Abstract
Background: This study aimed to investigate the acute changes in muscle and tendon viscoelastic properties in response to a progressive treadmill VO2max test among professional male soccer players. Methods: Bilateral assessments at five sites—the Achilles tendon (AT), biceps femoris, semitendinosus, rectus femoris (RF), and sternocleidomastoid (SCM)—measured tone (oscillation frequency), dynamic stiffness, logarithmic decrement (elasticity), stress relaxation time, and creep. Each site was probed five times and values averaged. Repeated-measures ANOVA (Time × Side) with Bonferroni correction tested pre- to post-exercise changes; Pearson’s r examined associations with VO2max. Results: Significant Time effects (all p < 0.05) were observed for RF frequency (ηp2 = 0.226), RF creep (ηp2 = 0.144), AT stiffness (ηp2 ≈ 0.035), AT frequency (ηp2 = 0.035), and SCM frequency (ηp2 = 0.037). Post-exercise, right AT stiffness fell by 65 ± 14 N/m (p = 0.015), while left AT stiffness rose by 22 ± 9 N/m (p = 0.015). RF stiffness decreased by 28 ± 6 N/m (p < 0.001) and tone by 1.2 ± 0.3 Hz (p < 0.001), with creep (+0.08 ± 0.02; p < 0.001) and relaxation time (+1.5 ± 0.7 ms; p < 0.001) increasing. SCM tone declined by 0.8 ± 0.4 Hz (p = 0.010). Baseline RF properties—frequency (r = −0.597), stiffness (r = −0.59), relaxation time (r = 0.53), and creep (r = 0.48)—correlated moderately with VO2max (all p < 0.05). Conclusions: These findings suggest that viscoelastic adaptations to exhaustive aerobic exercise are tissue- and side-specific, and that rectus femoris viscoelastic properties may serve as potential indicators of endurance readiness.
1. Introduction
The publication rate for research focused on human body stiffness in the literature of sports science and medicine is rapidly increasing [1,2], most likely because stiffness has been associated with increased athletic performance [1,3,4]. Stiffness traces back to Hooke’s law, which holds that the force required to deform an object equals the spring constant multiplied by the displacement distance [5,6]. Muscle and tendon stiffness indicate longitudinal tissue viscoelastic properties as assessed by tissue resistance to passive stretching [7]. It is primarily responsible for transferring and absorbing motion energy in a variety of sporting activities [8]. Furthermore, muscle and tendon stiffness can objectively indicate tissue condition [9] and fatigue [10]. For example, Yanagisawa et al. [9] utilized real-time ultrasound elastography to demonstrate that the stiffness of the gastrocnemius muscle increased immediately after dynamic exercise, indicating acute fatigue-related changes in tissue condition. Likewise, Andonian et al. [10] applied shear-wave elastography to track quadriceps tendon stiffness before, during, and after an extreme ultra-marathon, observing progressive increases in stiffness that paralleled the accumulation of muscle fatigue. Kalkhoven et al. found that increased medial gastrocnemius muscle stiffness appears to be beneficial for athletic performance in soccer players [11]. It is therefore important to accurately assess the stiffness of specific muscles and tendons based on the specificity of different sports disciplines in order to monitor changes in muscle and tendon stiffness both acutely and chronically.
Recent findings by Krzysztofik et al. [12] highlight the relevance of this assessment in the context of post-activation performance enhancement (PAPE). Their study demonstrated that various back squat activation protocols significantly affect both jumping performance and tissue stiffness. Specifically, reductions in AT and vastus lateralis stiffness were observed following different conditioning activities (e.g., high-load squats and velocity-based protocols), while jump height was acutely enhanced. These results emphasize that intensity, movement velocity, and training volume are critical variables when aiming to optimize performance without inducing excessive fatigue. Notably, a single set of squats at 60% 1RM with a 10% velocity loss proved effective in acutely improving jump performance, while limiting increases in training volume and preserving tendon integrity.
In professional soccer, athletes routinely subject their muscle-tendon units to extreme loads [13], placing them at elevated risk for both acute injury and cumulative fatigue that can compromise performance and career longevity [14,15]. Despite advances in training-load monitoring, real-time assessment of tissue mechanical properties remains largely confined to laboratory settings and chronic adaptations, and the immediate viscoelastic responses of muscle and tendon to maximal aerobic exercise—critical indicators of acute fatigue and tissue readiness [16,17,18]—have not been systematically investigated in elite players. To address this gap, we applied the MyotonPRO [19], a handheld myometer validated for in vivo measurement of muscle and tendon mechanics (Myoton AS, Tallinn, Estonia), during the post–VO2max window, to characterize acute, tissue- and side-specific viscoelastic changes in professional soccer players. We assessed tone, stiffness, elasticity, relaxation time and creep in the Achilles tendon, biceps femoris, semitendinosus, rectus femoris and sternocleidomastoid before and immediately after a progressive treadmill effort to exhaustion. Accordingly, the objective of the present study was to investigate the acute, tissue- and side-specific changes in muscle and tendon viscoelastic properties in professional male soccer players following a progressive treadmill VO2max test, as measured by the MyotonPRO device. We hypothesized that a VO2max test would induce heterogeneous, tissue- and side-specific alterations—with the rectus femoris and Achilles tendon showing the strongest associations between their baseline viscoelastic properties and VO2max—suggesting their potential as acute indicators of endurance readiness and informing strategies for injury prevention and performance optimization.
