Fs-Laser-Induced Micro- and Nanostructures on Polycarbonate and Cellulose Acetate Butyrate for Cell Alignment
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
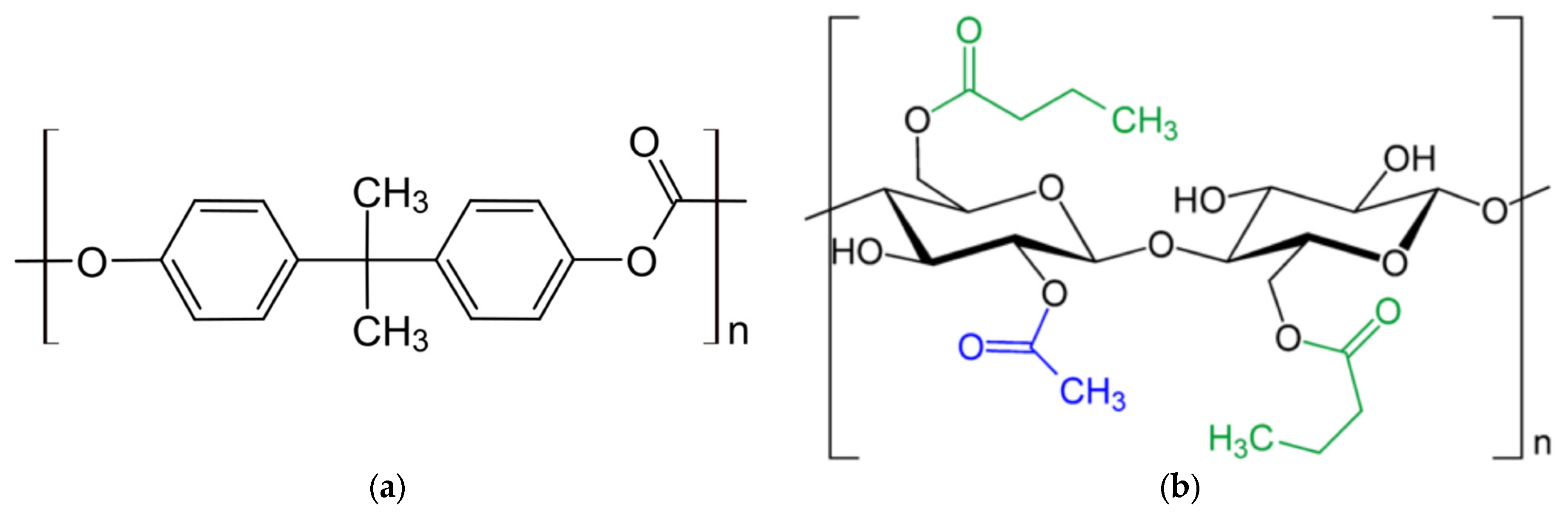
2.1. Polymer Samples
2.2. Laser-Structuring
2.3. Cell Cultivation
2.4. Preparation for Electron Microscope Investigation
3. Results
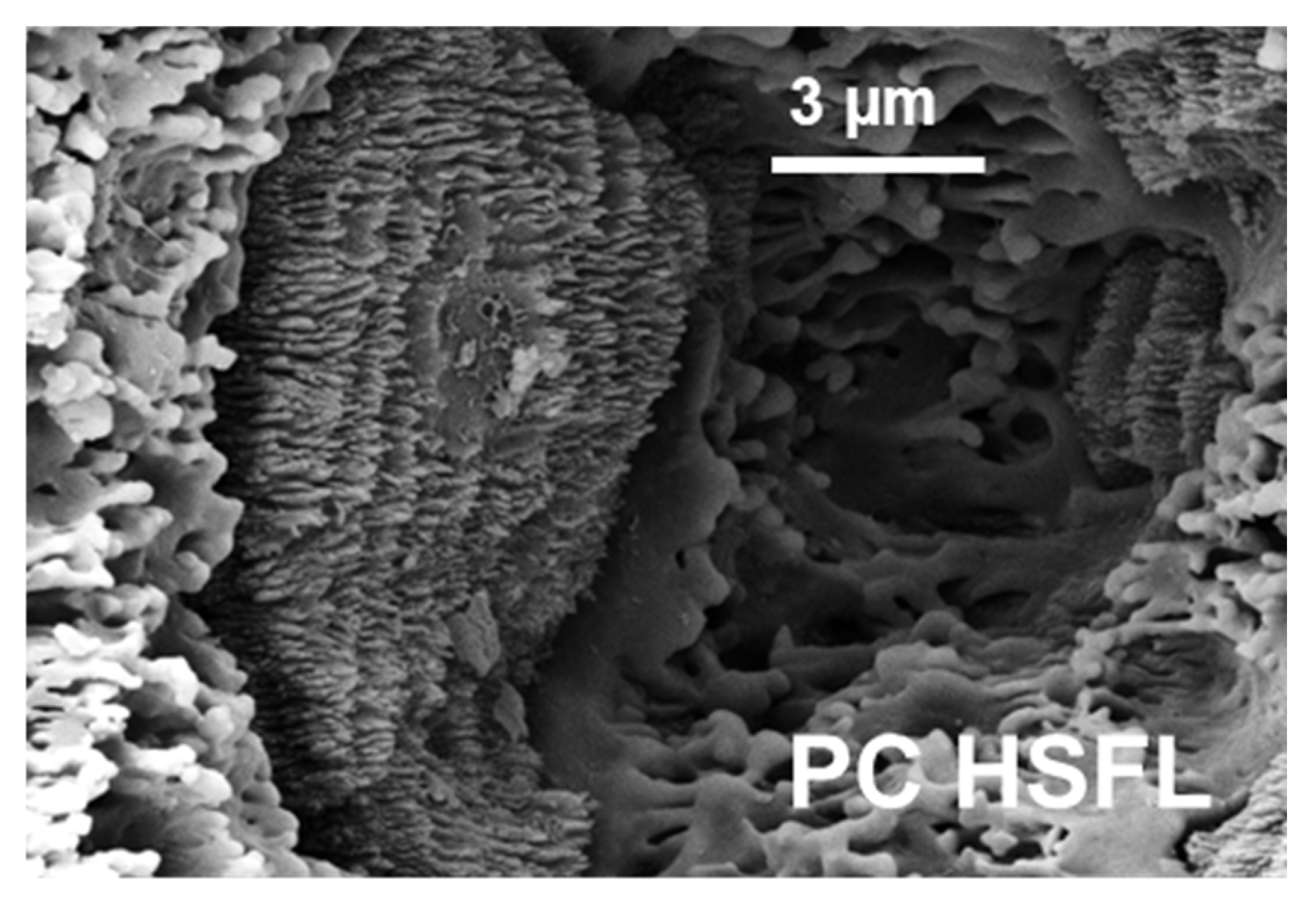
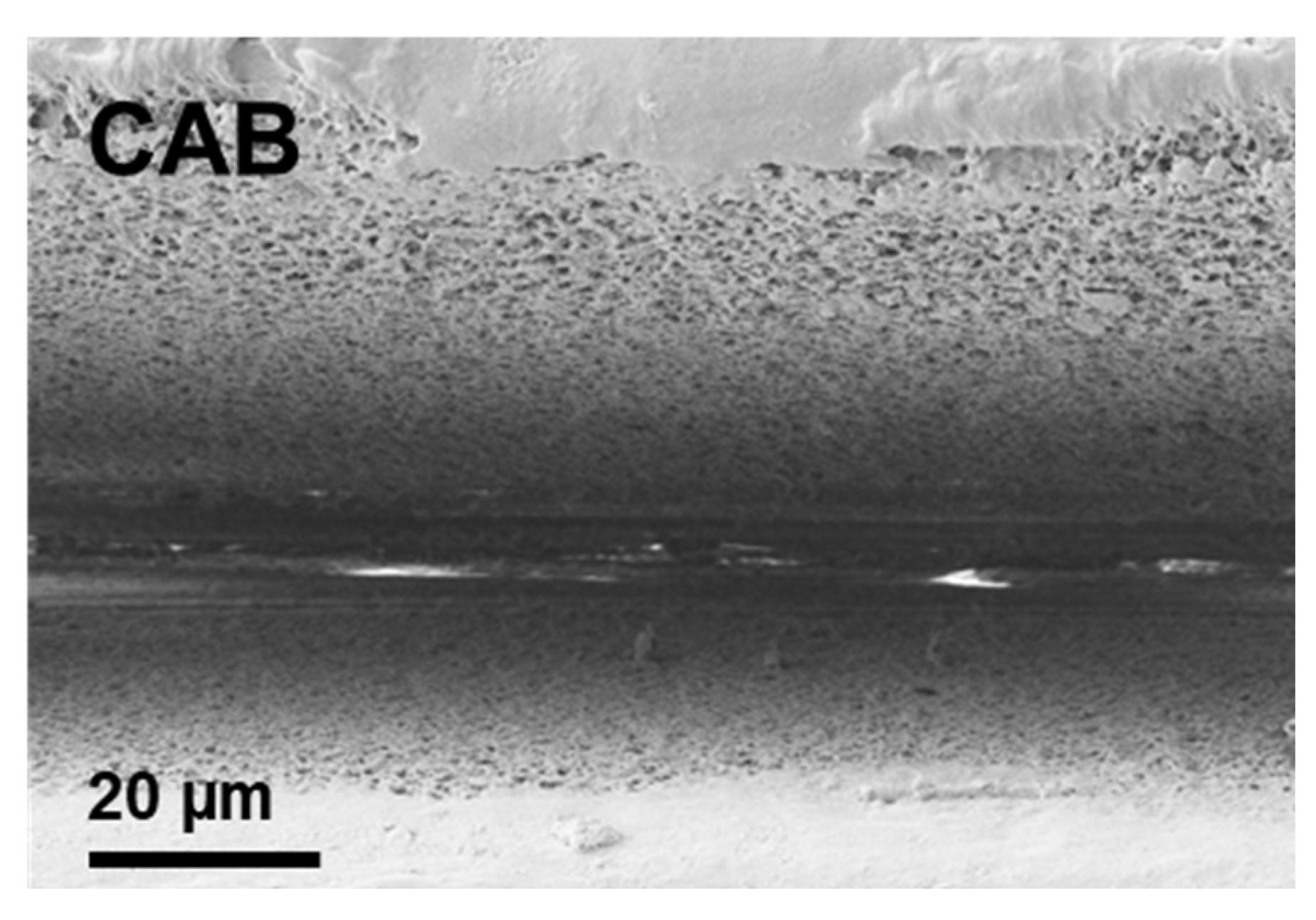
3.1. Single-Pulse-Burst Craters
3.2. Single- and Multiple-Overscan Lines
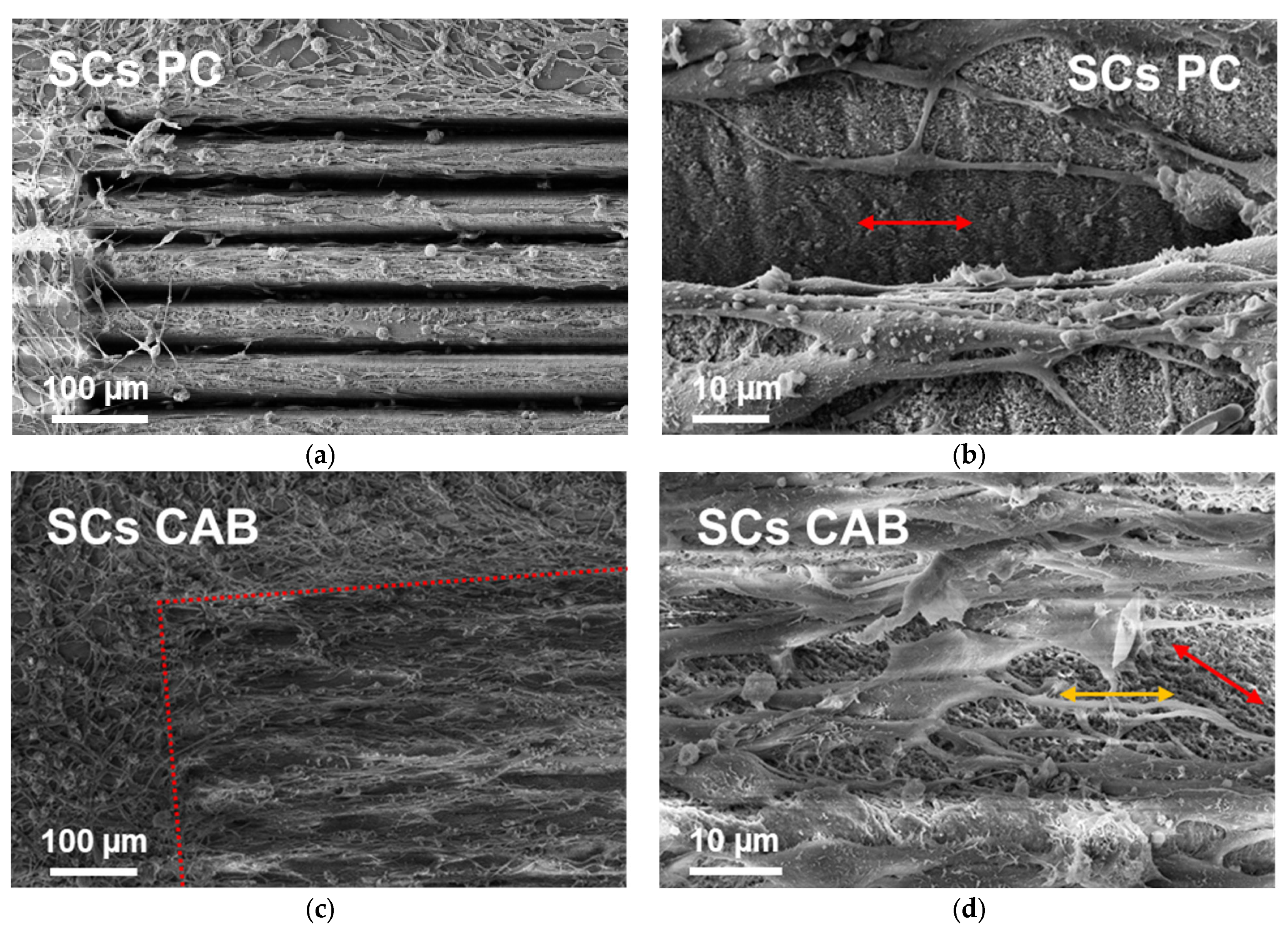
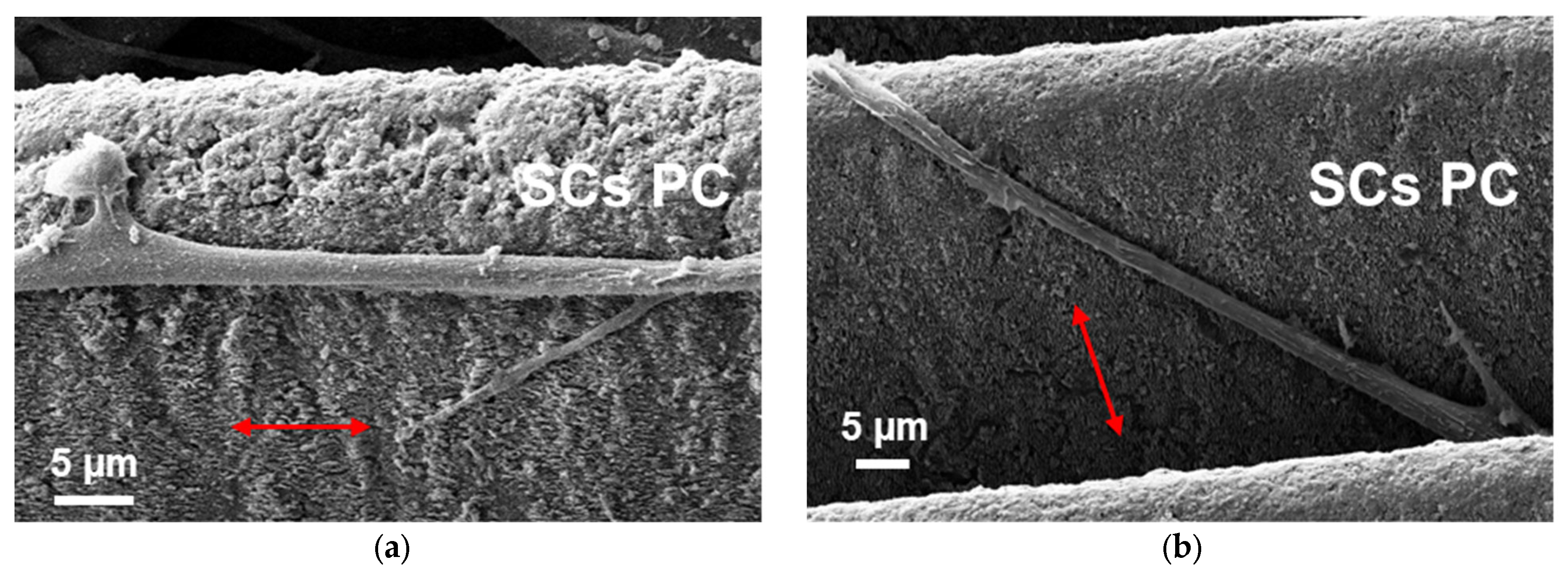
3.3. Schwann Cells Growing on Laser-Written Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflection |
| CAB | Cellulose acetate butyrate |
| CAGR | Compound annual growth rate |
| CDs | Compact disks |
| ECM | Extracellular matrix |
| FTIR | Fourier transform infrared |
| fs | Femtosecond |
| HMDS | Hexamethyldisilazane |
| HSF | High-spatial-frequency |
| HSFL | High-spatial-frequency LIPSS |
| KrF* | Krypton fluoride excimer |
| LIPSS | Laser-induced periodic surface structure |
| LSF | Low-special-frequency |
| LSFL | Low-special-frequency LIPSS |
| NIR | Near-infrared |
| ns | Nanosecond |
| PBS | Phosphate-buffered saline |
| PC | Polycarbonate |
| PET | Polyethylene terephthalate |
| ps | Picosecond |
| SCs | Schwann cells |
| SEM | Scanning electron microscopy |
