Effect of Contralateral Cervical Glide on the Suprascapular Nerve: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study (Anatomical Study)
2.1.1. Sample
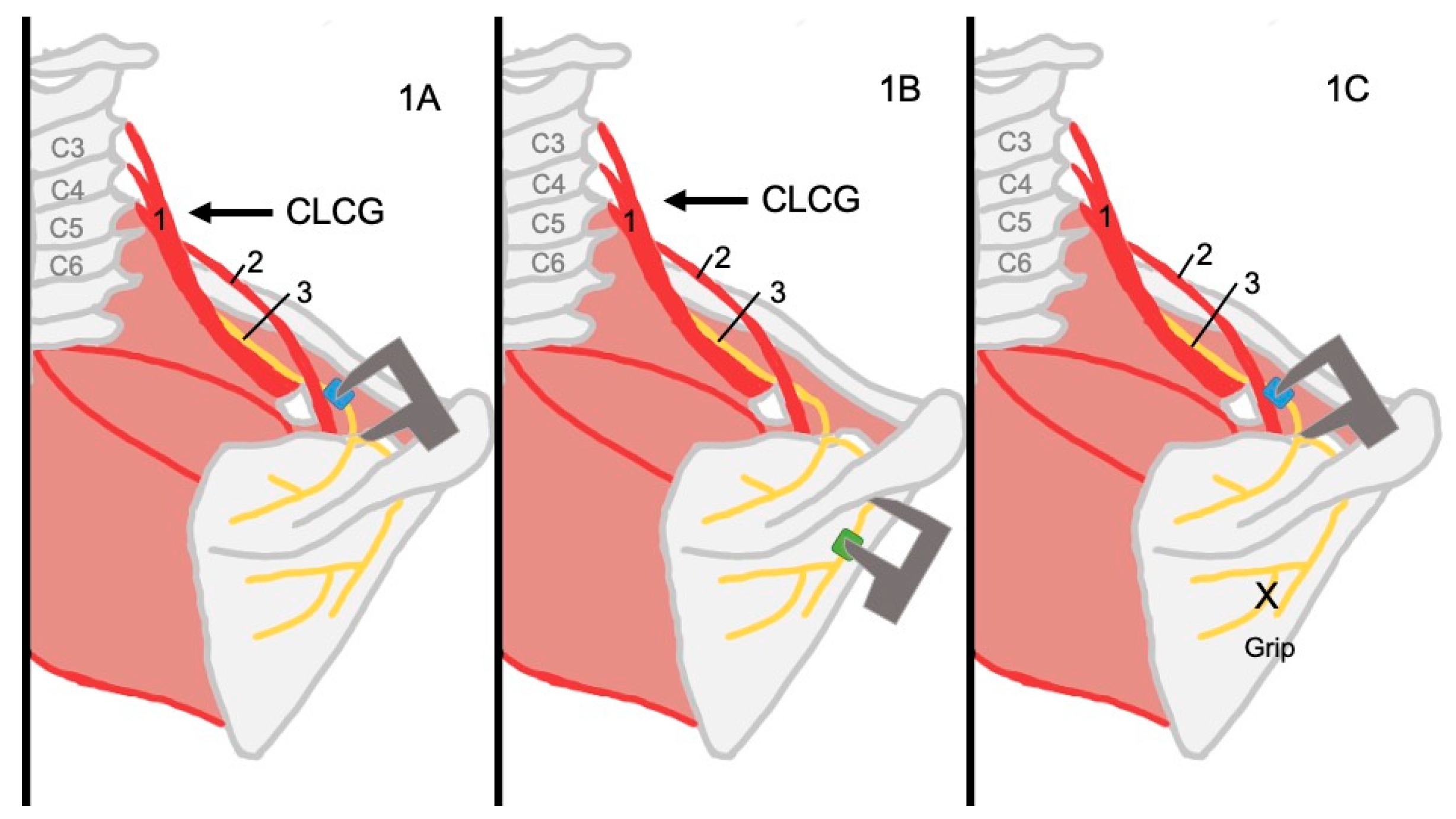
2.1.2. Procedure
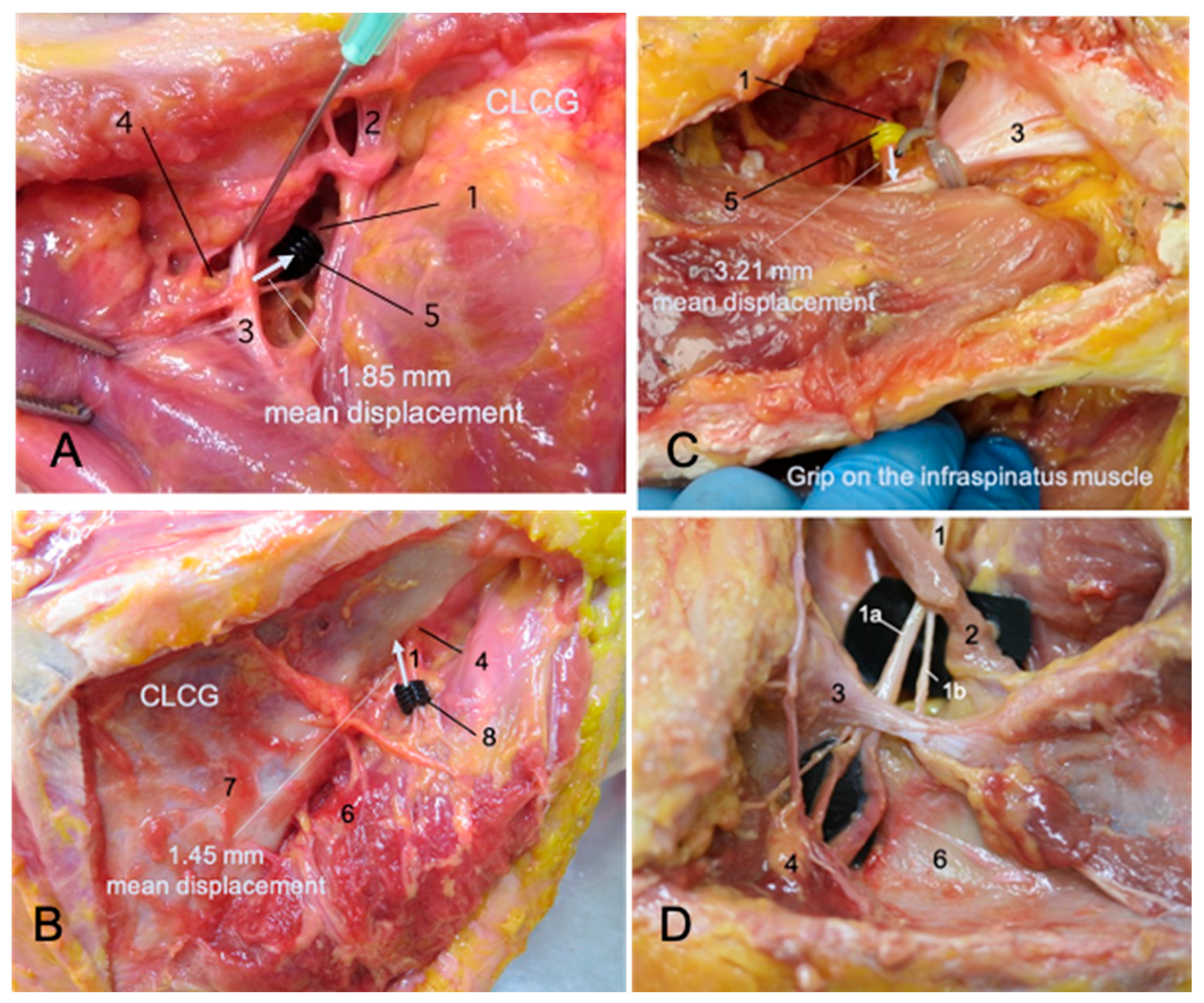
- The distance between the superior ring and the superior transverse scapular ligament (STSL) before and after a CLCG.
- The distance between the inferior ring and the SGN before and after a CLCG.
- The distance between the superior ring and the STSL before and after mimicking an infraspinatus muscle contraction.
- The difference between each pair of measurements was calculated to obtain the estimated displacement in millimeters of the silicone rings placed on the SNe.
