Enhancing Doxorubicin Efficacy in Hepatocellular Carcinoma: The Multi-Target Role of Muscari comosum Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatments
2.3. Viability Assay
2.4. Intracellular Reactive Oxygen Species (ROS) Assay
2.5. Real-Time Reverse Transcription PCR (qRT-PCR)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
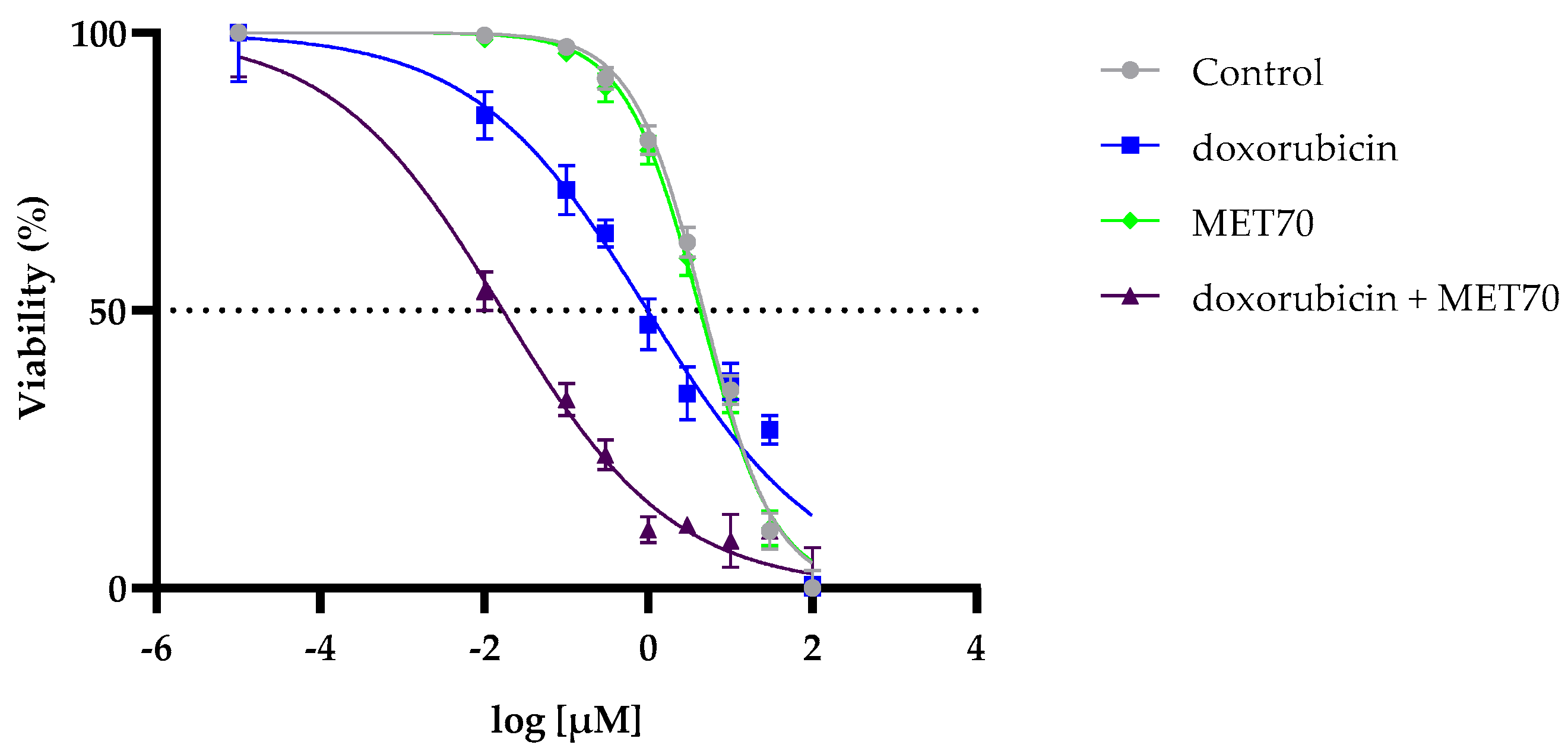
3.1. Enhanced Reduction in Hepg2 Cell Viability by Combined Treatment with Doxorubicin and Muscari comosum Extract (MET70)
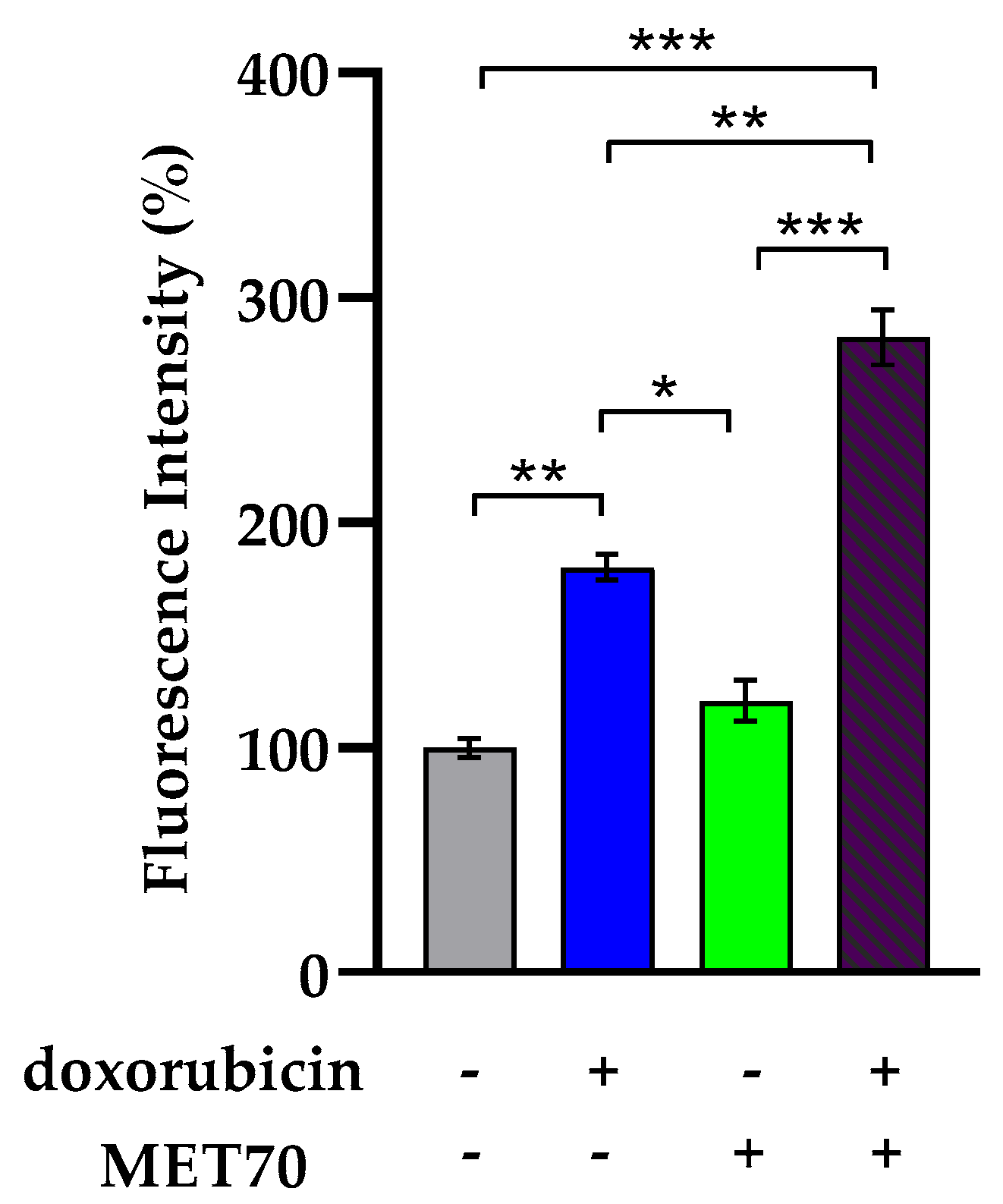
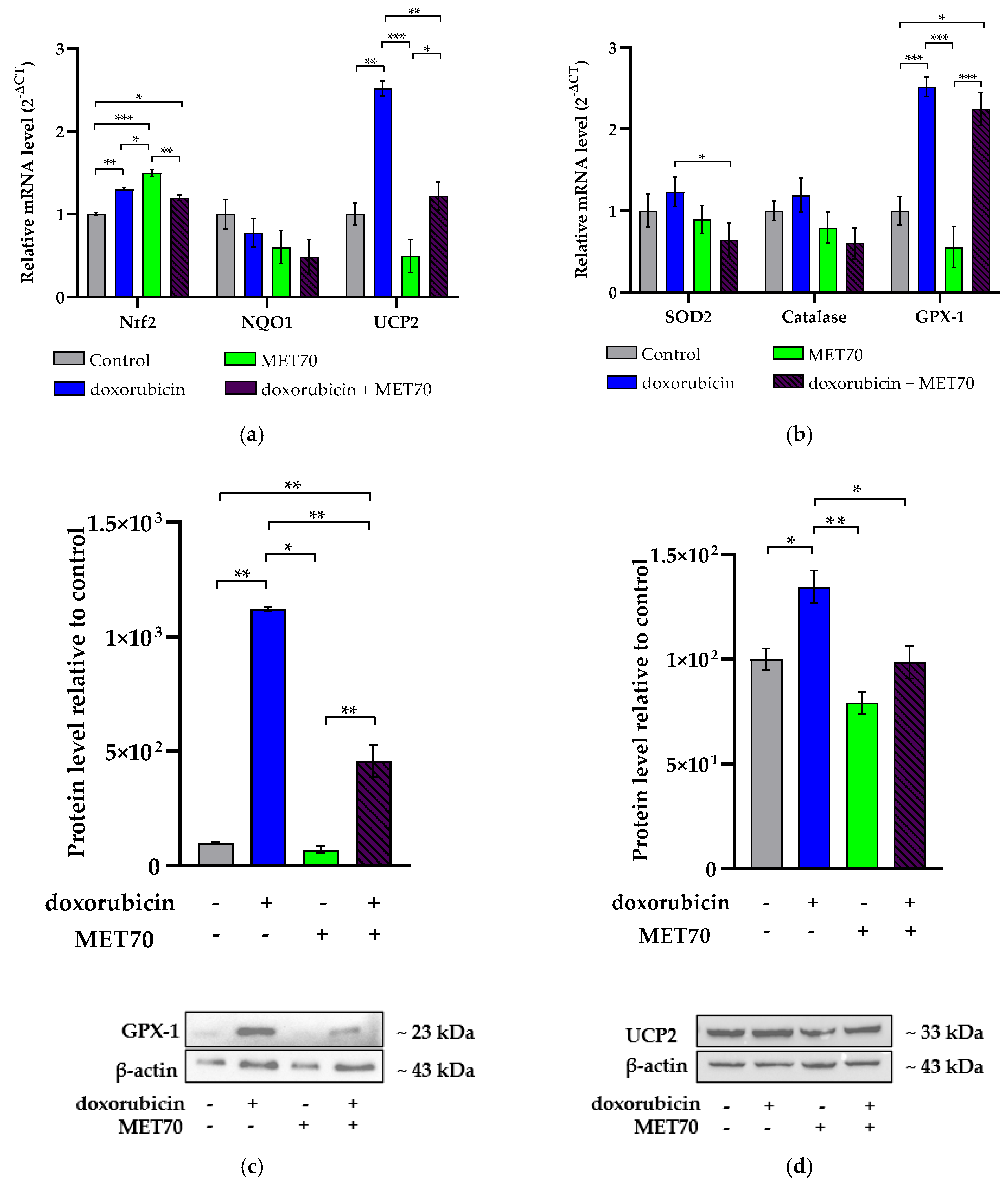
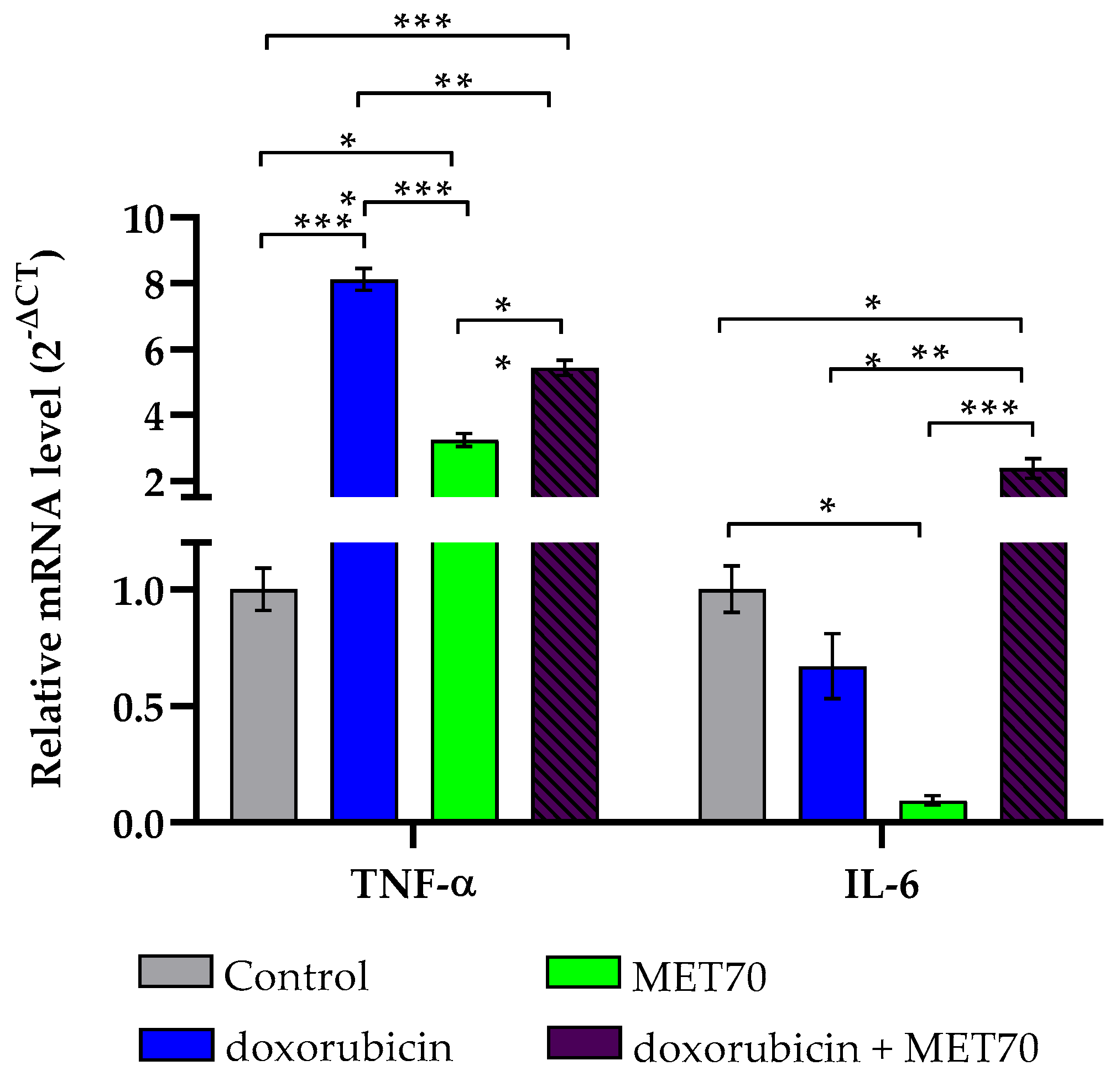
3.2. Impact of Combined Doxorubicin and Muscari comosum Extract (MET70) Treatment on Intracellular ROS Levels and Redox/Inflammatory Gene Expression
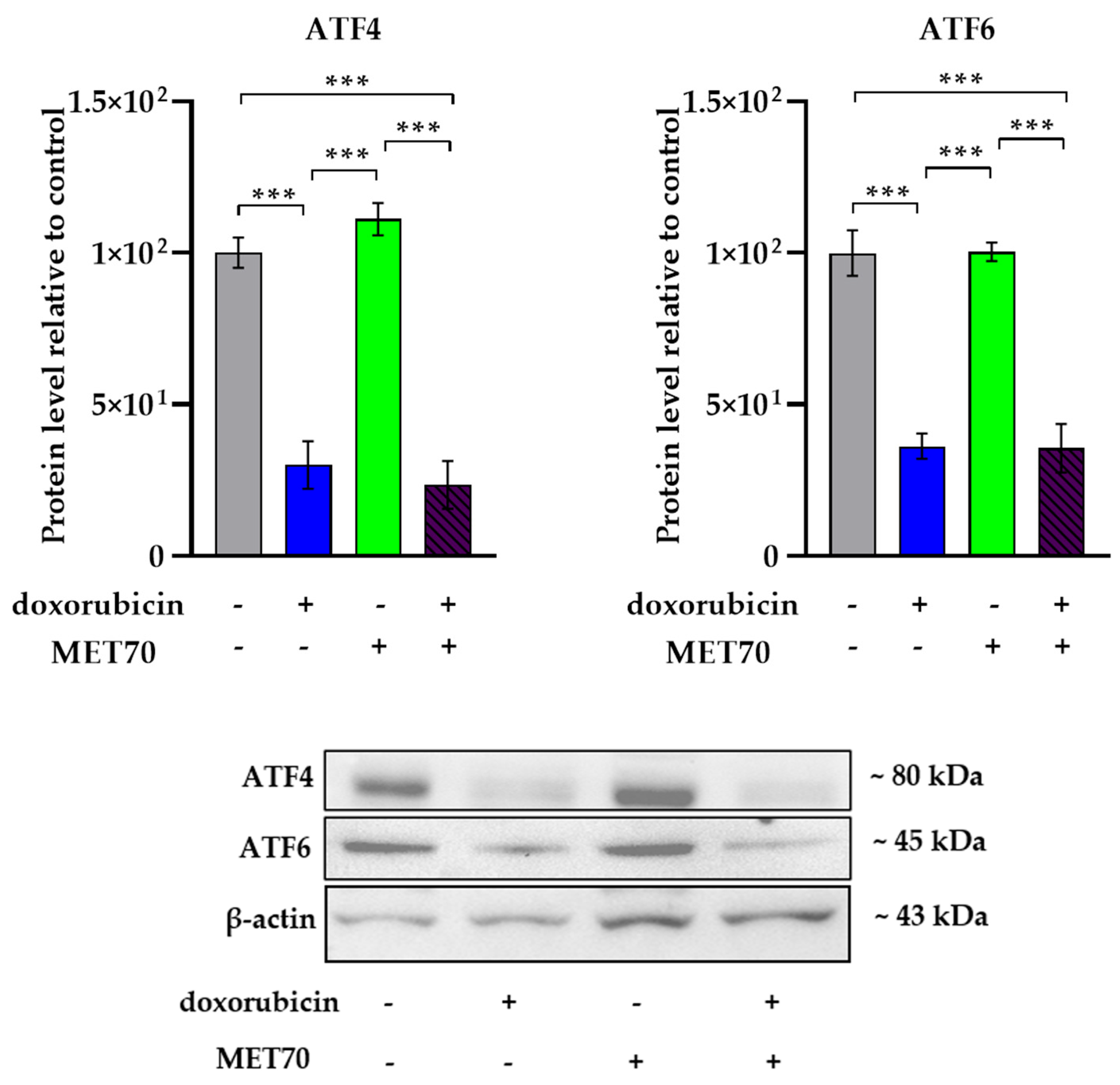
3.3. Effect of Doxorubicin-Muscari comosum Extract Treatment on the UPR Pathway
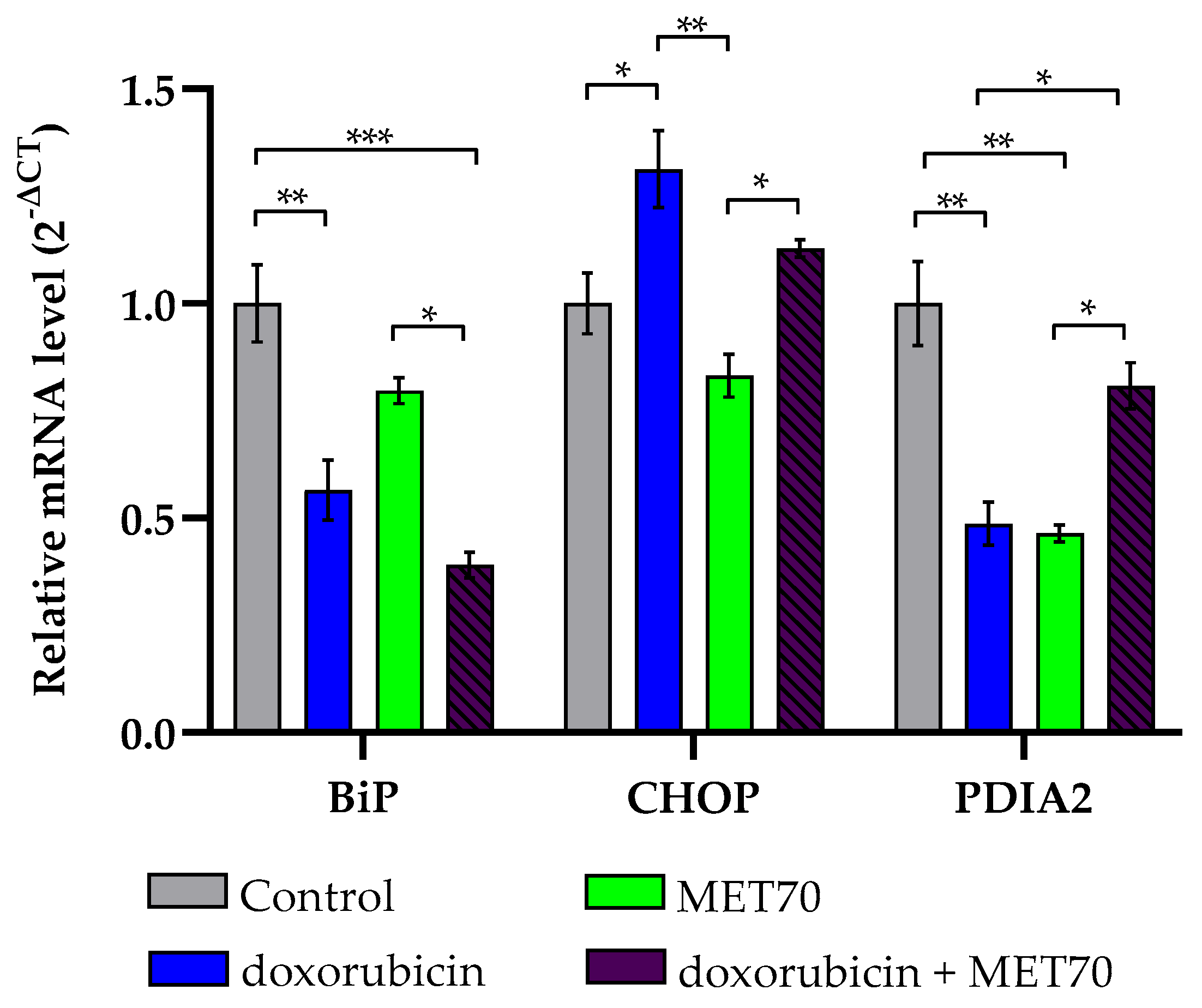
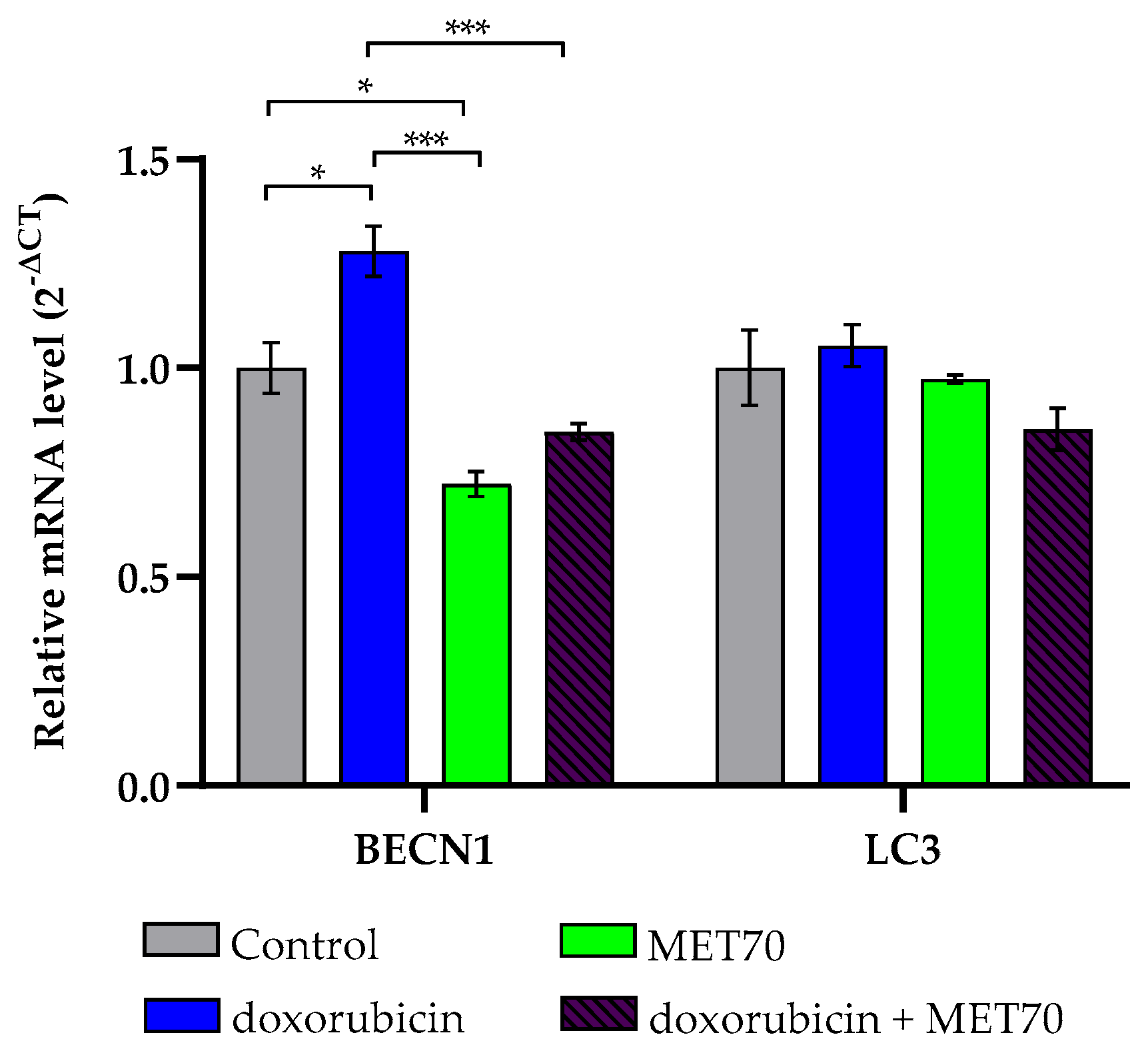
3.4. Modulation of Endoplasmic Reticulum Stress Markers by Combined Doxorubicin and Muscari comosum Extract (MET70) Treatment
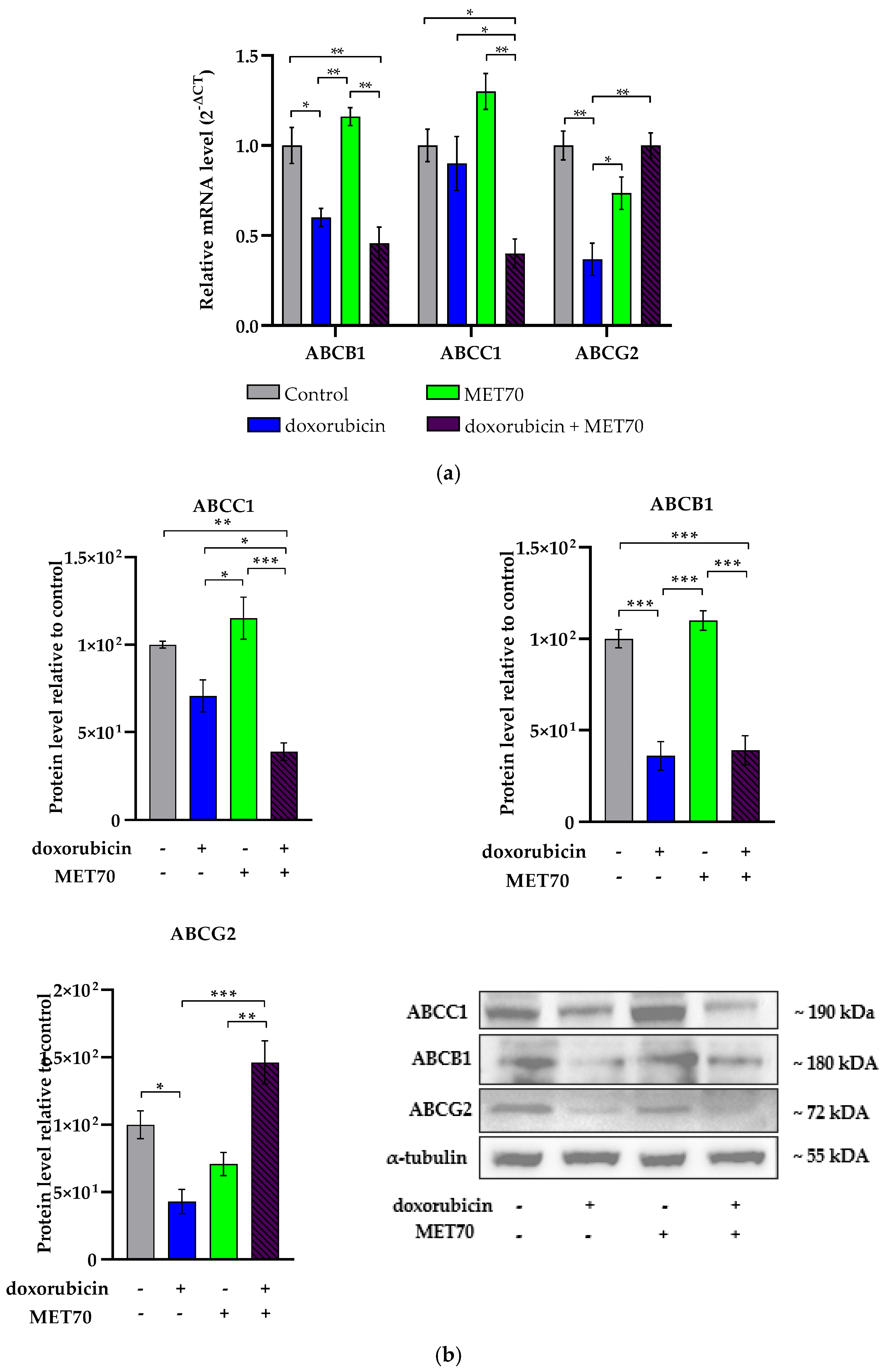