| USD | United States Dollar |
| UV | Ultraviolet |
References
- Lifka, S.; Plamadeala, C.; Weth, A.; Heitz, J.; Baumgartner, W. Oriented artificial nanofibers and laser induced periodic surface structures as substrates for Schwann cells alignment [version 3; peer review: 2 approved, 1 approved with reservations]. Open Res. Eur. 2024, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Manganas, P.; Kavatzikidou, P.; Kordas, A.; Babaliari, E.; Stratakis, E.; Ranella, A. The role of mechanobiology on the Schwann cell response: A tissue engineering perspective. Front. Cell. Neurosci. 2022, 16, 948454. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The success and failure of the Schwann cell response to nerve injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Peripheral Nerve Injury Market Size & Trends. Available online: https://www.grandviewresearch.com/industry-analysis/peripheral-nerve-injuries-market-report (accessed on 22 May 2025).
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021, 383, 617–644. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef]
- Millesi, F.; Weiss, T.; Mann, A.; Haertinger, M.; Semmler, L.; Supper, P.; Pils, D.; Naghilou, A.; Radtke, C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021, 35, e21196. [Google Scholar] [CrossRef]
- Stadlmayr, S.; Peter, K.; Millesi, F.; Rad, A.; Wolf, S.; Mero, S.; Zehl, M.; Mentler, A.; Gusenbauer, C.; Konnerth, J.; et al. Comparative Analysis of Various Spider Silks in Regard to Nerve Regeneration: Material Properties and Schwann Cell Response. Adv. Healthc. Mater. 2024, 13, e2302968. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Peter, K.; Stadlmayr, S.; Naghilou, A.; Ploszczanski, L.; Hofmann, M.; Riekel, C.; Liu, J.; Burghammer, M.; Gusenbauer, C.; Konnerth, J.; et al. Exploring the Unique Properties and Superior Schwann Cell Guiding Abilities of Spider Egg Sac Silk. ACS Appl. Bio Mater. 2025, 8, 1307–1319. [Google Scholar] [CrossRef]
- Huang, C.; Ouyang, Y.; Niu, H.; He, N.; Ke, Q.; Jin, X.; Li, D.; Fang, J.; Liu, W.; Fan, C.; et al. Nerve guidance conduits from aligned nanofibers: Improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl. Mater. Interfaces 2015, 7, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Bonse, J.; Höhm, S.; Kirner, S.V.; Rosenfeld, A.; Krüger, J. Laser-Induced Periodic Surface Structures—A Scientific Evergreen. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 9000615. [Google Scholar] [CrossRef]
- Fosodeder, P.; Baumgartner, W.; Steinwender, C.; Hassel, A.W.; Florian, C.; Bonse, J.; Heitz, J. Repellent rings at titanium cylinders against overgrowth by fibroblasts. Adv. Opt. Technol. 2020, 9, 113–120. [Google Scholar] [CrossRef]
