Abstract
In light of the increasing demand for laurel, driven by renewed interest in natural products and traditional medicinal usage of this plant, our study aimed to investigate the in vitro antimicrobial activity of essential oils from leaves and fruits of laurel (EOL and EOF, respectively) collected in the National Park Skadar Lake, Montenegro, as it related to their chemical composition, assessing the possibility of their usage in cosmetics and pharmaceuticals. Also, fatty oil from the remaining laurel fruit after EOF isolation was investigated as a possible source of bioactive compounds. The most abundant components in EOL and EOF were 1,8-cineol (35.1% and 33.3%, respectively) and α-terpinyl acetate (10.4% and 7.0%, respectively). Linalool (7.6%) was found in EOL, while α- pinene (5.8%) and β-elemene (5.7%) were present in significant amounts in EOF. Antibacterial and antifungal properties of EOL and EOF showed strong antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, and Bacillus subtilis, and potent antifungal effects against Candida albicans, opening the door for their application as antimicrobial agents. Chemical analysis of fatty oil unexpectedly revealed prominent content of sesquiterpene lactone dehydrocostunolide and phenylpropanoid derivative (E)-2-hexyl-cinnamaldehyde (21% and 5%, respectively), suggesting further investigations of this waste material as the source of valuable compounds with proven health benefits.
1. Introduction
Recently, essential oils (EOs) have been the subject of many studies due to their prominent antioxidant and antimicrobial properties. At the same time, the end of the 20th century has been characterized by a dramatic spread of pan-resistant pathogenic bacteria, resistant to all existing antibiotics. The World Health Organization (WHO) declared the spread of pan-resistant bacterial strains a “pan-resistance pandemic”, and so far there has been no specific solution to this phenomenon. Microbial resistance to classical antibiotics has raised serious concerns in treating infectious diseases. Hence, the development of rapid antibiotic resistance has directed researchers to focus on new antimicrobial agents originating from plants, which are both potent and not prone to developing resistance. Recent studies have revealed that phytochemicals exert potent antibacterial activities against sensitive and resistant pathogens via different mechanisms of action, with lower toxicity and fewer side effects. Adding to this the growing consumer demands for natural compounds as an alternative to synthetic substances, the new field of natural antimicrobial agent investigation was established [1,2,3,4]. Investigating non-antibiotic, plant-derived secondary metabolites with potential antibacterial properties has created opportunities for innovative therapeutic approaches [5]. Functional properties such as antimicrobial activity (antibacterial, antifungal, and insecticidal) of EOs are the basis of their possible application in processed food preservation, pharmaceuticals, cosmetics, alternative medicine, and natural therapies [6]. Replacing synthetic substances with natural ones, as well as adding natural substances beneficial for human health in products intended to prevent or alleviate existing health problems, is today one of the most current trends in the mentioned industries, both in the world and in our region.
Laurus nobilis L., Lauraceae (laurel), is a flowering, evergreen tree or large bush, often used as an aromatic, spicy, and medicinal plant. It is native to the southern Mediterranean region of Europe, especially Greece, Italy, Spain, France, Croatia, and Bulgaria. In European traditional medicine, laurel was used to treat earaches, sprains, and rheumatism, to promote perspiration, and for gastrointestinal disorders, such as flatulence, epigastric bloating, and impaired digestion, and as an antiseptic, insecticide, and stomachic [7]. In addition, it may be used to treat diabetes and prevent migraines [8]. These biological properties are mainly attributed to its phenolic compounds [9]. EO isolated from laurel leaves exhibits numerous pharmacological effects, including antibacterial [10], antifungal [11], insecticidal and repellent [12], larvicidal [13], antiproliferative [14], antioxidant [11,15], and anti-inflammatory [16]. Laurel leaves EO has been used in treating rheumatic pain, strained muscles, and digestive disorders [17]. In addition, this EO has been widely applied in cosmetics (creams, perfumes, soaps), in food and agriculture industries (as a preservative, spice, flavouring agent, and as a natural pesticide in postharvest crop protection). Recent studies have revealed its antitumor activity [18].
The fruits of L. nobilis are reported to have antiulcer, anticholinergic, anticonvulsant, antimicrobial, and antidiabetic effects. A fatty oil from the fruit is used externally to treat sprains and bruises and is sometimes used as ear drops to relieve pain [19]. Besides the above-mentioned applications, laurel has been reported as a potential source of biodiesel via compressed methanol transesterification [20].
Accordingly, the market demand for laurel EO has increased remarkably, considering that the global market size in 2023 was USD 56 million and is projected to touch USD 79.88 million by the end of 2032, exhibiting a compound annual growth rate of 3.9% during the forecast period. The growth in the market can be attributed to the renewed demand for natural and organic products, rising awareness about the benefits of laurel EO, and the growing use of laurel EO in various applications such as medical, personal care, home cleaning, and others (including the food and beverage and agriculture industries).