2.1.3. Data Analysis
2.2. In Vivo Study
2.2.1. Sample
2.2.2. Procedure
- -
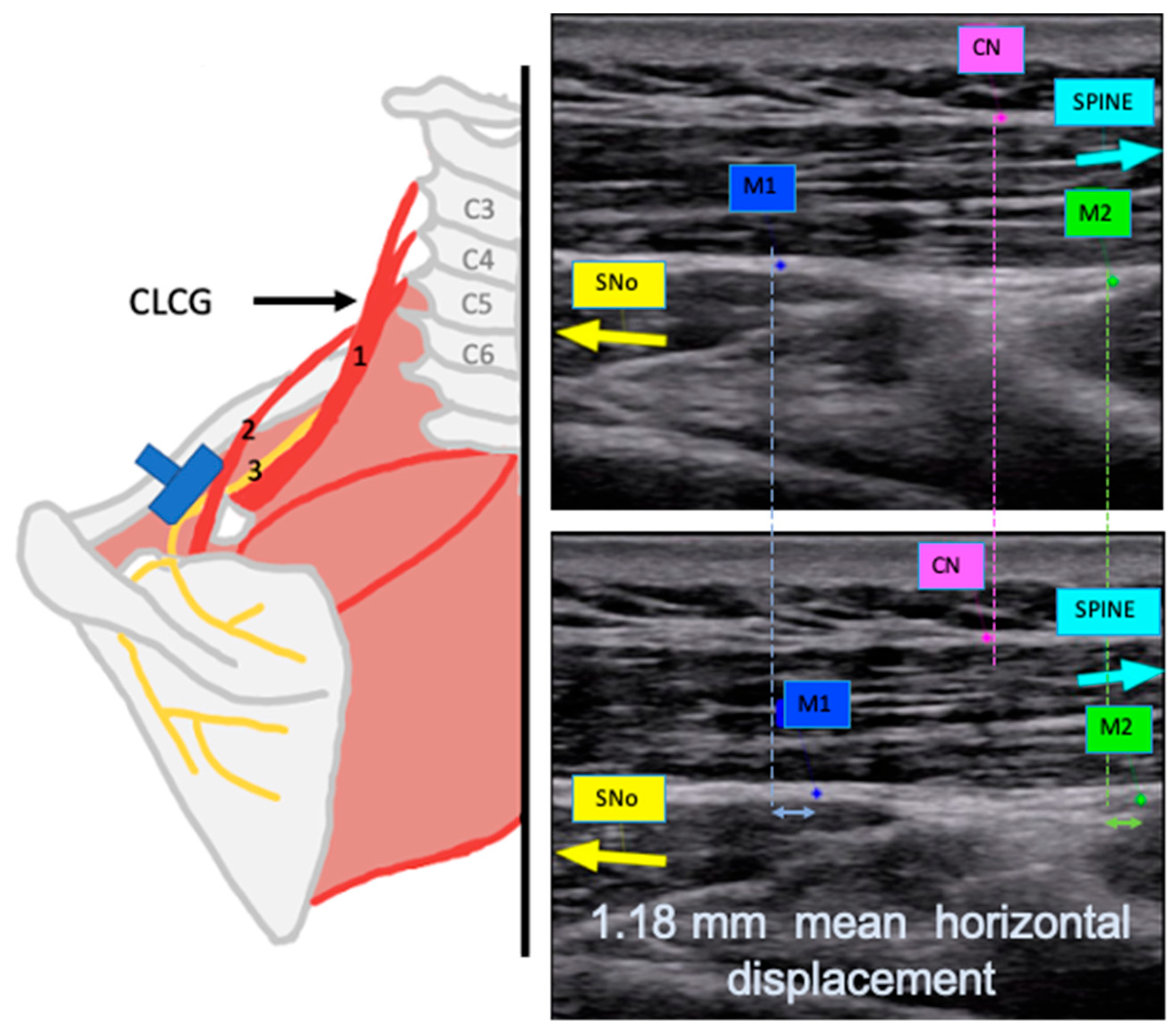
- For Video S1, the transducer was placed on the SNe as close as possible to the SNo. A CLCG was performed (see Figure 2A and the detailed explanation on Appendix B).
