Abstract
Background and Objectives: Cardiovascular diseases (CVDs) remain the leading cause of mortality worldwide. Regular physical activity (PA) represents a key modifiable factor in CVD prevention. Methods: Fifty-two healthy adult males participated in this study, divided into two groups: aged up to 45 years and over 45 years. The subjects performed a bicycle ergometer exercise and a standardised back muscle workload protocol. ECG, arterial blood pressure (ABP), and muscle oxygen saturation (StO2) measurements were obtained during workload and recovery. Results: During bicycle ergometer workload, heart rate (HR) at minute 2 was significantly lower in participants over 45 years of age compared to the younger group (126.8–109.8 bpm), while diastolic blood pressure (dBP) was significantly lower in the under-45 group during maximal workload (65.4–71.9 mmHg) and the first minute of recovery (54.6–69.3 mmHg). During workload for back muscles, the over-45 group showed significantly lower dBP at the third rest period (87–74.7 mmHg), while StO2 was significantly lower in the over-45 group compared to the under-45 group (54.4–77.8%). Conclusions: The findings of this study demonstrate that both bicycle ergometer exercise and standardised back muscle workload had a significant influence on cardiovascular system (CVS) responses, particularly when stratified by age. Participants over the age of 45 exhibited a higher incidence of functional myocardial ischaemia, reduced StO2 and more pronounced increases in HR during and following exertion.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death globally, accounting for nearly one-third of all deaths. In 2022, global deaths from CVDs reached 19.8 million [1]. There is an opportunity to improve efforts aligning with Sustainable Development Goal 3.4, aiming to decrease premature mortality from non-communicable CVDs by one third, and this objective corresponds with the World Heart Federation’s World Heart Vision 2030 [2]. Along with the increasing trend regarding sedentary lifestyles, there has been an increase in obesity and CVD morbidity; hence, the promotion of physical activity (PA) and regular exercise matters now more than ever [3]. PA can contribute to efforts to meet four major medical challenges: treatment, rehabilitation, prevention and health promotion. Health promotion and preservation are pertinent scientific and practical issues given the greater resources allocated to prevention compared with rehabilitation or treatment [4,5]. The European Society of Cardiology has highlighted an increase in PA as a top priority [6].
According to the World Health Organisation (WHO), engaging in 150–300 min of moderate-intensity regular PA per week including muscle-strengthening activities at least two days per week is recommended [7]. As a form of PA, exercise is generally dichotomised into endurance or aerobic training and resistance or strength training categories [8]. The general scientific consensus is that endurance exercise increases heart and respiratory rates for extended periods, enhancing cardiovascular fitness (strengthening the heart and lungs), improving circulation, and thus reducing the risk of heart disease, and traditional strength training has a positive influence on muscle mass, bone mineral density, and metabolic health [9,10]. Cycle ergometry is a valuable, non-invasive, and cost-effective tool for assessing aerobic capacity via ECG. It is instrumental in evaluating cardiovascular adaptations to endurance training and identifying physiological thresholds for optimised performance [11,12]. Meanwhile, back-strengthening exercises, such as planks, hyperextensions, and dynamic stabilisation routines, enhance core stability, improve posture, and reduce injury risk by targeting muscles like the erector spinae and transverse abdominis [13,14]. The combination of these exercises synergises with WHO recommendations by supporting both endurance and resistance training efficacy, ensuring balanced musculoskeletal health and functional longevity.
To advocate for health-promoting exercises, researchers prioritise the scientific evaluation of their effectiveness. In practice, various methods are used to evaluate changes in the body caused by physical exertion, but in the field of health sciences, evaluations of the functional state of the cardiovascular system (CVS) are considered one of the most important [15,16]. The timely and accurate diagnosis of CVDs based on ECG has a crucial role in mitigating premature cardiovascular mortality [17]. Consequently, the identification of specific cardiac deviations via the analysis of ECG signals has become imperative for diagnosing cardiac disorders.
CVS monitoring can be used to answer questions about the functional state of athletes, non-athletes, and patients with diseases. Older people who are starting to train, as well as those who train in their leisure time, are much less widely evaluated than high-performance athletes [18]. Well-structured training delivers adequate exercise stimuli to athletes while minimising the risk of prolonged non-functional overreaching or insufficient recovery. Regular athlete assessment can contribute to performance optimisation while helping to prevent injuries. Functional alterations in the body during exercise are conditioned by a sequence of complex interrelated processes. These processes cause physiological adaptation to physical load, which is generally defined as the development of such functionality, manifested in an individual’s greater tolerance to the physical workload, an increased amount of energy resources, and an optimisation of regulatory mechanisms [19]. When testing athletes at rest, it is often not possible to determine the functional state of organs or systems, the reserve capabilities of the body, functional failures, or pathological changes. Failure of reserve cardiac capabilities may only be evident when the heart is working at a higher power than usual [20]. As a result, cardiovascular functional capacity and its adaptability to PA are investigated and evaluated using functional testing.