3.5. Modulation of ABC Transporters Expression by Combined Doxorubicin and Muscari comosum Extract (MET70) Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022, 10, 1367–1401. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the Structure, Mechanism and Targeting of Chemoresistance-Linked ABC Transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Pote, M.S.; Gacche, R.N. ATP-Binding Cassette Efflux Transporters and MDR in Cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and Other Anthracyclines in Cancers: Activity, Chemoresistance and Its Overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Sun, Z.; Li, Y.; Gu, X.; Xu, H. Research Progress in Strategies to Improve the Efficacy and Safety of Doxorubicin for Cancer Chemotherapy. Expert Rev. Anticancer Ther. 2021, 21, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int. J. Mol. Sci. 2024, 25, 7477. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Limeres, M.J.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Cuestas, M.L. Thinking Small, Doing Big: Current Success and Future Trends in Drug Delivery Systems for Improving Cancer Therapy with Special Focus on Liver Cancer. Mater. Sci. Eng. C 2019, 95, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Koirala, M.; DiPaola, M. Overcoming Cancer Resistance: Strategies and Modalities for Effective Treatment. Biomedicines 2024, 12, 1801. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 8874003. [Google Scholar] [CrossRef]
- Kangra, K.; Kakkar, S.; Mittal, V.; Kumar, V.; Aggarwal, N.; Chopra, H.; Malik, T.; Garg, V. Incredible use of plant-derived bioactives as anticancer agents. RSC Adv. 2025, 15, 1721–1746. [Google Scholar] [CrossRef] [PubMed]
- Almilaibary, A. Phyto-therapeutics as anti-cancer agents in breast cancer: Pathway targeting and mechanistic elucidation. Saudi J. Biol. Sci. 2024, 31, 103935. [Google Scholar] [CrossRef]
- Pandey, P.; Lakhanpal, S.; Mahmood, D.; Kang, H.N.; Kim, B.; Kang, S.; Choi, J.; Choi, M.; Pandey, S.; Bhat, M.; et al. An updated review summarizing the anticancer potential of flavonoids via targeting NFkB pathway. Front. Pharmacol. 2025, 15, 1513422. [Google Scholar] [CrossRef]
- Hon, K.W.; Naidu, R. Synergistic Mechanisms of Selected Polyphenols in Overcoming Chemoresistance and Enhancing Chemosensitivity in Colorectal Cancer. Antioxidants 2024, 13, 815. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. The Bioactivity and Phytochemicals of Muscari comosum (Leopoldia comosa), a Plant of Multiple Pharmacological Activities. Int. J. Mol. Sci. 2024, 25, 2592. