Effects of Eight-Week Single-Set Resistance Training on Muscle Health, Metabolic Profile and Oxidative Stress in Individuals with Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Subjects’ Recruitment and Eligibility Criteria
2.3. Resistance Training Program
2.4. Blood Sample Collection and Processing
2.5. Blood Biochemical Analyses
2.5.1. CK Activity Assay
2.5.2. LDH Activity Assay
2.5.3. AST Activity Assay
2.5.4. ALT Activity Assay
2.5.5. Total Cholesterol
2.5.6. HDL Cholesterol
2.5.7. Triglycerides
2.5.8. LDL Cholesterol
2.5.9. Atherogenic Index
2.5.10. Glycated Hemoglobin
2.5.11. Estimated Average Glucose
2.5.12. Malondialdehyde
2.6. Statistical Analysis
3. Results
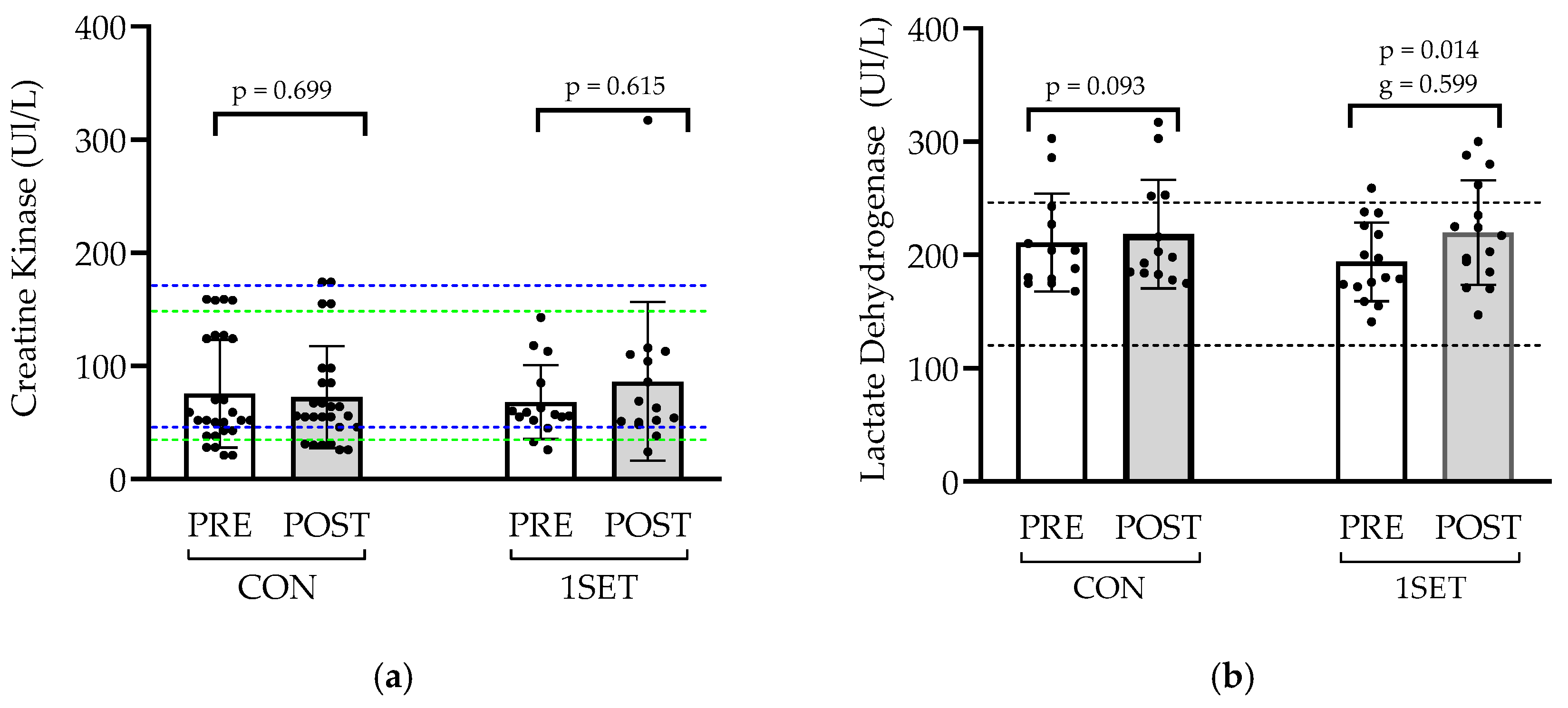
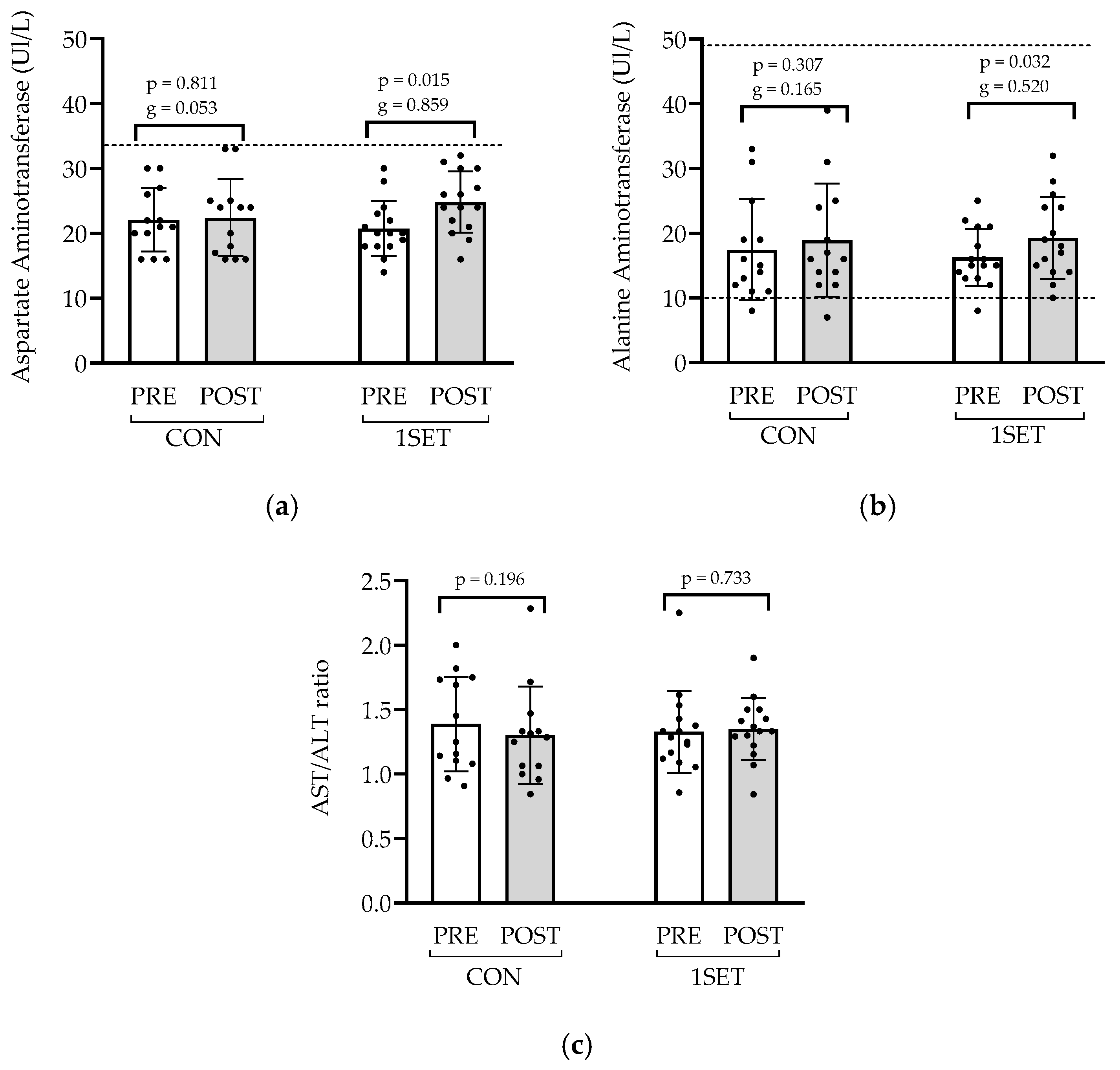
3.1. Effect of 8-Week Low-Volume RT Intervention on Muscle Health and Liver Function
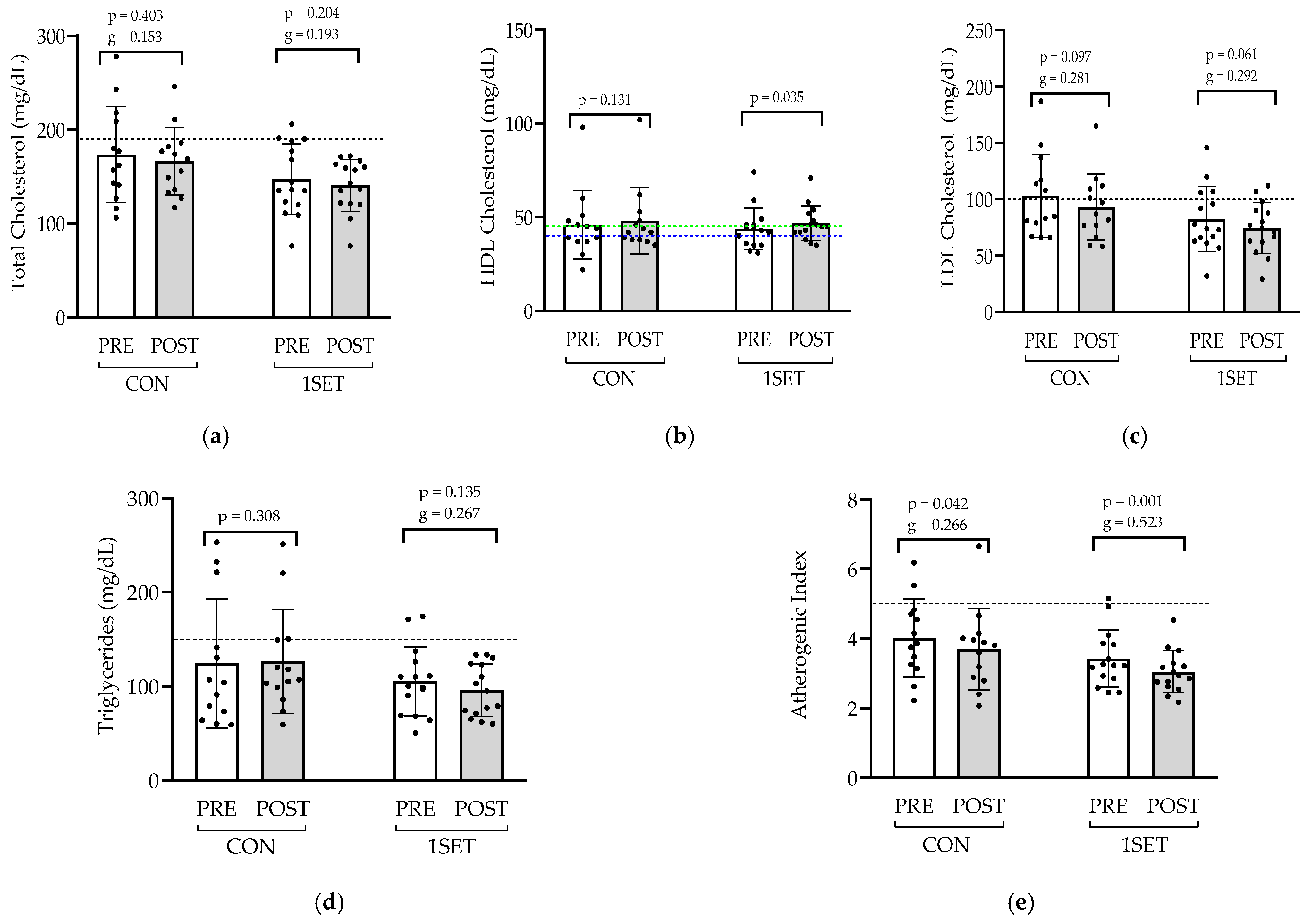
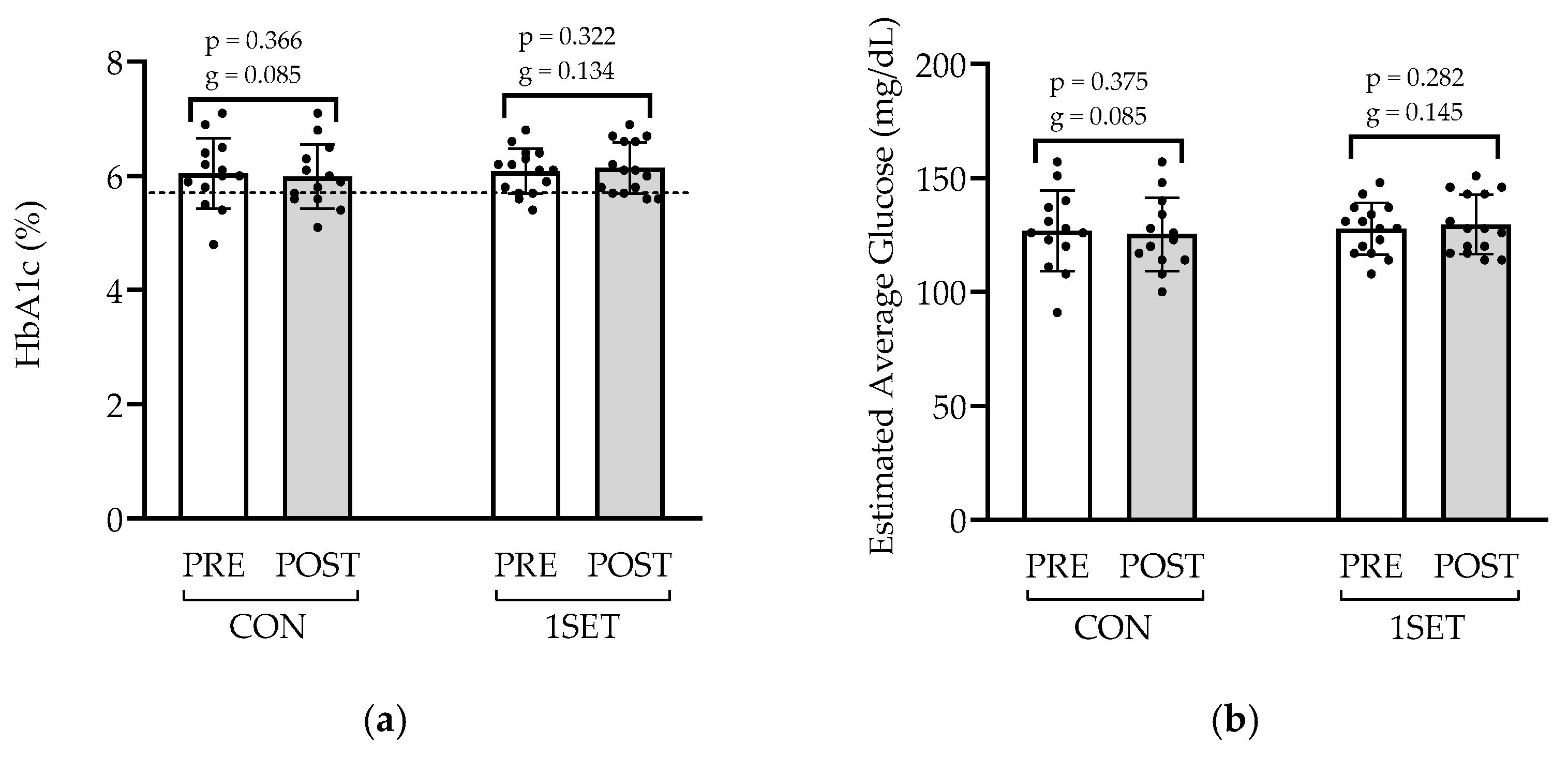
3.2. Effect of 8-Week Low-Volume RT Intervention on Lipid Profile and Glycemic Control
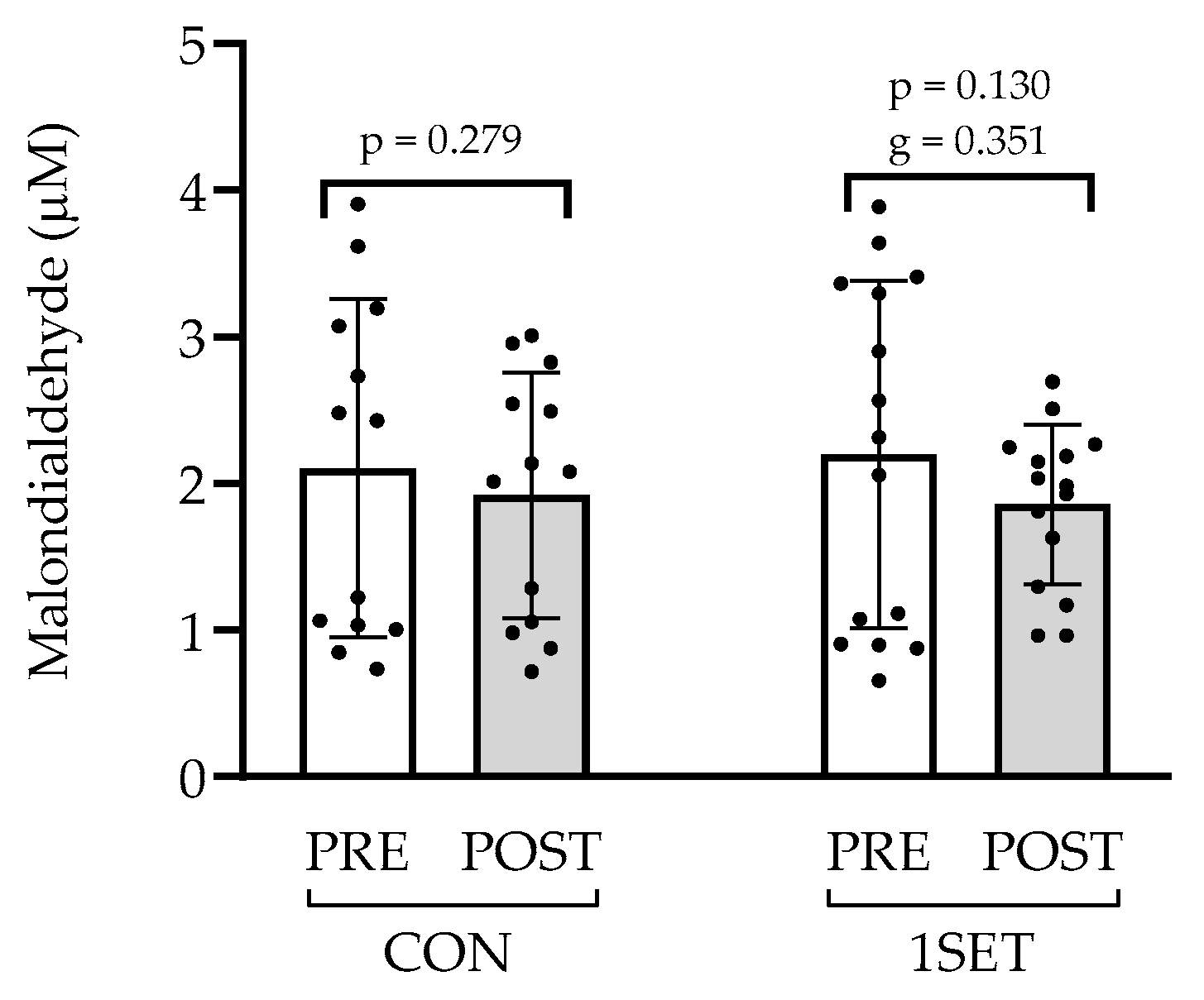
3.3. Effect of 8-Week Low-Volume RT Intervention on Oxidative Stress
4. Discussion
4.1. Muscle Damage and Liver Function Markers
4.2. Lipid Profile and Atherogenic Index
4.3. Glycemic Control
4.4. Oxidative Stress Marker
4.5. Methodological Considerations and Study Limitations
4.6. Summary and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | One-Repetition Maximum |
| ADAG | A1c-Derived Average Glucose |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| ATP | Adenosine Triphosphate |
| CK | Creatine Kinase |
| CON | Control |
| DRS-2 | Dementia Rating Scale-2 |
| HbA1c | Hemoglobin A1c |
| HDL | High-Density Lipoprotein |
| LDH | Lactate Dehydrogenase |
| LDL | Low-Density Lipoprotein |
| MDA | Malondialdehyde |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| R | Repetition |
| RT | Resistance Training |
| SD | Standard Deviation |
| 1SET | Single Set |
| SPPB | Short-Physical-Performance Battery |
| TBA | Thiobarbituric Acid |
| TBARS | Thiobarbituric Acid-Reactive Substances |
| TCA | Trichloroacetic Acid |