- Maalouf, M.; Abou Khalil, A.; Di Maio, Y.; Papa, S.; Sedao, X.; Dalix, E.; Peyroche, S.; Guignandon, A.; Dumas, V. Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation. Nanomaterials 2022, 12, 1619. [Google Scholar] [CrossRef]
- Muck, M.; Wolfsjäger, B.; Seibert, K.; Maier, C.; Lone, S.A.; Hassel, A.W.; Baumgartner, W.; Heitz, J. Femtosecond Laser-Processing of Pre-Anodized Ti-Based Bone Implants for Cell-Repellent Functionalization. Nanomaterials 2021, 11, 1342. [Google Scholar] [CrossRef]
- Yiannakou, C.; Simitzi, C.; Manousaki, A.; Fotakis, C.; Ranella, A.; Stratakis, E. Cell patterning via laser micro/nano structured silicon surfaces. Biofabrication 2017, 9, 025024. [Google Scholar] [CrossRef]
- Rebollar, E.; Frischauf, I.; Olbrich, M.; Peterbauer, T.; Hering, S.; Preiner, J.; Hinterdorfer, P.; Romanin, C.; Heitz, J. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008, 29, 1796–1806. [Google Scholar] [CrossRef]
- Mezera, M.; Bonse, J.; Römer, G.R.B.E. Influence of Bulk Temperature on Laser-Induced Periodic Surface Structures on Polycarbonate. Polymers 2019, 11, 1947. [Google Scholar] [CrossRef]
- Stratakis, E.; Vlahou, M.; Protopapa, N.; Maragkaki, S.; Tsibidis, G.D. Fabrication of Highly Uniform Laser-Induced Periodic Structures on Polycarbonate Via UV Femtosecond Pulses. Opt. Laser Technol. 2025, 189, 113121. [Google Scholar] [CrossRef]