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Mimaki, Y. Search for new steroidal glycosides with anti-cancer potential from natural resources. J. Nat. Med. 2024, 78, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Rizzuti, B.; Occhiuzzi, M.A.; Ioele, G.; Casacchia, T.; Gelmini, F.; Guzzi, R.; Garofalo, A.; Statti, G. Identification by Molecular Docking of Homoisoflavones from Leopoldia Comosa as Ligands of Estrogen Receptors. Molecules 2018, 23, 894. [Google Scholar] [CrossRef] [PubMed]
- Casacchia, T.; Sofo, A.; Casaburi, I.; Marrelli, M.; Conforti, F.; Statti, G.A. Antioxidant, Enzyme-Inhibitory and Antitumor Activity of the Wild Dietary Plant Muscari comosum (L.) Mill. Int. J. Plant Biol. 2017, 8, 6895. [Google Scholar] [CrossRef]
- Giglio, F.; Castiglione Morelli, M.A.; Matera, I.; Sinisgalli, C.; Rossano, R.; Ostuni, A. Muscari comosum L. Bulb Extracts Modulate Oxidative Stress and Redox Signaling in Hepg2 Cells. Molecules 2021, 26, 416. [Google Scholar] [CrossRef]
- Matera, I.; Miglionico, R.; Abruzzese, V.; Marchese, G.; Ventola, G.M.; Castiglione Morelli, M.A.; Bisaccia, F.; Ostuni, A. A Regulator Role for the ATP-Binding Cassette Subfamily C Member 6 Transporter in HepG2 Cells: Effect on the Dynamics of Cell-Cell and Cell-Matrix Interactions. Int. J. Mol. Sci. 2023, 24, 16391. [Google Scholar] [CrossRef]
