The Effects of Six Months of Exercise on Single- and Dual-Task Posture, Gait, and Functional Mobility Relative to Usual Care Alone Among People Living with Dementia: The ENABLED Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Approval and Consent/Assent to Participate
2.3. Recruitment
2.4. Eligibility Criteria
2.5. Sample Size Calculation
2.6. Randomization and Blinding
2.7. Procedures
2.8. Measures of Physical Functioning
2.9. Descriptive Measures at Baseline
2.10. Intervention: Adapted Otago Exercise Program
2.11. Usual Care (Control)
2.12. Data Analysis
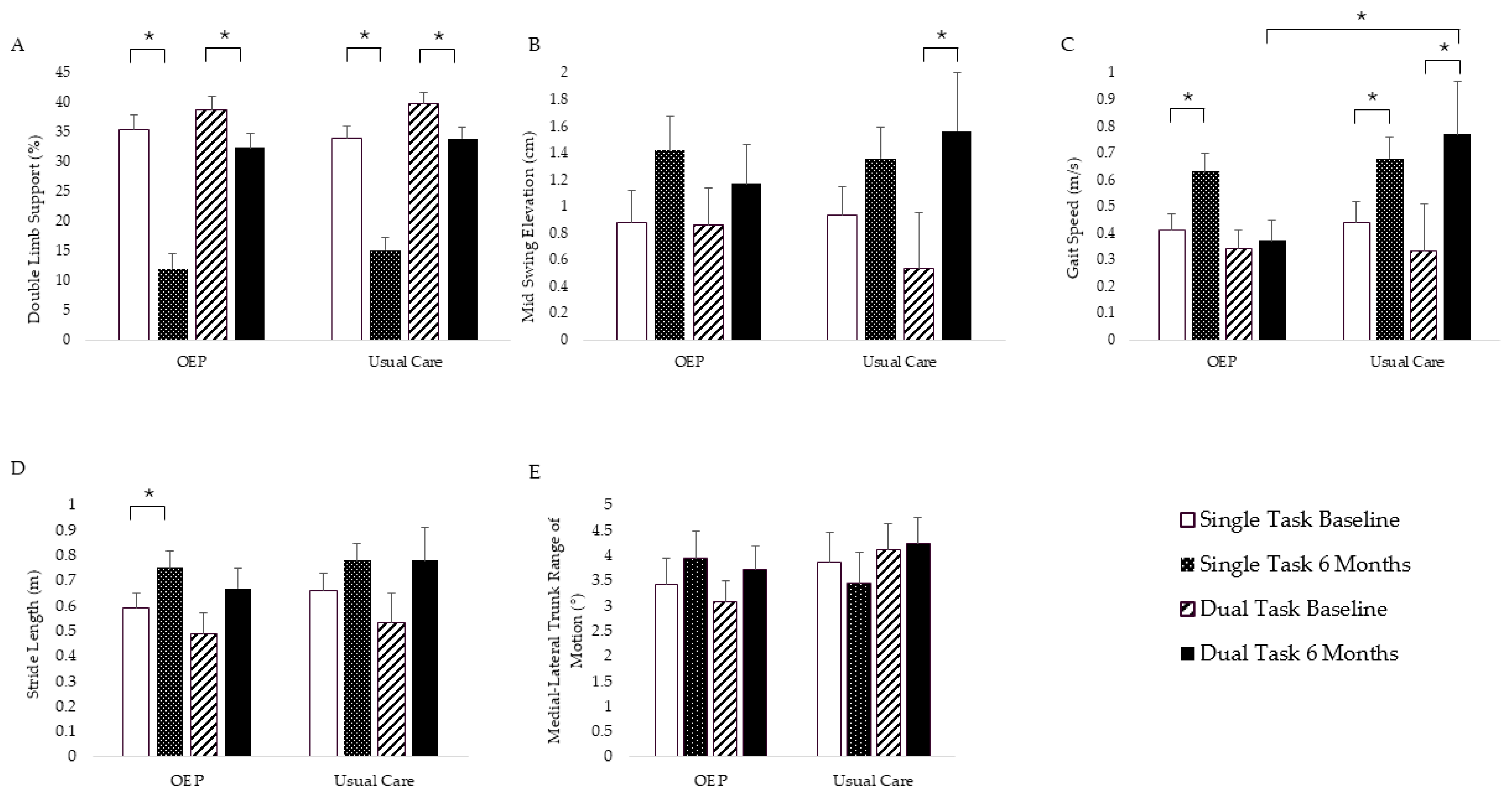
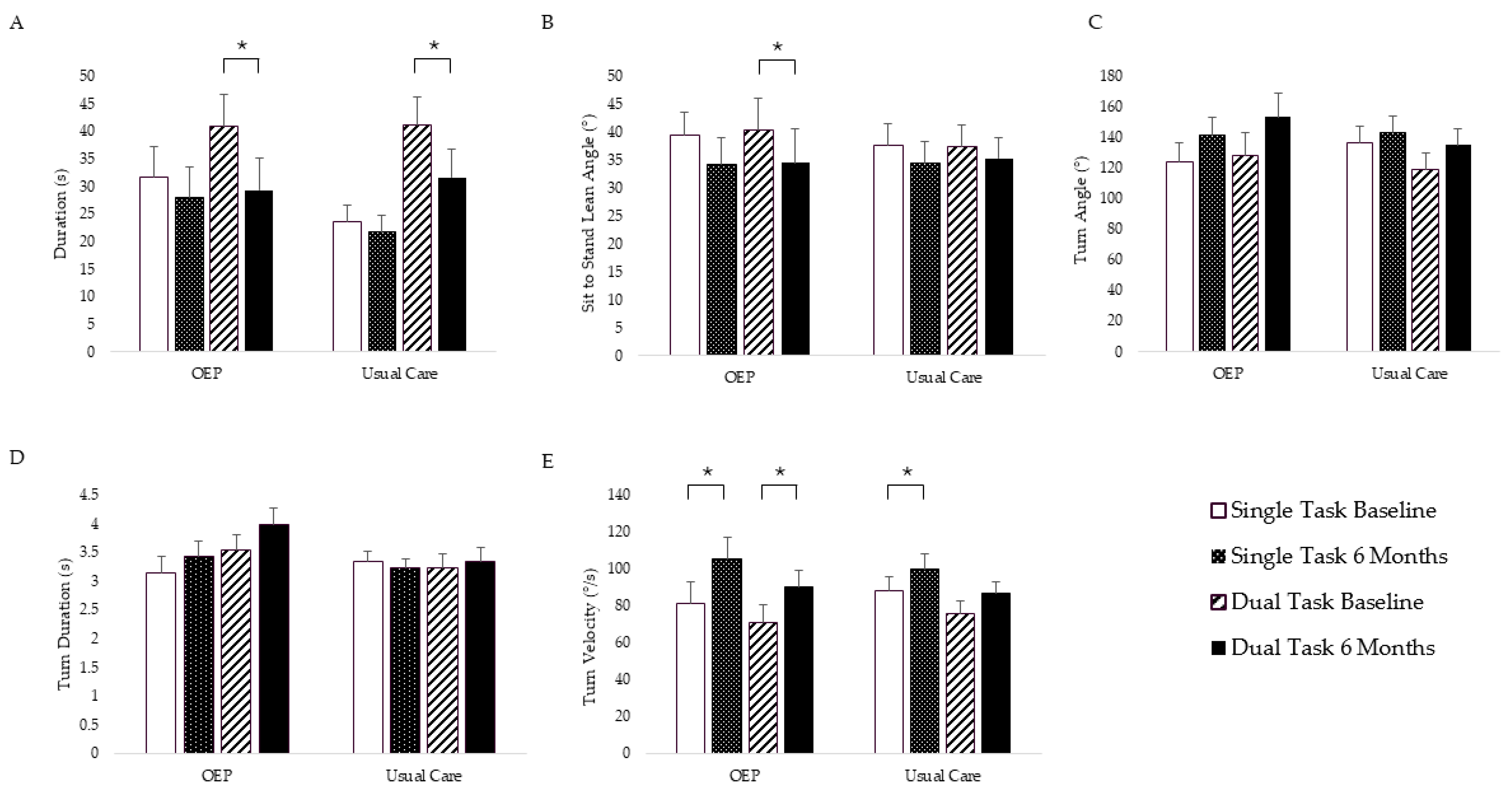
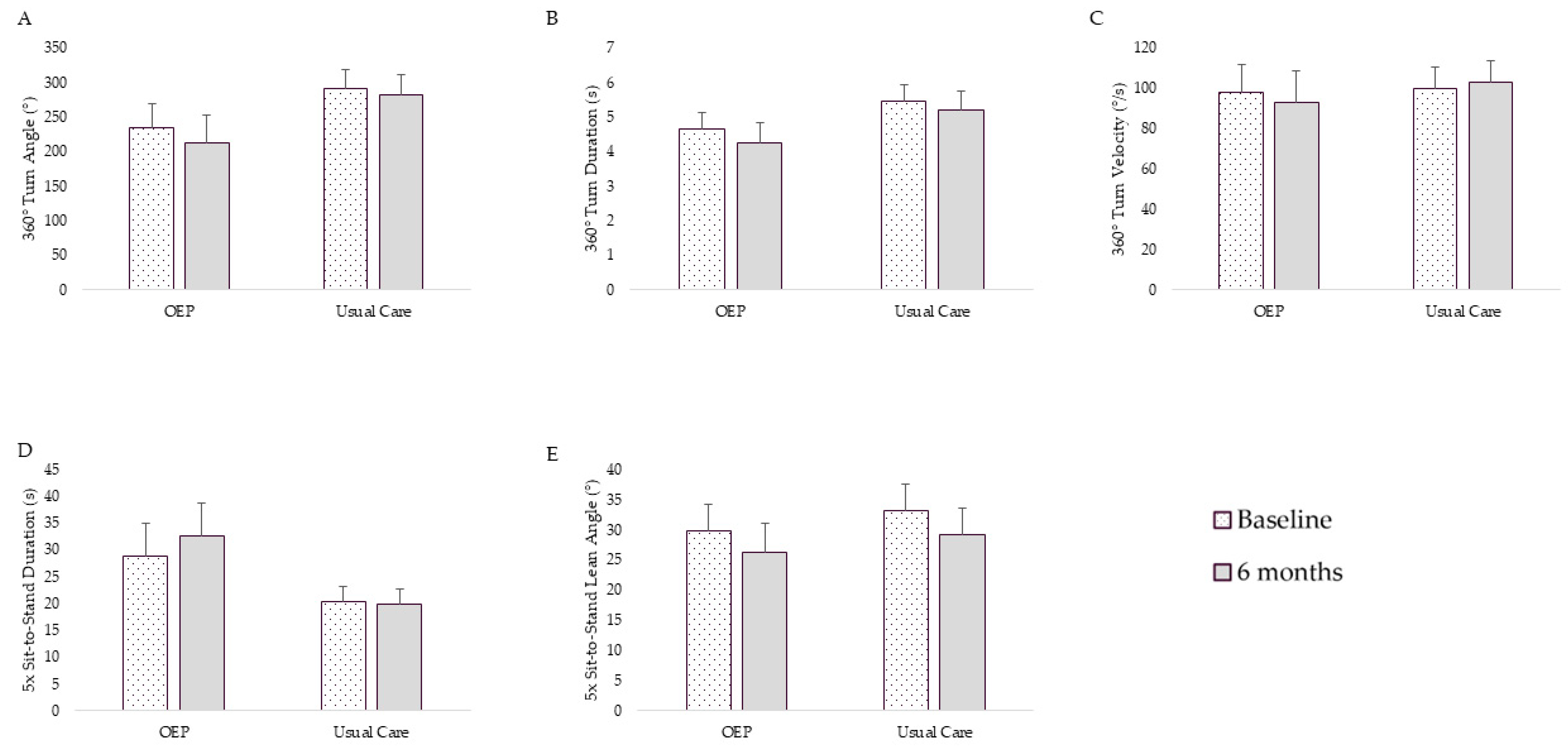
3. Results
3.1. Demographic Characteristics
3.2. Physical Function Outcomes
3.2.1. Adjusted Intent to Treat Analysis
3.2.2. Adjusted per Protocol Analysis
4. Discussion
4.1. Main Findings
4.2. Limitations
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PWD | People living with dementia |
| RCT | Randomized controlled trial |
| OEP | Otago Exercise Program |
| MoCA | Montreal Cognitive Assessment |
| TUG | Timed-up-and-go |
| APDM | Ambulatory Parkinson’s Disease Monitoring |
| ITT | Intent-to-treat |
Appendix A
| Variable | Least Squares Mean (SE) for Group x Time Interaction | F | p-Value | |||
|---|---|---|---|---|---|---|
| OEP Plus Usual Care | Usual Care | |||||
| Baseline | 6 Months | Baseline | 6 Months | |||
| Single-Task Postural Sway | ||||||
| Sway Area (m2/s4) ˅ | 0.40 (0.20) | 0.16 (0.23) | 0.16 (0.07) | 0.18 (0.07) | 0.78 | 0.39 |
| Centroidal Frequency (Hz) ˄ | 0.93 (0.08) | 1.02 (0.09) | 0.94 (0.06) | 1.03 (0.06) | 0.01 | 0.91 |
| Jerk (m2/s5) ˅ | 23.55 (12.09) | 12.79 (13.41) | 5.66 (4.58) | 12.17 (4.58) | 1.67 | 0.21 |
| Sway Velocity (m/s) ˅ | 0.45 (0.10) | 0.30 (0.12) | 0.25 (0.03) | 0.22 (0.03) | 0.48 | 0.50 |
| Dual-Task Postural Sway | ||||||
| Sway Area (m2/s4) ˅ | 0.35 (0.11) | 0.25 (0.14) | 0.26 (0.07) | 0.18 (0.07) | 0.01 | 0.91 |
| Centroidal Frequency (Hz) ˄ | 0.95 (0.08) | 1.01 (0.09) | 1.11 (0.06) | 1.13 (0.06) | 0.23 | 0.64 |
| Jerk (m2/s5) ˅ | 26.94 (10.95) | 8.45 (13.25) | 8.90 (2.32) | 9.75 (2.32) | 1.39 | 0.28 |
| Sway Velocity (m/s) ˅ | 0.40 (0.07) | 0.43 (0.08) | 0.25 (0.05) | 0.28 (0.05) | 0.00 | 0.99 |
| 360 Turn | ||||||
| Turn Angle (°) ˄ | 231.80 (27.21) | 217.80 (34.71) | 293.42 (21.61) | 280.55 (23.19) | 0.00 | 0.98 |
| Duration (s) ˅ | 4.62 (0.34) | 4.50 (0.47) | 5.42 (0.48) | 4.93 (0.51) | 0.21 | 0.65 |
| Turn Velocity (°/s) ˄ | 105.04 (10.92) | 90.35 (13.68) | 104.82 (9.37) | 110.83 (9.90) | 1.64 | 0.22 |
| 4 m Walk Single-Task | ||||||
| Double-Limb Support (%) ˅ | 33.90 (2.15) | 11.24 (2.53) | 33.01 (1.76) | 13.83 (1.83) | 0.58 | 0.46 |
| Mid-Swing Elevation (cm) ˄ | 1.17 (0.21) | 1.66 (0.25) | 1.18 (0.19) | 1.64 (0.19) | 0.01 | 0.92 |
| Gait Speed (m/s) ˄ | 0.48 (0.06) | 0.66 (0.07) | 0.50 (0.07) | 0.74 (0.07) | 0.22 | 0.64 |
| Stride Length (m) ˄ | 0.63 (0.06) | 0.75 (0.06) | 0.68 (0.06) | 0.81 (0.06) | 0.01 | 0.92 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.79 (0.42) | 4.25 (0.45) | 4.30 (0.55) | 3.84 (0.55) | 3.17 | 0.09 |
| 4 m Walk Dual-Task | ||||||
| Double-Limb Support (%) ˅ | 38.74 (1.72) | 32.06 (1.89) | 38.85 (1.84) | 32.80 (1.91) | 0.03 | 0.86 |
| Mid-Swing Elevation (cm) ˄ | 0.98 (0.20) | 1.45 (0.21) | 0.76 (0.40) | 1.71 (0.41) | 0.55 | 0.47 |
| Gait Speed (m/s) ˄ | 0.35 (0.05) | 0.49 (0.05) | 0.34 (0.18) | 0.75 (0.18) | 0.97 | 0.34 |
| Stride Length (m) ˄ | 0.50 (0.06) | 0.67 (0.06) | 0.54 (0.11) | 0.78 (0.11) | 0.13 | 0.73 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.35 (0.33) | 3.71 (0.36) | 4.17 (0.52) | 4.37 (0.52) | 0.11 | 0.74 |
| TUG Single-Task | ||||||
| Duration (s) ˅ | 30.88 (5.02) | 27.66 (5.14) | 23.10 (2.31) | 20.90 (2.31) | 0.16 | 0.70 |