- Parshin, A.; Gunyakov, V.; Zyryanov, V.; Shabanov, V. Domain Structures in Nematic Liquid Crystals on a Polycarbonate Surface. Int. J. Mol. Sci. 2013, 14, 16303–16320. [Google Scholar] [CrossRef]
- Technisches Datenblatt. Available online: https://www.sigmaaldrich.com/AT/de/specification-sheet/ALDRICH/419044 (accessed on 22 May 2025).
- Polycarbonate. Available online: https://de.wikipedia.org/wiki/Polycarbonate (accessed on 3 April 2025).
- Celluloseacetatbutyrat. Available online: https://de.wikipedia.org/wiki/Celluloseacetatbutyrat (accessed on 3 April 2025).
- Garcia-Lechuga, M.; Grojo, D. Simple and robust method for determination of laser fluence thresholds for material modifications: An extension of Liu’s approach to imperfect beams [version 2; peer review: 2 approved, 1 approved with reservations]. Open Res. Eur. 2021, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M. Simple technique for measurements of pulsed Gaussian-beam spot sizes. Opt. Lett. 1982, 7, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Diepens, M.; Gijsman, P. Photodegradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2007, 92, 397–406. [Google Scholar] [CrossRef]
- Rivaton, A.; Sallet, D.; Lemaire, J. The photochemistry of bisphenol-A polycarbonate reconsidered. Polym. Photochem. 1983, 3, 463–481. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Golizadeh, M.; Karimi, A.; Gandomi-Ravandi, S.; Vossoughi, M.; Khafaji, M.; Joghataei, M.T.; Faghihi, F. Evaluation of cellular attachment and proliferation on different surface charged functional cellulose electrospun nanofibers. Carbohydr. Polym. 2019, 207, 796–805. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, L.; Baumgartner, W.; Weth, A.; Lifka, S.; Heitz, J. Fs-Laser-Induced Micro- and Nanostructures on Polycarbonate and Cellulose Acetate Butyrate for Cell Alignment. Appl. Sci. 2025, 15, 6754. https://doi.org/10.3390/app15126754
Wagner L, Baumgartner W, Weth A, Lifka S, Heitz J. Fs-Laser-Induced Micro- and Nanostructures on Polycarbonate and Cellulose Acetate Butyrate for Cell Alignment. Applied Sciences. 2025; 15(12):6754. https://doi.org/10.3390/app15126754
Chicago/Turabian StyleWagner, Lukas, Werner Baumgartner, Agnes Weth, Sebastian Lifka, and Johannes Heitz. 2025. "Fs-Laser-Induced Micro- and Nanostructures on Polycarbonate and Cellulose Acetate Butyrate for Cell Alignment" Applied Sciences 15, no. 12: 6754. https://doi.org/10.3390/app15126754
APA StyleWagner, L., Baumgartner, W., Weth, A., Lifka, S., & Heitz, J. (2025). Fs-Laser-Induced Micro- and Nanostructures on Polycarbonate and Cellulose Acetate Butyrate for Cell Alignment. Applied Sciences, 15(12), 6754. https://doi.org/10.3390/app15126754