- Sagnelli, E.; Macera, M.; Russo, A.; Coppola, N.; Sagnelli, C. Epidemiological and Etiological Variations in Hepatocellular Carcinoma. Infection 2020, 48, 7–17. [Google Scholar] [CrossRef]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 Year Survival Rate in Hepatocellular Carcinoma Patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New Molecular and Biochemical Insights of Doxorubicin-Induced Hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef]
- Luby, A.; Alves-Guerra, M.-C. UCP2 as a Cancer Target through Energy Metabolism and Oxidative Stress Control. Int. J. Mol. Sci. 2022, 23, 15077. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Valle, A.; Oliver, J.; Roca, P. UCP2 Inhibition Sensitizes Breast Cancer Cells to Therapeutic Agents by Increasing Oxidative Stress. Free Radic. Biol. Med. 2015, 86, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Cabillic, F.; Corlu, A. Regulation of Transdifferentiation and Retrodifferentiation by Inflammatory Cytokines in Hepatocellular Carcinoma. Gastroenterology 2016, 151, 607–615. [Google Scholar] [CrossRef]
- Yu, L.-X.; Ling, Y.; Wang, H.-Y. Role of Nonresolving Inflammation in Hepatocellular Carcinoma Development and Progression. NPJ Precis Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, J.; Aslam, M.; Blumental-Perry, A.; Tew, K.D.; Townsend, D.M. Protein Disulfide Isomerase Family Mediated Redox Regulation in Cancer. Adv. Cancer Res. 2023, 160, 83–106. [Google Scholar] [CrossRef]
