Abstract
This work explores the effect of the incorporation of CeO2 into Co/SBA-15 catalysts in hydrogen production through acetic acid oxidative steam reforming as a bio-oil aqueous phase model compound. CeO2 was incorporated (5–30 wt.%) to improve the physicochemical properties of the catalyst. XRD analysis confirmed that the addition of CeO2 resulted in smaller Co0 mean crystallite sizes, while H2-TPR showed enhanced reducibility properties. The catalytic performance was evaluated in the 400–700 °C range, S/C molar ratio = 2, O2/C molar ratio = 0.0375, WHSV = 30.2 h−1, and P = 1 atm. Catalysts containing 10 and 20 wt.% of CeO2 exhibited the best catalytic performance, achieving nearly complete conversions and H2 yield values, approaching thermodynamic equilibrium at 550 °C. Both samples maintained an acetic acid conversion above 90% after 30 h of time-on-stream, with H2 yields above 55% along the steam reforming tests. This agrees with their lower coke formation rates (7.2 and 12.0 mgcoke·gcat−1·h−1 for Co/10CeO2-SBA15 and Co/20CeO2-SBA15, respectively).
1. Introduction
Traditional fuels have been the starting point of industrial and energy development worldwide. However, burning fossil fuels significantly contributes to the release of greenhouse gases, driving climate change. CO2 emissions and other pollutants increase global temperatures, leading to extreme climate, melting glaciers, and rising sea levels [1,2]. Given this alarming reality, it is imperative to implement robust environmental policies to lessen emissions and promote the transition in the direction of more sustainable sources of energy. To address these challenges, the established aim is to limit the global temperature increase to 1.5 °C until the end of the century [3,4,5]. Consequently, governments have implemented environmental policies that promote the utilization of renewable energies, the development of energy efficiency, and the integration of clean technologies [6]. In these circumstances, hydrogen has risen as a promising alternative, considering its high energetic potential and environmental advantages [7]. Hydrogen production from renewable resources, such as biomass, will be a sustainable alternative for managing organic waste and reducing the dependency on fossil fuels, playing a potentially key role in the industrial and transport sectors’ decarbonization [7,8].
Thermochemical processes, such as pyrolysis, use biomass to generate valuable products such as syngas, bio-char, and bio-oil. The pyrolysis bio-oil could be composed of organic as well as aqueous phases [8,9,10]. The organic phase consists of high-energy compounds, which could be further enhanced through hydrotreating to produce biofuels. However, the aqueous phase has a lower energy potential than the organic phase, given that it consists mainly of water and several oxygenated compounds with low and high molecular weight [8]. Despite having a lower energy value, the bio-oil aqueous phase could be revalorized via catalytic steam reforming, producing hydrogen [9,10,11,12,13]. Given the heterogeneity of the bio-oil aqueous phase composition, many authors focus their investigation on representative compounds, such as acetic acid, since it represents approximately 20 wt.% of these organic compounds [14,15,16,17].
In acetic acid steam reforming, steam reacts with this organic compound at high temperatures to produce hydrogen, as represented in Equation (1). This process is highly endothermic, requiring high energy consumption to maintain the high temperatures required.
In this scenario, some authors have reported that the inclusion of oxygen in the feed stream leads to oxidative steam reforming [18,19]. The primary advantage of this configuration is that exothermic oxidation reactions provide part of the energy requirements to drive forward the endothermic steam reforming reactions. An oxidizing environment would also reduce the coke deposited on the catalyst by its combustion. However, hydrogen generation will be slightly reduced, as shown in Equation (2).
Apart from the main reaction, secondary reactions can occur, such as a water–gas shift (Equation (3)), the thermal decomposition of acetic acid (Equation (4)), ketonization (Equation (5)), acetone steam reforming (Equation (6)), methane steam reforming (Equation (7)), coke formation (Equations (8) and (9)), and the Boudouard reaction (Equation (10)) [20].
The development of a suitable catalyst to promote selectivity to hydrogen formation is essential because it influences the reaction mechanism and thus is able to enhance the reaction rate toward hydrogen. Catalyst deactivation is still one of the principal issues in the scientific community when referring to reforming catalysts [21] due to the carbon formation or active phase sintering, and in the context of oxidative steam reforming, where an oxidizing environment is present, the active phase reoxidation [18].
Regarding the catalyst formulation, transition metals such as cobalt have been widely used as the active phase [15,22,23,24] in reforming reactions against noble metals, given their promising activity in breaking down C-C and C-H bonds and lower prices. Furthermore, the election of adequate support is crucial for enhancing the active phase dispersion. Previous works [16,21,25] have demonstrated that materials based on mesoporous SiO2, such as SBA-15, used as support, allow higher active phase dispersion due to their highly ordered mesoporous structure with a large surface area. Additionally, it has been reported that the incorporation of CeO2 into the catalyst formulation improved its activity and resistance toward coke deposition, by the capability of storing, releasing, and transporting oxygen [26].
CeO2-SiO2 supports have been previously studied in steam reforming reactions. In this respect, Calles et al. [27] researched CeO2-doped Ni/SiO2 (Ni/CS) and Ni/SiO2 (Ni/S) catalysts synthesized via incipient wet impregnation. In this study, it was demonstrated that the H2-TPR profiles indicated a slightly lower temperature peak corresponding to NiO reduction for the Ni/CS catalyst, due to the oxygen vacancies in CeO2, thus facilitating higher reducibility. In catalytic tests on ethanol steam reforming, it was demonstrated that a CeO2-doped Ni/SiO2 catalyst enhanced the catalytic activity, maintaining complete ethanol conversion over time. Cifuentes et al. [28] studied Rh-Pt-based catalysts supported on CeO2-SiO2 for ethanol steam reforming. The CeO2-SiO2 supports were synthesized by the co-precipitation method, and the active phase was incorporated by the incipient wetness co-impregnation method. They determined that the optimum Si content was 33 mol%, equivalent to a Si:Ce ratio of 2, whereby the highest catalytic yield was obtained, reaching 5.3 mol H2/mol EtOH at 700 °C. Ribeiro et al. [29] evaluated Ce0.65Me0.1Si0.25 catalysts (Me = V, Cr, Mn, Nb, W, Mo) in the production of oxygenates from ethanol/steam mixtures. They reported that in CeO2-SiO2 supports, the interactions between silica and surface ceria nanocrystals lead to Ce-O-Si unit formation, promoting increased surface area. Additionally, in Mn and Cr-doped catalysts, an improvement in reducibility was observed, attributed mainly to an increase in CeO2 oxygen mobility, thus enhancing the catalytic activity. Results showed an initial ethanol conversion close to 90%, with product distribution dominated by hydrogen and a suppressed ethylene formation, promoting a higher catalytic stability for 6 h.
Palma et al. [30] investigated the ethanol oxidative steam reforming reaction using Ni and Pt-Ni catalysts supported on CeO2-SiO2, achieving complete ethanol conversions and hydrogen yields close to thermodynamic equilibrium at temperatures above 450 °C. These works underscore the potential of CeO2-SiO2 supports, highlighting that the incorporation of CeO2 into the SiO2 matrix enhances oxygen mobility and strengthens metal–support interactions. This promotes the dispersion of the active phase and, likewise, improves the catalytic stability.
The current employment of mesostructured SiO2, such as SBA-15, enhances these structural and catalytic advantages. Several studies had explored CeO2-doped SBA-15 in the steam reforming of ethanol [31], methanol [32], propylene glycol [33], glycerol [34], and toluene [35], thus providing evidence of its viability for hydrogen production from oxygenated compounds. This raises potential applications in the upgrading of the aqueous phase of bio-oil, focusing on acetic acid conversion by reforming reactions.