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Asejeje, F.O.; Ogunro, O.B. Deciphering the Mechanisms, Biochemistry, Physiology, and Social Habits in the Process of Aging. Arch. Gerontol. Geriatr. Plus 2024, 1, 100003. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Fonseca, N.; Esteves, D.; Marques, D.L.; Faíl, L.B.; Sousa, T.; Pinto, M.P.; Neiva, H.P.; Izquierdo, M.; Marques, M.C. Acute and Time-Course Changes in Hemodynamic and Physical Performance Parameters Following Single and Multiple Resistance Training Sets in Cognitively Impaired Individuals: A Randomized Crossover Study. Exp. Gerontol. 2025, 201, 112688. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Vaquero-Cristóbal, R.; Huber, G. The Power of Resistance Training: Evidence-Based Recommendations for Middle-Aged and Older Women’s Health. Retos 2023, 51, 319–331. [Google Scholar] [CrossRef]
- Khodadad Kashi, S.; Mirzazadeh, Z.S.; Saatchian, V. A Systematic Review and Meta-Analysis of Resistance Training on Quality of Life, Depression, Muscle Strength, and Functional Exercise Capacity in Older Adults Aged 60 Years or More. Biol. Res. Nurs. 2023, 25, 88–106. [Google Scholar] [CrossRef]
- Fernandes, J.F.T.; Lamb, K.L.; Norris, J.P.; Moran, J.; Drury, B.; Borges, N.R.; Twist, C. Aging and Recovery After Resistance-Exercise-Induced Muscle Damage: Current Evidence and Implications for Future Research. J. Aging Phys. Act. 2021, 29, 544–551. [Google Scholar] [CrossRef]
- Swales, B.; Ryde, G.C.; Whittaker, A.C. A Randomized Controlled Feasibility Trial Evaluating a Resistance Training Intervention With Frail Older Adults in Residential Care: The Keeping Active in Residential Elderly Trial. J. Aging Phys. Act. 2022, 30, 364–388. [Google Scholar] [CrossRef]
- de Alencar Silva, B.S.; de Lira, F.S.; de Freitas, M.C.; Uzeloto, J.S.; dos Santos, V.R.; Freire, A.P.C.F.; Bertolini, G.N.; Gobbo, L.A. Traditional and Elastic Resistance Training Enhances Functionality and Lipid Profile in the Elderly. Exp. Gerontol. 2020, 135, 110921. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Sheikholeslami-Vatani, D. The Role of Resistance Training and Creatine Supplementation on Oxidative Stress, Antioxidant Defense, Muscle Strength, and Quality of Life in Older Adults. Front. Public Health 2023, 11, 1062832. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or Structural Brain Changes in Response to Resistance Exercises and Resistance Training Lead to Cognitive Improvements—A Systematic Review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Strategies to Optimize Recovery in Frail Patients with Cardiovascular Disease Through Exercise-Based Cardiac Rehabilitation. J. Lipid Atheroscler. 2025, 14, 159–173. [Google Scholar] [CrossRef]