2. Materials and Methods
2.1. Study Design
The study was designed to investigate changes in the muscle viscoelastic properties of the Achilles tendon, biceps femoris muscle, semitendinosus muscle, rectus femoris muscle, and sternocleidomastoid muscle, specifically tension (oscillation frequency in Hz) and stiffness (N/m), under the influence of progressive treadmill effort to exhaustion. Muscle viscoelastic properties for both legs were measured using a Myoton device at two time points: at rest before and immediately after the completion of the measurement of VO2max.
2.2. Participants
The study involved 21 highly trained male soccer players classified based on training status and performance caliber according to the classification of McKay et al. [20] (Table 1). The inclusion criteria were as follows: (a) male gender; (b) age between 18 and 40 years; (c) no cardiovascular diseases, such as unstable coronary artery disease, unstable arrhythmia, or severe heart failure; (d) no respiratory diseases, such as chronic obstructive pulmonary disease (COPD); (e) absence of muscle injuries (leading to absence from training for more than 4 weeks) for at least 6 months prior to the start of the study (based on a preliminary medical examination evaluated by the team doctor). The experimental session was conducted during the off-season period.

Table 1.
Descriptive characteristics of the study participants.
Participants were informed about potential risks and benefits associated with their participation in the project, as well as their right to withdraw from the study at any time without providing a reason for their decision. The participants signed a written consent form to participate in the study. All stages of the study were conducted at the Academy of Physical Education in Katowice, in the Human Functional Research Laboratory. The experimental project was approved by the Bioethics Committee for Scientific Research (3/2021, approval date 27 May 2021), following the ethical standards of the 1983 Helsinki Declaration. None of the participants withdrew from the study.
2.3. Testing Protocol
Participants were asked not to perform any resistance exercises for 24 h prior to the start of the experimental session. Additionally, they were advised to maintain their regular dietary habits and refrain from the consumption of any supplements or stimulants, except for habitual supplementation such as creatine in the week preceding the experiment. At the beginning of the experimental session, body composition was assessed using a multi-channel bioelectrical impedance analysis in a laboratory setting using the InBody 720 device (InBody, Tokyo, Japan). The first resting measurement of the muscle viscoelastic properties of the Achilles tendon, biceps femoris muscle, semitendinosus muscle, rectus femoris muscle, and sternocleidomastoid muscle, were directly taken before the commencement of VO2max measurement. All measurements of the viscoelastic properties of the muscles were performed in the same room as the VO2max measurement. All tests were conducted by the same person trained to operate the Myoton device.
The second sample was taken immediately after the completion of the VO2max measurement. The reliability between the trials (within the session) of a selected muscle group (2 trials per participant, N = 21, total measurements = 42) was tested using the intraclass correlation coefficient (ICC) model. Domholdt’s classification scales (1993) were applied to interpret the ICC: very high = 1.00–0.90; high = 0.89–0.70; moderate = 0.69–0.50; low = 0.49–0.26. This indicates that the ICC value across the time points was in a range of 0.71–0.94, with the exception of BF (0.56). A high reliability coefficient indicates that the applied tests represent a consistent measurement of data regarding the viscoelastic properties of muscles among soccer players.
2.4. Progressive Exercise on the Treadmill
The exercise was conducted on a Pulsar treadmill (HP-Cosmos, Nussdorf-Traunstein, Germany), starting at a speed of 6 km/h and 0 degrees incline. The treadmill speed was progressively increased by 2 km/h every 3 min of exercise until voluntary exhaustion [21]. During the test, heart rate, minute ventilation (VE), oxygen consumption (VO2), and carbon dioxide exhalation (CO2) were continuously measured breath-by-breath using a stationary ergo-spirometer, the Metalyzer 3B (Cortex, Isernhagen, Germany). VO2max was determined based on a reduced or plateaued VO2 with increasing speed (VO2 ≤ 150 mL/min above peak VO2). Capillary blood samples from the fingertip were collected to assess lactate concentration (LA) (Biosen C-line Clinic, EKF-Diagnostic GmbH, Barleben, Germany) during the 30-s break between treadmill speed increments, as well as at 3, 6, 9, and 12 min during the post-exercise recovery period to determine lactate utilization. Resting capillary blood samples and post-exercise blood samples were also used to determine acid-base balance and hemoglobin oxygen saturation (RapidLab 248, Bayer Diagnostics, Leverkusen, Germany).