Acetic acid oxidative steam reforming (AAOSR) has been explored mainly at autothermal conditions (ATR). Research such as that of Su et al. [36] evaluated the effect of Y-doping Ni/CeO2 catalysts in ATR at 700 °C, with a molar ratio of HAc/H2O/O2/N2 = 1/4/0.28/3, and GHSV = 50,600 mL/(g·h). Results showed that the NCY10 catalyst (14.4 wt% NiO, 75.7 wt% CeO2, 9.9 wt% YO1.5) achieved complete conversions during 10 h of reaction, with a 2.6 mol-H2/mol AcOH stable yield. In addition, for the catalyst used, TGA analysis did not show evidence of coke formation, suggesting a high stability of the system. Furthermore, Zhou et al. [37] synthesized mesoporous Ni-xSm-Al-O catalysts to evaluate the Sm doping effect on the catalyst. A high surface area, strong basicity and weak acidity, and highly dispersed Ni0 crystals with a mean crystallite size of 9.9 nm were observed after doping with 2% Sm. These characteristics enhanced the catalytic activity (T = 700 °C, P = 1 atm; GHSV = 30,000 mL/(g·h), and a molar ratio of HAc/H2O/O2/N2 = 1/4/0.28/3.90), with 100% acetic acid and H2 yield conversions of 2.6 mol-H2/mol AcOH achieved during 30 h of reaction. Some recent work by Megía et al. [25,38] was focused on nickel-based catalysts with different supports, highlighting the Ni/Ce-m catalyst, which achieved conversions around 97% during 5 h of reaction (T = 500 °C, P = 1 atm; GHSV = 11,000 h−1, and a molar ratio HAc/H2O/O2 = 1/2/0.08). Nevertheless, a coke formation of 120.6 mgcoke·gcat−1·h−1 was reported, thus leading to the La2O3 addition, enhancing the dispersion of the metallic phase as well as significantly lowering the carbon deposition to 62.3 mgcoke·gcat−1·h−1. Lastly, Co-Rh/CeO2 bimetallic catalysts [39] exhibited long-term stability during 25 h (T = 600 °C, P = 1 atm; WHSV = 30.1 h−1, and a molar ratio HAc/H2O/O2 = 1/2/0.08). The studies highlight the relevance of AAOSR and the importance of further optimization of the catalyst formulation to improve process efficiency, selectivity toward H2, and stability against deactivation.
Although CeO2 has been widely recognized for enhancing catalytic dispersion and coke resistance, most previous studies have focused on Ni-based catalysts on reforming reactions under autothermal conditions. Based on the above, the effect of the incorporation of CeO2 in the catalytic performance of a Co-based catalyst supported over SBA-15 in the AAOSR was evaluated. An optimal CeO2 proportion would enhance the cobalt reducibility, dispersion, and metal–support interaction, enhancing an efficient catalytic performance for hydrogen production.
2. Materials and Methods
2.1. Catalyst Synthesis
Commercial mesoporous silica (SBA-15) purchased from ACS Material was used as a support since its high surface area and hexagonal ordered structure allows better active phase dispersion than amorphous supports. CeO2-modified SBA-15 supports were synthesized following the incipient wetness impregnation technique using aqueous solutions of Ce(NO3)3·6H2O (99%, Sigma-Aldrich, St. Louis, MO, USA), as the Ce precursor, in different concentrations to obtain different Ce contents in the modified support: 5, 10, 20, and 30 wt.%. After that, materials were calcined in airflow at 550 °C for 5.5 h using a 1.8 °C/min heating rate.
The active phase was incorporated according to the same procedure explained above, using Co(NO3)2·6H2O (>98%, Sigma-Aldrich, St. Louis, MO, USA) solutions to get 7 wt.% of Co in the synthesized catalysts. The synthesized catalysts have been denoted as Co/xCeO2-SBA-15, where x indicates the Ce theoretical loading (wt.%).
2.2. Catalyst Characterization
All the samples were characterized by multiple procedures. ICP-OES analysis was performed to measure the Ce and Co contents in the synthesized catalysts, employing an Agilent ICP-OES 5800 VDV spectrophotometer (Agilent, Santa Clara, CA, USA). Before analysis, the catalysts were dissolved by acid digestion (H2SO4—HF mixture). The textural properties were evaluated based on N2 physisorption at 77 K using a Micromeritics TRISTAR 3000 sorptometer (Micromeritics, Norcross, GA, USA). Specific surface areas and pore sizes were estimated using the BET method and the BJH formula, respectively. X-ray diffractograms were acquired on a Philips X’PERT PRO diffractometer (Philips, Eindhoven, The Netherlands) equipped with Cu Kα radiation. Mean crystallite sizes were calculated using the Scherrer equation.
where “d (nm)” refers to the mean crystallite diameter, “K” is the Scherrer constant (0.9), “λ” is the X-ray wavelength (Cu-Kα at 0.15406 nm), “β” refers to the FWHM (full width at half maximum) of the highest intensity diffraction peak, and “θ” indicates the diffraction half-angle.
To assess the catalyst’s reducibility, H2-TPR analyses were performed on a Micromeritics in Situ Catalysts Characterization System (ICCS, Micromeritics, Norcross, GA, USA), where a reducing gas mixture flowed through the sample (10% H2/Ar, 60 mL/min) while heating at 10 °C/min up to 900 °C.
The morphology of the prepared materials was evaluated using TEM in a 120 kV JEOL JEM-1400 FLASH microscope (JEOL, Tokyo, Japan); the samples were previously dispersed in acetone. Using a Cary series UV-Vis-NIR spectrophotometer (Agilent, Santa Clara, CA, USA) at wavelength range of 200–800 nm, the CeO2 oxidation stages on the synthesized materials were evaluated. Finally, TGA and Raman spectra were used to assess the coke deposition on the used catalysts. For that purpose, a TGA-DSC Mettler Toledo thermobalance (Mettler Toledo, Greifensee, Switzerland) employing airflow at a 5 °C/min heating rate until 1000 °C and a Horiba LabRam HR evolution spectrometer (HORIBA Scientific, Kyoto, Japan) with a 532 nm laser beam (nominal power 200 mW) were utilized.
2.3. Catalytic Performance Tests
The AAOSR reactions (P= 1 atm, T = 400–700 °C) were carried out on a MICROACTIVITY-PRO unit (PID Eng&Tech. S.L., Alcobendas, Madrid, Spain), whose schematic diagram can be found elsewhere [25]. Co/xCeO2-SBA-15 catalysts (300 mg) were loaded inside the reactor and with an in situ reduction by pure hydrogen flow (30 mL/min) for 30 min at 600 °C before the reaction. After the catalyst activation, a pure nitrogen stream was fed into the reactor until the reaction conditions were reached. A Gilson 307 piston pump (Gilson, Middleton, WI, USA) was used to feed the acetic acid aqueous solution to the reactor (steam-to-carbon molar ratio = 2) at a WHSV of 30.2 h−1 (WHSV was defined as the mass flow rate of reactants—acetic acid, water, oxygen, and nitrogen—divided by the mass of the loaded catalyst in the reactor). The O2 supply was set to operate with an O2/C molar ratio of 0.0375, maintaining the nitrogen/oxygen mixture’s total flow at 60 mL/min. Condensable reaction vapors were condensed at 4 °C, collected, and subsequently analyzed on an Agilent 7820A GC System (Agilent, Santa Clara, CA, USA) while the non-condensable gaseous stream was analyzed in line on an Agilent MicroGC 490 (Agilent, Santa Clara, CA, USA). The mean value of three measurements of each gas sample was used. Figure 1 shows a simplified schematic of the Microactivity—Pro unit, focusing on the feed section, reaction section, and product analyses.
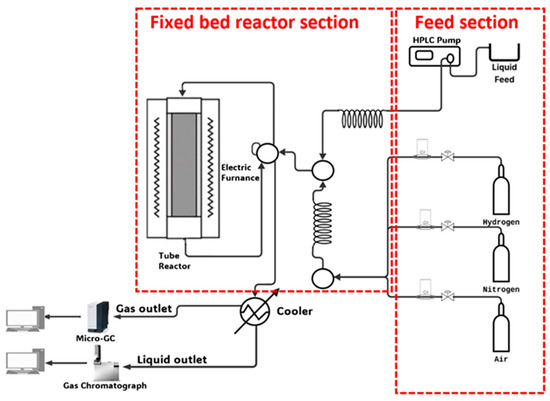
Figure 1.
The Microactivity—Pro unit simplified schematic diagram.
The reaction performance was evaluated through acetic acid conversion (X), H2 yield (YH2), and carbon-containing product selectivities (SCO, SCH4, SCO2, SC3H6O), calculated as shown in Equations (11)–(13):
where “F” refers to the molar flow rates of “i” species at either the inlet (in) or the outlet (out) of the reactor, “ni” represents the stoichiometric coefficient for maximum hydrogen generation, and “ai” is the stoichiometric factor for carbon between the acetic acid and each compound.
3. Results and Discussion
3.1. Catalyst Characterization
The elemental composition and the textural characteristics of the catalysts synthesized in the current research are summarized in Table 1. Concerning metal loadings calculated via ICP-OES, the calcined catalysts have Ce and Co contents nearing the nominal value established at the SBA-15 and Ce/SBA-15 impregnation, respectively.

Table 1.
Physical–chemical characteristics of Co/xCeO2-SBA-15 catalysts.