- Nuzzo, J.L.; Pinto, M.D.; Kirk, B.J.C.; Nosaka, K. Resistance Exercise Minimal Dose Strategies for Increasing Muscle Strength in the General Population: An Overview. Sports Med. 2024, 54, 1139–1162. [Google Scholar] [CrossRef]
- Wan, Y.; Su, Z. The Impact of Resistance Exercise Training on Glycemic Control Among Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res. Nurs. 2024, 26, 597–623. [Google Scholar] [CrossRef]
- Dos Santos, P.R.P.; Cavalcante, B.R.; Vieira, A.K.D.S.; Guimarães, M.D.; Da Silva, A.M.L.; Armstrong, A.D.C.; Carvalho, R.G.D.S.; De Carvalho, F.O.; De Souza, M.F. Improving Cognitive and Physical Function through 12-Weeks of Resistance Training in Older Adults: Randomized Controlled Trial. J. Sports Sci. 2020, 38, 1936–1942. [Google Scholar] [CrossRef]
- de Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 1–21. [Google Scholar] [CrossRef]
- Macaulay, T.R.; Fisher, B.E.; Schroeder, E.T. Potential Indirect Mechanisms of Cognitive Enhancement After Long-Term Resistance Training in Older Adults. Phys. Ther. 2020, 100, 907–916. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Donaldson, M.G. Exercise and Cognition in Older Adults: Is There a Role for Resistance Training Programmes? Br. J. Sports Med. 2009, 43, 25–27. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise Interventions for Cognitive Function in Adults Older than 50: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Milosevic, E.; Liu-Ambrose, T. How Much Will Older Adults Exercise? A Feasibility Study of Aerobic Training Combined with Resistance Training. Pilot. Feasibility Stud. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Bamman, M.; Vechin, F.C.; Conceicao, M.S.; Telles, G.; Longobardi, I.; Damas, F.; Lavin, K.M.; Drummer, D.J.; McAdam, J.S.; et al. Higher Resistance Training Volume Offsets Muscle Hypertrophy Nonresponsiveness in Older Individuals. J. Appl. Physiol. 2024, 136, 421–429. [Google Scholar] [CrossRef]
- Sayers, S.P. High-Speed Power Training: A Novel Approach to Resistance Training in Older Men and Women. A Brief Review and Pilot Study. J. Strength Cond. Res. 2007, 21, 518–526. [Google Scholar] [CrossRef]