2.5. Measurement of Muscle Viscoelastic Properties
MyotonPRO (Myoton AS, Tallinn, Estonia) is a non-invasive device monitoring superficial mechanical deformation of soft tissues by delivering a brief mechanical impulse and recording the resulting damped oscillations via an integrated accelerometer. It was used to bilaterally assess the following muscle viscoelastic properties: Achilles tendon, biceps femoris muscle, semitendinosus muscle, rectus femoris muscle, and sternocleidomastoid muscle. The participants were lying on their back, side, or stomach (depending on the command given by the specialist for proper sample collection) on a physiotherapy couch and rested for 3 min before the measurements of the viscoelastic properties of the muscles were taken (Figure 1). Measurement sites on each muscle were located using a measuring tape and marked with a skin-safe waterproof marker. A pillow was placed under the head, and a unique rolling cushion was placed under the lower leg and straightened foot to facilitate relaxation. One series of five individual Myoton measurements for each muscle group (5 points) was measured separately for the left and right leg. Additionally, to better understand the issue, the functionality of the dominant and non-dominant lower limbs was also determined. The dominant leg for a specific player was determined based on the information provided by the player in the questionnaire. Measurements comprised five dependent variables: dynamic stiffness (N·m−1), representing the resistance of the tissue to deformation; natural oscillation frequency (Hz), reflecting passive muscle tone; logarithmic decrement (unitless), indexing the rate of damping and thus tissue elasticity; mechanical stress relaxation time (ms), indicating the temporal viscous response under constant deformation; and creep (unitless), denoting progressive deformation under sustained load [22]. Myoton’s accelerometer was set to 3200 Hz, and the average value for analysis was obtained from five consecutive measurements (0.4 N for 15 ms) [2].

Figure 1.
Myotonometry of Achilles tendon procedure.
2.6. Statistical Analysis
All statistical analyses were performed using Jamovi (version 2.3.21; The Jamovi Project, Sydney, Australia). Data normality was assessed using the Shapiro–Wilk test, while the Levene test was used to examine the homogeneity of variances. To verify the assumption of homogeneity of covariance matrices, descriptive statistics are presented as means, standard deviations (SD), and 95% confidence intervals (CI). A 2-way repeated-measures ANOVA was conducted to assess differences in the variables of interest. Effect sizes for main effects and interactions were reported as partial eta squared (ηp2) and classified as small (0.01–0.059), moderate (0.06–0.137), and large (>0.137). A Bonferroni correction was applied to account for multiple comparisons. Correlation analyses were performed using Pearson’s linear correlation coefficient, with values from the dominant leg baseline included in the analysis. The magnitude of correlation was interpreted as follows: 0.00–0.10 (negligible), 0.10–0.39 (weak), 0.40–0.69 (moderate), 0.70–0.89 (strong), and 0.90–1.00 (very strong). The threshold of significance was set at α = 0.05. Statistical significance was set at p < 0.05 for all tests. The level of significance was interpreted as follows: p < 0.05 indicating moderate evidence against the null hypothesis; p < 0.01 providing strong evidence against the null hypothesis; p < 0.001 demonstrating very strong evidence against the null hypothesis. The results are presented in grouped charts, with 95% (CI) displayed as error bars. Correlation analyses are visualized using scatter plots, incorporating density marginals, a linear regression line, and standard error shading to illustrate the strength and direction of correlation between variables.
3. Results
A parametric analysis of variance was selected for the analysis as the Shapiro–Wilk test indicated p-values > 0.05 for almost all variables, confirming the normality of the distributions (Table 2). Additionally, the Levene’s test for homogeneity of variances of Side factor also yielded p-values > 0.05 across all variables, supporting the assumption of homogeneity. Since the repeated measures design included only two levels, the assumption of sphericity was inherently satisfied in this case.

Table 2.
Descriptive statistics and ANOVA results for CREEP Variable.
Significant differences were observed for the main effect of time for the CREEP variable for RF (F = 43.45; p < 0.001; ηp2 = 0.14). No significant interaction was found for TIME POINT × Side (F = 0.07; p = 0.79). The between-side analysis for the Side factor showed no significant differences (F = 2.68 × 10−4; p = 0.99). Post hoc comparisons with Bonferroni correction identified a statistically significant change in the variable from pre- to post-intervention, with a ∆ of −0.08 (t = −6.59; p < 0.001). Results are illustrated in Figure 2a.
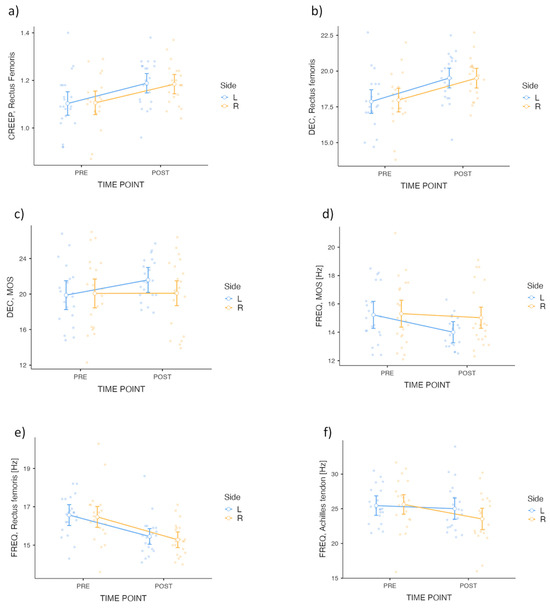
Figure 2.