2. Materials and Methods
2.1. Trial Design
The initial monitoring was conducted to inform participants about the study protocol. Each of the participants read and signed an informed consent form agreeing to engage in all testing procedures. Their PA patterns were assessed using the Global Physical Activity Questionnaire (GPAQ), and their body composition was evaluated. Before each assessment, participants were required to avoid smoking and eating for at least three hours. Throughout the intervention assessment period, participants were instructed to maintain their usual daily habits. The study involved two measurement phases, Study A and Study B, conducted within a one-week period. Both measurements followed the same standardised procedures and conditions to ensure consistency and reliability in data collection. After completing assessments, participants were categorised into two age groups: under 45 years (n = 24) and over 45 years (n = 28). The study protocol is shown in Figure 1.
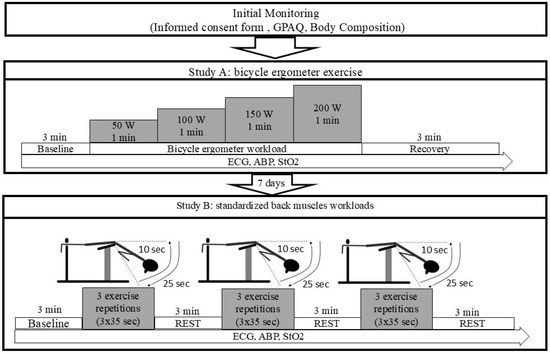
Figure 1.
Study protocol. GPAQ: Global Physical Activity Questionnaire; ECG: electrocardiogram; ABP: arterial blood pressure; StO2: oxygen saturation; BMI: Body Mass Index; W: watts; min: minutes; sec: seconds.
The study was approved by the Lithuanian Bioethics Committee in Approval to Conduct Biomedical Research (23 January 2020 No. L-20-1/1). The experiments were performed according to the ethical standards and principles of the Declaration of Helsinki.
2.2. Participants
Fifty-two physically active male participants (mean age: 41.2 ± 3.0 years; range: 22–62 years; height: 1.79 ± 0.01 m; weight: 82.6 ± 2.3 kg; BMI: 25.7 ± 0.6 kg/m2) were recruited through targeted convenience sampling. They were separated into two age-based groups: under 45 years of age (n = 24; age: 25.1 ± 0.8 years; height: 1.81 ± 0.01 m; weight: 80.7 ± 3.8 kg; BMI: 24.6 ± 0.9 kg/m2) and over 45 years of age (n = 28; age: 56.3 ± 1.2 years; height: 1.78 ± 0.01 m; weight: 84.4 ± 2.7 kg; BMI: 26.7 ± 0.7 kg/m2). All participants were engaged in regular physical activity, meeting the criteria for moderate to vigorous intensity as defined by the GPAQ. Each had attended a gym for at least one year and reported a minimum of three hours of physical activity per week, including both aerobic and resistance training. None were professional athletes. Inclusion criteria required that participants be male, aged 20 to 64 years, physically active, free from cardiovascular disease or other chronic health conditions, with normal resting blood pressure, and not taking any medications or dietary supplements. There were no significant differences between the two age groups in terms of BMI or habitual physical activity levels. Given the homogeneity of the sample with respect to sex, physical activity habits, BMI, and cardiovascular risk profile, no additional matching procedures were deemed necessary.
2.3. Assessments
2.3.1. Measures of Physical Activity
PA patterns were evaluated using the GPAQ developed by the WHO, which is known for its proven validity and reliability [21]. This tool assesses PA across three main areas: work-related activity, travel by active means, and recreational or leisure-time activity. The GPAQ includes 16 items aimed at identifying the frequency, duration, and intensity of PA. In this study, all participants indicated they engaged in PA of at least moderate or vigorous intensity.
2.3.2. Body Composition
For anthropometric evaluation, height and weight were recorded using calibrated equipment while participants were barefoot and dressed only in their underwear. A Seca® 213 stadiometer (Seca GmbH & Co, Hamburg, Germany) was employed for height measurement, and a Tanita® BC-545 scale (Tanita Corporation, Tokyo, Japan) was used to determine body weight. These measurements were essential for calculating the Body Mass Index (BMI).
2.3.3. Oxygen Saturation
Changes in muscle oxygen saturation (StO2) during and after the procedure were assessed by means of non-invasive near-infrared spectroscopy using a photosensor (Hutchinson Technology, Hutchinson, MN, USA). The photosensitive sensor was attached to the main muscle group performing the movement: Study A—vastus lateralis; Study B—erector spinae. The level of StO2 was recorded continuously throughout the exercise and for 5 min of recovery.