- -
- For Video S2, the transducer was placed in the same position as for Video S1. An isometric external rotation contraction was performed (see Figure 2B and the detailed explanation in Appendix B).
- -
- For Video S3, the transducer was placed along the inferior border of the spine of the scapula to scan the SNe distal to the SGN. A CLCG was performed (see Figure 2C and the detailed explanation in Appendix B).
2.2.3. Data Processing
2.2.4. Data Analysis
3. Results
3.1. Anatomical Study
3.2. In Vivo Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SNe | Suprascapular Nerve |
| SNo | Suprascapular Notch |
| STSL | Superior Transverse Scapular Ligament |
| CLCG | Contralateral Cervical Glide |
| SGN | Spinoglenoid Notch |
| ULNTT | Upper Limb Neural Tension Test |
| CI | Confidence Interval |
Appendix A
Detailed Analomical Study Procedure
- A contralateral cervical glide (CLCG) focusing on C5-C6 was performed. The maneuver was performed by the researcher gradually pressing the ulnar edge of the hand on the lateral surface of C5-C6 until an articular end-feel was obtained before returning to neutral position. The torso on the side contralateral to the measurement was stabilized, with the scapula being kept stable on the side that was being measured by the researcher who was performing the maneuver.
- The infraspinatus muscle fibers were gripped to bring the origin and insertion closer together to mimic a muscle contraction.
- The distance between the superior ring and the STSL before and after a CLCG.
- The distance between the inferior ring and the SGN before and after a CLCG.
- The distance between the superior ring and the STSL before and after mimicking an infraspinatus contraction.
Appendix B
- For Video S1, the transducer was placed in the horizontal plane on the anterolateral aspect of the neck to image the roots of C5-C6-C7 and was moved toward the acromion to locate the SNe as close as possible to the SNo. A CLCG was performed (see Maneuver Video S1), by the researcher gradually pressing the ulnar edge of their hand on the lateral surface of C5-C6 until an articular end-feel was obtained. The torso on the side contralateral to the measurement was stabilized, ensuring that the scapula was stable on the side being measured.
- For Video S2, the transducer was placed in the same position as for Video S1. An isometric external rotation contraction was performed (see Maneuver Video S2). The elbow was stabilized against the side of the trunk and a resistance was applied on the dorsal side of the wrist. An isometric contraction was chosen to prevent the ultrasound head from moving. A preliminary observation study was undertaken to determine the appropriate contraction intensity. Movement could be observed at the beginning of the contraction. No additional movement was observed when the contraction intensity increased. The SNe returned to its resting position when the contraction was released. Thus, it was decided that a mild isometric contraction would be used.
References
- Khosravi, F.; Amiri, Z.; Masouleh, N.A.; Kashfi, P.; Panjizadeh, F.; Hajilo, Z.; Shanayii, S.; Khodakarim, S.; Rahnama, L. Shoulder pain prevalence and risk factors in middle-aged women: A cross-sectional study. J. Bodyw. Mov. Ther. 2019, 23, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Thoomes-de Graaf, M.; Scholten-Peeters, G.G.M.; Duijn, E.; Karel, Y.H.J.M.; van den Borne, M.P.J.; Beumer, A.; Ottenheijm, R.P.G.; Dinant, G.J.; Tetteroo, E.; Lucas, C.; et al. Inter-professional agreement of ultrasound-based diagnoses in patients with shoulder pain between physical therapists and radiologists in the Netherlands. Man. Ther. 2014, 19, 478–483. [Google Scholar] [CrossRef]