ANOVA visualization for CREEP, DEC and FREQ variables of Rectus femoris, MOS and Achilles Tendon. Group means (±SEM) and individual data points for CREEP, DEC, and FREQ variables are presented for PRE and POST time points, separated by Side (Left [L], Right [R]). Each subplot corresponds to a specific variable and tissue: (a) CREEP of the Rectus femoris, (b) DEC of the Rectus femoris, (c) DEC of the MOS, (d) FREQ of the MOS [Hz], (e) FREQ of the Rectus femoris [Hz], and (f) FREQ of the Achilles tendon [Hz]. Data visualization includes individual data points and condition-specific means with standard error bars.

Table 3.
Descriptive statistics and ANOVA results for RT variable.
The analysis of the DEC variable for MOS revealed a significant main effect for TIME POINT (F = 6.67; p = 0.014; ηp2 = 0.039) (Table 4). The interaction between TIME POINT × Side approached significance (F = 4.05; p = 0.051; ηp2 = 0.024). The Side factor in the between-side analysis showed no significant effect (F = 0.37; p = 0.547). Post hoc comparisons confirmed a significant pre-post difference (∆ = −0.06; t = −2.58; p = 0.014). Results are presented in Figure 2c.

Table 4.
Descriptive statistics and ANOVA results for DEC variable.
The analysis of the FREQ variable for AT (Figure 2f) revealed a significant main effect for TIME POINT (F = 6.17; p = 0.017; ηp2 = 0.035) (Table 5). The interaction between TIME POINT × Side was not significant (F = 2.64; p = 0.112). The between-side analysis for the Side factor showed no significant effect (F = 0.54; p = 0.467). Post hoc comparisons confirmed a statistically significant pre-post difference (∆ = 1.27; t = 2.48; p = 0.017). The analysis of the FREQ variable for RF (Figure 2e) revealed a significant main effect for TIME POINT (F = 38.69; p < 0.001; ηp2 = 0.226). The interaction between TIME POINT × Side was not significant (F = 0.042; p = 0.838), and the between-side analysis showed no significant effect for the Side factor (F = 0.226; p = 0.637). Post hoc comparisons confirmed a significant improvement in the variable from pre- to post-intervention, with a ∆ of 1.15 (t = 6.22; p < 0.001). The analysis of the FREQ variable for MOS (Figure 2d) revealed a significant main effect for TIME POINT (F = 7.30; p = 0.010; ηp2 = 0.037). The interaction between TIME POINT × Side was not significant (F = 2.82; p = 0.101), and the between-side analysis showed no significant effect for the Side factor (F = 1.10; p = 0.301). Post hoc comparisons confirmed a statistically significant pre–post difference, with a ∆ of 0.75 (t = 2.70; p = 0.01).

Table 5.
Descriptive statistics and ANOVA results for FREQ Variable.
The analysis of variance for the STIFF variable for AT revealed a significant main effect for TIME POINT (F = 6.53; p = 0.015; ηp2 = 0.035) (Table 6). The interaction between TIME POINT × Side approached significance but was not statistically significant (F = 3.05; p = 0.089). The between-side analysis showed no significant effect for the Side factor (F = 0.270; p = 0.606). Post hoc comparisons confirmed a statistically significant improvement from pre- to post-intervention, with a ∆ of 38.6 (t = 2.55; p = 0.015). The analysis of variance for the STIFF variable for S revealed a significant main effect for TIME POINT (F = 5.15; p = 0.029; ηp2 = 0.014). The interaction between TIME POINT × Side was not significant (F = 0.010; p = 0.921), and the Side factor in the between-side analysis showed no significant effect (F = 1.45; p = 0.235). Post hoc comparisons confirmed a significant pre–post improvement, with a ∆ of 6.52 (t = 2.27; p = 0.029). The analysis of the STIFF variable for RF revealed a significant main effect for TIME POINT (F = 31.92; p < 0.001; ηp2 = 0.207). The interaction between TIME POINT × Side was not significant (F = 0.019; p = 0.892), and the Side factor in the between-side analysis also showed no significant effect (F = 0.01; p = 0.968). Post hoc comparisons confirmed a significant improvement, with a ∆ of 27.6 (t = 5.65; p < 0.001). Results for STIFF changes are presented in Figure 3.

Table 6.
Descriptive statistics and ANOVA results for STIFF variable.