In a recent paper [21], the authors highlighted the culinary usage of laurel leaves and fruits originating from Herceg Novi, Montenegro, pointing out differences in their essential oils’ chemical composition and antioxidant properties. Only one more work [22] presented an investigation of laurel wild species EO originating from Montenegro, Budva, stressing the chemical differences between essential oils obtained from different plant parts—leaves, stems, and shoots. Simić et al. [23] ascertained the chemical composition of laurel leaves essential oil and its antifungal properties, the chosen fungi belonging to soilborne pathogens, the food storage spoilage fungi, and mycotoxin producers, while the origin of the essential oil remained unspecified.
In light of the increasing demand for laurel EO, our study aimed to investigate the in vitro antimicrobial (antibacterial and antifungal) activity of the EOs isolated from leaves and fruits of laurel from Montenegro, as it related to their chemical composition, assessing the possibility of their use in cosmetic, pharmaceutical, and food industries as potential antimicrobial agents. In addition, after hydrodistillation of the EO, fatty oil from laurel fruit was investigated as a waste material representing a possible source of bioactive compounds prospectively applicable in the stated industries. To the best of our knowledge, no data exist on the chemical composition and antimicrobial activity of laurel leaves and fruit EOs from Montenegro.
2. Materials and Methods
2.1. Plant Material and Chemicals
Aerial parts of laurel leaves and fruits were collected in 2023 from the natural habitat at Kosmač island, one of the hidden treasures of Skadar Lake, Montenegro, at the following coordinates: latitude: 42°36′24.99″; longitude: 20°26′04″. The plant materials were identified and a voucher sample was deposited in the collection of the Institute for Medicinal Plant Research “Dr. Josif Pančić”. The leaves were collected at the beginning of July and the fruits at the end of October of the same year.
Petroleum ether (boiling range 40–60 °C, density at 15 °C 0.647–0.654 g/cm3, purity > 99.5%) was of analytical grade and purchased from Carlo Erba Reagents, Italy.
2.2. Microorganisms
The antimicrobial activity of tested EOs was evaluated against different standard strains of microorganisms: (1) three Gram (+) bacteria (Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, and Bacillus subtilis ATCC 6633), (2) four Gram (−) bacteria (Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Salmonella Abony NCTC 6017, and Pseudomonas aeruginosa ATCC 27853); and one strain of the yeast Candida albicans ATCC 10231. The selected bacteria used in this study are common causes of various human infections (skin, urinary, and digestive tract infections). The minimal inhibitory concentrations (MICs) of EOs were determined by the broth microdilution method (CLSI M07, 2018). In our study, we used Müller–Hinton Agar (MHA, Torlak, Serbia) to cultivate and maintain bacteria and Sabouraud dextrose agar (SDA, Torlak, Serbia) for C. albicans.
2.3. Chemical Analysis of Plant Material
The total phenolic (TP) content was evaluated using the Folin–Ciocalteu reagent, based on a slightly altered version of a previously established method [24]. Absorbance was measured at 725 nm, with gallic acid (GA) as the standard (0.01–0.1 mg/mL, calibration curve equation: y = 1.281x + 0.02).
The percentage of total tannins (TTs) content was determined using the method described in the European Pharmacopoeia 11.0 [25]. Absorbance was recorded using a UV–VIS Spectrophotometer HP 8453 (Agilent Technologies, Santa Clara, CA, USA) at a maximum wavelength of 760 nm.
The total flavonoid (TF) content was assessed using the procedure outlined in [25].
The percentage content of procyanidins was assessed using the method from [25]. In short, the laurel fruit was hydrolysed under reflux using a mixture of ethyl alcohol and HCl. Separation of procyanidins from the aqueous solution was performed using butyl alcohol. The absorbance was measured at a λmax of 545 nm. The content of procyanidins, expressed as cyanidin chloride, was calculated using the following expression:
where A was the absorbance at 545 nm, and m was the mass of the extracts to be examined in grams.
A × 500/(75 × m) [%],
2.4. Isolation of the Essential Oil
The dry leaves and fruit were cut into small pieces and separately subjected to hydrodistillation using a Clevenger-type apparatus (Medilab, Ambala Cantt, Haryana, India), each for 3 h [26]. The EOs (EOL and EOF) were extracted with diethyl ether and dried over anhydrous sodium sulfate. The EOs content was presented as the mean value ± SD of three experiments. The fatty oil was obtained from the laurel fruit by a continuous solvent extraction method with an organic solvent (petroleum ether) in a Soxhlet apparatus after the EO was removed by hydrodistillation, and the remaining plant material was dried at room temperature and protected from the light, while the organic solvent was evaporated on a rotary vacuum evaporator. The extraction yield was determined gravimetrically, expressed as a mean value ± SD of three experiments.
2.5. Chemical Analysis of Essential Oils
2.5.1. Gas Chromatography—GC
Gas chromatography analysis of the essential oils was carried out on an HP-5890 Series II GC apparatus [Hewlett-Packard, Waldbronn (Germany)], equipped with a split–splitless injector and automatic liquid sampler and fitted to a flame ionization detector (FID). The HP-5 column (25 m × 0.32 mm, 0.52 μm film thickness) was used, the flow rate of the carrier gas (H2) was 1 mL/min, with the injector temperature set at 250 °C, while the column temperature was linearly programmed from 40 °C to 260 °C (at a rate of 4 °C/min). The samples were dissolved in ethyl alcohol for essential oils. Area percent reports, obtained by standard processing of chromatograms, were used as a base for the quantification analysis.
