoo, M.; Ocen, D.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on the conformational, thermal and microscopic properties of gluten: Comparative studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014, 35, 238–246. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Mis, A.; Wilczewska, A.Z.; Markiewicz, K.H. Dietary fibre-induced changes in the structure and thermal properties of gluten proteins studied by Fourier transform—Raman spectroscopy and thermogravimetry. J. Agric. Food Chem. 2016, 64, 2094–2104. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins—A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Welc, R.; Luchowski, R.; Kłosok, K.; Gruszecki, W.I.; Nawrocka, A. How do phenolic acids change the secondary and tertiary structure of gliadin? Studies with an application of spectroscopic techniques. Int. J. Mol. Sci. 2022, 23, 6053. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Liu, X.; Hu, X.; Zhao, Y.; Tao, N.-P.; Wang, M. Dihydromyricetin as a Functional Additive to Enhance Antioxidant Capacity and Inhibit the Formation of Thermally Induced Food Toxicants in a Cookie Model. Molecules 2018, 23, 2184. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yao, X.; Chen, Z.; Ma, R.; Wen, Y.; Li, H.; Wang, J.; Bao, S. Interaction of high amylose corn starch with polyphenols: Modulating the stability of polyphenols with different structure against thermal processing. Food Chem. 2023, 437, 137708. [Google Scholar] [CrossRef] [PubMed]
- Bedrníček, J.; Jirotková, D.; Kadlec, J.; Laknerová, I.; Vrchotová, N.; Tříska, J.; Samková, E.; Smetana, P. Thermal stability and bioavailability of bioactive compounds after baking of bread enriched with different onion by-products. Food Chem. 2020, 319, 126562. [Google Scholar] [CrossRef]
- Blecker, C.; Chevalier, J.P.; Fougnies, C.; Van Herck, J.C.; Deroanne, C.; Paquot, M. Characterisation of different inulin samples by DSC: Influence of polymerisation degree on melting temperature. J. Therm. Anal. Calorim. 2003, 71, 215–224. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Tsivintzelis, I. On the thermodynamic thermal properties of quercetin and similar pharmaceuticals. Molecules 2022, 27, 6630. [Google Scholar] [CrossRef]
- Wiesser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2022, 100, 23–35. [Google Scholar] [CrossRef]
- Ma, W.; Yu, Z.; She, M.; Zhao, Y.; Islam, S. Wheat gluten protein and its impacts on wheat processing quality. Front. Agric. Sci. Eng. 2019, 6, 279–287. [Google Scholar] [CrossRef]
- Waga, J. Structure and allergenicity of wheat gluten proteins—A review. Pol. J. Food Nutr. Sci. 2004, 13/54, 327–338. [Google Scholar]
- Kieffer, R.; Schurer, F.; Köhler, P.; Wieser, H. Effect of hydrostatic pressure and temperature on the chemical and functional properties of wheat gluten: Studies on gluten, gliadin and glutenin. J. Cereal Sci. 2007, 45, 285–292. [Google Scholar] [CrossRef]