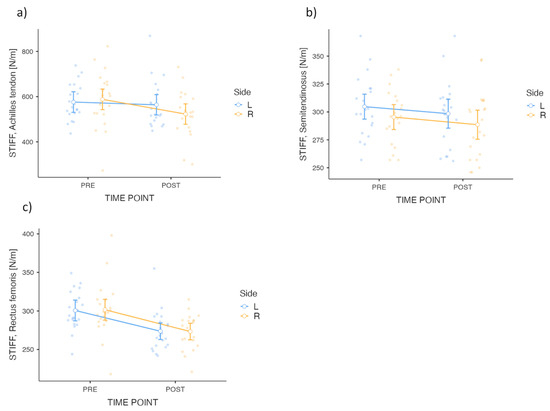
Figure 3.
ANOVA visualization STIFF variables of (a) Achilles tendon, (b) Semitendinosus and (c) Rectus femoris. Group means (±SEM) and individual data points for STIFF variables are presented for PRE and POST time points, separated by Side (Left [L], Right [R]). Each subplot corresponds to a specific variable and tissue: (a) Achilles tendon, (b) Semitendinosus and (c) Rectus femoris. Data visualization includes individual data points and condition-specific means with standard error bars.
The analysis revealed no statistically significant effects (p > 0.05) for all other variables. No meaningful changes over time, side-related differences, or interactions were observed. However, the DEC variable showed a significant effect for the Side factor in the between-side analysis for RF (p = 0.031; Figure 2b), indicating potential side-related differences in these measures. Despite some trends approaching significance, no conclusive differences were found for the remaining parameters.
The correlation analysis demonstrated significant relationships between VO2max and several RF Pre viscoelastic properties (Figure 4). Specifically, VO2max showed a moderate negative correlation with RF Pre FREQ (r = −0.60, p < 0.01) and RF Pre STIFF (r = −0.58, p < 0.01), indicating that higher aerobic capacity was associated with lower muscle frequency and stiffness. Conversely, VO2max was positively correlated with RF Pre RT (r = 0.53, p < 0.05) and RF Pre CREEP (r = 0.48, p < 0.05). All other correlations between VO2max and the examined variables were nonsignificant (p > 0.05).
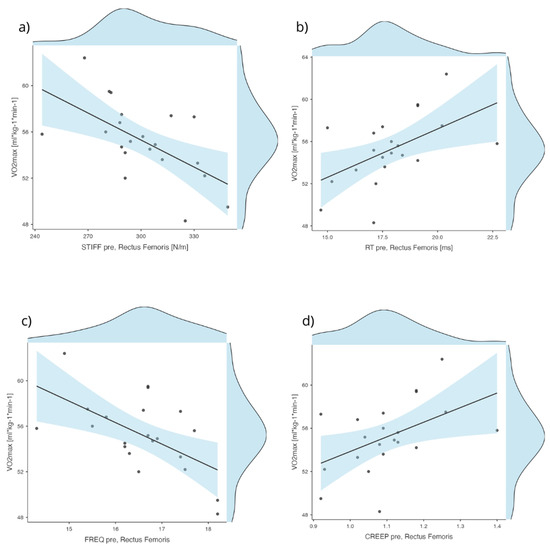
Figure 4.
Scatterplots for correlations assumptions. Scatterplots with regression lines and 95% confidence intervals displaying the relationship between VO2max (mL·kg−1·min−1) and various pre-intervention viscoelastic properties of the Rectus Femoris muscle. Each panel represents a bivariate correlation: (a) STIFF pre for [N/m], (b) RT [ms], (c) FREQ pre [H/z], (d) CREEP pre.
4. Discussion
The main finding of this study was that changes in stiffness were inhomogeneous across different muscle groups among professional soccer players following exhaustive treadmill exercise. Specifically, stiffness significantly increased in the left Achilles tendon (AT) and decreased in the right AT, left sternocleidomastoid (S), and left rectus femoris (RF). Moreover, there was a significant decrease in the state of tension—as indicated by natural oscillation frequency (FREQ)—in the left RF, left S, and right AT, along with a notable reduction in the ratio of relaxation to deformation time (CREEP) for both the left and right RF as well as the left S.
4.1. Heterogenity of Acute Alterations of Viscoelastic Properties
The heterogeneity of these changes, particularly in AT stiffness, is unexpected. For instance, Farris et al. [23] reported that a 30-min constant-intensity run did not significantly alter Achilles’ tendon stiffness. Relative strain and elongation remained constant during the run, and the tendon length while standing was unchanged post-exercise. In contrast, our study found a significant increase in left AT stiffness and a decrease in right AT stiffness, which may be partially attributable to the progressive increase in intensity until exhaustion. An increase in exercise intensity can impose greater stress on the tendon, potentially altering its post-exercise viscoelastic properties [24]. The decrease in right AT stiffness and its reduced oscillation frequency may signal a decline in the tendon’s viscoelastic properties [25]. Although the literature on tendon viscoelasticity is limited, fluid flow and structural interactions within the extracellular matrix during loading are likely to be key factors influencing this behavior [18,19]. Additionally, tendinopathy—characterized by reduced stiffness and elasticity—has been linked to diminished explosive performance in athletes [26]. The observed differences in tendon response between legs might also reflect varying viscoelastic conditions, possibly due to disturbed movement patterns or unilateral stress during treadmill running, though movement patterns were not assessed in the current study.