2.5.2. Gas Chromatography–Mass Spectrometry (GC–MS)
The same analytical conditions as those mentioned for GC–FID were employed for GC–MS analysis, along with column HP-5MS (30 m × 0.25 mm, 0.25 μm film thickness), using HP G 1800C Series II GCD system [Hewlett-Packard, Palo Alto, CA, USA]. Helium was used as a carrier gas. The transfer line was heated to 260 °C. Mass spectra were acquired in EI mode (70 eV), in the m/z range of 40–450. The components of the samples were identified by comparison of their spectra to those from the Wiley 275 and NIST/NBS libraries, using different search engines. Calibration was performed using a linear n-paraffin mixture (C6–C40) as a standard. The experimental values for retention indices were determined using the calibrated Automated Mass Spectral Deconvolution and Identification System Software (AMDIS version 2.1), compared to those from the available literature, and used as an additional tool to confirm the MS findings.
Extracted fatty oil was analysed using a Shimadzu GC-MSQP2010 ultra mass spectrometer (Shimadzu Corporation, Kyoto, Japan) fitted with a flame ionic detector and coupled with a GC-2010 gas chromatograph, as previously described in Tadic et al., 2021, with a slight modification [27]. The content of compounds was calculated based on the normalized peak area. The components of the oil were identified by comparison of their spectra to those from the Wiley 275 and NIST/NBS libraries. The experimental values for retention indices were determined by the use of calibrated Automated Mass Spectral Deconvolution and Identification System Software (AMDIS version 2.1), compared to those from available literature [28] and used as an additional tool to approve MS findings.
2.6. Preparation of Essential Oils for Antimicrobial Experiments
The essential oils were dissolved in 1% dimethylsulfoxide (DMSO, Sigma-Aldrich Company, Burlington, MA, USA) and diluted to the desired concentrations ranging from 31.2 to 1000 μg mL−1 with MHB for bacterial strains or Sabouraud dextrose broth (SDB) (HiMedia Laboratories Pvt. Ltd., Mumbai, India) for C. albicans.
2.7. Antibacterial Activity
MIC values of essential oils (EOL and EOF) were determined by the broth microdilution method according to Clinical and Laboratory Standards Institute guidelines (CLSI M07, 2018). Fresh overnight cultures of each bacterial strain were prepared in Müller–Hinton broth (MHB, Torlak, Belgrade, Serbia). Tests were performed in standard sterile microtiter plates. The final concentration of microorganisms in each well was adjusted to 2 × 106 CFU/mL, using a DEN-1 McFarland densitometer (Biosan, Riga, Lithuania). Two positive growth controls were included in the test. The results were interpreted after incubation of the plates in aerobic conditions for 24 h at 35 °C. All MIC determinations were performed in duplicate, and each test was repeated three times. The mean MIC values are presented.
2.8. Antifungal Activity
Antifungal activity was evaluated against a standard strain of the yeast Candida albicans ATCC 10231. Determination of MIC was performed by using a fresh overnight culture of Candida albicans in Sabouraud dextrose broth (SDB). The final concentration of yeast suspension in each well of microtiter plates was adjusted to 1 × 107 CFU/mL. The essential oils were dissolved as previously explained for antibacterial activity and diluted to the desired concentrations with SDB. The results were interpreted after incubation of the plates in aerobic conditions for 48 h at 35 °C. All tests were performed in duplicate and repeated three times. The two positive growth controls of C. albicans were included in each test. The lowest concentration of EO that inhibited the visible growth of microorganisms was determined to be MIC. In the results, the mean MIC values are presented.
2.9. Statistical Analysis
IBM SPSS Statistics 23.0 Desktop for Windows (IBM Corporation, Armonik, NY, USA) was used for statistical analysis. The results of the MIC value were determined by logit analysis.
3. Results
This research aimed to analyse the chemical composition and antibacterial and antifungal activity of the EO of wild laurel from leaves and fruits (EOL and EOF, respectively) of plants grown on Kosmač Island of Lake Skadar, part of the Montenegrin National Park. In addition, the fruit fatty oil was obtained from the fruit waste, and the extraction with petroleum ether was applied using the Soxhlet apparatus through continuous extraction. The uniqueness of this approach was that fruit that remained after the isolation of EO was dried and afterward used for fatty oil extraction.
3.1. Chemical Analysis
In our study, the EO yield was 1.67 ± 0.05% in the fruits and 1.11 ± 0.05% in the leaves. Fatty oil yield was 6.02 ± 0.07%. The laurel EOL was a light yellow, while EOF was slightly darker, both with a specific odour.