2.3.4. Arterial Blood Pressure Measurements
Arterial blood pressure (ABP) was assessed using a cuff-based auscultatory technique, utilising Korotkoff sounds for systolic (sBP) and diastolic (dBP) blood pressure determination (Omron Healthcare Co, Kyoto, Japan).
2.3.5. ECG Measurements
The selection of the two exercise modalities—intermittent back muscle loading and cycling—was based on their relevance to real-life physical activities involving postural and aerobic demands. During the procedure, a 12-lead ECG was recorded for later analysis by means of using the CardioScout Multi ECG recorder. The analysed parameters were sBP, dBP, heart rate (HR), and ST-segment depression (sum of negative values of 12 leads).
Study A (Figure 1): ECG signals were captured every 15 s at baseline, during graded exercise on a cycle ergometer at intensities of 50 W (light), 100 W (moderate), 150 W (heavy), and 200 W (maximal), and throughout a 3 min recovery phase. Prior to testing, participants were attached with ECG electrodes and instructed to remain at rest for 30 min to establish stable baseline readings. This was followed by an additional minute of rest while seated on the cycle ergometer. The exercise protocol required participants to pedal at a steady cadence of 60 rpm, with workload increasing by 50 W each minute, beginning at 50 W and progressing to 200 W. Post-exercise, participants remained seated for 3 min while ECG data were continuously collected to assess recovery. A 12-lead ECG and arm-cuff ABP readings were taken at the conclusion of each workload stage. Changes in muscle StO2 in the muscle (vastus lateralis) were also monitored continuously throughout the protocol. ECG and ABP measurements continued during the recovery period as well.
Study B (Figure 1): The subjects performed three sets of exercise for the back muscles, consisting of trunk extension exercise performed on a back extension bench supported by a trainer. Before the measurements began, the ECG electrodes were applied to the participant’s body and participants sat and rested for 30 min to assure stable baseline readings. Resting parameters were collected at the end of 30 min. After the test, the participants rested for 3 min while ECG data were continuously recorded for recovery analysis. The torso-stretching movement was performed for 10 s and the return to the starting position (torso bending movement) for 20–25 s (Figure 1), with three repetitions. Between each repetition, the subjects rested for 3 min while standing. After the exercise, the subjects rested for 3 min to monitor recovery. ECG measurements and the change in StO2 in the muscle (erector spinae) were recorded continuously throughout the study; ABP was measured at the end of each set.
2.3.6. Statistical Analysis
All statistical analyses were performed using SPSS Statistics software (version 27.0; IBM Corp., Armonk, NY, USA). Results are expressed as arithmetic means ± standard error of the mean (SEM). The normality of data distribution was assessed using the Kolmogorov–Smirnov test. To evaluate the effects of time (within-subject factor), age group (between-subject factor), and their interaction, a two-factor mixed ANOVA was conducted. To control for the risk of Type I error due to multiple comparisons, post hoc tests were adjusted using the Holm–Bonferroni correction. Effect sizes were calculated to estimate the magnitude of observed effects. A p-value of less than 0.05 was considered statistically significant.
3. Results
Figure 2 demonstrates that prior to and during the bicycle ergometer test (Study A), there were no statistically significant differences in HR between the two age groups. HR progressively increased with each workload level and subsequently decreased during the recovery phase in both groups. The highest HR was recorded at 200 W, reaching 156.1 ± 3.7 bpm in the under-45 group and 160.5 ± 3.7 bpm in the over-45 group. A statistically significant difference between groups emerged at the second minute of recovery: HR decreased to 126.8 ± 3.7 bpm in the younger group and to 117.0 ± 4.2 bpm in the older group (p < 0.05). Despite this reduction, HR values in both groups remained above baseline after three minutes of recovery. Based on HR data, participants under 45 showed a faster return to baseline levels after physical exertion compared to those over 45. Effect size analysis indicated a moderate difference in HR recovery at 2 and 3 min post-exercise, suggesting better cardiovascular recovery in the younger group.

Figure 2.
The HR dynamic during (A) the bicycle ergometer workload while the workload increased stepwise every minute and (B) the intermittent workloads applied to back muscles. Note. HR, heart rate; bpm, beats per minute; W, watts; min, minute; REP, repetition. * p < 0.05.