Supporting these observations, several studies have documented heterogeneous responses in viscoelastic properties across muscle groups. Farris et al. [23] underscored that the tendon’s response is highly sensitive to both exercise intensity and duration, while De Zee et al. [24] demonstrated that the dynamic viscoelastic behavior of lower extremity tendons is markedly influenced by the specific loading conditions during simulated running. These findings may help explain the asymmetrical changes observed between the dominant and non-dominant limbs in our investigation. The role of muscle fiber composition and neuromuscular recruitment patterns in mediating fatigue-induced changes in tissue stiffness is well documented. Fitts [27] detailed cellular mechanisms of muscle fatigue, indicating that muscles with a higher proportion of fast-twitch fibers might exhibit a different response to exhaustive exercise compared to those dominated by slow-twitch fibers. Similarly, Enoka and Duchateau [28] discussed how neuromuscular factors contribute to fatigue, potentially underpinning the differential stiffness alterations observed among various muscle groups. Clinically, the implications of altered tendon stiffness are significant. Morgan et al. [26] found that decreased tendon stiffness is associated with tendinopathic conditions, which can compromise explosive performance in athletes. These insights emphasize the need for precise post-exercise monitoring of tissue viscoelastic properties, particularly in elite athletes. Moreover, Andonian et al. [10] validated the use of stiffness measurements as objective markers of muscle fatigue, reinforcing the relevance of our methodological approach utilizing the MyotonPRO device.
In summary, the inhomogeneous changes observed in tissue stiffness across muscle groups suggest that factors such as exercise intensity, muscle fiber composition, and neuromuscular recruitment patterns may differentially influence the viscoelastic properties of muscles and tendons.
4.2. Viscoelastic Properties of the Rectus Femoris as an Indicator of Readiness for Exhaustive Exercise Testing
The present study revealed notable correlations between baseline MyotonPRO measurements and VO2max, suggesting that non-invasive assessments of muscle viscoelastic properties may serve as potential indicator of maximal endurance performance. Notably, these significant correlations were observed exclusively in the rectus femoris. Specifically, the rectus femoris demonstrated moderate negative correlations between VO2max and both natural oscillation frequency (r = −0.6, p < 0.01) and stiffness (r = −0.58, p < 0.01), while positive correlations were observed with relaxation time (RT; r = 0.53, p < 0.05) and creep (r = 0.484, p < 0.05). These results suggest that soccer players with greater VO2max exhibit significantly lower resting tone and stiffness in the rectus femoris, implying a more compliant muscle architecture that may enhance elastic energy storage and return during sustained running. This finding is consistent with recent work by Andonian et al. [10], who reported that baseline measures of muscle elasticity can serve as potential indicator of endurance training responsiveness—despite observing no meaningful changes in vastus lateralis viscoelasticity—underscoring the utility of rectus femoris stiffness as a non-invasive biomarker for aerobic performance potential.
The exclusive occurrence of these correlations in the rectus femoris suggests that this muscle group may serve as a particularly sensitive marker for assessing the interplay between muscle viscoelastic properties and endurance performance. Bizzini and Mannion [19] have demonstrated the reliability and sensitivity of the MyotonPRO for quantifying muscle stiffness and tone, underscoring the utility of such measurements. Similarly, Andonian et al. [10] reported that changes in muscle stiffness are closely associated with exercise-induced fatigue, reinforcing the notion that pre-test stiffness levels—particularly in the rectus femoris—could have predictive value regarding subsequent endurance performance. Additionally, foundational work by Fitts [27] and Enoka and Duchateau [28] has described the neuromuscular adaptations during fatigue, which may underpin the observed associations between viscoelastic muscle properties and VO2max [26,27]. Given these insights, incorporating MyotonPRO assessments of the rectus femoris prior to maximal endurance tests could provide coaches and sports scientists with a non-invasive method to gauge muscle readiness. Such pre-assessment might facilitate the optimization of warm-up protocols, more accurate monitoring of fatigue, and the tailoring of training regimens to enhance performance while reducing injury risk. Future research should further explore the predictive value of these correlations and investigate whether targeted interventions to modulate rectus femoris stiffness can positively influence endurance outcomes.
4.3. Limitations
While the current study provides novel insights into the viscoelastic responses of muscle and tendon tissues following exhaustive treadmill exercise, several limitations should be acknowledged. First, the relatively small sample size of professional male soccer players may limit the generalizability of the findings to other populations, including female athletes and individuals from different sports disciplines. Second, although the MyotonPRO device offers a reliable and non-invasive method for assessing tissue viscoelastic properties, its use may be subject to variability in probe placement and potential measurement error, particularly when evaluating deeper tissue layers. Third, the design of the study does not allow for the evaluation of recovery kinetics or long-term adaptations in viscoelastic properties post-exercise. Additionally, the absence of concurrent kinematic and electromyographic data restricts an understanding of the neuromuscular mechanisms underlying the observed asymmetrical changes between muscle groups and limbs.
