Abstract
Food-Induced Immediate Response of the Esophagus (FIRE) is a newly described syndrome observed in eosinophilic esophagitis (EoE) patients. It is defined by an immediate hypersensitivity reaction of the esophagus that occurs when specific foods and beverages interface with esophageal mucosa. The available data regarding this topic is scarce. Therefore, we aimed to review relevant publications in order to better characterize the main aspects of this syndrome and hypothesize about potential mechanisms underlying FIRE syndrome and possible future therapeutic approaches. We searched PubMed, Embase, and Web of Science databases for relevant articles published before February 1st, 2025. The results were narrowed down to four articles describing a total of 105 cases of FIRE syndrome. These patients had a distinct clinical presentation, characterized by retrosternal discomfort or pain, differentiating it from solid food dysphagia or pollen-food allergy syndrome (PFAS). Currently, diagnosis is based on clinical presentation, with no diagnostic tests or biomarkers available. Emerging evidence suggests that IgE-mediated hypersensitivity, mast cells, and neuroimmune interactions may play a central role in the pathogenesis of FIRE syndrome. The therapeutic approaches remain speculative, with trigger avoidance being the main option. This article brings to the forefront the need for continued research to address current knowledge gaps regarding FIRE syndrome, which is important for optimizing patient management.
1. Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune-driven esophageal condition associated with type 2 inflammation and characterized by esophageal eosinophilia. It is frequently associated with other type 2 atopic conditions such as atopic dermatitis, IgE-mediated food allergy, and rhinitis, and it can possibly be interpreted as a late-stage manifestation of the allergic march [1].
EoE is characterized by esophageal motility dysfunction symptoms associated with eosinophilic infiltrate of the esophageal mucosa in response to food or environmental allergens [2]. Its diagnosis consists of an eosinophil count of >15 eosinophils per high-power field (HPF) or 60 eosinophils/mm2 in esophageal biopsies, after excluding other common causes of esophageal eosinophilia, such as gastroesophageal reflux disease (GERD), infections, eosinophilic gastrointestinal diseases (EGIDs), drug hypersensitivity reactions, connective tissue diseases, and hypereosinophilic syndrome [2].
Since its initial reports in the literature, dating back to the late 1980s, EoE has been a subject of significant interest, positioned at the intersection of several medical specialties [3,4]. In recent years, there has been a growing focus on this condition, showing a marked increase in its recognition, with EoE being the leading cause of dysphagia in children and adults [5]. The pooled incidence estimated in a recent meta-analysis was 6.6 per 100,000 per year in children and 7.7 per 100,000 per year in adults, alongside a global prevalence of 34.4 per 100,000 individuals [6]. The increased awareness of EoE with a higher frequency of esophageal biopsies during esophagogastroduodenoscopy may explain this observed trend. However, similar to other atopic conditions, evidence suggests that its incidence is genuinely increasing, independent of improved diagnostic recognition [7,8].
Recognition and diagnosis of EoE occur in individuals of all ages, including both adults and children. Its clinical presentation is defined by esophageal dysfunction, with solid food dysphagia and food impaction being the hallmark symptoms of this disease. The phenotypic expression of EoE seems to be highly age-dependent, considering that non-specific symptoms such as abdominal pain, vomiting, regurgitation, or feeding difficulties are more common in children [8,9]. A recent systematic review reported that 13% to 75.3% of EoE patients suffered from feeding difficulties [10]. These patients developed modified eating behaviors, for instance, frequently drinking during meals, eating in small portions, or chewing excessively, all of which contributed to delayed diagnosis [8,11,12]. The mean diagnostic delay in patients with EoE has been found to be around 4 years, with longstanding inflammation leading to complications such as esophageal fibrosis with strictures. There is evidence suggesting that each additional year of untreated EoE increases the risk of stricture by 9% [13,14,15].
Recent publications describe that a subset of EoE patients experience an immediate hypersensitivity-like response upon exposure to different foods or beverages, which appears to be distinct from the chronic delayed Th2-driven symptoms typically seen in EoE [16]. This novel entity has been characterized as Food-Induced Immediate Response of the Esophagus (FIRE) syndrome. FIRE syndrome presents with acute retrosternal pain, food impaction leading to a choking sensation, and dysphagia, which is different from classical dysphagia seen in EoE [16]. Unlike classic EoE, where symptoms worsen gradually over time, FIRE syndrome involves a rapid-onset, reproducible esophageal reaction upon exposure to different foods or beverages, raising important questions about its pathophysiology and optimal management [16,17]. Given the lack of standardized diagnostic criteria and the low recognition of this syndrome in EoE population, further research is needed in order to identify it, understand the mechanisms that drive its clinical presentation, and develop tailored therapeutic approaches.
2. Reported Cases of FIRE Syndrome: Insights from the Literature
2.1. Methods
FIRE syndrome has recently been documented as a distinct clinical entity in EoE patients [16]. Consequently, data on this subgroup remain limited. Several cases have been described in the literature, providing insights into its symptomatology, triggers, and impact. The aim of this paper is to review the available literature data on FIRE syndrome and to propose some hypotheses regarding its pathophysiology and management strategies.