- Müller, S.; Wieser, H. The location of disulphide bonds in monomeric γ-type gliadins. J. Cereal Sci. 1997, 26, 169–176. [Google Scholar] [CrossRef]
- Lagrain, B.; Thewissen, B.G.; Brijs, K.; Delcour, J.A. Mechanism of gliadin–glutenin cross-linking during hydrothermal treatment. Food Chem. 2008, 107, 753–760. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Awika, J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020, 9, 2164–2199. [Google Scholar] [CrossRef]
- Li, M.; Yue, Q.; Liu, C.; Zheng, X.; Hong, J.; Li, L.; Bian, K. Effect of gliadin/glutenin ratio on pasting, thermal, and structural properties of wheat starch. J. Cereal Sci. 2020, 93, 102973. [Google Scholar] [CrossRef]

| Polyphenol | Polyphenol Content | Weight Loss (%) | Td1 (°C) | Td2 (°C) | Td3 (°C) | Polyphenol | Polyphenol Content | Weight Loss (%) | Td1 (°C) | Td2 (°C) | Td3 (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| glia_control | 0 | 68.7 (2.4) de | 69 (1) b | 310 (3) c | glu_control | 0 | 70.8 (2.1) d | 72 (2) b | 301 (3) d | ||
| glia_QUE | 0.05 | 79.3 (1.6) i | 76 (1) c | 310 (2) c | glu_QUE | 0.05 | 75.6 (2.8) d | 69 (3) c | 301 (1) d | ||
| 0.1 | 72.4 (1.1) efg | 69 (1) b | 319 (2) d | 0.1 | 72.5 (2.1) d | 76 (3) b | 301 (3) d | ||||
| 0.2 | 75.4 (1.9) fghi | 67 (2) b | 319 (1) d | 0.2 | 73.9 (2.4) d | 59 (1) b | 301 (4) d | ||||
| 100 | 48.5 (2.5) a | 336 (3) e | 100 | 48.5 (2.2) a | 336 (3) f | ||||||
| glia_RUTG | 0.05 | 77.4 (1.5) hi | 67 (2) b | 310 (1) c | glu_RUTG | 0.05 | 73.1 (1.5) d | 75 (2) b | 301 (3) d | ||
| 0.1 | 77.3 (1.8) hi | 59 (2) a | 310 (3) c | 0.1 | 72.2 (3.2) d | 75 (2) a | 301 (2) d | ||||
| 0.2 | 77.3 (1.4) hi | 66 (2) b | 318 (1) d | 0.2 | 74.4 (2.2) d | 68 (2) b | 301 (2) d | ||||
| 100 | 61.5 (2.1) bc | 128 (2) f | 275 (2) a | 427 (2) c | 100 | 61.5 (2.8) bc | 128 (3) f | 275 (2) a | 427 (3) a | ||
| glia_NAR | 0.05 | 77.0 (2.0) hi | 74 (2) c | 318 (1) d | glu_NAR | 0.05 | 72.9 (2.8) d | 68 (3) c | 301 (3) d | ||
| 0.1 | 72.2 (1.9) ef | 66 (2) b | 318 (3) d | 0.1 | 74.3 (1.0) d | 72 (2) b | 301 (2) d | ||||
| 0.2 | 74.5 (1.8) fgh | 74 (1) c | 318 (3) d | 0.2 | 73.2 (2.5) d | 76 (3) c | 301 (4) d | ||||
| 100 | 57.2 (1.9) b | 318 (3) d | 100 | 57.2 (2.7) b | 318 (4) e | ||||||
| glia_NARG | 0.05 | 73.8 (2.2) fgh | 59 (2) a | 318 (2) d | glu_NARG | 0.05 | 73.1 (3.1) d | 61 (3) a | 301 (3) d | ||
| 0.1 | 74.4 (1.3) fgh | 59 (1) a | 320 (2) d | 0.1 | 71.5 (2.9) d | 75 (3) a | 319 (3) e | ||||
| 0.2 | 75.4 (1.7) fghi | 59 (2) a | 318 (2) d | 0.2 | 72.6 (1.6) d | 71 (3) a | 293 (4) c | ||||
| 100 | 64.4 (1.8) cd | 93 (2) d | 284 (3) b | 375 (2) b | 100 | 64.4 (2.5) c | 93 (2) d | 284 (2) b | 375 (2) b | ||