| Sit-to-Stand Lean Angle (°) ˄ | 36.37 (3.17) | 30.99 (3.72) | 35.94 (3.74) | 32.91 (3.75) | 0.21 | 0.65 |
| Turn Angle (°) ˅ | 129.43 (9.62) | 149.28 (9.90) | 143.74 (7.68) | 149.53 (7.68) | 1.99 | 0.18 |
| Turn Duration (s) ˅ | 3.06 (0.25) | 3.51 (0.26) | 3.38 (0.16) | 3.19 (0.16) | 3.37 | 0.09 |
| Turn Velocity (°/s) ˄ | 84.96 (9.84) | 105.33 (9.92) | 88.67 (6.46) | 101.13 (6.46) | 1.69 | 0.21 |
| TUG Dual-Task | ||||||
| Duration (s) ˅ | 37.35 (4.54) | 26.49 (4.80) | 37.10 (4.39) | 28.17 (4.33) | 0.19 | 0.67 |
| Sit-to-Stand Lean Angle (°) ˄ | 36.26 (4.19) | 30.03 (4.93) | 34.83 (3.48) | 34.50 (3.43) | 0.99 | 0.34 |
| Turn Angle (°) ˅ | 119.34 (12.21) | 148.61 (13.21) | 117.31 (8.65) | 135.04 (8.65) | 0.55 | 0.47 |
| Turn Duration (s) ˅ | 3.20 (0.22) | 3.86 (0.25) | 3.13 (0.23) | 3.23 (0.23) | 1.88 | 0.19 |
| Turn Velocity (°/s) ˄ | 76.33 (7.64) | 90.45 (7.76) | 76.60 (5.92) | 89.21 (5.98) | 0.05 | 0.82 |
| 5x Sit-to-Stand | ||||||
| Duration (s) ˅ | 27.43 (5.52) | 32.26 (5.72) | 20.12 (2.23) | 19.36 (2.23) | 3.09 | 0.11 |
| Sit-to-Stand Lean Angle (°) ˄ | 33.61 (3.26) | 29.44 (3.81) | 35.51 (4.09) | 32.48 (4.09) | 0.04 | 0.84 |
| Variable | Least Squares Mean (SE) for Group x Time Interaction | Effect Size ηp2 | F | p-Value | |||
|---|---|---|---|---|---|---|---|
| OEP Plus Usual Care | Usual Care | ||||||
| Baseline | 6 Months | Baseline | 6 Months | ||||
| Single-Task Postural Sway | |||||||
| Sway Area (m2/s4) ˅ | 0.43 (0.21) | 0.14 (0.24) | 0.15 (0.10) | 0.19 (0.10) | 0.0019 | 1.24 | 0.28 |
| Centroidal Frequency (Hz) ˄ | 0.97 (0.10) | 1.04 (0.10) | 0.96 (0.07) | 1.06 (0.07) | 0.0012 | 0.06 | 0.81 |
| Jerk (m2/s5) ˅ | 28.86 (12.42) | 14.35 (13.58) | 7.01 (5.72) | 15.12 (5.87) | 0.0035 | 2.61 | 0.12 |
| Sway Velocity (m/s) ˅ | 0.47 (0.11) | 0.30 (0.12) | 0.26 (0.05) | 0.24 (0.05) | 0.0035 | 0.74 | 0.40 |
| Dual-Task Postural Sway | |||||||
| Sway Area (m2/s4) ˅ | 0.29 (0.14) | 0.19 (0.16) | 0.20 (0.09) | 0.12 (0.09) | 0.0035 | 0.02 | 0.90 |
| Centroidal Frequency (Hz) ˄ | 0.96 (0.10) | 1.03 (0.11) | 1.13 (0.08) | 1.15 (0.08) | 0.0028 | 0.26 | 0.62 |
| Jerk (m2/s5) ˅ | 27.14 (11.25) | 7.99 (13.43) | 8.53 (3.22) | 9.48 (3.23) | 0.0052 | 1.39 | 0.26 |
| Sway Velocity (m/s) ˅ | 0.42 (0.09) | 0.46 (0.10) | 0.25 (0.07) | 0.28 (0.07) | 0.0000 | 0.00 | 0.96 |
| 360 Turn | |||||||
| Turn Angle (°) ˄ | 234.95 (33.91) | 212.50 (39.72) | 291.68 (27.25) | 283.15 (28.85) | 0.0013 | 0.09 | 0.77 |
| Duration (s) ˅ | 4.67 (0.48) | 4.24 (0.61) | 5.46 (0.49) | 5.22 (0.53) | 0.0014 | 0.04 | 0.84 |
| Turn Velocity (°/s) ˄ | 98.03 (13.58) | 93.28 (15.58) | 100.01 (10.43) | 102.80 (10.97) | 0.0042 | 0.20 | 0.66 |
| 4 m Walk Single-Task | |||||||
| Double-Limb Support (%) ˅ | 35.51 (2.41) | 11.94 (2.65) | 34.04 (2.05) | 15.07 (2.13) | 0.0042 | 0.97 | 0.33 |
| Mid-Swing Elevation (cm) ˄ | 0.88 (0.24) | 1.42 (0.26) | 0.93 (0.22) | 1.36 (0.23) | 0.0014 | 0.10 | 0.75 |
| Gait Speed (m/s) ˄ | 0.41 (0.06) | 0.63 (0.07) | 0.44 (0.08) | 0.68 (0.08) | 0.0014 | 0.02 | 0.88 |
| Stride Length (m) ˄ | 0.59 (0.06) | 0.75 (0.07) | 0.66 (0.07) | 0.78 (0.07) | 00009 | 0.11 | 0.75 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.43 (0.51) | 3.94 (0.54) | 3.86 (0.61) | 3.45 (0.62) | 0.0404 | 2.60 | 0.12 |
| 4 m Walk Dual-Task | |||||||
| Double-Limb Support (%) ˅ | 38.69 (2.34) | 32.37 (2.32) | 39.78 (1.86) | 33.88 (1.92) | 0.0404 | 0.01 | 0.91 |
| Mid-Swing Elevation (cm) ˄ | 0.86 (0.28) | 1.17 (0.29) | 0.53 (0.42) | 1.56 (0.44) | 0.0404 | 1.12 | 0.30 |
| Gait Speed (m/s) ˄ | 0.34 (0.07) | 0.37 (0.08) | 0.33 (0.18) | 0.77 (0.20) | 0.0404 | 1.24 | 0.28 |