4.4. Practical Implications
The findings of this study have several practical implications for sports scientists, coaches, and clinicians. The use of non-invasive tools such as the MyotonPRO to assess the viscoelastic properties—particularly of the rectus femoris—can serve as a strategic pre-assessment measure to determine an athlete’s readiness for exhaustive exercise. By identifying baseline tissue characteristics that correlate with performance metrics like VO2max, practitioners may tailor warm-up protocols and training regimens to enhance neuromuscular efficiency and reduce the risk of overuse injuries. Furthermore, regular monitoring of muscle and tendon stiffness could inform the design of recovery strategies, ensuring that athletes maintain optimal tissue properties for peak performance. Ultimately, integrating viscoelastic assessments into routine performance evaluations could provide actionable feedback, allowing for timely adjustments to training loads and preventive measures against potential injuries.
5. Conclusions
This study demonstrated that exhaustive treadmill exercise induces heterogeneous changes in the viscoelastic properties of muscle and tendon tissues in professional soccer players. The alterations were both tissue-specific and side-dependent, with significant changes observed particularly in the rectus femoris, Achilles’ tendon, and semitendinosus muscles. Among the measured variables, stiffness, frequency, and creep showed the most notable modifications post-exercise. The rectus femoris exhibited significant associations with VO2max, suggesting its viscoelastic properties may serve as potential indicators of aerobic readiness. These findings highlight the relevance of using non-invasive tools such as the MyotonPRO for the assessment of muscle–tendon mechanics in elite athletes. The data also suggest that monitoring viscoelastic properties prior to maximal endurance testing may provide valuable insights into fatigue, asymmetries, and performance potential. Future research should explore long-term adaptations to training and recovery using this methodology and assess whether interventions targeting specific muscle-tendon units can enhance performance or mitigate injury risk.
Author Contributions
Conceptualization, K.S. and J.J.; methodology, J.J., K.S. and A.T.; software, A.T.; validation, A.T., K.S. and R.R.; formal analysis, M.K.; investigation, M.D.; resources, M.D. and J.J.; data curation, A.T. and R.R.; writing—original draft preparation, K.S. and A.T.; writing—review and editing, K.S., A.T. and Ł.R.; visualization, A.T. and K.S.; supervision, M.K. and R.R.; project administration, M.K.; funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved (number: 3/2021, approval date 27 May 2021) by the Institutional Review Board at the Academy of Physical Education in Katowice.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AT | Achilles Tendon |
| BF | Biceps Femoris |
| CI | Confidence Interval |
| DEC | Logarithmic Decrement (measure of elasticity) |
| FREQ | Natural Oscillation Frequency (Hz), characterizing muscle tone |
| HR | Heart rate |
| ICC | Intraclass Correlation Coefficient |
| LA | Lactate |
| MOS | Sternocleidomastoid |
| PAPE | Post-Activation Performance Enhancement |
| PRE/POST | Pre-exercise/Post-exercise |
| RF | Rectus Femoris |
| R/L | Right/Left |
| RL | Relaxation Time (ms) |
| S | Semitendinosus |
| SD | Standard Deviation |
| VO2 | Oxygen consumption |
| VO2max | Maximal Oxygen Uptake |
| VE | Minute Ventilation |
References
- Serpell, B.G.; Ball, N.B.; Scarvell, J.M.; Smith, P.N. A review of models of vertical, leg, and knee stiffness in adults for running, jumping or hopping tasks. J. Sports Sci. 2012, 30, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, J.; Gaweł, D.; Krzysztofik, M.; Zając, A.; Tsoukos, A.; Bogdanis, G.C.; Wilk, M. Effects of blood flow restriction on viscoelastic properties of the rectus femoris muscle at rest. Front. Physiol. 2023, 14, 1244376. [Google Scholar] [CrossRef] [PubMed]
- Bret, C.; Rahmani, A.; Dufour, A.B.; Messonnier, L.; Lacour, J.R. Leg strength and stiffness as ability factors in 100 m sprint running. J. Sports Med. Phys. Fitness 2002, 42, 274–281. [Google Scholar]
- Hobara, H.; Kimura, K.; Omuro, K.; Gomi, K.; Muraoka, T.; Iso, S.; Kanosue, K. Determinants of difference in leg stiffness between endurance- and power-trained athletes. J. Biomech. 2008, 41, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.P.; Garrett, G.E.; Tiberio, D. Effect of added mass on human unipedal hopping. Percept. Mot. Ski. 2002, 94, 834–840. [Google Scholar] [CrossRef]