During the local intermittent loading protocol (Study B), HR dynamics differed. Compared to exercise, HR increased during recovery periods. Statistically significant differences between age groups were observed from the first to the last recovery stage (p < 0.05). At the first rest interval, HR in the under-45 group was 92.4 ± 4.0 bpm, while the over-45 group reached 106.0 ± 5.0 bpm (p < 0.05). During the second repetition, HR decreased slightly to 89.7 ± 2.4 bpm and 100.0 ± 3.7 bpm in the younger and older groups, respectively (p < 0.05). A subsequent increase was observed during the second rest period, with HR reaching 95.3 ± 4.0 bpm in the under-45 group and 108.0 ± 4.6 bpm in the over-45 group (p < 0.05). This upward trend continued in the third repetition and rest phase. HR during the third repetition increased in the under-45 group to 94.4 ± 2.7 bpm and 103.0 ± 2.2 bpm in the over-45 group (p < 0.05). The third rest period exhibited the highest HR values of this protocol: 99.1 ± 3.4 bpm in the under-45 group and 109.5 ± 3.5 bpm in the over-45 group (p < 0.05).
Figure 3 shows that at the onset of exercise, sBP in Study A progressively increased with each stage of the bicycle ergometer test and decreased during the recovery period. In Study B, sBP values returned close to baseline during the rest intervals between repetitions. No statistically significant differences in sBP were observed between age groups at any stage of testing or recovery. At baseline, sBP in Study A was 127.3 ± 2.6 mmHg in participants under 45 years of age and 124.0 ± 2.9 mmHg in those over 45. The highest values were recorded at the 200 W workload, reaching 188.8 ± 5.9 mmHg in the younger group and 197.2 ± 4.0 mmHg in the older group.
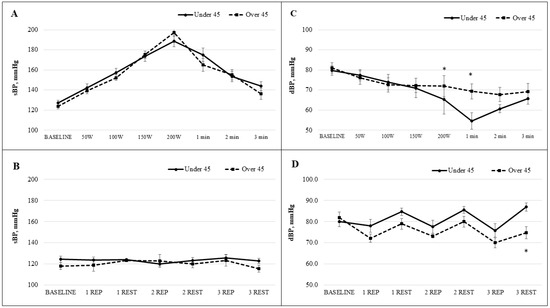
Figure 3.
The sBP (A) and dBP (C) dynamic during the bicycle ergometer workload while the workload increased stepwise every minute and sBP (B) and dBP (D) dynamic during the the intermittent workloads applied to back muscles. Note. sBP, systolic blood pressure; dBP, diastolic blood pressure; mmHg, millimetres of mercury; W, watts; min, minute; REP, repetition. * p < 0.05.
During the local intermittent exercise protocol targeting the back muscles (Study B), sBP values during recovery were lower than baseline in both age groups. Baseline sBP was 124.4 ± 3.1 mmHg in the under-45 group and 118.0 ± 3.4 mmHg in the over-45 group. After the third repetition, sBP peaked during exercise, reaching 125.8 ± 3.1 mmHg in the younger group and 123.3 ± 5.1 mmHg in the older group. The lowest values were observed during the third rest phase: 122.9 ± 2.4 mmHg in the under-45 group and 115.7 ± 3.4 mmHg in the over-45 group. dBP at baseline in Study A was 79.7 ± 2.4 mmHg for the younger group and 81.0 ± 2.5 mmHg for the older group. A statistically significant difference between groups was observed at the 200 W workload: dBP decreased to 65.4 ± 7.3 mmHg in the under-45 group and to 71.9 ± 5.3 mmHg in the over-45 group (p < 0.05). One minute into recovery, dBP further dropped to 54.6 ± 4.1 mmHg and 69.3 ± 3.7 mmHg, respectively, again showing a statistically significant difference (p < 0.05). After three minutes of recovery, values did not return to baseline. In Study B, dBP at baseline was 80.0 ± 2.4 mmHg in the under-45 group and 82.0 ± 2.5 mmHg in the over-45 group. Throughout the intermittent exercise protocol, dBP decreased during repetitions and increased during rest in both groups. Notably, during the final repetition, dBP decreased more in the over-45 group (70.0 ± 2.3 mmHg) compared with the under-45 group (75.8 ± 3.2 mmHg). During the third rest period, a statistically significant difference emerged: dBP increased to 87.0 ± 2.0 mmHg in the younger group and only to 74.7 ± 2.9 mmHg in the older group (p < 0.05). Age has a moderate effect on diastolic blood pressure during and after exercise, with the over-45 group showing slightly higher values. Effect sizes range from 0.25 to 0.67, indicating small to moderate differences between age groups.
Figure 4 illustrates that in Study A, ST-segment depression increased significantly in both age groups as the workload approached 200 W. At this intensity, the ST-segment depression was measured at 0.38 ± 0.09 mV in participants aged under 45 years of age and at 0.43 ± 0.1 mV in those over 45 years of age. However, the difference between the groups was not statistically significant. In Study B, during localised repeated loading of the back muscles, ST-segment depression values progressively increased in response to rising workload and were further elevated during the recovery phase. Higher ST-segment depression values were recorded in the group aged over 45 years, particularly during recovery. Following the third repetition, ST-segment depression during recovery was 0.17 ± 0.06 mV in the older group and 0.10 ± 0.02 mV in the younger group; however, this difference also did not reach statistical significance.