| glia_HET | 0.05 | 75.4 (1.9) fghi | 76 (2) c | 319 (3) d | glu_HET | 0.05 | 73.7 (2.8) d | 72 (2) c | 301 (4) d | ||
| 0.1 | 74.6 (1.1) fgh | 66 (3) b | 319 (2) d | 0.1 | 73.9 (3.2) d | 71 (2) b | 301 (4) d | ||||
| 0.2 | 74.4 (2.4) fgh | 75 (1) c | 319 (1) d | 0.2 | 72.7 (1.2) d | 59 (3) c | 301 (3) d | ||||
| 100 | 61.7 (2.5) bc | 371 (2) f | 100 | 61.7 (2.3) bc | 371 (3) g | ||||||
| glia_HEDG | 0.05 | 72.3 (1.8) efg | 69 (2) b | 319 (3) d | glu_HEDG | 0.05 | 72.4 (2.9) d | 67 (1) b | 301 (3) d | ||
| 0.1 | 76.8 (2.1) ghi | 69 (1) b | 319 (3) d | 0.1 | 71.4 (3.0) d | 72 (1) b | 301 (2) d | ||||
| 0.2 | 71.4 (2.1) ef | 69 (1) b | 319 (1) d | 0.2 | 73.7 (2.5) d | 76 (2) b | 301 (3) d | ||||
| 100 | 64.1 (2.4) c | 101 (1) e | 284 (2) b | 354 (3) a | 100 | 64.1 (1.0) c | 101 (1) e | 284 (2) b | 354 (3) a |
| Polyphenol | T1 (°C) | ΔH1 (J·g−1) | T2 (°C) | ΔH2 (J·g−1) | T3 (°C) | ΔH3 (J·g−1) | T4 (°C) | ΔH4 (J·g−1) | T5 (°C) | ΔH5 (J·g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| glia_control | 76 (2) bcd | −179 (2) f | 308 (2) fg | −263 (2) e | ||||||
| glia_QUE | 80 (2) def | −180 (2) ef | 267 (3) b | 136 (2) g | 324 (1) l | 44 (2) l | ||||
| 81 (1) ef | −196 (2) c | 262 (2) b | 89 (1) f | 320 (2) jkl | 60 (1) m | |||||
| 76 (2) bcd | −190 (3) d | 249 (3) e | 41 (2) e | 308 (1) fg | 55 (2) ł | |||||
| 105 (2) g | −21 (2) j | 322 (2) kl | −154 (2) i | 347 (1) b | 68 (2) a | |||||
| glia_RUTG | 81 (2) ef | −215 (2) a | 305 (3) ef | −240 (2) g | ||||||
| 81 (2) ef | −203 (2) b | 305 (1) ef | −272 (2) d | |||||||
| 80 (2) def | −181 (2) ef | 312 (3) ghi | −267 (2) e | |||||||
| 155 (2) a | −104 (2) a | 220 (3) c | −26 (1) d | 301 (3) de | 9 (2) j | |||||
| glia_NAR | 80 (3) def | −203 (3) b | 308 (2) fg | −286 (1) c | ||||||
| 75 (1) bc | −170 (1) g | 312 (3) ghi | −266 (1) e | |||||||
| 73 (2) b | −191 (2) d | 284 (1) a | −252 (0) f | |||||||
| 255 (1) a | −172 (2) a | 316 (3) hij | 204 (2) n | |||||||
| glia_NARG | 73 (2) b | −180 (1) ef | 292 (3) b | −248 (2) f | ||||||
| 74 (2) bc | −177 (2) f | 311 (2) gh | −291 (2) b | |||||||
| 66 (2) a | −141 (1) h | 291 (2) b | −219 (1) h | |||||||
| 102 (2) g | −108 (2) i | 165 (2) b | −13 (1) c | 283 (3) a | 28 (2) k | |||||
| glia_HET | 80 (2) def | −180 (1) ef | 298 (2) cd | −338 (2) a | ||||||
| 82 (1) ef | −180 (2) ef | 312 (2) ghi | 344 (2) o | |||||||
| 78 (2) cde | −193 (2) cd | 298 (2) cd | 537 (2) p | |||||||
| 230 (2) d | −161 (2) b | 342 (3) a | 112 (1) b | |||||||
| glia_HEDG | 83 (2) f | −203 (2) b | 312 (2) ghi | −264 (2) e | ||||||
| 72 (2) b | −184 (2) e | 317 (2) ijk | −291 (1) b | |||||||
| 83 (2) f | −177 (2) f | 294 (2) bc | −291 (1) b | |||||||
| 197 (1) c | −101 (2) b | 257 (3) a | −122 (2) c | 283 (3) a | 51 (2) ł | |||||
| glu_control | 91 (2) e | −190 (2) d | 302 (3) bc | 29 (2) efg | ||||||
| glu_QUE | 92 (1) ef | −152 (1) hi | 303 (2) c | 48 (2) l | ||||||