| Stride Length (m) ˄ | 0.49 (0.08) | 0.67 (0.08) | 0.53 (0.12) | 0.78 (0.13) | 0.0404 | 0.14 | 0.72 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.07 (0.44) | 3.72 (0.46) | 4.11 (0.52) | 4.23 (0.53) | 0.0151 | 0.79 | 0.38 |
| TUG Single-Task | |||||||
| Duration (s) ˅ | 31.60 (5.58) | 27.93 (5.65) | 23.56 (3.14) | 21.73 (3.14) | 0.0068 | 0.47 | 0.51 |
| Sit-to-Stand Lean Angle (°) ˄ | 39.39 (4.21) | 34.34 (4.61) | 37.70 (3.87) | 34.40 (3.89) | 0.0020 | 0.10 | 0.76 |
| Turn Angle (°) ˅ | 124.23 (12.37) | 141.19 (12.10) | 136.51 (10.80) | 143.38 (10.81) | 0.0174 | 0.98 | 0.34 |
| Turn Duration (s) ˅ | 3.15 (0.29) | 3.42 (0.28) | 3.35 (0.16) | 3.23 (0.16) | 0.0256 | 1.62 | 0.23 |
| Turn Velocity (°/s) ˄ | 81.23 (11.96) | 105.24 (11.80) | 87.88 (7.97) | 100.14 (7.99) | 0.0259 | 3.10 | 0.10 |
| TUG Dual-Task | |||||||
| Duration (s) ˅ | 40.86 (5.80) | 29.32 (5.88) | 41.13 (5.18) | 31.68 (5.03) | 0.0027 | 0.15 | 0.70 |
| Sit-to-Stand Lean Angle (°) ˄ | 40.40 (5.66) | 34.57 (6.02) | 37.40 (3.82) | 35.31 (3.67) | 0.0069 | 0.35 | 0.56 |
| Turn Angle (°) ˅ | 127.99 (15.38) | 153.26 (15.38) | 119.12 (10.79) | 135.27 (10.85) | 0.0025 | 0.25 | 0.62 |
| Turn Duration (s) ˅ | 3.53 (0.28) | 3.98 (0.28) | 3.23 (0.24) | 3.35 (0.24) | 0.0076 | 0.59 | 0.45 |
| Turn Velocity (°/s) ˄ | 71.19 (9.21) | 90.15 (9.14) | 75.97 (6.60) | 86.46 (6.63) | 0.0071 | 1.78 | 0.20 |
| 5x Sit-to-Stand | |||||||
| Duration (s) ˅ | 28.82 (6.07) | 32.57 (6.16) | 20.40 (2.82) | 19.96 (2.83) | 0.0227 | 1.66 | 0.22 |
| Sit-to-Stand Lean Angle (°) ˄ | 29.74 (4.39) | 26.16 (4.82) | 33.12 (4.52) | 29.13 (4.54) | 0.0002 | 0.00 | 0.95 |
| Variable | Least Squares Mean (SE) for Group x Time Interaction | F | p-Value | |||
|---|---|---|---|---|---|---|
| OEP Plus Usual Care | Usual Care | |||||
| Baseline | 6 Months | Baseline | 6 Months | |||
| Single-Task Postural Sway | ||||||
| Sway Area (m2/s4) ˅ | 0.67 (0.37) | 0.20 (0.37) | 0.16 (0.07) | 0.18 (0.07) | 1.05 | 0.34 |
| Centroidal Frequency (Hz) ˄ | 0.87 (0.09) | 1.01 (0.09) | 0.94 (0.06) | 1.03 (0.06) | 0.24 | 0.63 |
| Jerk (m2/s5) ˅ | 34.77 (22.97) | 19.20 (22.97) | 5.66 (4.58) | 12.17 (4.58) | 1.19 | 0.31 |
| Sway Velocity (m/s) ˅ | 0.56 (0.19) | 0.32 (0.19) | 0.25 (0.03) | 0.22 (0.03) | 0.55 | 0.48 |
| Dual-Task Postural Sway | ||||||
| Sway Area (m2/s4) ˅ | 0.26 (0.09) | 0.24 (0.09) | 0.26 (0.07) | 0.18 (0.07) | 0.34 | 0.57 |
| Centroidal Frequency (Hz) ˄ | 0.89 (0.07) | 1.01 (0.07) | 1.11 (0.06) | 1.13 (0.06) | 0.86 | 0.37 |
| Jerk (m2/s5) ˅ | 12.32 (3.48) | 8.66 (3.48) | 8.90 (2.32) | 9.75 (2.32) | 1.25 | 0.30 |
| Sway Velocity (m/s) ˅ | 0.45 (0.11) | 0.42 (0.11) | 0.25 (0.05) | 0.28 (0.05) | 0.14 | 0.71 |
| 360 Turn | ||||||
| Turn Angle (°) ˄ | 209.72 (39.15) | 229.94 (43.79) | 293.42 (21.61) | 280.55 (23.19) | 0.48 | 0.50 |
| Duration (s) ˅ | 4.57 (0.52) | 4.89 (0.63) | 5.43 (0.48) | 4.93 (0.51) | 0.68 | 0.42 |
| Turn Velocity (°/s) ˄ | 96.25 (15.99) | 84.90 (17.93) | 104.82 (9.37) | 110.83 (9.90) | 0.84 | 0.38 |
| 4 m Walk Single-Task | ||||||
| Double-Limb Support (%) ˅ | 34.92 (2.77) | 13.16 (2.91) | 33.01 (1.76) | 13.83 (1.83) | 0.19 | 0.67 |
| Mid-Swing Elevation (cm) ˄ | 1.00 (0.26) | 1.47 (0.29) | 1.18 (0.19) | 1.63 (0.19) | 0.00 | 0.98 |
| Gait Speed (m/s) ˄ | 0.46 (0.07) | 0.63 (0.08) | 0.50 (0.07) | 0.74 (0.07) | 0.26 | 0.62 |
| Stride Length (m) ˄ | 0.60 (0.07) | 0.74 (0.08) | 0.68 (0.06) | 0.81 (0.06) | 0.00 | 0.95 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 4.14 (0.61) | 4.26 (0.63) | 4.30 (0.55) | 3.84 (0.55) | 1.25 | 0.28 |
| 4 m Walk Dual-Task | ||||||
| Double-Limb Support (%) ˅ | 37.02 (2.29) | 31.48 (2.29) | 38.85 (1.85) | 32.8 (1.91) | 0.02 | 0.88 |
| Mid-Swing Elevation (cm) ˄ | 1.00 (0.24) | 1.32 (0.24) | 0.77 (0.40) | 1.71 (0.41) | 0.94 | 0.34 |
| Gait Speed (m/s) ˄ | 0.38 (0.07) | 0.51 (0.07) | 0.34 (0.18) | 0.75 (0.18) | 1.07 | 0.31 |