Figure 2A and Figure S1 show the N2 physisorption isotherms of the calcined catalysts and supports, respectively. These isotherms exhibited a type IV isotherm curve with an H1-type hysteresis loop according to the IUPAC classification, which is typical for mesoporous material consisting of well-defined cylindrical pore channels [40]. These results demonstrate that the characteristic porous structure of bare SBA-15 was preserved after the support modification with ceria and the cobalt active phase incorporation. Notably, some differences in the isotherm shape can be appreciated with the increase in Ce content, indicating a slight modification of the mesoporous structure. This effect is particularly evident for Co/20CeO2-SBA-15 and Co/30CeO2-SBA-15 samples because of their higher Ce content, resulting in 38.2% and 50.8% decreases in BET surface area, respectively, compared to the Co/SBA-15 catalyst. In line with this assumption, the pore volume, BET surface area, and mean pore diameter displayed in Table 1 and Table S1, corresponding to catalyst and supports, respectively, became smaller as the CeO2 amount increased, which can be ascribed to partial filling of SBA-15 channels. Similar behavior was observed after cobalt incorporation into the ceria-modified silica supports.
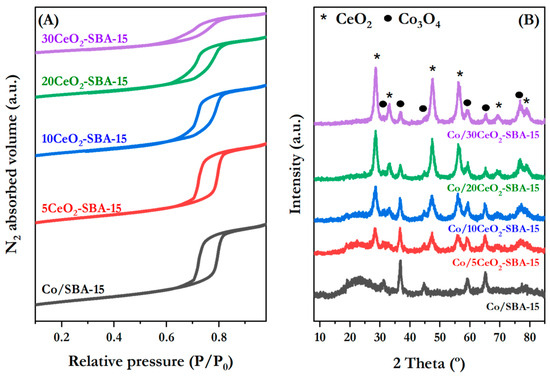
Figure 2.
N2 physisorption isotherms at 77 K (A) and XRD diffraction patterns (B) of calcined catalysts.
The UV-Vis diffuse reflectance spectra of the supports are shown in Figure S2. The UV-Vis spectra of the CeO2-SBA-15 supports show a broad absorption band centered at about 288 nm, ascribed to charge transfer transitions from O2− to Ce4+ [41,42]. An additional shoulder around 255 nm is noted in particular for the 5CeO2-SBA-15 support, possibly indicating the presence of Ce3+ species.
Band gap energy was estimated by the Tauc method, calculated from the intercept with the abscissa in a linear regression of the (F(R)·hυ)1/n vs. hυ representation, considering a direct electronic transition (n = ½) [43] (Figure S3). The obtained values exhibit a slight variation, 3.7 eV (5CeO2-SBA-15), 3.5 eV (10CeO2-SBA-15), 3.6 eV (20CeO2-SBA-15), and 3.7 eV (30CeO2-SBA-15). Such differences indicate possible alterations in the electronic structure of the supports, linked to the presence of structural defects. These defects are probably caused by the formation of oxygen vacancies. In particular, the decrease in the band gap observed in the 10CeO2-SBA-15 and 20CeO2-SBA-15 supports is likely related to an improved density of oxygen vacancies [44].
The crystalline phases of ceria and cobalt oxides supported on SBA-15 material were analyzed by XRD, and their diffraction patterns are illustrated in Figure 2B. All the catalysts show a broad peak between 20 and 30°, ascribed to the amorphous SiO2 on the SBA-15 framework [45]. The intensity of the peak becomes smaller as the CeO2 content increases. Peaks related to the Co3O4 cubic phase (JCPDS 01-071-4921) appear in all the patterns of the materials after calcination, showing reflections at 2θ = 31.2, 36.8, 44.8, 59.5, 65.4, and 77.7°, referring to the (220), (311), (400), (511), (440), and (533) diffraction planes, respectively. In addition to Co3O4 diffraction lines, Co/xCeO2-SBA-15 samples also show reflections at 2θ = 28.5, 32.9, 47.5, 56.4, 66.5, and 79.1° according to the planes (111), (200), (220), (311), (400), and (420) of the cubic CeO2 structure (JCPDS 01-089-8436), respectively. Comparing these XRD patterns, it is possible to observe that, as the CeO2 content increases, Co3O4 peaks become broader and less intense, suggesting more dispersed Co-species over the xCeO2-SBA-15-modified supports [46]. This effect was evidenced by the mean crystallite sizes on the Co3O4 main diffraction line. Over xCeO2-SBA-15, smaller crystallite sizes were achieved (see Table 1) than over Co/SBA-15, thus indicating higher metal dispersion in the modified CeO2 samples. Similarly, Jiménez-Morales et al. developed a variety of Ni/SBA-15 catalysts for the hydrogenolysis of glycerol, while studying the impact of Ce incorporation, and concluded that high active phase dispersion was achieved as a consequence of the interactions between metallic oxide particles and the cerium oxide ones [47].
The reducibility was assessed for catalysts produced using H2-TPR analysis, the reducing profiles of which are depicted in Figure 3. In all cases, several reduction features can be distinguished. The Co/SBA-15 catalyst displayed three reduction stages at 344 °C, 393 °C, and 472 °C. The first two are associated with the reduction of Co3+ to Co2+ and afterwards to Co0, while the third one, centered at 472 °C, is related to the existence of Co oxide species with a strong engagement with the SBA-15 support [34]. Cerium-modified catalysts showed a low-temperature reduction peak between 347 °C and 361 °C, which could be described as a simultaneous Co3+ reduction to Co2+ and Co2+ reduction to Co0 [48]. This reduction performance could be correlated with the redox properties of CeO2, which has a well-known oxygen storage and mobilization capacity [26]; this characteristic allowed it to capture and release oxygen easily, generating vacancies in the structure. Incorporating CeO2 into the SBA-15 support facilitates the surface migration and oxygen transfer from cobalt oxides, thus favoring the reduction processes. Furthermore, in these samples, the peak centered at 472 °C shifts toward slightly higher temperatures, suggesting a stronger metal–support interaction (MSI) between the cobalt oxide species and the support. It is associated with the smaller Co crystallite formation, increasing the interfacial contact area, leading to the surface chemical bonding [49]. This highlights the significant role of metal–support interaction in catalyst design since a suitable support will interact effectively with the metallic species, minimizing its surface migration and preventing sinterization. In contrast, scarce metal–support interaction would promote particle agglomeration, leading to a negative impact on the catalytic performance [50]. The Co/10CeO2-SBA-15 catalyst exhibited the most strongly pronounced reduction feature associated with strong metal–support interactions, while this peak was almost absent in the Co/30CeO2-SBA-15 catalyst. It could be attributed to the slight modification of the mesoporous structure. A further decrease in surface area could probably promote CeO2 agglomeration, hindering the effective cobalt oxide species dispersion. Consequently, the metal–support interaction effect between the cobalt phase and the SBA-15 structure was reduced. Additionally, reduction features at higher temperatures (735–769 °C) arise in all Co/xCeO2-SBA-15 catalysts, ascribed to the partial reduction of CeO2 to CeO2−x [25,51]. The reduction degree of Co species was assessed by quantifying the H2 consumption throughout the TPR analysis by comparing the experimental and theoretical H2 uptake (Table 2). The theoretical hydrogen uptake was calculated from the full reduction stoichiometry of the cobalt oxide loading (measured by ICP-OES). Meanwhile, the H2 experimental uptake was obtained by the H2-TPR profiles’ integration of the hydrogen uptake peaks, corresponding to the Co3O4 reduction. All the catalysts exhibited a reduction degree above 95%, with slightly higher values observed for the Ce-doped samples, which is in agreement with the shift in the reduction profile toward lower temperatures for Co species. This slight increase in the reducibility can be attributed to the oxygen vacancy generation caused by the incorporation of ceria [26]. In this respect, the Co/10CeO2-SBA-15 sample reached the highest reduction level, with its experimental uptake, in terms of μmol H2·gcat−1, closely matching the theoretical value.
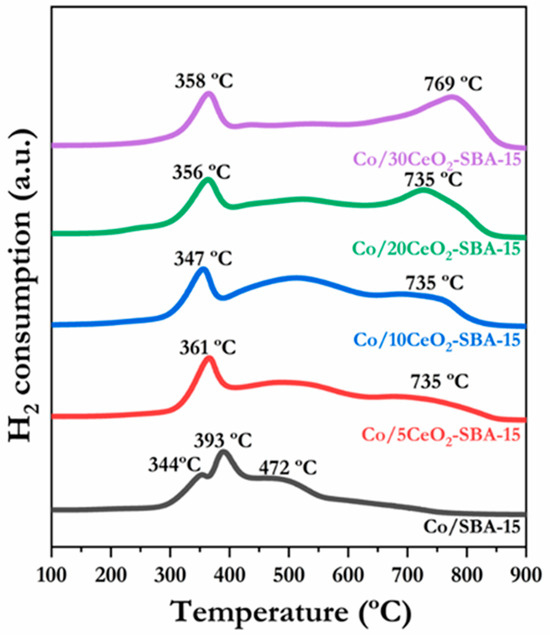
Figure 3.
H2-TPR profiles of calcined catalysts.

Table 2.