| 96 (2) fg | −145 (2) j | 303 (2) c | 35 (2) ij | |||||||
| 90 (1) de | −128 (3) k | 301 (2) bc | 31 (2) fgh | |||||||
| 105 (2) i | −21 (2) n | 321 (2) d | −154 (2) a | 347 (4) b | 69 (3) a | |||||
| glu_RUTG | 90 (1) de | −178 (1) f | 300 (2) bc | 34 (1) hij | ||||||
| 85 (2) bc | −150 (1) i | 300 (3) bc | 29 (2) efg | |||||||
| 83 (2) abc | −160 (2) g | 301 (1) bc | 85 (2) m | |||||||
| 157 (2) a | −104 (2) a | 220 (2) b | −26 (1) d | 301 (2) bc | 9 (2) b | |||||
| glu_NAR | 86 (2) cd | −219 (2) b | 296 (1) b | 117 (2) b | ||||||
| 90 (2) de | −202 (2) c | 302 (3) bc | 55 (2) ł | |||||||
| 99 (2) gh | −159 (2) g | 301 (3) bc | 32 (1) ghi | |||||||
| 255 (1) a | −173 (2) a | 318 (3) d | 205 (2) n | |||||||
| glu_NARG | 93 (2) ef | −156 (2) gh | 300 (3) bc | 40 (2) k | ||||||
| 81 (2) ab | −236 (2) a | 300 (3) bc | 18 (2) cd | |||||||
| 91 (1) e | −151 (1) i | 300 (2) bc | 37 (1) jk | |||||||
| 102 (3) hi | −108 (2) i | 165 (2) b | −13 (2) b | 283 (3) a | 28 (1) ef | |||||
| glu_HET | 91 (1) e | −183 (3) e | 301 (2) bc | 35 (3) ij | ||||||
| 92 (1) ef | −114 (2) l | 301 (3) bc | 33 (1) hi | |||||||
| 92 (2) ef | −112 (2) lł | 300 (4) bc | 32 (2) ghi | |||||||
| 232 (3) c | −161 (2) b | 341 (2) a | 109 (3) b | |||||||
| glu_HEDG | 85 (2) bc | −216 (2) b | 301 (3) bc | 15 (1) c | ||||||
| 90 (2) de | −94 (2) m | 301 (2) bc | 27 (1) e | |||||||
| 80 (2) a | −233 (2) a | 300 (3) bc | 20 (1) d | |||||||
| 197 (3) c | −101 (2) a | 257 (2) a | −122 (2) c | 280 (3) a | 51 (2) l |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krekora, M.; Markiewicz, K.H.; Wilczewska, A.Z.; Nawrocka, A. The Thermal Properties of Gliadins and Glutenins Fortified with Flavonoids and Their Glycosides Studied via Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC). Appl. Sci. 2025, 15, 7303. https://doi.org/10.3390/app15137303
Krekora M, Markiewicz KH, Wilczewska AZ, Nawrocka A. The Thermal Properties of Gliadins and Glutenins Fortified with Flavonoids and Their Glycosides Studied via Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC). Applied Sciences. 2025; 15(13):7303. https://doi.org/10.3390/app15137303
Chicago/Turabian StyleKrekora, Magdalena, Karolina Halina Markiewicz, Agnieszka Zofia Wilczewska, and Agnieszka Nawrocka. 2025. "The Thermal Properties of Gliadins and Glutenins Fortified with Flavonoids and Their Glycosides Studied via Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC)" Applied Sciences 15, no. 13: 7303. https://doi.org/10.3390/app15137303
APA StyleKrekora, M., Markiewicz, K. H., Wilczewska, A. Z., & Nawrocka, A. (2025). The Thermal Properties of Gliadins and Glutenins Fortified with Flavonoids and Their Glycosides Studied via Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC). Applied Sciences, 15(13), 7303. https://doi.org/10.3390/app15137303