| Stride Length (m) ˄ | 0.50 (0.08) | 0.68 (0.08) | 0.54 (0.11) | 0.78 (0.11) | 0.08 | 0.77 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.32 (0.46) | 4.14 (0.46) | 4.17 (0.52) | 4.37 (0.52) | 1.17 | 0.30 |
| TUG Single-Task | ||||||
| Duration (s) ˅ | 33.01 (8.12) | 31.36 (8.16) | 23.10 (2.31) | 20.90 (2.31) | 0.05 | 0.84 |
| Sit-to-Stand Lean Angle (°) ˄ | 38.19 (4.64) | 25.98 (4.92) | 35.94 (3.75) | 32.91 (3.75) | 3.38 | 0.10 |
| Turn Angle (°) ˅ | 133.03 (11.57) | 149.25 (11.10) | 143.74 (7.68) | 149.53 (7.68) | 1.09 | 0.32 |
| Turn Duration (s) ˅ | 3.32 (0.34) | 3.46 (0.31) | 3.38 (0.16) | 3.19 (0.16) | 0.72 | 0.43 |
| Turn Velocity (°/s) ˄ | 80.16 (13.88) | 105.76 (13.78) | 88.67 (6.46) | 101.13 (6.46) | 4.63 | 0.051 |
| TUG Dual-Task | ||||||
| Duration (s) ˅ | 40.29 (6.82) | 28.79 (6.82) | 37.10 (4.39) | 28.17 (4.33) | 0.24 | 0.63 |
| Sit-to-Stand Lean Angle (°) ˄ | 40.52 (5.26) | 28.16 (5.26) | 34.83 (3.48) | 34.50 (3.43) | 4.26 | 0.08 |
| Turn Angle (°) ˅ | 129.07 (16.57) | 153.77 (14.59) | 117.31 (8.65) | 135.04 (8.65) | 0.12 | 0.74 |
| Turn Duration (s) ˅ | 3.56 (0.24) | 4.06 (0.23) | 3.13 (0.23) | 3.23 (0.23) | 0.65 | 0.44 |
| Turn Velocity (°/s) ˄ | 73.10 (10.32) | 85.99 (10.19) | 76.60 (5.92) | 89.21 (5.92) | 0.00 | 0.97 |
| 5x Sit-to-Stand | ||||||
| Duration (s) ˅ | 23.96 (5.45) | 30.46 (5.59) | 20.12 (2.23) | 19.36 (2.23) | 3.69 | 0.09 |
| Sit-to-Stand Lean Angle (°) ˄ | 29.95 (3.98) | 25.06 (4.41) | 35.51 (4.09) | 32.48 (4.09) | 0.07 | 0.80 |
| Variable | Least Squares Mean (SE) for Group x Time Interaction | Effect Size ηp2 | F | p-Value | |||
|---|---|---|---|---|---|---|---|
| OEP Plus Usual Care | Usual Care | ||||||
| Baseline | 6 Months | Baseline | 6 Months | ||||
| Single-Task Postural Sway | |||||||
| Sway Area (m2/s4) ˅ | 0.69 (0.36) | 0.16 (0.36) | 0.15 (0.10) | 0.19 (0.10) | 0.0132 | 1.32 | 0.29 |
| Centroidal Frequency (Hz) ˄ | 0.89 (0.11) | 1.01 (0.11) | 0.96 (0.07) | 1.06 (0.07) | 0.0007 | 0.02 | 0.89 |
| Jerk (m2/s5) ˅ | 38.15 (21.65) | 18.85 (21.60) | 7.23 (5.84) | 15.70 (6.03) | 0.0003 | 1.67 | 0.23 |
| Sway Velocity (m/s) ˅ | 0.58 (0.18) | 0.32 (0.18) | 0.26 (0.05) | 0.24 (0.05) | 0.0003 | 0.69 | 0.43 |
| Dual-Task Postural Sway | |||||||
| Sway Area (m2/s4) ˅ | 0.24 (0.10) | 0.19 (0.10) | 0.21 (0.08) | 0.13 (0.08) | 0.0009 | 0.05 | 0.83 |
| Centroidal Frequency (Hz) ˄ | 0.87 (0.09) | 1.01 (0.09) | 1.13 (0.07) | 1.15 (0.08) | 0.0245 | 0.88 | 0.37 |
| Jerk (m2/s5) ˅ | 12.45 (4.01) | 8.10 (4.00) | 8.93 (2.99) | 9.93 (3.000) | 0.0150 | 2.02 | 0.20 |
| Sway Velocity (m/s) ˅ | 0.47 (0.13) | 0.44 (0.13) | 0.24 (0.07) | 0.27 (0.07) | 0.0073 | 0.13 | 0.72 |
| 360 Turn | |||||||
| Turn Angle (°) ˄ | 217.12 (46.60) | 224.36 (51.05) | 298.38 (28.10) | 289.02 (29.68) | 0.0048 | 0.10 | 0.75 |
| Duration (s) ˅ | 4.52 (0.62) | 4.44 (0.74) | 5.43 (0.51) | 5.23 (0.55) | 0.0006 | 0.01 | 0.91 |
| Turn Velocity (°/s) ˄ | 94.02 (18.59) | 90.40 (20.20) | 101.42 (10.70) | 103.49 (11.26) | 0.0016 | 0.09 | 0.77 |
| 4 m Walk Single-Task | |||||||
| Double-Limb Support (%) ˅ | 35.72 (2.74) | 13.68 (2.97) | 33.80 (2.03) | 14.90 (2.12) | 0.0016 | 0.30 | 0.60 |
| Mid-Swing Elevation (cm) ˄ | 0.83 (0.26) | 1.29 (0.29) | 0.95 (0.22) | 1.37 (0.22) | 0.0000 | 0.01 | 0.91 |
| Gait Speed (m/s) ˄ | 0.41 (0.06) | 0.60 (0.07) | 0.46 (0.08) | 0.68 (0.08) | 0.0000 | 0.07 | 0.80 |
| Stride Length (m) ˄ | 0.57 (0.07) | 0.74 (0.07) | 0.67 (0.07) | 0.78 (0.07) | 0.0037 | 0.26 | 0.62 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.78 (0.56) | 3.86 (0.59) | 3.72 (0.65) | 3.29 (0.66) | 0.0180 | 0.80 | 0.38 |
| 4 m Walk Dual-Task | |||||||
| Double-Limb Support (%) ˅ | 37.39 (3.06) | 31.60 (2.99) | 39.83 (1.90) | 34.28 (1.97) | 0.0001 | 0.00 | 0.95 |