Figure 4.
The ST-segment dynamic during (A) the bicycle ergometer test while the workload increased stepwise every minute and (B) the intermittent workloads applied to back muscles. Note. mV, millivolts; W, watts; min, minute; REP, repetition.
Figure 5 presents the dynamics of StO2 during both the exercise and recovery phases. In Study A, as the workload on the bicycle ergometer increased incrementally each minute, a decline in StO2 was observed in both age groups. Upon reaching a workload of 150 W, the mean StO2 value in participants under 45 years of age was 77.8 ± 5.3%, whereas in those over 45 years of age it was 54.4 ± 7.7%, with a statistically significant difference between the groups (p < 0.05). During the recovery phase, StO2 values in both groups exceeded baseline levels. In the younger group (<45 years), the peak value was recorded in the second minute post-exercise (120.7 ± 4.6%), while in the older group (≥45 years), the highest value occurred in the third minute of recovery (135.1 ± 7.1%). The study results showed that StO2 levels were higher in participants over the age of 45 compared to younger individuals. The calculated effect sizes indicate a strong age-related impact on muscle oxygenation dynamics both during physical exertion and in the recovery period.
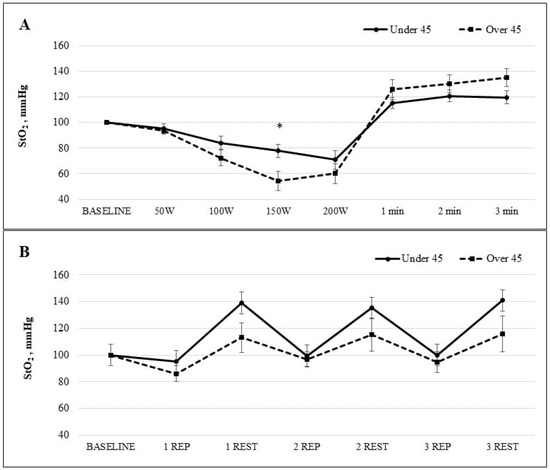
Figure 5.
The StO2 dynamic during (A) the bicycle ergometer test while the workload increased stepwise every minute and (B) the intermittent workloads applied to back muscles. Note. StO2, muscle oxygen saturation; mmHg, millimetres of mercury; W, watts; min, minute; REP, repetition. * p < 0.05.
In Study B, involving intermittent loading of the back muscles, StO2 levels decreased during exercise repetitions and increased during recovery periods. However, the difference in StO2 values between the age groups was not statistically significant. The study revealed differences in StO2 dynamics between age groups during exercise and recovery. Participants over 45 showed more efficient oxygen recovery after each repetition. The effect size indicates a moderate age-related impact, especially during recovery phases.
4. Discussion
In the context of health promotion, the evaluation of health parameters constitutes a fundamental component for the identification of determinants and the formulation of evidence-based interventions. Many methods of assessing the response of the CVS to workload are presented in the scientific literature [22], and cyclical exercise tests are commonly described [23]; however, CVS responses to intermittent workload for the back muscles are not sufficiently investigated. Strengthening the back muscles is an integral part of physical health, and the connection between active working muscles and the CVS is complex. This complexity results from the fact that the muscles do not work in isolation but together with other muscle groups, internal organs, and all other physiological systems [24,25]. Therefore, in our study, two different workloads helped us to monitor CVS responses to back muscle exercises and differences in this dynamic response by age.
HR and ABP are well-known and well-studied indicators of CVS responses to workload [26]. An increase in HR during physical exertion is linked to a reduction in parasympathetic influence and a simultaneous rise in sympathetic nervous system activity [27]. In our study, the dynamic changes in HR during the ergometer testing were consistent with the findings of previous research. It is notable that although the differences between age groups were not statistically significant, HR values in the over-45 group remained consistently higher throughout the test. This result may be associated with age-related changes in the autonomic nervous system, slower recovery of parasympathetic tone, and decreased physical fitness in older adults [28]. During intermittent workloads applied to the back muscles, HR increased more during the recovery phases than during exercise repetitions. Throughout the testing procedure, participants’ postural changes from supine to standing positions could be related to the HR and ST-segment depression responses to workload. HR changes caused by postural provocations are widely described in the literature [29]. Increased HR immediately after a repetition could also be attributed to the calf muscle pump activation during exercise; in the literature, this mechanism has been referred to as the body’s ‘second heart’ due to its role in enhancing systemic blood circulation [30]. Notably, age-related differences were observed: participants aged 45 and older exhibited a more pronounced HR response to the workload, with HR increasing more noticeably during the testing procedure compared with younger individuals.