TP content was 9.75 ± 0.01 mg GA/g in the analysed leaves sample, while the content of TF and TT were 0.43 ± 0.20% and 1.51 ± 0.05%, respectively. The investigated fruit contained 7.78 ± 0.15 mg GAE/g TP, TT was 0.03 ± 0.010%, while TF and total procyanidins were present with percentages of 0.02 ± 0.00% and 0.04 ± 0.00%, respectively.
The data regarding the chemical composition of EOL and EOF from laurel leaves and fruits, respectively, with the fatty oil obtained from fruits, the Kovats retention indices (KIs), and the relative percentages of the identified compounds are given in Table 1 and Figure 1.

Table 1.
Comparative chemical profile of the analysed L. nobilis essential oil from leaves and fruit (EOL and EOF, respectively) and fatty oil obtained from fruit waste after essential oil extraction.
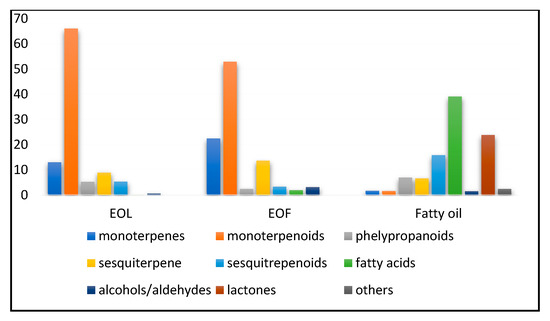
Figure 1.
The main groups of identified components in the investigated samples of EOL, EOF, and fatty oil (obtained from laurel fruits after EO extraction).
Based on the GC–MS analysis a total of 63 components were identified in the EOL, representing 99.1% of all present components. Oxygenated monoterpenes were found to have the highest contribution to the EOL composition (Figure 1, 66.0%). In specific, 1,8-cineole (eucalyptol, Table 1) was the main oxygenated monoterpene detected, accounting for 35.1% of the total EOL content. Aside from 1,8-cineole, the main constituents in EOL were (Table 1) α-terpinyl acetate (10.4%), linalool (7.6%), trans-sabinene hydrate (4.2%), and α-terpineol (2.3%). Within monoterpene hydrocarbons (13.0%), the largest percentages were ascribed to sabinene (6.5%), α-pinene (2.7%), and β-pinene (1.9%). Phenylpropanoids were presented by methyl eugenol (3.1%) and eugenol (2.0%). In the sesquiterpene group (8.9%), γ-(E)-bisabolene (2.3%), trans-(E)-caryophyllene (1.4%), and β-elemene (1.1%) were the most prominent compounds. Oxygenated sesquiterpenes were present in a lesser percentage (5.2%), of which spathulenol and α-cadinol (0.9%) appeared at an equal rate.
The chemical analysis of the EOF composition revealed the presence of 106 components, representing 99.6% of the total content. The main constituents in the EOF were oxygenated monoterpenes (Figure 1, 52.9%), with 1,8-cineole (33.3%), α-terpinyl acetate (7.0%), and α-terpineol (3.9%) as the main compounds. The second largest group was represented by monoterpene hydrocarbons (22.4%), with α-pinene (5.8%), sabinene (5.3%), and β-pinene (4.0%) being most abundant. Within sesquiterpenes (13.6%), β-elemene (5.7%) was predominant.
In addition to the leaf and fruit EOs, fatty oil obtained from the fruit material after hydrodistillation and EO separation was investigated (Figure 1). Results showed that 88 constituents representing 99.2% of the total content were identified in the fatty oil. The chemical composition revealed that the main constituents in the fatty oil were sesquiterpene lactone dehydrocostuslactone (21.7%), saturated fatty acids lauric acid (13.8%), and palmitic acid (8%), and nonsaturated fatty acids with short-chain, monounsaturated ω-9 fatty acid—oleic acid (5.4%), and short-chain polyunsaturated fatty acids (SC-PUFA), ω-6 linoleic acid (3.1%). In addition, the phenylpropanoid derivative of cinnamic acid, (E)-2-hexyl-cinnamaldehyde was detected (5.0%).
3.2. Antibacterial Activity
The results of the antibacterial activity of EOL and EOF are presented in Table 2 and Figure 2. The strong activity of both EOL and EOF was observed against S. aureus, E. faecalis, B. subtilis, while only EOF exerted strong activity against S. abony. The rest of the investigated bacterial strains exhibited moderate sensitivity against both tested EOs.

Table 2.
Antibacterial activity of laurel (Laurus nobilis L.) leaves (EOL) and fruits (EOF) essential oil.
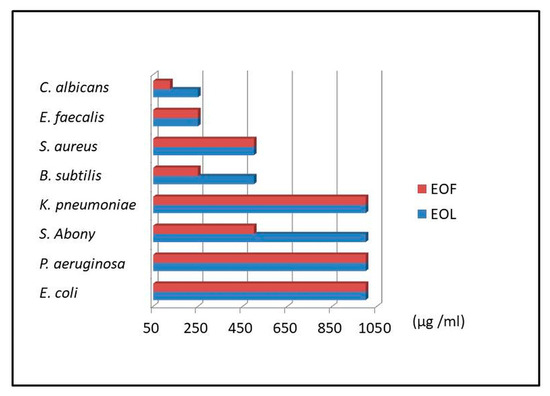
Figure 2.