- Tao, J.; Yin, L.; Wu, A.; Zhang, J.; Zhang, J.; Shi, H.; Liu, S.; Niu, L.; Xu, L.; Feng, Y.; et al. PDIA2 Bridges Endoplasmic Reticulum Stress and Metabolic Reprogramming During Malignant Transformation of Chronic Colitis. Front. Oncol. 2022, 12, 836087. [Google Scholar] [CrossRef]
- Hashemi, M.; Nadafzadeh, N.; Imani, M.H.; Rajabi, R.; Ziaolhagh, S.; Bayanzadeh, S.D.; Norouzi, R.; Rafiei, R.; Koohpar, Z.K.; Raei, B.; et al. Targeting and Regulation of Autophagy in Hepatocellular Carcinoma: Revisiting the Molecular Interactions and Mechanisms for New Therapy Approaches. Cell Commun. Signal. 2023, 21, 32. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural Products as Multidrug Resistance Modulators in Cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.R.; Prasad, N.R. Phytochemicals Reverse P-Glycoprotein Mediated Multidrug Resistance via Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC Transporters and the Hallmarks of Cancer: Roles in Cancer Aggressiveness beyond Multidrug Resistance. Cancer Biol. Med. 2020, 17, 253. [Google Scholar] [CrossRef] [PubMed]
| Gene | Accession Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| β-actin | NM_001101.3 | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