- Crookes, T.; Wall, C.; Byrnes, J.; Johnson, T.; Gill, D. Chronic shoulder pain. Aust. J. Gen. Pract. 2023, 52, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Karel, Y.H.J.M.; Scholten-Peeters, G.G.M.; Thoomes-de Graaf, M.; Duijn, E.; van Broekhoven, J.B.; Koes, B.W.; Verhagen, A.P. Physiotherapy for patients with shoulder pain in primary care: A descriptive study of diagnostic- and therapeutic management. Physiotherapy 2017, 103, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Uhl, T.L.; Smith-Forbes, E.V.; Nitz, A.J. Factors influencing final outcomes in patients with shoulder pain: A retrospective review. J. Hand Ther. 2017, 30, 200–207. [Google Scholar] [CrossRef]
- Kooijman, M.; Swinkels, I.; Van Dijk, C.; De Bakker, D.; Veenhof, C. Patients with shoulder syndromes in general and physiotherapy practice: An observational study. BMC Musculoskelet. Disord. 2013, 14, 128. [Google Scholar] [CrossRef]
- Lucas, J.; van Doorn, P.; Hegedus, E.; Lewis, J.; van der Windt, D. A systematic review of the global prevalence and incidence of shoulder pain. BMC Musculoskelet. Disord. 2022, 23, 1073. [Google Scholar] [CrossRef]
- Memon, M.; Kay, J.; Ginsberg, L.; Simunovic, N.; Bak, K.; Lapner, P.; Ayeni, O.R. Arthroscopic management of suprascapular neuropathy of the shoulder improves pain and functional outcomes with minimal complication rates. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 240–266. [Google Scholar] [CrossRef]
- Ohanisian, L.; Brown, N.; White, S.D.; Rubay, D.; Schwartz, P.M. Persistent Shoulder Pain Due to a Suprascapular Nerve Injury in the Setting of Trauma. Cureus 2019, 11, e4224. [Google Scholar] [CrossRef]
- Bachasson, D.; Singh, A.; Shah, S.; Lane, J.G.; Ward, S.R. The Role of the Peripheral and Central Neurvous Systems inRotator Cuff Disease. J. Shoulder Elb. Surg. 2015, 24, 1322–1335. [Google Scholar] [CrossRef]
- Laumonerie, P.; Dalmas, Y.; Tibbo, M.E.; Robert, S.; Faruch, M.; Chaynes, P.; Bonnevialle, N.; Mansat, P. Sensory innervation of the human shoulder joint: The three bridges to break. J. Shoulder Elb. Surg. 2020, 29, e499–e507. [Google Scholar] [CrossRef] [PubMed]
- Polguj, M.; Rożniecki, J.; Sibiński, M.; Grzegorzewski, A.; Majos, A.; Topol, M. The variable morphology of suprascapular nerve and vessels at suprascapular notch: A proposal for classification and its potential clinical implications. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.H.; Lee, D.G. Effectiveness of new nerve blocks method on the articular branches of the suprascapular and subscapular nerves to treat shoulder pain. Medicine 2020, 99, e22050. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, H.; Podgórski, M.; Wysiadecki, G.; Olewnik, Ł.; De Caro, R.; Macchi, V.; Polguj, M. Morphological Aspects in Ultrasound Visualisation of the Suprascapular Notch Region: A Study Based on a New Four-Step Protocol. J. Clin. Med. 2018, 7, 491. [Google Scholar] [CrossRef]
- Polguj, M.; Synder, M.; Borowski, A.; Wojciechowski, M.; Wysiadecki, G.; Topol, M. Anterior Coracoscapular Ligament as a Factor Predisposing to or Protective for Suprascapular Neuropathy. Biomed Res. Int. 2016, 2016, 4134280. [Google Scholar] [CrossRef]
- Tasaki, A.; Nimura, A.; Mochizuki, T.; Yamaguchi, K.; Kato, R.; Sugaya, H.; Akita, K. Anatomic observation of the running space of the suprascapular nerve at the suprascapular notch in the same direction as the nerve. Knee Surg. Sport. Traumatol. Arthrosc. 2015, 23, 2667–2673. [Google Scholar] [CrossRef]