- Meredith, S.J.; Cox, N.J.; Ibrahim, K.; Higson, J.; McNiff, J.; Mitchell, S.; Rutherford, M.; Wijayendran, A.; Shenkin, S.D.; Kilgour, A.H.M.; et al. Factors That Influence Older Adults’ Participation in Physical Activity: A Systematic Review of Qualitative Studies. Age Ageing 2023, 52, afad145. [Google Scholar] [CrossRef]
- Healy, S.; Patterson, F.; Biddle, S.; Dumuid, D.; Glorieux, I.; Olds, T.; Woods, C.; Bauman, A.E.; Gába, A.; Herring, M.P.; et al. It’s about Time to Exercise: Development of the Exercise Participation Explained in Relation to Time (EXPERT) Model. Br. J. Sports Med. 2024, 58, 1131–1144. [Google Scholar] [CrossRef]
- Radaelli, R.; Rech, A.; Molinari, T.; Markarian, A.M.; Petropoulou, M.; Granacher, U.; Hortobágyi, T.; Lopez, P. Effects of Resistance Training Volume on Physical Function, Lean Body Mass and Lower-Body Muscle Hypertrophy and Strength in Older Adults: A Systematic Review and Network Meta-Analysis of 151 Randomised Trials. Sports Med. 2025, 55, 167–192. [Google Scholar] [CrossRef]
- Abreu, F.; Rodrigues, A.; Baptista, F. Low-Volume Resistance Training: A Feasible, Cost-Effective Strategy for Musculoskeletal Frailty in Older Adults Attending Daycare Centers. Front. Sports Act. Living 2025, 7, 1542188. [Google Scholar] [CrossRef]
- Hong, H.; Jiang, K.; Ye, H.; Xue, H.; Chen, M. The Aspartate Aminotransferase to Alanine Aminotransferase Ratio: A Novel Indicator for Skeletal Muscle Mass in Chinese Community Adults. Nutrition 2024, 117, 112214. [Google Scholar] [CrossRef]
- Lee, B.T.; Chintamaneni, K.; Kaplowitz, N. Markedly Elevated Serum Aspartate Aminotransferase to Alanine Aminotransferase Ratio: A Clue to Hepatic Neoplasia. Hepatol. Commun. 2020, 4, 1099–1101. [Google Scholar] [CrossRef]
- He, Y.; Su, Y.; Duan, C.; Wang, S.; He, W.; Zhang, Y.; An, X.; He, M. Emerging Role of Aging in the Progression of NAFLD to HCC. Ageing Res. Rev. 2023, 84, 101833. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, S.; Jurica, P.J.; Mattis, S.; Pinto, A.T. DRS-2: Escala de Avaliação de Demência—2; CEGOC-TE: Lisboa, Portugal, 2011. [Google Scholar]