| ABCB1 | NM_000927.5 | 5′-CCTTCAGGGTTTCACATTTGG-3′ | 5′-ACTCACATCCTGTCTGAGCA-3′ |
| ABCC1 | NM_004996.4 | 5′-GATCATGCTCACTTTCTGGC-3′ | 5′-TGGGCATCCTCTTTTAAGGC-3′ |
| ABCG2 | NM_004827.2 | 5′-ATCACTGATCCTTCCATCTTG-3′ | 5′-GCTTAGACATCCTTTTCAGG-3′ |
| BECN1 | NM_003766.5 | 5′-AGCTGCCGTTATACTGTTCTG-3′ | 5′-ACTGCCTCCTGTGTCTTCAATCTT -3′ |
| BiP | NM_005347.5 | 5′-GAATCGCCTGACACCTGAAGA-3′ | 5′-GTTTGCTGATAATTGGTTGAACA-3′ |
| CAT | NM_001752.4 | 5′-GAATCGCCTGACACCTGAAGA-3′ | 5′-GTTGAATCTCCGCACTTCTCCAG-3′ |
| CHOP | NM_001195053.1 | 5′-GTACCTATGTTTCACCTCCTG-3′ | 5′-TCTCCTTCATGCGCTGCTTTC-3′ |
| GPX-1 | NM_000581.4 | 5‘-CAGTCGGTGTATGCCTTCTCG-3′ | 5′-CTCGTTCATCTGGGTGTAGTCC-3′ |
| IL-6 | NM_000600.5 | 5′-GGATTCAATGAG GAGACTTG-3′ | 5′-CTACTCTCAAATCTGTTCTGG-3′ |
| LC3 | NM_032514.4 | 5′-GAGAGCAGCATCCAACCAAAA-3′ | 5′- CCGTTCACCAACAGGAAGAAGG-3′ |
| NQO1 | NM_000903 | 5′-GGTGGTGGAGTCGGACCTCTA-3′ | 5′-AGGGTCCTTCAGTTTACCTGTGAT-3′ |
| NRF2 | NM_00114541.3 | 5′-AACTACTCCCAGGTTGCCCA-3′ | 5′-CATTGTCATCTACAAACGGGAA-3′ |
| PDIA2 | NM_006849.4 | 5′-ACGGAGTACCCTACGCTCAAGTT-3′ | 5′-CGTCCCGTGGTCCTGTGTA-3′ |
| SOD2 | NM_000636.4 | 5′-CCGACCTGCCCTACGACTAC-3′ | 5′-AACGCCTCCTGGTACTTCTCC-3′ |
| TNF-α | NM_000594.4 | 5′-GCAGTCAGATCATCTTCTCG-3′ | 5′-TGAAGAGGACCTGGCAGTAG-3′ |