Experimental and theoretic H2 consumption and Co reduction degree for Co/xCeO2-SBA-15 calcined catalysts.
After the catalysts’ reduction step at 600 °C, samples were characterized by XRD analysis, and the resultant diffraction patterns are displayed in Figure 4. As can be discerned, no diffraction peaks corresponding to Co oxides can be distinguished, confirming that the reduction step proceeded successfully. In this sense, peaks arising at 2θ = 44.1 and 76.4° are attributed to the cubic phase of Co0 (JCPDS-15-0806). The Co0 mean crystal sizes in the reduced catalysts were determined based on the principal Co0 diffraction plane (111) using the Scherrer equation (Table 1). Notably, no diffraction features corresponding to Co0 can be observed for Co/20CeO2-SBA-15 and Co/30CeO2-SBA-15 catalysts, which reached a mean crystallite size lower than the detectable limit of the technique. This suggests the metallic phase was widely dispersed on the SBA-15 support modified with 20–30 wt.% of ceria.
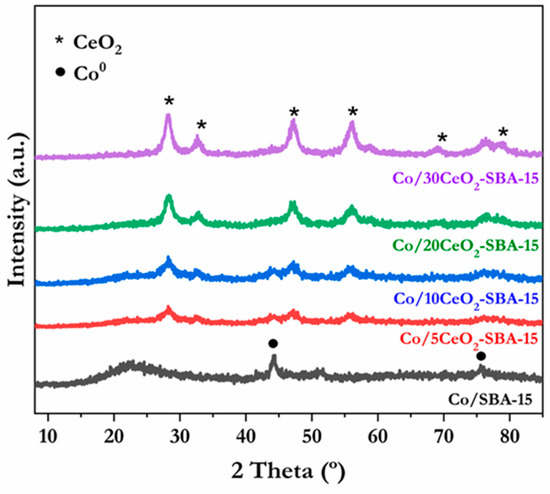
Figure 4.
XRD patterns obtained from catalysts reduced at 600 °C.
TEM micrographs for the reduced catalysts are presented in Figure 5, in which the well-ordered mesoporous structure typical for SBA-15 can be distinguished, indicating that the mesoporous structure is preserved. This observation aligns with the N2 physisorption results obtained for the calcined samples. In all cases, dark zones can be distinguished on the SBA-15 channels corresponding to Co0 and CeO2 particles, as corroborated by EDX. Furthermore, in the micrograph of Co/SBA-15 (Figure 5A), particles on the external surface of the SBA-15 are clearly distinguishable, suggesting some Co0 particle clustering, as reported before [52]. Moreover, not all of the SBA-15 surface or channels were filled with cobalt particles. On the other hand, in CeO2-doped catalysts (Figure 5B–E), smaller crystallites can be differentiated within SBA-15 channels, corroborating the higher active phase dispersion expected from the mean crystallite sizes shown in Table 1.
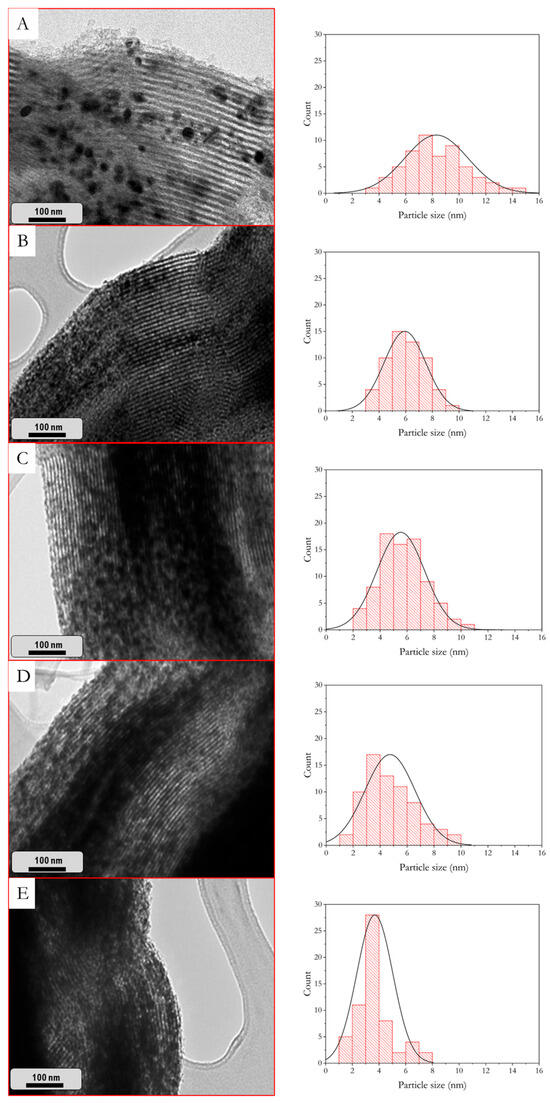
Figure 5.
TEM micrographs and particle size distribution histograms of the reduced catalysts at 600 °C. (A) Co/SBA15, (B) Co/5CeO2-SBA15, (C) Co/10CeO2-SBA15, (D) Co/20CeO2-SBA15, and (E) Co/30CeO2-SBA15.
From the TEM micrographs, particle size distribution histograms of the reduced catalysts were determined. For Co/SBA-15, the distribution was broader and centered at about 8 nm, indicating a higher particle size, in agreement with the agglomerate formation on the surface. With the CeO2 incorporation, a decrease in the particle size was observed along with a narrower distribution, with values around 3 nm attained for the Co/30CeO2-SBA-15 catalyst. This is consistent with the Co0 crystal size decrease determined by XRD (Table 1). Furthermore, even though Co0 and CeO2 particles were not distinguishable in the TEM micrographs, both were included in the measurement distribution, given that the CeO2 mean crystallite size ranges from 5.4 nm (Co/5CeO2-SBA-15) to 7.3 nm (Co/30CeO2-SBA-15), which overlaps with the distribution range.
3.2. Oxidative Steam Reforming of Acetic Acid
The effect of the CeO2 content on the catalytic activity of Co/xCeO2-SBA-15 catalysts in AAOSR was evaluated in the range of 400 to 700 °C. In this regard, Figure 6a,b display the results of catalytic activity concerning H2 yield and acetic acid conversion as a function of the reaction temperature, respectively. As predicted, given the endothermic characteristics of the reforming reactions, an increase in the reaction temperature led to an improvement in the catalytic activity for all the catalysts [53]. Concerning the H2 yield, low values were achieved at temperatures below 500 °C. However, increasing the reaction temperature from 500 °C to 550 °C significantly enhanced the H2 yield, with values around 30% higher reached for the catalysts supported over modified CeO2-SBA-15. Beyond this temperature, ceria-containing samples considerably increased the H2 yield as compared to Co/SBA-15, reaching H2 yields up to 70–75% in all cases, near thermodynamic equilibrium values. Therefore, the effect of Ce content was less remarkable with further temperature rises.

Figure 6.
Effect of the CeO2 addition on the H2 yield (a) and the acetic acid conversion (b) at different temperatures (400–700 °C). Reaction conditions: S/C molar ratio = 2, O2/C molar ratio = 0.0375, WHSV = 30.2 h−1, P = 1 atm.
To analyze the efficacy of the incorporation of CeO2 into the Co/SBA-15 catalysts, concerning acetic acid conversion, it is required to focus on temperatures below 550 °C. Over this range, the Co/5CeO2-SBA-15 catalyst achieved approximately 5% greater acetic acid conversion than Co/SBA-15. Similarly, the acetic acid conversion increased by almost 20% for Ce contents over 10 wt.% in the catalyst formulation. Likewise, acetic acid conversion increased by over 40% when increasing the temperature from 500 to 550 °C, reaching almost complete acetic acid conversions for catalysts Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15. At temperatures above 600 °C, almost complete conversion was reached for all catalysts. These findings align with those mentioned by Bie et al. [26], who stated that cerium’s incorporation onto Co/SBA-15 catalysts for CO oxidation activated the oxygen in the surface lattice of SBA-15 (Ce3+/4+-O-Si) and generated oxygen vacancies. In this way, the behavior of CeO2-based materials has been related to ceria redox properties, which can release, capture, and store oxygen according to the following redox reaction:
where Ox is the lattice oxygen at the CeO2 surface [51]. This fact can promote the activity performance toward reactions such as the WGS (Equation (3)), which may increase the hydrogen yield [34]. Moreover, this lattice oxygen could induce a decrease in carbon deposition, promoting coke gasification [16,51]. During the AAOSR tests, the Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 samples showed similar H2 yield and acetic acid conversion values. In contrast, the Co/30CeO2-SBA-15 catalyst showed a slightly lower catalytic performance than the latter ones. The catalytic activity observed in the Co/30CeO2-SBA-15 catalyst can be attributed to its textural properties, and lower oxygen vacancy. The Co/30CeO2-SBA-15 catalyst exhibits the smallest BET surface area (240 m2/g) and pore volume (0.50 cm3/g), thus indicating a partial blockage of the mesoporous structure, which may hinder the diffusion of reactants and products and limit the accessibility to active sites in comparison with Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15. Consequently, Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 catalysts demonstrate potential as suitable catalysts for hydrogen production through AAOSR.