- de Vos, N.J.; Singh, N.A.; Ross, D.A.; Stavrinos, T.M.; Orr, R.; Fiatarone Singh, M.A. Optimal Load for Increasing Muscle Power during Explosive Resistance Training in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.L.; Neiva, H.P.; Marinho, D.A.; Pires, I.M.; Nunes, C.; Marques, M.C. Load-Power Relationship in Older Adults: The Influence of Maximal Mean and Peak Power Values and Their Associations with Lower and Upper-Limb Functional Capacity. Front. Physiol. 2022, 13, 1007772. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Castelli, W.P.; Abbott, R.D.; McNamara, P.M. Summary Estimates of Cholesterol Used to Predict Coronary Heart Disease. Circulation 1983, 67, 730–734. [Google Scholar] [CrossRef]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J. Translating the A1C Assay Into Estimated Average Glucose Values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses After Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef]
- Lavin, K.M.; Roberts, B.M.; Fry, C.S.; Moro, T.; Rasmussen, B.B.; Bamman, M.M. The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology 2019, 34, 112–122. [Google Scholar] [CrossRef]
- Halonen, P.I.; Konttinen, A. Effect of Physical Exercise on Some Enzymes in the Serum. Nature 1962, 193, 942–944. [Google Scholar] [CrossRef]
- Loll, H.; Hilscher, A. Change of Substrate Concentration and Enzyme Activity in Serum by Physical Exercise. Arztl. Forsch. 1958, 12, II/85–II/86. [Google Scholar]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular Exercise Can Cause Highly Pathological Liver Function Tests in Healthy Men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Lira, F.S.; Rossi, F.E. Role of Metabolic Stress for Enhancing Muscle Adaptations: Practical Applications. World J. Methodol. 2017, 7, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Osto, E.; von Eckardstein, A. The Endothelium Is Both a Target and a Barrier of HDL’s Protective Functions. Cells 2021, 10, 1041. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Poggio, P.; Valerio, V.; Cabaro, S.; Campana, P.; Comentale, G.; Attena, E.; Russo, V.; Pilato, E.; et al. Inflammation and Cardiovascular Diseases in the Elderly: The Role of Epicardial Adipose Tissue. Front. Med. 2022, 9, 844266. [Google Scholar] [CrossRef]