ABP is one of the most extensively used haemodynamic variables studied following exercise. In this study, ABP data confirm the well-known hypothesis [31] that during exercise, sBP increases and dBP decreases at the same moment. In this study, ABP returned to initial values after the intermittent workloads applied to back muscles. After ergometer exercise, 3 min was too short a time for ABP values to return to baseline values. Similar results were found by other investigators [26]. However, dBP responses varied with age. Participants over the age of 45 demonstrated more stable diastolic values throughout the ergometer test, while younger individuals showed a statistically significant decrease in dBP. This age-related difference may be attributed to reduced arterial elasticity in older adults, which limits the ability of blood vessels to accommodate increased cardiac output [32]. Similar results were observed when evaluating dBP dynamics during back muscle exercises, with older individuals exhibiting smaller fluctuations in this parameter.
ST-segment depression is a widely discussed ECG parameter, which, for many decades, has been recognised as an indicator of myocardial ischaemia [33]. Deviation in this parameter is usually measured at its junction with the end of the QRS complex [34]. In our study, ST-segment depression increased during the bicycle test and decreased at recovery. During intermittent workloads applied to the back muscles, ST-segment depression reacted to workload in a different way from that most commonly described in the literature [35], with the values increasing more during recovery than during exercise. We attribute this observation to the reasons described above. This increasing dynamic is not significant in the study group over 45 years of age. In this case, ST-segment depression increased in every exercise repetition and also during recovery. Studies have shown that an increase in ST-segment depression of more than 0.1 mV during or after exercise might predict coronary artery disease, based on an ST-segment depression of more than 0.2 mV as the super-positive standard [36]. In our study, the highest value during exercises to the back muscles reached −0.16 ± 0.1 mV. The observed dynamics in these indicators could be related to the recovery period being too short to allow a return to baseline values. Previous studies reported that return of ST-segment depression to baseline after workload required a prolonged recovery phase of at least 5 min [37]. Similar dynamics were observed in the group aged under 45, but in this case, changes in ST-segment depression values were smaller and were not significant. Thus, the emergence of these functional ischaemic episodes during exercise can determine the capacity of the functional state of the CVS in older age. Therefore, analysing the ST segment during back muscle training can help to identify early signs of ischemia [38].
Changes in StO2 in muscle tissue are associated with changes in arterial blood flow intensity [39]. In the event of fatigue in the working muscles, the oxygen saturation begins to decrease, which is especially noticeable in the case of the highest workloads. During exercise, these changes determine muscle blood flow intensity and StO2 for muscle work [40]. In our study, during the bicycle ergometer exercise when workload reached 150 W, the muscles received a lower supply of oxygen in participants over 45 years age. At recovery, the StO2 of these participants was higher than that seen for participants under 45 years age, which may be caused by vasodilation of the peripheral blood vessels and severe intensification of blood flow in the calf. These phenomena are widely described in the literature [41]. During the intermittent workloads to the back muscles, StO2 monitored in the muscle tissue was not significantly different between the groups, which may result from the intensity and specific nature of the workload.
Age-related alterations in the autonomic nervous system play a crucial role in explaining the differences observed between various age groups. Older adults typically show decreased parasympathetic activity alongside an increased influence of the sympathetic nervous system [42]. This shift results in a higher resting heart rate, a stronger heart rate response during physical exercise, and a slower recovery afterward. Furthermore, vascular ageing—marked by reduced arterial elasticity and endothelial dysfunction—impairs the blood vessels’ ability to dilate (vasodilation) and adjust to the elevated cardiac output required during physical activity [43]. Thus, these physiological changes together affect not only heart rate dynamics and blood pressure regulation but also muscle oxygenation and myocardial oxygen demand. Therefore, older participants in the study showed a more pronounced increase in heart rate, a more stable but higher diastolic blood pressure, and a slower recovery phase of muscle oxygen saturation and ST-segment changes. These mechanisms help us to better understand why age is an important factor in assessing cardiovascular responses to exercise.
A limitation of this study is the small group size. Further studies could be performed with a larger number of participants to better reveal the CVS parameter responses to other exercises. One more limitation of this study is the lack of a longitudinal design, which would be valuable to investigate how cardiovascular responses to various types of physical exertion change over a longer period. Additionally, future cohorts should consider stratification by sex, activity level, and CVD risk profile to provide a more comprehensive understanding of these factors’ influence on cardiovascular responses.