Product distribution includes not only hydrogen but also several by-products detected in the output stream. In this context, the relations between (a) acetone, (b) methane, (c) carbon dioxide, and (d) carbon monoxide selectivities and the reaction temperature are graphically displayed in Figure 7. Acetone (Figure 7a) is an intermediate product that mainly was obtained at temperatures between 400 °C and 550 °C. Acetone would have been formed by adding acetyl (CH3CO*) and methyl (CH3*) groups that come from the acetic acid decomposition on the Co surface, according to Li et al. [54]. Acetone selectivity was significantly enhanced after the addition of CeO2 to the Co/SBA-15 catalyst, reaching values between 65% and 70% at 400 °C with Co/10CeO2-SBA-15, Co/20CeO2-SBA-15, and Co/30CeO2-SBA-15 catalysts. These results align with the findings reported by Davidson et al. [55] and Basagiannis et al. [56], who experimented with CeO2-supported catalysts in the steam reforming (Equation (1)) and ketonization of acetic acid (Equation (5)); they concluded that CeO2 promotes the ketonization of acetic acid. The mechanism of acetic acid ketonization proposed by Bossola et al. [57] suggests that acetone formation takes place via the ketene intermediate, proceeding from acetic acid decomposition to the acyl group, which occurs on the CeO2 support surface, predominating at low reaction temperatures (<250–350 °C). This would suggest that the acetic acid ketonization pathway has been thermodynamically unfavored, given that it is an exothermic reaction (ΔH25 °C =16.7 KJ/mol). Moreover, by increasing the reaction temperature, the acetone selectivity decreases, dropping to near 0% above 550 °C alongside increasing CO2 (Figure 7c) and CO (Figure 7d) selectivity, which could imply that acetone steam reforming is undergoing.

Figure 7.
(a) Acetone, (b) methane, (c) CO2, and (d) CO selectivity at different temperatures (400–700 °C). Reaction conditions: S/C molar ratio = 2, O2/C molar ratio = 0.0375, WHSV = 30.2 h−1, P = 1 atm.
Accordingly, above 550 °C, a slight enhancement in CO2 selectivity alongside a CO selectivity decrease was observed on Ce-doped catalysts. Conversely, in the Co/SBA-15 catalyst, the CO selectivity increased alongside a slight CO2 selectivity decrease. One of the possible reaction pathways for the Co/SBA-15 catalyst is the reverse water–gas shift (RWGS), which was thermodynamically favored as the temperature increased. By contrast, Ce-doped catalysts would be kinetically favoring the gas–water shift reaction (WGS) (Equation (3)), as stated above. Specifically, CeO2 incorporation enhances the oxygen vacancy density on the catalyst surface [51]; thus, these vacancies promote the WGS reaction efficiently, oxidizing CO into CO2. In addition, the improved cobalt dispersion leads to a greater number of active sites, evenly distributed, which further facilitates the CO oxidation.
Finally, the methane selectivity (Figure 7b) remained at very low values (<5%) from 400 to 700 °C, suggesting that the thermal decomposition of acetic acid to methane (Equation (4)) is not a kinetically or thermodynamically relevant pathway. Sun et al. [58] reported that one possible pathway to methane generation is the hydrogenation of CH3* species, proceeding from the breaking of C-C bonds, which could be kinetically disfavored when using cobalt-based catalysts as well as catalysts supported on metal oxides such as CeO2. There has been speculation that this effect could be caused by the ease of cleavage of C-H bonds, thus limiting the formation of CH3* species, or also by the oxidation of CH3* species to COx due to the oxygen mobility.
Given that the Co/xCeO2-SBA-15 catalyst with 10–20 wt.% of CeO2 exhibited the best catalytic performance, its stability was evaluated through a long-term test at 550 °C.
3.3. Stability Test on Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 Catalysts
Avoiding catalyst deactivation caused by harsh operating conditions has been a primary issue in the steam reforming process. Hence, the development of a stable catalyst plays a main role in hydrogen formation via AAOSR. The catalytic activity of Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 catalysts, with respect to acetic acid conversion and H2 yield, has been investigated at 550 °C and under the same reaction conditions for long time-on-stream periods (50 h). Figure 8 displays the results that were achieved. The initial H2 yield for the Co/10CeO2-SBA-15 catalyst was 66.8%, and it was slightly lower (62%) for the Co/20CeO2-SBA-15 sample. These two catalysts showed a slightly decreasing yield throughout the reaction time (50 h), reaching approximately 55% at 50 h. Similarly, the acetic acid conversion curves started at around 98% for both catalysts and ended at approximately 85%. H2 yields close to the thermodynamic equilibrium value (67.4%) and high conversion values were achieved. These results highlight their capacity to maintain suitable catalytic performance over a prolonged period, constituting a fundamental requirement for continuous hydrogen production.

Figure 8.
Evolution over time-on-stream of H2 yield and conversion using Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 in the AAOSR. Reaction conditions: T = 550 °C, P = 1 atm, S/C ratio = 2, O2/C molar ratio = 0.0375, and WHSV = 30.2 h−1.
To compare the findings of this study with the previously reported studies of catalytic systems for acetic acid oxidative steam reforming, the stability and catalytic performance were compared to recent data reported in the literature (Table 3). In this way, a reliable reference framework for analyzing the feasibility of the proposed catalysts was made available.

Table 3.
Catalytic performance of several catalysts in acetic acid oxidative steam reforming.
Table 3 shows that the Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 catalysts achieved notable H2 yields, in spite of being tested under thermodynamically and kinetically less favorable conditions (550 °C). In the literature, most of the research on acetic acid oxidative steam reforming has focused on Ni-based catalysts, frequently operating in autothermal conditions at 700 °C [37,59,60,61,63,64,65], reaching H2 yields of 62-65%. Although a straightforward comparison becomes difficult due to differences in experimental conditions, the use of a standardized H2 yield expression allows a more objective assessment. A competitive performance is demonstrated by Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 catalysts. Furthermore, a comparison with our studies performed under similar conditions [25,38,39] showed that both catalysts exhibited excellent long-term stability, maintaining their activity over 50 h time-on-stream.
3.4. Characterization of Used Catalysts After Stability Tests
Coke formation is widely recognized as a main deactivation factor in reforming catalysts, along with sintering and/or oxidation [66]. Thus, the used catalysts, after 50 h of time-on-stream, were characterized through XRD, TEM, Raman spectroscopy, and TGA techniques. Figure 9 displays the XRD patterns of the two spent catalysts. The presence of a new diffraction feature at 2θ = 26.31° is noticeable, as compared with the calcined and reduced catalysts, attributed to graphitic carbon (JCPDS 01-074-2329), in both catalysts. The Co/20CeO2-SBA-15 catalyst displayed a more intense diffraction peak, suggesting that a higher coke deposition was taking place during the reaction. Furthermore, despite the oxidizing atmosphere during the oxidative steam reforming reaction, no Co3O4 peaks were observed in either sample, indicating that the active Co0 species did not oxidize after 50 h of reaction. Similar to the calcined and reduced catalysts, the mean crystallite sizes of metallic Co species were calculated using the Scherrer equation, with mean crystallite sizes of 5.0 for Co/10CeO2-SBA-15 and 4.2 nm for Co/20CeO2-SBA-15 obtained. Taking into account the crystallite sizes of the reduced catalysts (Table 1), a small increase in the mean crystallite size can be inferred, suggesting a moderate sintering effect; according to Ochoa et al. [66], the sintering route is significantly affected by the size of the crystal.
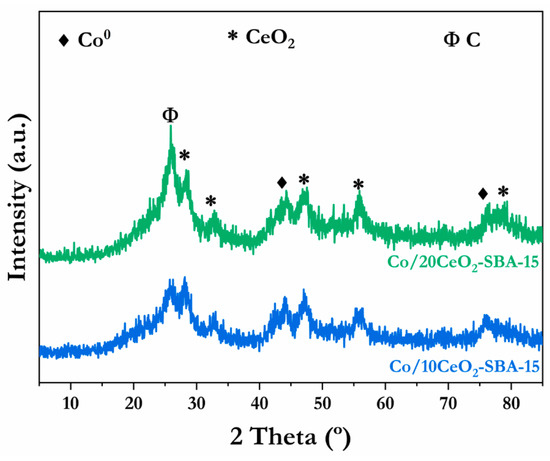
Figure 9.