| Mid-Swing Elevation (cm) ˄ | 0.98 (0.31) | 1.26 (0.32) | 0.65 (0.44) | 1.61 (0.45) | 0.0001 | 0.95 | 0.34 |
| Gait Speed (m/s) ˄ | 0.37 (0.10) | 0.53 (0.11) | 0.35 (0.19) | 0.77 (0.20) | 0.0001 | 0.81 | 0.38 |
| Stride Length (m) ˄ | 0.49 (0.09) | 0.73 (0.09) | 0.57 (0.13) | 0.79 (0.14) | 0.0001 | 0.01 | 0.94 |
| Medial–Lateral Trunk Range of Motion (°) ˄ | 3.06 (0.55) | 4.00 (0.56) | 3.99 (0.56) | 4.12 (0.57) | 0.0350 | 1.63 | 0.22 |
| TUG Single-Task | |||||||
| Duration (s) ˅ | 32.71 (8.47) | 30.54 (8.50) | 22.88 (3.13) | 21.16 (3.14) | 0.0020 | 0.03 | 0.86 |
| Sit-to-Stand Lean Angle (°) ˄ | 40.82 (5.55) | 29.24 (5.72) | 37.42 (4.12) | 34.18 (4.14) | 0.0513 | 2.42 | 0.15 |
| Turn Angle (°) ˅ | 129.39 (11.71) | 145.80 (11.18) | 136.42 (10.37) | 142.96 (10.38) | 0.0199 | 0.85 | 0.39 |
| Turn Duration (s) ˅ | 3.37 (0.39) | 3.41 (0.37) | 3.34 (0.16) | 3.24 (0.16) | 0.0096 | 0.24 | 0.64 |
| Turn Velocity (°/s) ˄ | 81.36 (15.23) | 109.63 (15.04) | 89.05 (8.04) | 101.23 (8.07) | 0.0710 | 5.92 | 0.03 |
| TUG Dual-Task | |||||||
| Duration (s) ˅ | 40.63 (7.55) | 28.44 (7.43) | 39.73 (5.39) | 30.64 (5.23) | 0.0058 | 0.26 | 0.62 |
| Sit-to-Stand Lean Angle (°) ˄ | 43.45 (6.26) | 31.91 (6.14) | 37.50 (3.89) | 35.49 (3.76) | 0.0687 | 3.07 | 0.11 |
| Turn Angle (°) ˅ | 133.69 (18.76) | 158.23 (16.62) | 119.97 (11.19) | 135.48 (11.30) | 0.0042 | 0.17 | 0.69 |
| Turn Duration (s) ˅ | 3.56 (0.31) | 4.05 (0.27) | 3.15 (0.25) | 3.29 (0.25) | 0.0166 | 0.49 | 0.51 |
| Turn Velocity (°/s) ˄ | 70.59 (11.41) | 88.34 (11.36) | 76.07 (6.78) | 86.56 (6.79) | 0.0045 | 1.39 | 0.26 |
| 5x Sit-to-Stand | |||||||
| Duration (s) ˅ | 25.02 (5.59) | 30.42 (5.69) | 21.12 (2.85) | 20.57 (2.86) | 0.0580 | 2.84 | 0.13 |
| Sit-to-Stand Lean Angle (°) ˄ | 27.81 (4.64) | 23.85 (5.31) | 33.84 (4.48) | 29.91 (4.48) | 0.0000 | 0.00 | 0.99 |
References
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D. Epidemiology and Pathophysiology of Dementia-Related Psychosis. J. Clin. Psychiatry 2020, 81, AD19038BR1C. [Google Scholar] [CrossRef] [PubMed]
- Felton, N.; Deave, T. The Lived Experience of Healthcare Workers in Preventing Falls in Community Dwelling Individuals with Dementia. Geriatrics 2022, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Jehu, D.A.; Langston, R.; Sams, R.; Young, L.; Hamrick, M.; Zhu, H.; Dong, Y. The Impact of Dual-Tasks and Disease Severity on Posture, Gait, and Functional Mobility among People Living with Dementia in Residential Care Facilities: A Pilot Study. Sensors 2024, 24, 2691. [Google Scholar] [CrossRef] [PubMed]
- Plummer, P.; Eskes, G.; Wallace, S.; Giuffrida, C.; Fraas, M.; Campbell, G.; Clifton, K.L.; Skidmore, E.R. Cognitive-motor interference during functional mobility after stroke: State of the science and implications for future research. Arch. Phys. Med. Rehabil. 2013, 94, 2565–2574.e6. [Google Scholar] [CrossRef]
- Leone, C.; Feys, P.; Moumdjian, L.; D’Amico, E.; Zappia, M.; Patti, F. Cognitive-motor dual-task interference: A systematic review of neural correlates. Neurosci. Biobehav. Rev. 2017, 75, 348–360. [Google Scholar] [CrossRef]
- Longhurst, J.K.; Rider, J.V.; Cummings, J.L.; John, S.E.; Poston, B.; Landers, M.R. Cognitive-motor dual-task interference in Alzheimer’s disease, Parkinson’s disease, and prodromal neurodegeneration: A scoping review. Gait Posture 2023, 105, 58–74. [Google Scholar] [CrossRef]
- Rogojin, A.; Gorbet, D.J.; Hawkins, K.M.; Sergio, L.E. Cognitive-Motor Integration Performance Is Affected by Sex, APOE Status, and Family History of Dementia. J. Alzheimers Dis. 2019, 71, 685–701. [Google Scholar] [CrossRef]
- Hyndman, D.; Ashburn, A. Stops walking when talking as a predictor of falls in people with stroke living in the community. J. Neurol Neurosurg. Psychiatry 2004, 75, 994–997. [Google Scholar] [CrossRef]