- Ghafari, M.; Faramarzi, M.; Baghurst, T. Effect of Exercise Training on Lipid Profiles in the Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Shahrekord Univ. Med. Sci. 2020, 22, 200–207. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Ribeiro, A.S.; Souza, M.F.; Schiavoni, D.; Schoenfeld, B.J.; Venturini, D.; Barbosa, D.S.; Landucci, K.; Sardinha, L.B.; Cyrino, E.S. Resistance Training Improves Inflammatory Level, Lipid and Glycemic Profiles in Obese Older Women: A Randomized Controlled Trial. Exp. Gerontol. 2016, 84, 80–87. [Google Scholar] [CrossRef]
- Yun, H.; Su, W.; Zhao, H.; Li, H.; Wang, Z.; Cui, X.; Xi, C.; Gao, R.; Sun, Y.; Liu, C. Effects of Different Exercise Modalities on Lipid Profile in the Elderly Population: A Meta-Analysis. Medicine 2023, 102, e33854. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Moraes, G.K.; Sugihara, P.; Barbosa, D.S.; Venturini, D.; Cyrino, E.S. Resistance Training Performed with Single-Set Is Sufficient to Reduce Cardiovascular Risk Factors in Untrained Older Women: The Randomized Clinical Trial. Active Aging Longitudinal Study. Arch. Gerontol. Geriatr. 2019, 81, 171–175. [Google Scholar] [CrossRef]
- Hurley, B.F.; Hanson, E.D.; Sheaff, A.K. Strength Training as a Countermeasure to Aging Muscle and Chronic Disease. Sports Med. 2011, 41, 289–306. [Google Scholar] [CrossRef]
- Misra, A.; Alappan, N.K.; Vikram, N.K.; Goel, K.; Gupta, N.; Mittal, K.; Bhatt, S.; Luthra, K. Effect of Supervised Progressive Resistance-Exercise Training Protocol on Insulin Sensitivity, Glycemia, Lipids, and Body Composition in Asian Indians with Type 2 Diabetes. Diabetes Care 2008, 31, 1282–1287. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Roubenoff, R.; Tucker, K.L.; Nelson, M.E. A Randomized Controlled Trial of Resistance Exercise Training to Improve Glycemic Control in Older Adults with Type 2 Diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.; Layne, J.E.; Gordon, P.L.; Roubenoff, R.; Nelson, M.E.; Castaneda-Sceppa, C. Strength Training Improves Muscle Quality and Insulin Sensitivity in Hispanic Older Adults with Type 2 Diabetes. Int. J. Med. Sci. 2006, 4, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; De Courten, M.; Shaw, J.; Zimmet, P. High-Intensity Resistance Training Improves Glycemic Control in Older Patients with Type 2 Diabetes. Diabetes Care 2002, 25, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, J.; Izquierdo, M.; Argüelles, I.; Forga, L.; Larrión, J.L.; García-Unciti, M.; Idoate, F.; Gorostiaga, E.M. Twice-Weekly Progressive Resistance Training Decreases Abdominal Fat and Improves Insulin Sensitivity in Older Men with Type 2 Diabetes. Diabetes Care 2005, 28, 662–667. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, S.; Hossen, M.A.A.; Mia, M.; Ferdous, K. A Review: Assessment of HbA1c as A Diagnostic Tool in Diabetes Mellitus. Mol. Mech. Res. 2023, 1. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Alikhani, S.; Sheikholeslami-Vatani, D. Oxidative Stress and Anti-oxidant Responses to Regular Resistance Training in Young and Older Adult Women. Geriatr. Gerontol. Int. 2019, 19, 419–422. [Google Scholar] [CrossRef]
- Carru, C.; Da Boit, M.; Paliogiannis, P.; Zinellu, A.; Sotgia, S.; Sibson, R.; Meakin, J.R.; Aspden, R.M.; Mangoni, A.A.; Gray, S.R. Markers of Oxidative Stress, Skeletal Muscle Mass and Function, and Their Responses to Resistance Exercise Training in Older Adults. Exp. Gerontol. 2018, 103, 101–106. [Google Scholar] [CrossRef]
- Bobeuf, F.; Labonte, M.; Dionne, I.J.; Khalil, A. Combined Effect of Antioxidant Supplementation and Resistance Training on Oxidative Stress Markers, Muscle and Body Composition in an Elderly Population. J. Nutr. Health Aging 2011, 15, 883–889. [Google Scholar] [CrossRef]
- Vincent, K.R.; Vincent, H.K.; Braith, R.W.; Lennon, S.L.; Lowenthal, D.T. Resistance Exercise Training Attenuates Exercise-Induced Lipid Peroxidation in the Elderly. Eur. J. Appl. Physiol. 2002, 87, 416–423. [Google Scholar] [CrossRef]
- McBride, J.M.; Kraemer, W.J.; Triplett-McBride, T.; Sebastianelli, W. Effect of Resistance Exercise on Free Radical Production. Med. Sci. Sports Exerc. 1998, 30, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Im, J.; Kang, J.; Maresh, C.M.; Kraemer, W.J.; French, D.; Nioka, S.; Kime, R.; Rundell, K.W.; Ratamess, N.A.; et al. Comparison of Low- and High-Intensity Resistance Exercise on Lipid Peroxidation: Role of Muscle Oxygenation. J. Strength Cond. Res. 2007, 21, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H.; McKenzie, M.J. Oxidative Stress Response to Aerobic Exercise: Comparison of Antioxidant Supplements. Med. Sci. Sports Exerc. 2006, 38, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. BioMed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]

| Exercises | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 |