XRD diffraction patterns of the spent catalysts after stability tests for 50 h and at 550 °C.
The mass loss profiles together with the thermogravimetric derivative curves of each spent catalyst are displayed in Figure 10. In both samples, the mass percentage started decreasing when the temperature reached around 450 °C, with most of the weight loss in the temperature range 450–600 °C. The coke deposition rate was calculated as 7.2 mgcoke·gcat−1·h−1 for the Co/10CeO2-SBA-15 catalyst and 12.0 mgcoke·gcat−1·h−1 for Co/20CeO2-SBA-15. The DTG profiles display two peaks, a lower-intensity peak centered at 460–480 °C, and a high-amplitude and intensity peak at 550–570 °C. According to the literature, these peaks correspond to the oxidation of deposited carbon nanofilaments with differing ordering degrees. At low temperatures (460–480 °C), filamentous carbon species oxidation took place, which was related to the coke deposition on the metal surface. At higher temperatures (550–570 °C), carbon species were described as aged filamentous carbon, which would be deposited on the catalyst support [51,67]. Coke deposited on the metallic sites would block the active sites directly, affecting the catalytic activity. In contrast, coke deposited on the support would mainly affect the reactants’ diffusion. On both catalysts, the coke deposited on the support (higher temperature peak) was predominant. A higher intensity for Co/20CeO2-SBA-15 was observed, indicating a high carbon total accumulation.
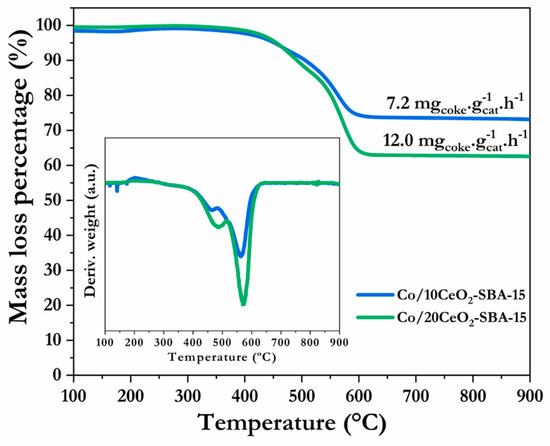
Figure 10.
TG and DTG of the spent catalysts after stability test at 50 h, 550 °C.
The spent samples were also characterized by TEM, as shown in Figure 11. The SBA-15 porous mesostructure was preserved after carrying out the catalytic activity tests. Well-dispersed dark rounded particles can be appreciated, which correspond to Co0 and CeO2. Concerning coke deposition, nanofilamentous coke can be appreciated for both samples, in agreement with DTG results. According to the literature [66], this coke type is generally ascribed to transition metals, such as Co, Ni, or Fe.
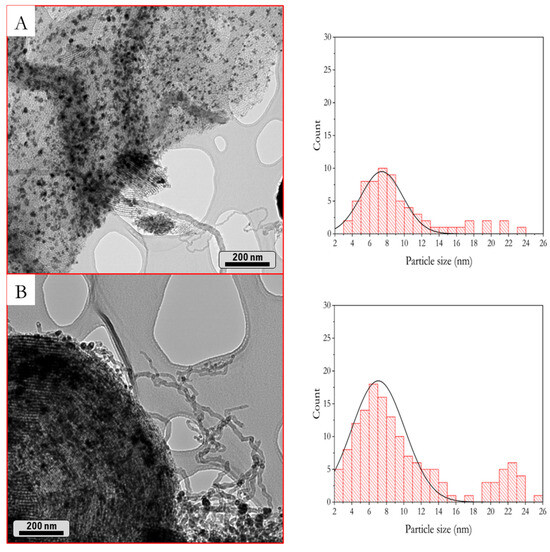
Figure 11.
TEM micrographs and particle size distribution histograms of used catalysts at 50 h, 550 °C. (A) Co/10CeO2-SBA15 and (B) Co/20CeO2-SBA15.
The particle size distribution histograms were obtained from TEM micrographs (Figure 11). The histograms show a shift in the particle size distribution to higher particle sizes in comparison with the reduced catalysts. Particularly, the Co/20CeO2-SBA-15 catalyst presents a higher frequency of particles above 16 nm in size, deviating with respect to the Gaussian distribution profile. These higher particle sizes are related to sintering phenomena caused by the coke filament formation. These filaments would cause the detachment of the Co0 crystals from the mesoporous structure, favoring their agglomeration [21,66].
Finally, Figure 12 displays the Raman spectra of the spent catalysts. These spectra exhibit two predominant bands: the D band (~1340 cm−1) and the G band (~1590 cm−1), both attributable to different carbon structures [68,69]. The ratio of these two bands, represented as the ID/IG ratio, has been commonly applied as an indicator of the degree of structural order in the deposited carbon species [68]. In this research, TEM and TGA/DTG analysis demonstrated that the coke was composed mainly of carbonaceous nanostructures, as filaments with different degrees of ordering. Such structures are associated with a higher intensity in the D band [68]; therefore, an increase in the ID/IG ratio could be associated with a greater proportion of them. Accordingly, the Co/20CeO2-SBA-15 catalyst exhibited an ID/IG ratio of 1.4, higher than the 0.8 of Co/10CeO2-SBA-15, suggesting a greater amount of filamentous carbon with a less-ordered structure and, possibly, more tendency for coke accumulation.
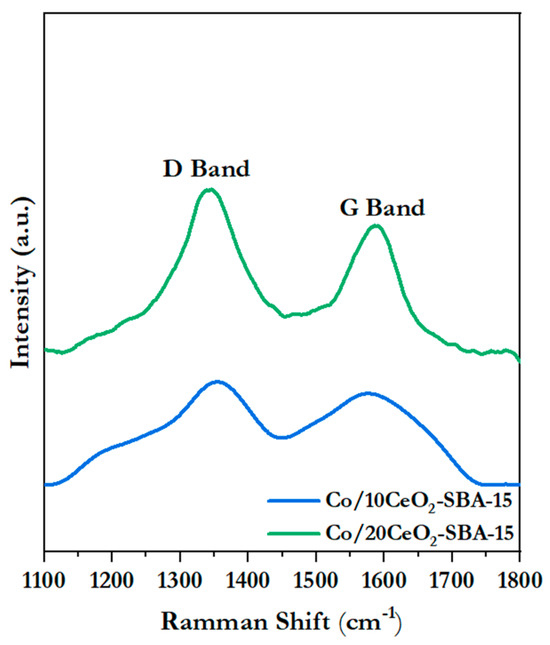
Figure 12.
Raman spectra of the spent catalyst in AAOSR.
4. Conclusions
This study highlights the significant potential of CeO2-doped Co/SBA-15 catalysts for AAOSR, as bio-oil aqueous phase representative compounds. The characterization findings revealed that Ce-doped catalysts preserved the well-ordered mesostructure of the SBA-15 support while increasing the catalyst’s reducibility. Additionally, the incorporation of CeO2 improved cobalt dispersion, as evidenced by smaller crystallite sizes, thus enhancing the catalytic performance and reducing the coke formation rate. Catalysts with 10–20 wt.% CeO2 exhibited the best catalytic performance, reaching H2 yields close to thermodynamic equilibrium and nearly complete acetic acid conversion at 550 °C. Furthermore, these catalysts maintained the H2 yield above 55% and high acetic acid conversion with a low coke formation rate. These findings underscore the importance of using CeO2 as a promoter in the catalyst formulation, highlighting that Co/10CeO2-SBA-15 and Co/20CeO2-SBA-15 catalysts are promising for reforming purposes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15116376/s1, Figure S1: N2 physisorption isotherms at 77 K of calcined supports; Figure S2: UV-vis spectra of calcined supports; Figure S3: Application of Tauc method for the determination of band-gap energy in A. 5CeO2-SBA-15, B. 10CeO2-SBA-15, C. 20CeO2-SBA-15, and D. 30CeO2-SBA-15 supports; Table S1: Physical–chemical characteristics of xCeO2-SBA-15 supports; Table S2: Hydrogen yield in acetic acid steam oxidative reforming; Table S3: Acetic acid conversion in oxidative steam reforming.
Author Contributions
C.A.C.: Investigation, Formal analysis, Data curation, Writing—original draft, Writing—review and editing. Á.M.d.l.C.: Investigation. P.J.M.: Methodology, Formal analysis, Data curation, Writing—review and editing. A.J.V.: Formal analysis, Writing—review and editing, Funding acquisition. J.A.C.: Conceptualization, Writing—review and editing, Funding acquisition. A.C.: Conceptualization, Formal analysis, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financially supported by the Spanish Ministry of Science and Innovation (Projects TED2021-131499B-I00 and PID2020-117273RB-I00).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IEA. Global Energy Review: CO2 Emissions in 2021. Available online: https://iea.blob.core.windows.net/assets/c3086240-732b-4f6a-89d7-db01be018f5e/GlobalEnergyReviewCO2Emissionsin2021.pdf (accessed on 9 December 2023).