- Yoo, Y.S.; Jang, S.W.; Kim, Y.S.; Choi, J.A.; Oh, J.H.; Jeong, J.Y. Does the Suprascapular Nerve Move within the Suprascapular Notch? Biomechanical Perspective Using the Finite Element Method. Yonsei Med. J. 2022, 63, 657–664. [Google Scholar] [CrossRef]
- Kostretzis, L.; Theodoroudis, I.; Boutsiadis, A.; Papadakis, N.; Papadopoulos, P. Suprascapular Nerve Pathology: A Review of the Literature. Open Orthop. J. 2017, 11, 140–153. [Google Scholar] [CrossRef]
- Shacklock, M. Neurodynamics. Physiotherapy 1995, 81, 9–16. [Google Scholar] [CrossRef]
- Basson, C.A.; Stewart, A.; Mudzi, W.; Musenge, E. Effect of neural mobilization on nerve-related neck and arm pain: A randomized controlled trial. Physiother. Can. 2020, 72, 408–419. [Google Scholar] [CrossRef]
- Brochwicz, P.; von Piekartz, H.; Zalpour, C. Sonography assessment of the median nerve during cervical lateral glide and lateral flexion. Is there a difference in neurodynamics of asymptomatic people? Man. Ther. 2013, 18, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Paquette, P.; El Khamlichi, Y.; Lamontagne, M.; Higgins, J.; Gagnon, D.H. Comparison of longitudinal excursion of a nerve-phantom model using quantitative ultrasound imaging and motion analysis system methods: A convergent validity study. Ultrasound 2017, 25, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Bellot, N.; Ridehalgh, C.; Brismée, J.-M.; Crawford, M.; St-Pierre, M.-O.; Effatparvar, M.R.; Lavoie, F.-A.; Sobczak, S. Neurodynamic testing of the suprascapular nerve: An observational cadaveric study. Clin. Biomech. 2025, 121, 106380. [Google Scholar] [CrossRef] [PubMed]
- Beddaa, H.; Kably, B.; Marzouk, B.; Mouhi, I.; Marfak, A.; Azemmour, Y.; Alaoui, I.B.; Birouk, N. The effectiveness of the median nerve neurodynamic mobilisation techniques in women with mild or moderate bilateral carpal tunnel syndrome: A single-blind clinical randomised trial. S. Afr. J. Physiother. 2022, 78, 1823. [Google Scholar] [CrossRef]
- Joshi, K.C.; Eapen, C.; Kumar, S.P. Normal sensory and range of motion (ROM) responses during Thoracic Slump Test (ST) in asymptomatic subjects. J. Man. Manip. Ther. 2013, 21, 24–32. [Google Scholar] [CrossRef]
- Shacklock, M. Clinical Neurodynamics in Sports Injuries. Sport. Rehabil. 2014, 3, 50–56. [Google Scholar]
- Coppieters, M.W.; Alshami, A.M.; Babri, A.S.; Souvlis, T.; Kippers, V.; Hodges, P.W. Strain and excursion of the sciatic, tibial and plantar nerves during a modified straight leg raising test. J. Orthop. Res. 2006, 24, 1883–1889. [Google Scholar] [CrossRef]
- Coppieters, M.W.; Butler, D.S. Do “sliders” slide and “tensioners” tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008, 13, 213–221. [Google Scholar] [CrossRef]
- Ellis, R.; Carta, G.; Andrade, R.J.; Coppieters, M.W. Neurodynamics: Is tension contentious? J. Man. Manip. Ther. 2022, 30, 3–12. [Google Scholar] [CrossRef]
- Carta, G.; Gambarotta, G.; Fornasari, B.E.; Muratori, L.; El Soury, M.; Geuna, S.; Raimondo, S.; Fregnan, F. The neurodynamic treatment induces biological changes in sensory and motor neurons in vitro. Sci. Rep. 2021, 11, 13277. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; La Touche, R.; Varangot-Reille, C.; Sardinoux, M.; Bahier, J.; Suso-Martí, L.; Fernández-Carnero, J. Effects of Neural Mobilization on Pain Intensity, Disability, and Mechanosensitivity: An Umbrella Review With Meta-Meta-Analysis. Phys. Ther. 2022, 102, pzac040. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.M.; Nery, E.; Cruz, E.B.; Afreixo, V.; Silva, A.G. Effectiveness of Neural Mobilisation on Pain Intensity, Functional Status, and Physical Performance in Adults with Musculoskeletal Pain—A Systematic Review with Meta-Analysis. Clin. Rehabil. 2024, 38, 145–183. [Google Scholar] [CrossRef] [PubMed]