|---|---|---|---|---|---|---|---|---|
| Leg press (R × %1RM) | 12 × 50 | 12 × 50 | 10 × 60 | 10 × 60 | 8 × 65 | 8 × 65 | 6 × 70 | 6 × 70 |
| Chest press (R × %1RM) | 12 × 40 | 12 × 40 | 10 × 50 | 10 × 50 | 8 × 55 | 8 × 55 | 6 × 60 | 6 × 60 |
| Chair squat (R) | 12 | 12 | 10 | 10 | 8 | 8 | 6 | 6 |
| Medicine ball throw (R × kg) | 12 × 1 | 12 × 1 | 10 × 1 | 10 × 1 | 8 × 1 | 8 × 1 | 6 × 1 | 6 × 1 |
| CON (n = 13) | 1SET (n = 15) | ||
|---|---|---|---|
| Age (years) | Mean ± SD | 90.00 ± 10.39 | 81.00 ± 9.66 |
| Min–max | 57–95 | 58–93 | |
| Sex (n) | Female | 9 | 6 |
| Male | 4 | 9 | |
| Height (m) | 1.58 ± 0.08 | 1.53 ± 0.09 | |
| Body mass (kg) | 66.40 ± 11.21 | 67.90 ± 10.74 | |
| Cognitive Impairment (DRS-2, n) | Mild | 2 | 4 |
| Moderate | 1 | 4 | |
| Severe | 10 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, M.; Marques, M.C.; Fonseca, N.; Marques, D.L.; Nunes, C.; Marinho, D.A.; Neiva, H.P.; Izquierdo, M.; Esteves, D.; Fonseca, C.P. Effects of Eight-Week Single-Set Resistance Training on Muscle Health, Metabolic Profile and Oxidative Stress in Individuals with Cognitive Impairment. Appl. Sci. 2025, 15, 7091. https://doi.org/10.3390/app15137091
Lopes M, Marques MC, Fonseca N, Marques DL, Nunes C, Marinho DA, Neiva HP, Izquierdo M, Esteves D, Fonseca CP. Effects of Eight-Week Single-Set Resistance Training on Muscle Health, Metabolic Profile and Oxidative Stress in Individuals with Cognitive Impairment. Applied Sciences. 2025; 15(13):7091. https://doi.org/10.3390/app15137091
Chicago/Turabian StyleLopes, Mariana, Mário C. Marques, Nuno Fonseca, Diogo L. Marques, Célia Nunes, Daniel A. Marinho, Henrique P. Neiva, Mikel Izquierdo, Dulce Esteves, and Carla P. Fonseca. 2025. "Effects of Eight-Week Single-Set Resistance Training on Muscle Health, Metabolic Profile and Oxidative Stress in Individuals with Cognitive Impairment" Applied Sciences 15, no. 13: 7091. https://doi.org/10.3390/app15137091
APA StyleLopes, M., Marques, M. C., Fonseca, N., Marques, D. L., Nunes, C., Marinho, D. A., Neiva, H. P., Izquierdo, M., Esteves, D., & Fonseca, C. P. (2025). Effects of Eight-Week Single-Set Resistance Training on Muscle Health, Metabolic Profile and Oxidative Stress in Individuals with Cognitive Impairment. Applied Sciences, 15(13), 7091. https://doi.org/10.3390/app15137091