- Zhumadilova, A.; Zhigitova, S.; Turalina, M. The Impact of Greenhouse Gases on Climate Change. Sci. Horiz. 2023, 26, 97–109. [Google Scholar] [CrossRef]
- Paris Agreement to the United Nations Framework Convention on Climate Change, 12 December 2015, T.I.A.S. No. 16-1104. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 23 May 2025).
- IRENA. World Energy Transitions Outlook 2023: 1.5 °C Pathway. Available online: https://www.irena.org/Publications/2023/Jun/World-Energy-Transitions-Outlook-2023 (accessed on 9 December 2023).
- Global Carbon Budget Fossil CO2 Emissions at Record High in 2023. Available online: https://globalcarbonbudget.org/fossil-co2-emissions-at-record-high-in-2023/ (accessed on 9 December 2023).
- United Nations Climate Change Annual Report 2022. Available online: https://unfccc.int/sites/default/files/resource/UNClimateChange_AnnualReport_2022.pdf (accessed on 11 December 2023).
- Kobina, M.; Gil, S. Green Hydrogen: A Key Investment for the Energy Transition. Available online: https://blogs.worldbank.org/ppps/green-hydrogen-key-investment-energy-transition (accessed on 11 December 2023).
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X.; Liu, Q. A Mini Review of the Specialties of the Bio-Oils Produced from Pyrolysis of 20 Different Biomasses. Renew. Sustain. Energy Rev. 2019, 114, 109313. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Aziz, M.A.A.; Abdullah, S.; Teh, L.P.; Jusoh, R. Hydrogen Production from Catalytic Steam Reforming of Biomass Pyrolysis Oil or Bio-Oil Derivatives: A Review. Int. J. Hydrogen Energy 2020, 45, 18376–18397. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Q.; Zhang, F.; Li, X.; Zhang, L.; Luo, Z. Hydrogen Production via Catalytic Reforming of the Bio-Oil Model Compounds: Acetic Acid, Phenol and Hydroxyacetone. Int. J. Hydrogen Energy 2014, 39, 18675–18687. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Thermodynamic Analysis of Hydrogen Production via Steam Reforming of Selected Components of Aqueous Bio-Oil Fraction. Int. J. Hydrogen Energy 2007, 32, 212–223. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Wang, Y. Catalysts for Steam Reforming of Bio-Oil: A Review. Ind. Eng. Chem. Res. 2017, 56, 4627–4637. [Google Scholar] [CrossRef]
- Souza, I.C.A.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen Production from Steam Reforming of Acetic Acid over Pt–Ni Bimetallic Catalysts Supported on ZrO2. Biomass Bioenergy 2022, 156, 106317. [Google Scholar] [CrossRef]
- Cortese, M.; Ruocco, C.; Palma, V.; Megía, P.J.; Carrero, A.; Calles, J.A. On the Support Effect and the Cr Promotion of Co Based Catalysts for the Acetic Acid Steam Reforming. Catalysts 2021, 11, 133. [Google Scholar] [CrossRef]
- Megía, P.J.; Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Effect of the Incorporation of Reducibility Promoters (Cu, Ce, Ag) in Co/CaSBA-15 Catalysts for Acetic Acid Steam Reforming. Int. J. Energy Res. 2020, 45, 1685–1702. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Resasco, D.E.; Jensen, A.D. Steam Reforming of Light Oxygenates. Catal. Sci. Technol. 2013, 3, 3292–3302. [Google Scholar] [CrossRef]
- Arandia, A.; Remiro, A.; García, V.; Castaño, P.; Bilbao, J.; Gayubo, A.G. Oxidative Steam Reforming of Raw Bio-Oil over Supported and Bulk Ni Catalysts for Hydrogen Production. Catalysts 2018, 8, 322. [Google Scholar] [CrossRef]
- Remiro, A.; Arandia, A.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Stability of a Rh/CeO2-ZrO2 Catalyst in the Oxidative Steam Reforming of Raw Bio-Oil. Energy Fuels 2018, 32, 3588–3598. [Google Scholar] [CrossRef]
- Goicoechea, S.; Ehrich, H.; Arias, P.L.; Kockmann, N. Thermodynamic Analysis of Acetic Acid Steam Reforming for Hydrogen Production. J. Power Sources 2015, 279, 312–322. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Ruiz-Abad, M.; Calles, J.A.; Carrero, A. Coke Evolution in Simulated Bio-Oil Aqueous Fraction Steam Reforming Using Co/SBA-15. Catal. Today 2021, 367, 145–152. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Hydrogen Production by Steam Reforming of Ethanol over Co/CeO2 Catalysts: Effect of Cobalt Content. J. Energy Inst. 2019, 92, 222–238. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Jalil, A.A.; Firmansyah, L.; Triwahyono, S. Production of Hydrogen via Steam Reforming of Acetic Acid over Ni and Co Supported on La2O3 Catalyst. Int. J. Hydrogen Energy 2017, 42, 8975–8985. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Miralles-Martínez, A.; García-Muñoz, B.; Maestro-Cuadrado, S.; Chica, A. Ni and Co-Based Catalysts Supported on ITQ-6 Zeolite for Hydrogen Production by Steam Reforming of Ethanol. Int. J. Hydrogen Energy 2023, 48, 26518–26525. [Google Scholar] [CrossRef]
- Megía, P.J.; Morales, A.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Oxidative Steam Reforming of Acetic Acid on Ni Catalysts: Influence of the La Promotion on Mesostructured Supports. Int. J. Hydrogen Energy 2024, 52, 1136–1145. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, J.; Xiao, P.; Zhao, Y. Cobalt Oxide Supported on Cex-SBA-15 for CO Oxidation: Effect of Ce Addition. Chem. Lett. 2016, 45, 1359–1361. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; Lindo, M. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Catalysts 2015, 5, 58–76. [Google Scholar] [CrossRef]
- Cifuentes, B.; Hernández, M.; Monsalve, S.; Cobo, M. Hydrogen Production by Steam Reforming of Ethanol on a RhPt/CeO2/SiO2 Catalyst: Synergistic Effect of the Si:Ce Ratio on the Catalyst Performance. Appl. Catal. A Gen. 2016, 523, 283–293. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Rabelo-Neto, R.C.; Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. A Relationship between the Production of Oxygenates from Ethanol/Steam Mixtures and the Oxygen Mobility in Transition Metal Oxide Doped CeO2·SiO2 Catalysts. J. Phys. Chem. C 2014, 118, 28007–28016. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Oxidative Steam Reforming of Ethanol on Mesoporous Silica Supported PtNi/CeO2 Catalysts. Int. J. Hydrogen Energy 2017, 42, 1598–1608. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Ce and La Modification of Mesoporous Cu–Ni/SBA-15 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Microporous Mesoporous Mater. 2009, 119, 200–207. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y. Influence of Ceria Existence Form on Deactivation Behavior of Cu-Ce/SBA-15 Catalysts for Methanol Steam Reforming. Int. J. Hydrogen Energy 2023, 48, 1323–1336. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Neuberg, S.; Hessel, V.; Kolb, G. Effect of Ceria and Zirconia Promotors on Ni/SBA-15 Catalysts for Coking and Sintering Resistant Steam Reforming of Propylene Glycol in Microreactors. Appl. Catal. B 2017, 203, 859–869. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen Production through Glycerol Steam Reforming Using Co Catalysts Supported on SBA-15 Doped with Zr, Ce and La. J. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, L.; Dong, C.; Lu, Q.; Du, X.; Dahlquist, E. Catalytic Steam Reforming of Toluene as a Model Compound of Biomass Gasification Tar Using Ni-CeO2/SBA-15 Catalysts. Energies 2013, 6, 3284–3296. [Google Scholar] [CrossRef]
- Su, Y.; Shu, C.; Ding, C.; Chen, Q.; Xu, Y.; Sheng, J.; Huang, L. Solid Solution of Ce1−xYxO2−δ Supported Nickel-Based Catalysts for Auto-Thermal Reforming of Acetic Acid with High Resistance to Coking. J. Energy Inst. 2024, 114, 101589. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhong, X.; Xie, X.; Jia, X.; Chen, B.; Wang, N.; Huang, L. Auto-Thermal Reforming of Acetic Acid for Hydrogen Production by Ordered Mesoporous Ni-XSm-Al-O Catalysts: Effect of Samarium Promotion. Renew. Energy 2020, 145, 2316–2326. [Google Scholar] [CrossRef]