Antimicrobial activity of laurel leaves and fruits essential oil, EOL and EOF, respectively (MIC values were given in μg/mL).
3.3. Antifungal Activity
Both essential oils, EOL and EOF, showed strong antifungal activity against C. albicans, with MIC values of 250 µg/mL and 125 µg/mL, respectively. Although interesting, the presented results of the antifungal activity were achieved by testing a single standard strain of C. albicans. Future studies should include other fungal species as well as clinical isolates.
4. Discussion
4.1. Chemical Analysis
The results of the qualitative assessment of EOL were similar to those found in several published papers dealing with laurel leaves EO, pointing out abundance in 1,8-cineole, followed by α-terpinyl acetate, sabinene, and linalool [7,11,13,15,30]. Besides the investigations of EOs obtained using the classical hydrodistillation method, recently Zeković et al. obtained laurel leaves isolates by supercritical fluid extraction (SFE) at different pressures/temperatures of 100 bar/40 °C and 250 bar/40 °C, revealing that the main component was also 1,8-cineole. However, when a higher temperature was employed (the condition of SFE set at 100 bar/60 °C), α−terpineol acetate was a dominant constituent [8].
EO from laurel fruit, EOF, exhibited a similar chemical composition pattern compared to EOL, with a slight difference in the relative percentage of 1,8-cineol, being more abundant in EOL (35.1% and 33.3% in EOL and EOF, respectively), but with α- and β-pinenes and fatty acids detected in a higher percentage (Table 1, Figure 3). The obtained results were in line with the published data, with differences that might be ascribed to the different geographical origins of the investigated samples used in our study [31]. In addition, in EOF phenylpropanoid (E)-2-hexyl-cinnamaldehyde was detected (1.7%), but methyl eugenol, present in EOL, was not detected in EOF (Table 1). Data from the literature revealed that the main compounds of the fruit EOs in previous studies were 1,8-cineole (8.10–48.0%), α-terpinyl acetate (3.67–10.4%), sabinene (4.49–11.4%), α-phellandrene, eugenol, methyl eugenol, α-pinene, β-ocimene, and β-pinene (3.91–12.8%) [32].
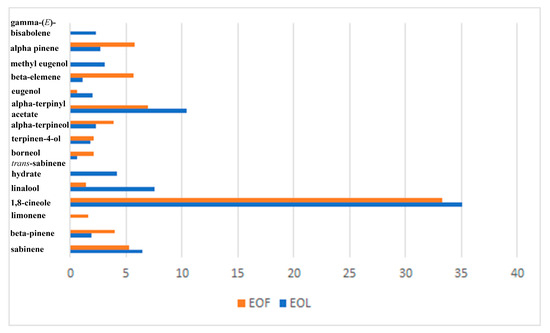
Figure 3.
The comparative presentation of the main constituents present in the investigated essential oils, EOL and EOF.
The differences between the Montenegro laurel EOs composition in this study and those from other countries reported in the literature were probably due to the different plant genotypes and climatic and ecological conditions in the respective locality of the National Park where the plants were grown but also to the plant parts processed and extracted, including methods of harvesting, processing, drying, and optimization of technological processes for obtaining EOs [13].
Abu-Dahab et al. performed an investigation regarding the chemical composition and the possible anti-proliferative activity of leaves and fruits EOs [14]. Similarly, Fidan et al. dealt with both the chemical profile and antimicrobial activity of EOs from laurel leaves and fruits originating from Bulgaria, emphasizing the influence of various factors, e.g., growth environment, harvest season, plant parts used, and extraction method on the yield and chemical composition [15].
Compared to other already performed studies, the novelty of our research was in the investigation of fatty oil obtained from the fruit after EO was extracted by hydrodistillation as a waste material, representing a possible source of bioactive compounds. Obtained fatty oil contained high amounts of saturated fatty acids, namely lauric and palmitic acids, which was in accordance with the already published data. The contents of oleic acid and linoleic acid (Table 1) were lower in comparison to results presented in previous works [31,33,34,35], while the most abundant component detected in our fatty oil was sesquiterpene lactone dehydrocostuslactone (21.7%). Phenylpropanoid (E)-2-hexyl-cinnamaldehyde was detected in significant amounts in our sample (relative percentage of 5%), but the literature survey revealed that this compound was not mentioned in the previously conducted research. Hence in the analysed fatty oil, in addition to fatty acids, the presence of sesquiterpene lactones (dehydrocostuslactone, costunolide, and dehydrocostunolide) was of particular interest. Namely, Batturini et al. revealed in their work that dehydrocostuslactone and costunolide affected the intracellular glutathione level, which triggered the inhibition of the activator of transcription 3 (STAT3) tyrosine phosphorylation [36]. As the number of inflammation-correlated diseases such as Crohn’s disease, pleurisy, and psoriasis are characterized by hyperactivation of STAT3, any treatment counteracting the hyper-expression or -activation of STAT3 might present a new strategy to treat these pathologies which are increasing worldwide. In addition, the study of Wang et al. revealed that dehydrocosuslactone can rapidly cross the blood–brain barrier, enabling its effects against glioma. Furthermore, the anti-cancer activity of this lactone may be mediated through inhibition of the NF-κB/COX-2 signalling pathway by targeting IKKβ in vitro and in vivo [37].