| UCP2 | NM_001381943.1 | 5′-GCTGGAGGTGGTCGGAGATA-3′ | 5′-TTACGAGCAACATTGGGAGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistone, A.; Matera, I.; Abruzzese, V.; Castiglione Morelli, M.A.; Rosa, M.; Ostuni, A. Enhancing Doxorubicin Efficacy in Hepatocellular Carcinoma: The Multi-Target Role of Muscari comosum Extract. Appl. Sci. 2025, 15, 6509. https://doi.org/10.3390/app15126509
Pistone A, Matera I, Abruzzese V, Castiglione Morelli MA, Rosa M, Ostuni A. Enhancing Doxorubicin Efficacy in Hepatocellular Carcinoma: The Multi-Target Role of Muscari comosum Extract. Applied Sciences. 2025; 15(12):6509. https://doi.org/10.3390/app15126509
Chicago/Turabian StylePistone, Alessandro, Ilenia Matera, Vittorio Abruzzese, Maria Antonietta Castiglione Morelli, Martina Rosa, and Angela Ostuni. 2025. "Enhancing Doxorubicin Efficacy in Hepatocellular Carcinoma: The Multi-Target Role of Muscari comosum Extract" Applied Sciences 15, no. 12: 6509. https://doi.org/10.3390/app15126509
APA StylePistone, A., Matera, I., Abruzzese, V., Castiglione Morelli, M. A., Rosa, M., & Ostuni, A. (2025). Enhancing Doxorubicin Efficacy in Hepatocellular Carcinoma: The Multi-Target Role of Muscari comosum Extract. Applied Sciences, 15(12), 6509. https://doi.org/10.3390/app15126509