- Echlin, H.V.; Gorbet, D.J.; Sergio, L.E. Assessment of a Cognitive-Motor Training Program in Adults at Risk for Developing Dementia. Can. Geriatr. J. 2020, 23, 190–198. [Google Scholar] [CrossRef]
- Gaßner, H.; Trutt, E.; Seifferth, S.; Friedrich, J.; Zucker, D.; Salhani, Z.; Adler, W.; Winkler, J.; Jost, W.H. Treadmill training and physiotherapy similarly improve dual task gait performance: A randomized-controlled trial in Parkinson’s disease. J. Neural Transm. 2022, 129, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.; Huang, M.Z.; Liao, L.R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Zieschang, T.; Englert, S.; Grewal, G.; Najafi, B.; Hauer, K. Improvements in gait characteristics after intensive resistance and functional training in people with dementia: A randomised controlled trial. BMC Geriatr. 2014, 14, 73. [Google Scholar] [CrossRef]
- Ball, H.A.; McWhirter, L.; Ballard, C.; Bhome, R.; Blackburn, D.J.; Edwards, M.J.; Fleming, S.M.; Fox, N.C.; Howard, R.; Huntley, J.; et al. Functional cognitive disorder: Dementia’s blind spot. Brain 2020, 143, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Balbim, G.M.; Falck, R.S.; Barha, C.K.; Starkey, S.Y.; Bullock, A.; Davis, J.C.; Liu-Ambrose, T. Effects of exercise training on the cognitive function of older adults with different types of dementia: A systematic review and meta-analysis. Br. J. Sports Med. 2022, 56, 933–940. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Cai, X.; Zhang, X.; Zhang, J.; Peng, M.; Xiao, D.; Ouyang, H.; Yan, F. Effects of physical activity interventions on executive function in older adults with dementia: A meta-analysis of randomized controlled trials. Geriatr. Nurs. 2023, 51, 369–377. [Google Scholar] [CrossRef]
- Braz de Oliveira, M.P.; Moreira Padovez, R.F.C.; Serrão, P.; de Noronha, M.A.; Cezar, N.O.C.; Andrade, L.P. Effectiveness of physical exercise at improving functional capacity in older adults living with Alzheimer’s disease: A systematic review of randomized controlled trials. Disabil. Rehabil. 2023, 45, 391–402. [Google Scholar] [CrossRef]
- Bardopoulou, M.S.; Patsaki, I.; Chondronikola, C.; Chryssanthopoulos, C.; Cherouveim, E.D.; Lakoniti, K.O.; Maridaki, M.; Papageorgiou, S.G.; Koutsilieris, M.; Philippou, A. Effect of a 36-Week Supervised Exercise Training Program on Physical and Cognitive Function in Older Patients With Dementia. Vivo 2024, 38, 286–294. [Google Scholar] [CrossRef]
- Bracco, L.; Pinto-Carral, A.; Hillaert, L.; Mourey, F. Tango-therapy vs physical exercise in older people with dementia; a randomized controlled trial. BMC Geriatr. 2023, 23, 693. [Google Scholar] [CrossRef]
- Ghadiri, F.; Bahmani, M.; Paulson, S.; Sadeghi, H. Effects of fundamental movement skills based dual-task and dance training on single- and dual-task walking performance in older women with dementia. Geriatr. Nurs. 2022, 45, 85–92. [Google Scholar] [CrossRef]
- Lloyd-Hazlegreaves, P.; Hayes, L.; Pearce, M.S. Associations between physical inactivity and dementia prevalence: Ecological study using global data. Public Health 2023, 225, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.A.; Pullen, M.E. Alzheimer’s Disease and Physical Activity. ACSM’s Health Fit. J. 2021, 25, 5–10. [Google Scholar] [CrossRef]
- Jehu, D.A.; Bek, J.; Bennett, C.; Hackney, M.E. Group and partnered dance for people living with dementia: An overview of intervention design and measurement considerations. Front. Psychol. 2025, 16, 1500688. [Google Scholar] [CrossRef] [PubMed]
- NIH. Exercising With Chronic Conditions. 2020. Available online: https://www.nia.nih.gov/health/exercise-and-physical-activity/exercising-chronic-conditions (accessed on 1 April 2025).