5. Conclusions
The findings of this study demonstrate that both bicycle ergometer exercise and standardised back muscle workload have a significant influence on CVS responses, particularly when stratified by age. Participants over the age of 45 exhibited a higher incidence of functional myocardial ischaemia, reduced muscle StO2, and more pronounced increases in HR during and following exertion. These alterations are likely attributable to age-related changes in autonomic nervous system regulation and vascular function. While differences in blood pressure and StO2 between age groups were more evident during bicycle ergometer exercise, the standardised back muscle workload also revealed a meaningful tendency, especially in the recovery phase for older individuals. These results underscore the importance of developing age-specific PA and health promotion strategies. Further research with larger sample sizes is warranted to validate these observed patterns.
Author Contributions
Conceptualisation, R.B., K.M., K.P., E.T. and Z.K.; methodology, R.B., K.M., K.P., E.T. and Z.K.; software, R.B. and Z.K.; validation, R.B., K.M. and Z.K.; formal analysis, Z.K.; investigation, R.B.; resources, Z.K.; data curation Z.K.; writing—original draft preparation, R.B.; writing—review and editing, R.B.; visualisation, K.M.; supervision, Z.K.; project administration, Z.K.; funding acquisition, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for all procedures in this study was obtained from the The Group of Biomedical Research Experts of the Lithuanian Bioethics Committee (Approval No. 23-01-2020 23 January 2020, Ref. L-20-1/2) The study adhered to the ethical standards and principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Walther, O. New study reveals latest data on global burden of cardiovascular disease. J. Am. Coll. Cardiol. 2023. Available online: https://www.acc.org/About-ACC/Press-Releases/2023/12/11/18/48/New-Study-Reveals-Latest-Data-on-Global-Burden-of-Cardiovascular-Disease (accessed on 17 June 2025).
- Di Cesare, M.; Bixby, H.; Gaziano, T.; Hadeed, L.; Kabudula, C.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Perel, P.; Piñeiro, D.; et al. World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023. [Google Scholar]
- Albalak, G.; Stijntjes, M.; van Bodegom, D.; Jukema, J.W.; E Atsma, D.; van Heemst, D.; Noordam, R. Setting your clock: Associations between timing of objective physical activity and cardiovascular disease risk in the general population. Eur. J. Prev. Cardiol. 2023, 30, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary behavior, exercise, and cardiovascular health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Abou Sawan, S.; Nunes, E.A.; Lim, C.; McKendry, J.; Phillips, S.M. The health benefits of resistance exercise: Beyond hypertrophy and big weights. Exerc. Sport Mov. 2023, 1, e00001. [Google Scholar] [CrossRef]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong endurance exercise and its relation with coronary atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Currier, B.S.; Lowisz, C.V.; Phillips, S.M. The influence of resistance exercise training prescription variables on skeletal muscle mass, strength, and physical function in healthy adults: An umbrella review. J. Sport Health Sci. 2024, 13, 47–60. [Google Scholar] [CrossRef]
- Daanen, H.A.M.; Lamberts, R.P.; Kallen, V.L.; Jin, A.; Van Meeteren, N.L.U. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int. J. Sports Physiol. Perform. 2012, 7, 251–260. [Google Scholar] [CrossRef]
- Matabuena, M.; Vidal, J.C.; Hayes, P.R.; Huelin Trillo, F. A 6-minute sub-maximal run test to predict VO2 max. J. Sports Sci. 2018, 36, 2531–2536. [Google Scholar] [CrossRef]
- Borisovskaya, A.; Chmelik, E.; Karnik, A. Exercise and Chronic Pain. In Physical Exercise for Human Health; Springer: Singapore, 2020; pp. 233–253. [Google Scholar] [CrossRef]
- Peng, M.S.; Wang, R.; Wang, Y.Z.; Chen, C.C.; Wang, J.; Liu, X.C.; Song, G.; Guo, J.B.; Chen, P.J.; Wang, X.Q. Efficacy of therapeutic aquatic exercise vs physical therapy modalities for patients with chronic low back pain: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2142069. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Vafa, M. Dietary restriction, cardiovascular aging and age-related cardiovascular diseases: A review of the evidence. Rev. Biomark. Stud. Aging Anti-Aging Res. 2019, 1178, 113–127. [Google Scholar]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 408204. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.E.; Zegkos, T.; Efthimiadis, G.; Tsaklidis, G. Analysis of digitalized ECG signals based on artificial intelligence and spectral analysis methods specialized in ARVC. Int. J. Numer. Method. Biomed. Eng. 2022, 38, e3644. [Google Scholar] [CrossRef]