- Shou, M.K.; Study, C.; Lateral, C.; Sliders, N.; Syndrome, P. Comparative Study of Contralateral Cervical Lateral Glide and Neural Sliders in Athletes with Cervicobrachial Pain Syndrome. Int. J. Progress. Res. Sci. Eng. 2022, 3, 18–28. [Google Scholar]
- Rodríguez-Sanz, D.; Calvo-Lobo, C.; Unda-Solano, F.; Sanz-Corbalán, I.; Romero-Morales, C.; López-López, D. Cervical lateral glide neural mobilization is effective in treating Cervicobrachial pain: A randomized waiting list controlled clinical trial. Pain Med. 2017, 18, 2492–2503. [Google Scholar] [CrossRef]
- Shacklock, M.; Giménez Donoso, C.; Lucha López, M. Hacia un enfoque clínico- científico en el diagnóstico con test neurodinámicos (tensión neural). Fisioterapia 2017, 29, 288–297. [Google Scholar] [CrossRef]
- Zamorano, E. Capitulo 10: Manual de pruebas de provocación neural. In Movilización Neuromeníngea: Tratamiento de los Trastornos Mecanosensitivos del Sistema Nervioso; Editorial Médica Panamericana S.A.: Madrid, Spain, 2013; pp. 203–223. ISBN 8479039701. [Google Scholar]
- Boudier-Revéret, M.; Gilbert, K.K.; Allégue, D.R.; Moussadyk, M.; Brismée, J.M.; Sizer, P.S.; Feipel, V.; Dugailly, P.M.; Sobczak, S. Effect of neurodynamic mobilization on fluid dispersion in median nerve at the level of the carpal tunnel: A cadaveric study. Musculoskelet. Sci. Pract. 2017, 31, 45–51. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ahbouch, A.; Qadah, R.M.; Kim, M.; Alrawaili, S.M.; Moustafa, I.M. Impact of the Order of Movement on the Median Nerve Root Function: A Neurophysiological Study with Implications for Neurodynamic Exercise Sequencing. J. Clin. Med. 2024, 13, 913. [Google Scholar] [CrossRef]
- Bueno-Gracia, E.; Pérez-Bellmunt, A.; Estébanez-de-Miguel, E.; López-de-Celis, C.; Caudevilla-Polo, S.; Shacklock, M.; González-Rueda, V. Effect of cervical contralateral lateral flexion on displacement and strain in the median nerve and flexor digitorum superficialis at the wrist during the ULNT1—Cadaveric study. Musculoskelet. Sci. Pract. 2020, 50, 102244. [Google Scholar] [CrossRef]
- Bueno-Gracia, E.; Fanlo-Mazas, P.; Malo-Urriés, M.; Rodriguez-Mena, D.; Montaner-Cuello, A.; Ciuffreda, G.; Shacklock, M.; Estébanez-de-Miguel, E. Diagnostic accuracy of the upper limb neurodynamic test 1 using neurodynamic sequencing in diagnosis of carpal tunnel syndrome. Musculoskelet. Sci. Pract. 2024, 69, 102897. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; López-de-Celis, C.; Estébanez-de-Miguel, E.; Pérez-Rey, J.; Shacklock, M.; Ortiz-Miguel, S.; Bueno-Gracia, E. Effect of cervical contralateral lateral flexion on the median nerve and fascia at the wrist—Cadaveric study. Musculoskelet. Sci. Pract. 2024, 73, 103146. [Google Scholar] [CrossRef]