- Butler, R.J.; Crowell, H.P.; Davis, I.M. Lower extremity stiffness: Implications for performance and injury. Clin. Biomech. 2003, 18, 511–517. [Google Scholar] [CrossRef]
- Akagi, R.; Takahashi, H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med. Sci. Sports Exerc. 2013, 45, 1348–1354. [Google Scholar] [CrossRef]
- Borges, P.R.; Santos, T.R.; Procópio, P.R.; Chelidonopoulos, J.H.; Zambelli, R.; Ocarino, J.M. Passive stiffness of the ankle and plantar flexor muscle performance after Achilles’ tendon repair: A cross-sectional study. Braz. J. Phys. Ther. 2017, 21, 51–57. [Google Scholar] [CrossRef]
- Yanagisawa, O.; Niitsu, M.; Kurihara, T.; Fukubayashi, T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: A feasibility study. Clin. Radiol. 2011, 66, 815–819. [Google Scholar] [CrossRef]
- Andonian, P.; Viallon, M.; Le Goff, C.; de Bourguignon, C.; Tourel, C.; Morel, J.; Giardini, G.; Gergele, L.; Millet, G.P.; Croisille, P. Shear-Wave Elastography Assessments of Quadriceps Stiffness Changes prior to, during and after Prolonged Exercise: A Longitudinal Study during an Extreme Mountain Ultra-Marathon. PLoS ONE 2016, 11, e0161855. [Google Scholar]
- Kalkhoven, J.T.; Watsford, M.L. The relationship between viscoelastic stiffness and athletic performance markers in sub-elite footballers. J. Sports Sci. 2018, 36, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Krzysztofik, M.; Wilk, M.; Pisz, A.; Kolinger, D.; Tsoukos, A.; Zając, A.; Bogdanis, G.C. Acute effects of varied back squat activation protocols on muscle-tendon stiffness and jumping performance. J. Strength Cond. Res. 2023, 37, 1419–1427. [Google Scholar] [CrossRef]
- Jones, D.A.; Newham, D.J.; Clarkson, P.M. Skeletal muscle stiffness and pain following eccentric exercise of the elbow flexors. Pain 1987, 30, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Balci, B.P. Spasticity Measurement. Noro Psikiyatr Ars 2018, 55, S49–S53. [Google Scholar]
- Brandenburg, J.E.; Eby, S.F.; Song, P.; Zhao, H.; Brault, J.S.; Chen, S.; An, K.N. Ultrasound elastography: The new frontier in direct measurement of muscle stiffness. Arch. Phys. Med. Rehabil. 2014, 95, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Zwahlen, A.K.; Casartelli, N.C.; Item-Glatthorn, J.F.; Maffiuletti, N.A. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef]
- Pożarowszczyk, B.; Pawlaczyk, W.; Smoter, M.; Zarzycki, A.; Mroczek, D.; Kumorek, M.; Witkowski, K.; Adam, K. Effects of Karate Fights on Achilles Tendon Stiffness Measured by Myotonometry. J. Hum. Kinet. 2017, 56, 93–97. [Google Scholar] [CrossRef]
- Orner, S.; Kratzer, W.; Schmidberger, J.; Grüner, B. Quantitative tissue parameters of Achilles tendon and plantar fascia in healthy subjects using a handheld myotonometer. J. Bodyw. Mov. Ther. 2018, 22, 105–111. [Google Scholar] [CrossRef]
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Thron, M.; Woll, A.; Klos, L.; Härtel, S.; Ruf, L.; Kloss, C.; Altmann, S. Overestimation of maximal aerobic speed by the Université de Montréal track test and a 1500-m-time trial in soccer. Front. Physiol. 2022, 13, 1023257. [Google Scholar] [CrossRef] [PubMed]
- Salagas, A.; Tsoukos, A.; Terzis, G.; Paschalis, V.; Katsikas, C.; Krzysztofik, M.; Wilk, M.; Zajac, A.; Bogdanis, G.C. Effectiveness of either short-duration ischemic pre-conditioning, single-set high-resistance exercise, or their combination in potentiating bench press exercise performance. Front. Physiol. 2022, 13, 1030109. [Google Scholar] [CrossRef] [PubMed]
- Farris, D.J.; Trewartha, G.; McGuigan, M.P. The effects of a 30-min run on the mechanics of the human Achilles tendon. Eur. J. Appl. Physiol. 2012, 112, 653–660. [Google Scholar] [CrossRef] [PubMed]
- De Zee, M.; Bojsen-Moller, F.; Voigt, M. Dynamic viscoelastic behavior of lower extremity tendons during simulated running. J. Appl. Physiol. 2000, 89, 1352–1359. [Google Scholar] [CrossRef]
- Joo, J.M. Use of the Logarithmic decrement to assess the damping in oscillations. Rev. Investig. Fis. 2016, 19, 161901551. [Google Scholar]
- Morgan, G.E.; Martin, R.; Williams, L.; Pearce, O.; Morris, K. Objective assessment of stiffness in Achilles tendinopathy: A novel approach using the MyotonPRO. BMJ Open Sport Exerc. Med. 2018, 4, e000446. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).