- Lollgen, H.; Leyk, D. Exercise Testing in Sports Medicine. Dtsch. Arztebl. Int. 2018, 115, 409–416. [Google Scholar] [CrossRef]
- Weakley, J.; Schoenfeld, B.J.; Ljungberg, J.; Halson, S.L.; Phillips, S.M. Physiological responses and adaptations to lower load resistance training: Implications for health and performance. Sports Med. Open 2023, 9, 28. [Google Scholar] [CrossRef]
- Lee, B.A.; Oh, D.J. The effects of long-term aerobic exercise on cardiac structure, stroke volume of the left ventricle, and cardiac output. J. Exerc. Rehabil. 2016, 12, 37. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Onofre, T.; Oliver, N.; Carlos, R.; Felismino, A.; Corte, R.C.; Silva, E.; Bruno, S.; Barbosa, T.M. Oxygen uptake efficiency slope as a useful measure of cardiorespiratory fitness in morbidly obese women. PLoS ONE 2017, 12, e0172894. [Google Scholar] [CrossRef]
- Olsson, K.; Salier Eriksson, J.; Rosdahl, H.; Schantz, P. Are heart rate methods based on ergometer cycling and level treadmill walking interchangeable? PLoS ONE 2020, 15, e0237388. [Google Scholar] [CrossRef]
- Torrents, C.; Balague, N. Dynamic Systems Theory and Sports Training. Educ. Phys. Train. Sport 2006, 60, 72–88. [Google Scholar] [CrossRef]
- Lambert, E.V.; St Clair Gibson, A.; Noakes, T.D. Complex systems model of fatigue: Integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br. J. Sports Med. 2005, 39, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Hammami, A.; Kasmi, S.; Farinatti, P.; Fgiri, T.; Chamari, K.; Bouhlel, E. Blood pressure, heart rate and perceived enjoyment after small-sided soccer games and repeated sprint in untrained healthy adolescents. Biol. Sport 2017, 34, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Javorka, M.; Zila, I.; Balharek, T.; Javorka, K. Heart rate recovery after exercise: Relations to heart rate variability and complexity. Braz. J. Med. Biol. Res. 2002, 35, 991–1000. [Google Scholar] [CrossRef]
- Giunta, S.; Xia, S.; Pelliccioni, G.; Olivieri, F. Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. Geroscience 2024, 46, 113–127. [Google Scholar] [CrossRef]
- Aponte-Becerra, L.; Novak, P. Tilt Test: A Review. J. Clin. Neurophysiol. 2021, 38, 279–286. [Google Scholar] [CrossRef]
- Raju, S.; Fredericks, R.; Lishman, P.; Neglén, P.; Morano, J. Observations on the calf venous pump mechanism: Determinants of postexercise pressure. J. Vasc. Surg. 1993, 17, 459–469. [Google Scholar] [CrossRef]
- Baev, V.M.; Kudryavtseva, E.N. Adaptation to physical load and the state of the autonomic nervous system in young women with low blood pressure. Patol. Fiziol. Eksp. Ter. 2015, 59, 97–100. [Google Scholar]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac changes associated with vascular aging. Clin. Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef]
- Hopenfeld, B. ST segment depression: The possible role of global repolarization dynamics. Biomed. Eng. Online 2007, 6, 6. [Google Scholar] [CrossRef][Green Version]
- Lilaonitkul, M.; Robinson, K.; Roberts, M. Wellens’ syndrome: Significance of ECG pattern recognition in the emergency department. Emerg. Med. J. 2009, 26, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Teo, S.-G.; Poh, K.-K. ST-segment changes with exercise stress. Singap. Med. J. 2016, 57, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Shao, G. A comparative study on coronary angiography and exercise ECG testing. Zhonghua Nei Ke Za Zhi 1996, 35, 107–109. [Google Scholar] [PubMed]
- Lanza, G.A.; Mustilli, M.; Sestito, A.; Infusino, F.; Sgueglia, G.A.; Crea, F. Diagnostic and prognostic value of ST segment depression limited to the recovery phase of exercise stress test. Heart 2004, 90, 1417–1421. [Google Scholar] [CrossRef]
- Campero Jurado, I.; Fedjajevs, A.; Vanschoren, J.; Brombacher, A. Interpretable assessment of ST-segment deviation in ECG time series. Sensors 2022, 22, 4919. [Google Scholar] [CrossRef]
- Hnatkova, K.; Johannesen, L.; Vicente, J.; Malik, M. Heart rate dependency of JT interval sections. J. Electrocardiol. 2017, 50, 814–824. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Sarelius, I.; Pohl, U. Control of muscle blood flow during exercise: Local factors and integrative mechanisms. Acta Physiol. 2010, 199, 349–365. [Google Scholar] [CrossRef]
- Pfeifer, M.A.; Weinberg, C.R.; Cook, D.; Best, J.D.; Reenan, A.; Halter, J.B. Differential changes of autonomic nervous system function with age in man. Am. J. Med. 1983, 75, 249–258. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).