- Ries, J.D.; Carroll, M. Feasibility of a small group otago exercise program for older adults living with dementia. Geriatrics 2022, 7, 23. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, W.; Zhang, X.; Zhou, J.; Wang, Z. The effectiveness of Otago exercise program in older adults with frailty or pre-frailty: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 114, 105083. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Davis, J.C.; Best, J.R.; Dian, L.; Madden, K.; Cook, W.; Hsu, C.L.; Khan, K.M. Effect of a Home-Based Exercise Program on Subsequent Falls Among Community-Dwelling High-Risk Older Adults After a Fall: A Randomized Clinical Trial. JAMA 2019, 321, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Hsu, C.L.; Barha, C.; Jehu, D.A.; Chan, P.; Ghag, C.; Jacova, P.; Adjetey, C.; Dian, L.; Parmar, N.; et al. Comparing the cost-effectiveness of the Otago Exercise Programme among older women and men: A secondary analysis of a randomized controlled trial. PLoS ONE 2022, 17, e0267247. [Google Scholar] [CrossRef]
- Papamichail, P.; Sagredaki, M.L.; Bouzineki, C.; Kanellopoulou, S.; Lyros, E.; Christakou, A. The Effectiveness of an Exercise Program on Muscle Strength and Range of Motion on Upper Limbs, Functional Ability and Depression at Early Stage of Dementia. J. Clin. Med. 2024, 13, 4136. [Google Scholar] [CrossRef]
- Trapuzzano, A.; McCarthy, L.; Dawson, N. Investigating the effects of an OTAGO-based program among individuals living with dementia. Phys. Occup. Ther. Geriatr. 2020, 38, 185–198. [Google Scholar] [CrossRef]
- Taylor, M.E.; Wesson, J.; Sherrington, C.; Hill, K.D.; Kurrle, S.; Lord, S.R.; Brodaty, H.; Howard, K.; O’Rourke, S.D.; Clemson, L.; et al. Tailored Exercise and Home Hazard Reduction Program for Fall Prevention in Older People With Cognitive Impairment: The i-FOCIS Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 655–665. [Google Scholar] [CrossRef]
- Jehu, D.A.; Dong, Y.; Zhu, H.; Huang, Y.; Soares, A.; Patel, C.; Aden, Z.; Hergott, C.; Ange, B.; Waller, J.L.; et al. The effects of strEngth aNd BaLance exercise on Executive function in people living with Dementia (ENABLED): Study protocol for a pilot randomized controlled trial. Contemp. Clin. Trials 2023, 130, 107220. [Google Scholar] [CrossRef] [PubMed]
- Langston, R.L.; Dong, Y.; Zhu, H.; Soares, A.; Patel, C.; Hergott, C.; Waller, J.L.; Young, L.; Robinson-Johnson, D.; Carrick, R.M.; et al. The effects of six months of exercise on single- and dual-task gait and functional mobility relative to usual care alone among people living with dementia: A pilot randomized controlled trial. In Proceedings of the Alzheimer’s Association International Conference, Philadelphia, PA, USA, 27–31 July 2024. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Obstet. Gynecol. 2010, 115, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Charlton, J.M.; Tse, C.T.F.; Esculier, J.F.; Graffos, A.; Krowchuk, N.M.; Thatcher, D.; Hunt, M.A. Validity and reliability of wearable inertial sensors in healthy adult walking: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devices 2016, 13, 455–462. [Google Scholar] [CrossRef]
- Lezak, M.; Howieson, D.; Bigler, E.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Lee, H.S.; Ko, M.; Park, S.W.; Braden, H. Concurrent validity of the Groningen Meander Walking and Timed Up and Go tests in older adults with dementia. Physiother. Theory Pract. 2020, 36, 1432–1437. [Google Scholar] [CrossRef]
- Ries, J.D.; Echternach, J.L.; Nof, L.; Gagnon Blodgett, M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys. Ther. 2009, 89, 569–579. [Google Scholar]
- Nightingale, C.J.; Mitchell, S.N.; Butterfield, S.A. Validation of the Timed Up and Go Test for Assessing Balance Variables in Adults Aged 65 and Older. J. Aging Phys. Act. 2019, 27, 230–233. [Google Scholar] [CrossRef]
- Groll, D.L.; To, T.; Bombardier, C.; Wright, J.G. The development of a comorbidity index with physical function as the outcome. J. Clin. Epidemiol. 2005, 58, 595–602. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Pan, I.M.Y.; Lau, M.S.; Mak, S.C.; Hariman, K.W.; Hon, S.K.H.; Ching, W.K.; Cheng, K.M.; Chan, C.F. Staging of Dementia Severity With the Hong Kong Version of the Montreal Cognitive Assessment (HK-MoCA)’s. Alzheimer Dis. Assoc. Disord. 2020, 34, 333–338. [Google Scholar] [CrossRef]
- Davis, J.C.; Robertson, M.C.; Ashe, M.C.; Liu-Ambrose, T.; Khan, K.M.; Marra, C.A. Does a home-based strength and balance programme in people aged > or =80 years provide the best value for money to prevent falls? A systematic review of economic evaluations of falls prevention interventions. Br. J. Sports Med. 2010, 44, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Robertson, M.C.; Gardner, M.M.; Norton, R.N.; Buchner, D.M. Falls prevention over 2 years: A randomized controlled trial in women 80 years and older. Age Ageing 1999, 28, 513–518. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C.; Gardner, M.M.; Norton, R.N.; Tilyard, M.W.; Buchner, D.M. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997, 315, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.C.; Gardner, M.M.; Devlin, N.; McGee, R.; Campbell, A.J. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ 2001, 322, 701–704. [Google Scholar] [CrossRef]
- Robertson, M.C.; Campbell, A.J.; Gardner, M.M.; Devlin, N. Preventing injuries in older people by preventing falls: A meta-analysis of individual-level data. J. Am. Geriatr. Soc. 2002, 50, 905–911. [Google Scholar] [CrossRef]
- Hauer, K.; Becker, C.; Lindemann, U.; Beyer, N. Effectiveness of physical training on motor performance and fall prevention in cognitively impaired older persons: A systematic review. Am. J. Phys. Med. Rehabil. 2006, 85, 847–857. [Google Scholar] [CrossRef]
- Clarke, D.E.; van Reekum, R.; Simard, M.; Streiner, D.L.; Conn, D.; Cohen, T.; Freedman, M. Apathy in dementia: Clinical and sociodemographic correlates. J. Neuropsychiatry Clin. Neurosci. 2008, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Khan, K.; Mansournia, M.A.; Khosravi, A.; Rhodes, R.E.; Chan, P.; Zhao, M.; Jehu, D.A.; Parmar, N.; Liu-Ambrose, T. A ‘case-mix’ approach to understand adherence trajectories for a falls prevention exercise intervention: A longitudinal cohort study. Maturitas 2021, 147, 1–6. [Google Scholar] [CrossRef]