4.2. Antibacterial Activity
The antibacterial activity of the EOL and EOF was subjected to screening against seven strains of bacteria using the broth dilution method. The inhibitory activity of the extracts in Gram-positive was higher than in Gram-negative bacteria (Table 2, Figure 2). The EOF possessed inhibitory activity against all investigated Gram-positive bacteria; especially E. faecalis and B. subtilis were sensitive to the investigated EO with MICs of 250 μg/mL. In the case of B. subtilis, EOF showed greater activity compared to EOL (250 μg/mL vs. 500 μg/mL). In addition, EOF showed greater activity against S. abony in comparison to EOL (500 μg/mL vs. 1000 μg/mL). Both tested EOs exhibited the same MIC of 500 μg/mL against S. aureus (Table 2, Figure 2).
S. aureus is a major pathogen responsible for a wide range of skin and soft-tissue infections and also nasal colonization and lower respiratory tract infections [38,39]. Notably, S. aureus ranks among the most frequently isolated infectious microorganisms from wound infections [40], and based on our findings, the tested laurel EOs were revealed to be promising natural alternatives for combating infections caused by this bacterium. Although B. subtilis is generally considered a non-pathogenic, Gram-positive bacterium, commonly found in the human gastrointestinal tract, it may act as an opportunistic pathogen, especially among immunocompromised patients [41].
Despite the commensal nature of E. faecalis, it is a clinically significant opportunistic pathogen, particularly in hospital settings, causing healthcare-associated infections of bacteremia, endocarditis [42], urinary tract, intra-abdominal, and pelvic infections [43]. The clinical relevance of E. faecalis is amplified by its resistance to multiple antibiotics, such as vancomycin [44].
C. albicans exhibited the greatest sensitivity to the investigated EOs, EOL and EOF, with MICs of 250 and 125 μg/mL, respectively. The increasing resistance of C. albicans to commonly used antifungal agents is a significant therapeutic challenge that highlights the need for more effective antifungal strategies [45]. In this context, EOs derived from laurel leaves and fruits demonstrated promising antifungal potential.
The qualitative analysis of EOL and EOF revealed their similarities. Chemical analyses of both EOs revealed the presence of components responsible for antimicrobial properties (1,8-cineol, α- and β-pinene, α-terpinyl acetate, α-terpineol, sabinene, eugenol, and in the case of EOF, the presence of (E)-2-hexyl-cinnamaldehyde). Namely, plants containing these constituents encompass a diverse range of biological activities, stressing their potential as antimicrobial agents. Juergens et al. published a review on 1,8-cineole, describing its inhibitory effects on the microbial pathogens (S. aureus, E. coli, Moraxella catarrhalis) causing chronic rhinosinusitis. In addition, the same authors claimed that 1,8-cineole might be used as an adjunctive therapy and a therapeutic agent for inflammatory airway disease control [46]. 1,8-cineol is also one of the active ingredients of the marketed medicinal product Rowatinex® capsules (in addition to α- and β-pinene, camphene, borneol, fenchone, and anethole) used for dissolving or excreting concernments in the urinary tract due to weakening of muscle spasm, which reduces the pain caused by renal and urinary colic while increasing the blood flow and reducing inflammation associated with the presence of kidney stones [47]. Bearing in mind the amount of 1,8-cineol determined in EOL and EOF (more than 30%) on the one hand and the amount of this compound in the marketed drugs (3 mg in Rowatinex® and up to 200 mg in SoledumTM), it could be assumed that this compound, present in high amounts in the tested Eos, may be expected to exert certain health benefits, being potentially used as an antimicrobial agent, taking into consideration the results of the antimicrobial activity assessment. Considering the previously stated clinical relevance of the tested bacteria/fungus, EOL and EOF could be used in dermocosmetic products for the treatment of fungal infections and wound healing, as well as in dietetic supplements/herbal preparations for the prevention and/or treatment of urinary and respiratory infections.
The results obtained for EOL antimicrobial potential and 1,8-cineole as its main constituent were in accordance with previous studies. Namely, Caputo et al. showed the antimicrobial activity of laurel leaves EO against several microorganisms to be more pronounced in comparison to the antimicrobial activity of its main compound 1,8-cineole, confirming that the complex EOs composition represents the base for strong antimicrobial properties [11]. Similarly, Sırıken et al. investigated the antibacterial activity of laurel leaves EO, hypothesizing that the synergy between terpenes (linalool), lactones, oxides (1,8-cineole), and monoterpenes (α-pinene) might be responsible for the observed effect, suggesting its potential application as an alternative to antibiotics [5]. Based on the compounds found in EOL and EOF in amounts higher that 5%, which have been reported to exert potent antimicrobial effects, such as linalool [48], sabinene [49], and α- and β-pinene [50], the synergistic effect between 1,8-cineol and the stated compounds in the exerted antimicrobial potential could be assumed as well in the EOs tested in this study. Nevertheless, further studies should address the clinical isolates of the investigated bacterial and fungal strains, as well as experiments regarding synergy testing and cytotoxicity assays.