- Ciuffreda, G.; Bueno-Gracia, E.; Albarova-Corral, I.; Montaner-Cuello, A.; Pérez-Rey, J.; Pardos-Aguilella, P.; Malo-Urriés, M.; Estébanez-de-Miguel, E. In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Santilli, G.; Ciccarelli, A.; Martino, M.; Pacini, P.; Agostini, F.; Bernetti, A.; Giuliani, L.; Del Gaudio, G.; Mangone, M.; Colonna, V.; et al. Pain, Function, and Elastosonographic Assessment After Shockwave Therapy in Non-Calcific Supraspinatus Tendinopathy: A Retrospective Observational Study. J. Funct. Morphol. Kinesiol. 2025, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Snoj, Ž.; Wu, C.H.; Taljanovic, M.S.; Dumić-Čule, I.; Drakonaki, E.E.; Klauser, A.S. Ultrasound Elastography in Musculoskeletal Radiology: Past, Present, and Future. Semin. Musculoskelet. Radiol. 2020, 24, 156–166. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J.; Nightingale, E.; Pourkazemi, F.; Sman, A.; Hiller, C.; et al. Normative reference values for strength and flexibility of 1000 children and adults. Neurology 2017, 88, 36–43. [Google Scholar] [CrossRef]
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Basson, A.; Olivier, B.; Ellis, R.; Coppieters, M.; Stewart, A.; Mudzi, W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: A systematic review and meta-Analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 593–615. [Google Scholar] [CrossRef]
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef]
- Marks, M.; Schöttker-Königer, T.; Probst, A. Efficacy of cervical spine mobilization versus peripheral nerve slider techniques in cervicobrachial pain syndrome—A randomized clinical trial. J. Phys. Ther. 2011, 4, 9–17. [Google Scholar]
- Davis, F.B.; Katsuura, Y.; Dorizas, J.A. A retrospective review of 112 patients undergoing arthroscopic suprascapular nerve decompression. J. Orthop. 2020, 19, 31–35. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Barbosa, M.A.; Sousa, A.L.L.; Ramos, G.C. Bloqueio do Nervo Supraescapular: Procedimento Importante na Prática Clínica. Rev. Bras. Anestesiol. 2012, 62, 96–104. [Google Scholar] [CrossRef]
- Leider, J.D.; Derise, O.C.; Bourdreaux, K.A.; Dierks, G.J.; Lee, C.; Varrassi, G.; Sherman, W.F.; Kaye, A.D. Treatment of suprascapular nerve entrapment syndrome. Orthop. Rev. 2021, 13, 25554. [Google Scholar] [CrossRef]



| Cadaveric Study | In Vivo Study | ||
|---|---|---|---|
| Contralateral cervical glide observed at the suprascapular notch. | 1.85 (1.73, 1.98) mm | Horizontal | 1.18 (0.79, 1.31) mm |
| Vertical | 0.39 (0.23, 0.54) mm | ||
| Infraspinatus muscle contraction observed at the suprascapular notch (simulated in the dissection study). | 3.21 (2.93, 3.46) mm | Horizontal | 1.34 (1.05, 1.75) mm |
| Vertical | 0.75 (0.47, 1.02) mm | ||
| Contralateral cervical glide observed at the spinoglenoid notch. | 1.45 (1.35, 1.56) mm | Horizontal | 1.65 (1.08, 2.00) mm |
| Vertical | 0.34 (0.22, 0.54) mm | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montané-Blanchart, M.; Miguel-Pérez, M.; Rodero-de-Lamo, L.; Navarro-Cano, P.; Pérez-Bellmunt, A. Effect of Contralateral Cervical Glide on the Suprascapular Nerve: An In Vitro and In Vivo Study. Appl. Sci. 2025, 15, 6987. https://doi.org/10.3390/app15136987
Montané-Blanchart M, Miguel-Pérez M, Rodero-de-Lamo L, Navarro-Cano P, Pérez-Bellmunt A. Effect of Contralateral Cervical Glide on the Suprascapular Nerve: An In Vitro and In Vivo Study. Applied Sciences. 2025; 15(13):6987. https://doi.org/10.3390/app15136987
Chicago/Turabian StyleMontané-Blanchart, Marta, Maribel Miguel-Pérez, Lourdes Rodero-de-Lamo, Pasqual Navarro-Cano, and Albert Pérez-Bellmunt. 2025. "Effect of Contralateral Cervical Glide on the Suprascapular Nerve: An In Vitro and In Vivo Study" Applied Sciences 15, no. 13: 6987. https://doi.org/10.3390/app15136987
APA StyleMontané-Blanchart, M., Miguel-Pérez, M., Rodero-de-Lamo, L., Navarro-Cano, P., & Pérez-Bellmunt, A. (2025). Effect of Contralateral Cervical Glide on the Suprascapular Nerve: An In Vitro and In Vivo Study. Applied Sciences, 15(13), 6987. https://doi.org/10.3390/app15136987