- Davis, J.C.; Rhodes, R.E.; Khan, K.M.; Mansournia, M.A.; Khosravi, A.; Chan, P.; Zhao, M.; Jehu, D.A.; Liu-Ambrose, T. Cognitive Function and Functional Mobility Predict Exercise Adherence in Older Adults Who Fall. Gerontology 2021, 67, 350–356. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Sanders, L.M.J.; Hortobágyi, T.; Karssemeijer, E.G.A.; Van der Zee, E.A.; Scherder, E.J.A.; van Heuvelen, M.J.G. Effects of low- and high-intensity physical exercise on physical and cognitive function in older persons with dementia: A randomized controlled trial. Alzheimers Res. Ther. 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Zieschang, T.; Oster, P.; Hauer, K. Dual-task performances can be improved in patients with dementia: A randomized controlled trial. Neurology 2010, 74, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.U.; Gunter, K.B.; Costello, M.; Aum, H.; MacDonald, S.; White, K.N.; Snow, C.M.; Hayes, W.C. Stride width discriminates gait of side-fallers compared to other-directed fallers during overground walking. J. Aging Health 2007, 19, 200–212. [Google Scholar] [CrossRef]
- Jehu, D.A.; Davis, J.C.; Falck, R.S.; Bennett, K.J.; Tai, D.; Souza, M.F.; Cavalcante, B.R.; Zhao, M.; Liu-Ambrose, T. Risk factors for recurrent falls in older adults: A systematic review with meta-analysis. Maturitas 2021, 144, 23–28. [Google Scholar] [CrossRef]
- O’Keeffe, S.T.; Kazeem, H.; Philpott, R.M.; Playfer, J.R.; Gosney, M.; Lye, M. Gait disturbance in Alzheimer’s disease: A clinical study. Age Ageing 1996, 25, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Annweiler, C.; Dubost, V.; Allali, G.; Kressig, R.W.; Bridenbaugh, S.; Berrut, G.; Assal, F.; Herrmann, F.R. Stops walking when talking: A predictor of falls in older adults? Eur. J. Neurol. 2009, 16, 786–795. [Google Scholar] [CrossRef]
- Wu, Y.T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time - current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef]
- Cohen, J.A.; Verghese, J. Gait and dementia. Handb. Clin. Neurol. 2019, 167, 419–427. [Google Scholar]
- Livingston, G.; Huntley, J.; Liu, K.; Costafreda, S.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Dorris, H.; Oh, J.; Jacobson, N. Wearable movement data as a potential digital biomarker for chronic pain: An investigation using deep learning. Phys. Act. Health 2024, 8, 83–92. [Google Scholar] [CrossRef]
- Jehu, D.A.; Davis, J.C.; Gill, J.; Oke, O.; Liu-Ambrose, T. The effect of exercise on falls in people living with dementia: A systematic review. J. Alzheimers Dis. 2023, 92, 1199–1217. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, H.; Quan, W.; Jiang, X.; Liang, M.; Li, S.; Ugbolue, U.C.; Baker, J.S.; Gusztav, F.; Ma, X.; et al. A new method proposed for realizing human gait pattern recognition: Inspirations for the application of sports and clinical gait analysis. Gait Posture 2024, 107, 293–305. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Participants (n = 42) | OEP (ITT n = 21) | OEP (Per Protocol n = 9) | Usual Care (n = 21) |
|---|---|---|---|---|
| Age, years | 82.1 ± 8.1 (60–98) | 80.7 ± 7.5 (68.0–94.0) | 81.9 ± 8.3 (68.0–93.0) | 83.5 ± 8.6 (60.0–98.0) |
| Male; Female Sex, n (%) | 27 (64.3); 15 (35.7) | 14 (66.7); 7 (33.3) | 5 (55.6); 4 (44.4) | 13 (61.9); 8 (38.1) |
| Race: Black, White, Other n (%) | 7 (16.7), 35 (83.3), 0 (0) | 4 (9.5), 17 (40.5), 0 (0) | 2 (22.2), 7 (77.8), 0 (0) | 3 (7.1), 18 (42.9), 0 (0) |
| Ethnicity: Hispanic, Non-Hispanic, n (%) | 1 (2.4), 41 (97.6) | 1 (4.8), 20 (95.2) | 0 (0), 9 (100) | 0 (0), 21 (100) |
| BMI, kg/m2 | 26.6 ± 5.5 (17.6–41.8) | 27.0 ± 5.7 (17.6–36.2) | 28.0 ± 6.5 (19.2–36.2) | 26.2 ± 5.5 (19.1–41.8) |
| FCI, total number | 3.7 ± 2.0 (1–9) | 4.0 ± 2.1 (1–8) | 3.6 ± 2.5 (1–8) | 3.4 ± 2.0 (1–9) |
| Medications, total number | 12.5 ± 5.6 (0–26) | 13.6 ± 6.3 (6–26) | 14.7 ± 6.7 (6–26) | 11.4 ± 4.8 (0–24) |
| Fall History in the Last 6 Months | 2.8 ± 12.2 (0–78) | 4.3 ± 16.9 (0–78) | 0.4 ± 0.7 (0–2) | 1.3 ± 2.3 (0–9) |
| Morse Fall Scale, points | 45.6 ± 23.9 (15–90) | 47.6 ± 24.9 (15–90) | 46.1 ± 22.6 (15–80) | 43.6 ± 23.4 (15–80) |
| MoCA, points | 10.0 ± 5.9 (0–19) | 10.6 ± 6.3 (0–19) | 9.3 ± 7.4 (0–18) | 9.5 ± 5.5 (1–19) |
| Dementia Onset, years | 3.5 ± 5.2 (0.2 to 26.6) | 2.1 ± 1.5 (0.4 to 5.2) | 1.4 ± 1.2 (0.4 to 3.0) | 5.0 ± 7.2 (0.2 to 26.6) |
| Dementia Type, n (%) | ||||
| Alzheimer’s Disease | 16 (38.1) | 7 (33.3) | 3 (33.3) | 9 (42.9) |
| Vascular Dementia | 3 (7.1) | 1 (4.8) | 0 (0.0) | 2 (9.5) |
| PD Dementia | 1 (2.4) | 1 (4.8) | 1 (11.1) | 0 (0.0) |
| Alcohol-Induced Dementia | 1 (2.4) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Unspecified, Without BD | 21 (50.0) | 12 (57.1) | 5 (55.6) | 9 (42.9) |
| Site | ||||
| Nursing Home, n (%) | 24 (57.1) | 13 (31.0) | 5 (55.6) | 11 (26.2) |
| Memory Care, n (%) | 7 (16.7) | 5 (11.9) | 3 (33.3) | 2 (4.8) |
| Assisted Living, n (%) | 11 (26.2) | 3 (7.1) | 1 (11.1) | 8 (19.0) |
| Mobility Device, n (%) | ||||
| Wheelchair | 5 (11.9) | 4 (9.5) | 0 (0) | 1 (2.4) |
| Walker | 10 (23.8) | 6 (14.3) | 3 (33.3) | 4 (9.5) |
| Cane | 11 (26.2) | 5 (11.9) | 3 (33.3) | 6 (14.3) |
| None | 16 (38.1) | 6 (14.3) | 3 (33.3) | 10 (23.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehu, D.A.; Langston, R.; Patel, C.; Soares, A.; Waller, J.L.; Carrick, R.M.; Hergott, C.; Young, L.; Hall, W.; Robinson-Johnson, D.; et al. The Effects of Six Months of Exercise on Single- and Dual-Task Posture, Gait, and Functional Mobility Relative to Usual Care Alone Among People Living with Dementia: The ENABLED Pilot Randomized Controlled Trial. Appl. Sci. 2025, 15, 6624. https://doi.org/10.3390/app15126624
Jehu DA, Langston R, Patel C, Soares A, Waller JL, Carrick RM, Hergott C, Young L, Hall W, Robinson-Johnson D, et al. The Effects of Six Months of Exercise on Single- and Dual-Task Posture, Gait, and Functional Mobility Relative to Usual Care Alone Among People Living with Dementia: The ENABLED Pilot Randomized Controlled Trial. Applied Sciences. 2025; 15(12):6624. https://doi.org/10.3390/app15126624
Chicago/Turabian StyleJehu, Deborah A., Ryan Langston, Charmi Patel, Andre Soares, Jennifer L. Waller, Ryan M. Carrick, Colleen Hergott, Lufei Young, William Hall, Dawnchelle Robinson-Johnson, and et al. 2025. "The Effects of Six Months of Exercise on Single- and Dual-Task Posture, Gait, and Functional Mobility Relative to Usual Care Alone Among People Living with Dementia: The ENABLED Pilot Randomized Controlled Trial" Applied Sciences 15, no. 12: 6624. https://doi.org/10.3390/app15126624
APA StyleJehu, D. A., Langston, R., Patel, C., Soares, A., Waller, J. L., Carrick, R. M., Hergott, C., Young, L., Hall, W., Robinson-Johnson, D., Allen, C., Sams, R., Hamrick, M., Huang, Y., Zhu, H., & Dong, Y. (2025). The Effects of Six Months of Exercise on Single- and Dual-Task Posture, Gait, and Functional Mobility Relative to Usual Care Alone Among People Living with Dementia: The ENABLED Pilot Randomized Controlled Trial. Applied Sciences, 15(12), 6624. https://doi.org/10.3390/app15126624