- Megía, P.J.; Morales, A.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production through Oxidative Steam Reforming of Acetic Acid over Ni Catalysts Supported on Ceria-Based Materials. Catalysts 2022, 12, 1526. [Google Scholar] [CrossRef]
- Megía, P.J.; Soria, M.A.; Cerqueira, P.; Vizcaíno, A.J.; Carrero, A.; Calles, J.A.; Madeira, L.M. Influence of Rh Addition to Transition Metal-Based Catalysts in the Oxidative Steam Reforming of Acetic Acid. Catal. Today 2024, 429, 114479. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Synthesis, Characterization and Catalytic Performances of Ce-SBA-15 Supported Nickel Catalysts for Methane Dry Reforming to Hydrogen and Syngas. Int. J. Hydrogen Energy 2012, 37, 19–30. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, L.; Xu, J.; Zheng, Q.; Pan, L.; Zou, J.; Zhang, X.; Li, G. Boosting Hydrogen Production from Steam Reforming of Ethanol on Nickel by Lanthanum Doped Ceria. Appl. Catal. B 2021, 286, 119884. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, C.; Li, P.; Wang, D.; Zhang, X.; Wang, L.; Zou, J.J.; Li, G. Engineering Oxygen Vacancies on Tb-Doped Ceria Supported Pt Catalyst for Hydrogen Production through Steam Reforming of Long-Chain Hydrocarbon Fuels. Chin. J. Chem. Eng. 2024, 68, 181–192. [Google Scholar] [CrossRef]
- Vaja, F.; Oprea, O.; Ficai, D.; Ficai, A.; Guran, C. Synthesis of CeO2 Nanoparticles on the Mesoporous Silica Support via Nanocasting. Dig J Nanomater Biostruct 2014, 9, 187–195. [Google Scholar]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Wang, J.; Prasad, S.; Ahamed, M.; Cheng, C.; Byrappa, K. CeO2 Nanostructures Enriched with Oxygen Vacancies for Photocatalytic CO2 Reduction. ACS Appl. Nano Mater. 2020, 3, 138–148. [Google Scholar] [CrossRef]
- Munasir, M.; Hidayat, N.; Kusumawati, D.H.; Putri, N.P.; Taufiq, A.; Sunaryono, S. Amorphous-SiO2nanoparticles for Water Treatment Materials. AIP Conf. Proc. 2020, 2251, 040030. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete Oxidation of Benzene with Cobalt Oxide and Ceria Using the Mesoporous Support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of Glycerol to Obtain 1,2-Propanediol on Ce-Promoted Ni/SBA-15 Catalysts. Appl. Catal. B 2012, 117–118, 253–259. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S. Temperature Programme Reduction (TPR) Studies of Cobalt Phases in -Alumina Supported Cobalt Catalysts. J. Pet. Technol. Altern. Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of Metal-Support Interactions in Heterogeneous Catalysts to Enhance Activity and Selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Safari, M.; Haghtalab, A.; Roghabadi, F.A. Tuning the Strong Metal Support Interaction of the Fischer-Tropsch Synthesis Silica-Coated Cobalt-Based Nano-Catalyst. Int. J. Hydrogen Energy 2024, 65, 348–361. [Google Scholar] [CrossRef]
- Pu, J.; Ikegami, F.; Nishikado, K.; Qian, E.W. Effect of Ceria Addition on NiRu/CeO2Al2O3 Catalysts in Steam Reforming of Acetic Acid. Int. J. Hydrogen Energy 2017, 42, 19733–19743. [Google Scholar] [CrossRef]
- Rodrigues, J.J.; Fernandes, F.A.N.; Rodrigues, M.G.F. Study of Co/SBA-15 Catalysts Prepared by Microwave and Conventional Heating Methods and Application in Fischer–Tropsch Synthesis. Appl. Catal. A Gen. 2013, 468, 32–37. [Google Scholar] [CrossRef]
- Haynes, D.J.; Shekhawat, D. Oxidative Steam Reforming. Fuel Cells Technol. Fuel Process. 2011, 129–190. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhu, Y.; Yang, G.; Zheng, P. DFT Study of Bio-Oil Decomposition Mechanism on a Co Stepped Surface: Acetic Acid as a Model Compound. Int. J. Hydrogen Energy 2015, 40, 330–339. [Google Scholar] [CrossRef]
- Davidson, S.D.; Spies, K.A.; Mei, D.; Kovarik, L.; Kutnyakov, I.; Li, X.S.; Lebarbier Dagle, V.; Albrecht, K.O.; Dagle, R.A. Steam Reforming of Acetic Acid over Co-Supported Catalysts: Coupling Ketonization for Greater Stability. ACS Sustain. Chem. Eng. 2017, 5, 9136–9149. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Reforming Reactions of Acetic Acid on Nickel Catalysts over a Wide Temperature Range. Appl. Catal. A Gen. 2006, 308, 182–193. [Google Scholar] [CrossRef]
- Bossola, F.; Recchia, S.; Santo, V.D. Catalytic Steam Reforming of Acetic Acid: Latest Advances in Catalysts Development and Mechanism Elucidation. Curr. Catal. 2017, 7, 89–98. [Google Scholar] [CrossRef]
- Sun, J.; Mei, D.; Karim, A.M.; Datye, A.K.; Wang, Y. Minimizing the Formation of Coke and Methane on Co Nanoparticles in Steam Reforming of Biomass-Derived Oxygenates. ChemCatChem 2013, 5, 1299–1303. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Q.; Hu, X.; Ding, C.; Huang, L.; Wang, N. Mullite-like SmMn2O5-Derived Composite Oxide-Supported Ni-Based Catalysts for Hydrogen Production by Auto-Thermal Reforming of Acetic Acid. Materials 2024, 17, 2490. [Google Scholar] [CrossRef]
- Chen, Q.; Liao, F.; Ding, C.; Hu, X.; Xu, Y.; Cheng, P.; Zheng, Z.; Huang, L.; Wang, N. Reaction Induced Ni/MgTi2O5 Interface Promotes the Resistance to Sintering and Oxidation in Auto-Thermal Reforming of Acetic Acid. Catal. Today 2024, 432, 114635. [Google Scholar] [CrossRef]
- Gan, M.; Liao, F.; Chen, Q.; Pang, F.; Xu, Y.; Su, Y.; Huang, L. Y2Ti2O7 Pyrochlore Supported Nickel-Based Catalysts for Hydrogen Production by Auto-Thermal Reforming of Acetic Acid. Mater. Sci. Eng. B 2024, 302, 117264. [Google Scholar] [CrossRef]
- Hu, X.; Ding, C.; Wang, Q.; Chen, H.; Jia, X.; Huang, L. Preparation of Co-Ce-O Catalysts and Its Application in Auto-Thermal Reforming of Acetic Acid. Inorg. Chem. Commun. 2022, 141, 109537. [Google Scholar] [CrossRef]
- Ding, C.; Hu, X.; Sun, W.; Hailili, R.; Liao, F.; Shu, C.; Huang, J.; Huang, L.; Wang, N. Interface of Ni-MgCr2O4 Spinel Promotes the Autothermal Reforming of Acetic Acid through Accelerated Oxidation of Carbon-Containing Intermediate Species. ACS Catal. 2023, 13, 4560–4574. [Google Scholar] [CrossRef]
- Cheng, P.; Gan, M.; Shu, C.; Ding, C.; Chen, Q.; Xu, Y.; Huang, L.; Wang, N. Layered Perovskite-Derived Ni/La2−2xPr2xO3 Catalysts for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid. Int. J. Hydrogen Energy 2025, 104, 13–22. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Wang, N.; Chen, B.; Xie, X.; Wang, Q.; Huang, L. Auto-Thermal Reforming of Acetic Acid over Hydrotalcites-Derived Co-Based Catalyst: A Stable and Anti-Coking Co/Sr-Alx-O Catalyst. Appl. Catal. B 2020, 267, 118370. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Coke Formation and Deactivation during Catalytic Reforming of Biomass and Waste Pyrolysis Products: A Review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-Promoted Ni/SBA-15 Catalysts for Ethanol Steam Reforming with Enhanced Activity and Resistance to Deactivation. Appl. Catal. B 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Megía, P.J.; Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen Production from Steam Reforming of Acetic Acid as a Model Compound of the Aqueous Fraction of Microalgae HTL Using Co-M/SBA-15 (M: Cu, Ag, Ce, Cr) Catalysts. Catalysts 2019, 9, 1013. [Google Scholar] [CrossRef]
- Landa, L.; Valecillos, J.; Remiro, A.; Valle, B.; Bilbao, J.; Gayubo, A.G. Comparison of the NiAl2O4 Derived Catalyst Deactivation in the Steam Reforming and Sorption Enhanced Steam Reforming of Raw Bio-Oil in Packed and Fluidized-Bed Reactors. Chem. Eng. J. 2023, 458, 141494. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).