5. Conclusions
This research evaluated the qualitative and quantitative composition of the laurel leaf and fruit EOs (EOL and EOF, respectively) and fatty oil obtained from the waste after EOF isolation. To our knowledge, this is the first study of laurel essential and fatty oils originating from the Montenegro area. Besides the chemical fingerprint, the antimicrobial activity of EOL and EOF was determined. The most prominent activity of EOL and EOF was detected against the fungus C. albicans, while the strong antibacterial potential was validated against S. aureus, E. faecalis, and B. subtilis, opening the door for their application as prospective antimicrobial agents of natural origin in the cosmetic, pharmaceutical, and food industries, representing alternatives for synthetic substances. Fatty oil from the fruit obtained as waste after EOF production was characterized by the usual constituents of fatty oils, like saturated and unsaturated fatty acids. In addition, a significant percentage of dehydrocostuslactone and (E)-2-hexyl-cinnamaldehyde was quantified, providing the added value of this oil, bearing in mind the proven beneficial effects of stated compounds that could be of interest for the prevention and/or treatment of health conditions of inflammatory aetiology. Keeping in mind the volatility and versatility of EOs in general, the encapsulation of EOL and EOF, facilitating their gradual release over an extended period and increasing their efficiency and stability, might present a starting point for further investigations of this plant material.
Author Contributions
Conceptualization, D.B. and V.M.T. methodology, D.B. and V.M.T.; software, A.Ž.; validation, D.B., M.Š. and M.T.M. formal analysis, M.Š., V.M.T. and M.T.M. investigation, D.B.; resources, V.M.T.; data curation I.L.; writing—original draft preparation, D.B., V.M.T. and I.L. writing—review and editing, A.Ž.; visualization, I.L.; supervision, V.M.T.; project administration, V.M.T. and D.B.; funding acquisition, V.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia, grant number 451-03-136/2025-03/200003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest. Aleksandra Stolić Jovanović (Author) was employed by the “Filly Farm Pharmacy”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EOL | leaves essential oil |
| EOF | fruits essential oil |
| EOs | essential oils |
| WHO | World Health Organization |
| ATCC | American-type culture collection |
| NCIMB | National Collection of Industrial, Food, and Marine Bacteria |
| MIC | minimal inhibitory concentration |
| TP | total phenolic |
| GA | gallic acid |
| SD | standard deviation |
| TT | total tannins |
| PVPP | polyvinylpolypyrrolidone |
| TF | total flavonoid |
| GC | gas chromatography |
| CLSI | Clinical and Laboratory Standards Institute |
| MHB | Müller–Hinton broth |
| RI | retention indices |
| SFE | supercritical fluid extraction |
| FID | flame ionization detector |
References
- Ng, W.J.; Shit, C.S.; Ee, K.Y.; Chai, T.T. Plant natural products for mitigation of antibiotic resistance. In Sustainable Agriculture Reviews 49. Sustainable Agriculture Reviews; Panwar, H., Sharma, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 49, pp. 57–91. [Google Scholar]
- Angelini, P. Plant-derived antimicrobials and their crucial role in combating antimicrobial resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Sırıken, B.; Yavuz, C.; Güler, A. Antibacterial Activity of Laurus nobilis: A review of literature. Med. Sci. Discov. 2018, 5, 374–379. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food. Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- El, S.N.; Karagozlu, N.; Karakaya, S.; Sahın, S. Antioxidant and antimicrobial activities of essential oils extracted from Laurus nobilis L. leaves by using solvent-free microwave and hydrodistillation. Food Nutr. Sci. 2014, 5, 97–106. [Google Scholar] [CrossRef]
- Zeković, Z.P.; Lepojević, Ž.D.; Mujić, I.O. Laurel extracts obtained by steam distillation, supercritical fluid and solvent extraction. J. Nat. Prod. 2009, 2, 104–109. [Google Scholar]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef]
- Moghtader, M.; Farahmand, A. Evaluation of the antibacterial effects of essential oil from the leaves of Laurus nobilis L. in Kerman Province. J. Microbiol. Antimicrob. 2013, 5, 13–17. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Jemâa, J.M.B.; Tersim, N.; Toudert, K.T.; Khouja, M.L. Insecticidal activities of essential oils from leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and comparative chemical composition. J. Stored Prod. Res. 2012, 48, 97–104. [Google Scholar] [CrossRef]
- Fernandez, C.M.M.; da Rosa, M.F.; Fernandez, A.C.A.M.; Lorenzetti, F.B.; Raimundo, K.F.; Cortez, D.A.G.; Gonçalves, J.E.; Simões, M.R.; Colauto, N.B.; Lobo, V.D.S.; et al. Larvicidal activity against Aedes aegypti of essential oil of Laurus nobilis leaves obtained at different seasons. J. Essent. Oil Res. 2018, 30, 379–387. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Kasabri, V.; Afifi, F.U. Evaluation of the Volatile Oil Composition and Antiproliferative Activity of Laurus nobilis L. (Lauraceae) on Breast Cancer Cell Line Models. Rec. Nat. Prod. 2014, 8, 136–147. [Google Scholar]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 181–187. [Google Scholar]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef]
- Leyel, C.F. A Modern Herbal; Grieve, M., Ed.; Penguin Books: Harmondsworth, UK, 1984; pp. 154–196. [Google Scholar]
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; pp. 171–178. [Google Scholar]
- Ilić, Z.S.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. Chemical Profiling of Essential Oils from Main Culinary Plants—Bay (Laurus nobilis L.) and Rosemary (Rosmarinus officinalis L.) from Montenegro. Horticulturae 2024, 10, 1249. [Google Scholar] [CrossRef]
- Kovacevic, N.N.; Simic, M.D.; Ristic, M.S. Essential oil of Laurus nobilis from Montenegro. Chem. Nat. Compd. 2007, 43, 408–411. [Google Scholar] [CrossRef]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopeia, 11th ed.; Council of Europe: Strasbourg, France, 2024. [Google Scholar]
- Jugoslovenska Farmakopeja IV SFRJ [Pharmacopoea Jugoslavica], 4th ed.; Savezni Zavod za Zdravstvenu Zaštitu: Belgrade, Serbia, 1984. (In Serbian)
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced skin performance of emulgel vs. cream as systems for topical delivery of herbal actives (immortelle extract and hemp oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Michielin, E.M.; Salvador, A.A.; Riehl, C.A.; Smânia, A., Jr.; Smânia, E.F.; Ferreira, S.R. Chemical composition and antibacterial activity of Cordia verbenacea extracts obtained by different methods. Bioresour. Technol. 2009, 100, 6615–6623. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis leaves and fruits: A review of metabolite composition and interest in human health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- Petkova, Z.; Stefanova, G.; Girova, T.; Antova, G.; Stoyanova, M.; Damianova, S.; Gochev, V.; Stoyanova, A.; Zheljazkov, V.D. Phytochemical investigations of laurel fruits (Laurus nobilis). Nat. Prod. Commun. 2019, 14, 1934578X19868876. [Google Scholar] [CrossRef]
- Türkmen, M.; Koçer, O. Variation of components in laurel (Laurus nobilis L.) fixed oil extracted by different methods. Int. J. Chem. Technol. 2021, 5, 167–171. [Google Scholar] [CrossRef]
- Castilho, P.C.; Costa, M.D.C.; Rodrigues, A.; Partidário, A. Characterization of laurel fruit oil from Madeira Island, Portugal. J. Am. Oil Chem. Soc. 2005, 82, 863–868. [Google Scholar] [CrossRef]
- Nurbas, M.; Bal, Y. Recovery of fixed and volatile oils from Laurus nobilis L. fruit and leaves by solvent extraction method. Eng. Arch. Fac. EskişehirOsmangazi Univ. 2005, 18, 15–24. [Google Scholar]
- Butturini, E.; Cavalieri, E.; Carcereri de Prati, A.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; et al. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ. Am. J. Cancer Res. 2017, 7, 1270. [Google Scholar] [PubMed]
- Boucher, H.; Miller, L.G.; Razonable, R.R. Serious Infections Caused by Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2010, 51, S183–S197. [Google Scholar] [CrossRef]
- Tilahun, B.; Faust, A.C.; McCorstin, P.; Ortegon, A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am. J. Crit. Care. 2015, 24, 8–12. [Google Scholar] [CrossRef]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of Antimicrobial Activity of Some Plant Extracts against Antibiotic Susceptible and Resistant Bacterial Strains Causing Wound Infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Tsonis, I.; Karamani, L.; Xaplanteri, P.; Kolonitsiou, F.; Zampakis, P.; Gatzounis, G.; Marangos, M.; Assimakopoulos, S.F. Spontaneous cerebral abscess due to Bacillus subtilis in an immunocompetent male patient: A case report and review of literature. World J. Clin. Cases. 2018, 6, 1169. [Google Scholar] [CrossRef]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S.; et al. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef]
- Choi, U.; Kim, E.J.; Lyu, D.H.; Park, B.H.; Chung, H.; Han, C.H.; Bae, S. Ureteral stent induced urinary tract infection and microbial inconsistency between bladder and renal pelvis. Urogenit. Tract Infect. 2021, 16, 61–66. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans Natural Products, Sources of New Antifungal Drugs: A Review. J. Mycol. Méd. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1, 8-cineole in COPD and asthma: Review on the new therapeutic approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and production of sabinene: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.L.D.; Jayaweera, S.A.; Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).