To support this aim, a literature search was conducted to identify and summarize relevant data on FIRE syndrome published before 1 February 2025. Relevant studies were identified through a search in three major databases: PubMed, Embase, and Web of Science. We used Medical Subject Heading (MeSH) and keywords including “food-induced immediate response of the esophagus”, “FIRE syndrome”, “eosinophilic esophagitis”, and “esophageal immediate hypersensitivity”. Boolean operators (AND/OR) were applied to refine the search strategy. Additionally, we manually reviewed the reference lists of all included studies to identify further relevant publications. Conference abstracts, studies unrelated to the scope of this narrative review, and articles not available in English were excluded. Databases such as Scopus and Google Scholar were not included, as they were found to have substantial content overlap with the sources consulted.
The search yielded 24 initial entries. After removing duplicate records and screening for relevance based on titles and abstracts, a total of 4 papers were included for review. These comprised two original articles, one case series, and one case report [16,17,18,19]. Study selection and data synthesis were conducted by two authors as part of this narrative review. For transparency reasons, the article selection process is illustrated in Figure 1.
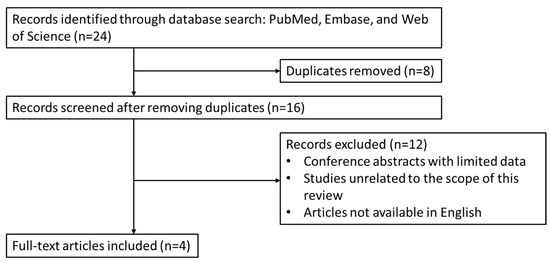
Figure 1.
Overview of article identification and selection process for this review.
The current evidence base is limited by the low number of published studies, all of which implemented observational or descriptive designs. This poses essential limitations in terms of methodological rigor, generalizability and strength of the conclusions. However, as these reports represent the only available data on FIRE syndrome, we aimed to critically evaluate and synthesize them in order to highlight the key characteristics and identify knowledge gaps for future research.
2.2. The Main Characteristics of Reported Cases
The majority of current understanding regarding FIRE syndrome originates from the researchers who first described this phenomenon. In their study, Biedermann et al. [16] used a questionnaire-based survey among EoE experts and patients to better characterize FIRE syndrome patients’ profiles. It was estimated that symptoms consistent with FIRE syndrome occur in less than 5% to up to 20% for the adult EoE population. In contrast, patient-reported symptoms suggest a possible higher prevalence of almost 40% [16]. These estimates are based on subjective assessments rather than epidemiologic data, and should be interpreted as preliminary insights. Importantly, all FIRE syndrome cases have so far been self-reported by patients, and no data are currently available concerning the use of oral food challenges to formally assess this syndrome [16,17,18,19]. As evidence is still emerging, available epidemiological data remain limited to small cohorts. For instance, Koken et al.’s study reported a prevalence of 1.2% in their cohort of pediatric patients, suggesting a potential age-related difference [19]. Given the small number of patients being surveyed and the observational nature of these case series, it remains unclear whether this difference is truly age-dependent. The following section aims to summarize key findings from the reported cases, highlighting the defining features observed in these patients. A total of 105 cases have been reported so far across the four articles included in the research [16,17,18,19]. Their key characteristics are presented in Table 1.

Table 1.
Currently reported cases of FIRE syndrome.
Of the total 105 patients, 103 were adults reported in two studies: 95 patients with a mean age of 46 years in the first study, and 8 patients with a mean age of 35 years in the second study [16,17]. Furthermore, there were two cases reported in the pediatric EoE population of 9 and 16 years of age, respectively [18,19]. The occurrence of FIRE symptoms appears to be linked to an earlier age of EoE diagnosis. Additionally, this syndrome seems to be more common among male patients with a history of food bolus impactions necessitating endoscopic intervention [16].
Regarding the correlation with EoE activity, FIRE syndrome symptoms appear to develop at variable stages in the course of the disease and may not be directly linked to active eosinophilic inflammation. In Biedermann et al.’s patient cohort, only 28.4% reported having FIRE syndrome symptoms only in relation to active EoE, while almost 39% described this phenomenon independently of EoE activity [16].
Examining the temporal association between the initial FIRE syndrome manifestations and EoE diagnosis, data from the same study showed that in 31.6% of cases FIRE syndrome symptoms preceded EoE’s clinical expression, in 24.2% they appeared after EoE onset, and only 15.8% of FIRE syndrome patients reported symptoms concomitant with EoE. Notably, 24.2% were unable to clearly define the timeline [16]. These observations suggest a heterogenous pattern, with a potential trend toward FIRE syndrome symptoms occurring early in the disease course, in some cases preceding EoE diagnosis. This temporal trend warrants further investigation as there are still insufficient data to establish FIRE syndrome as a predictive marker for EoE. If validated, FIRE syndrome could serve as a potential clinical red flag, prompting earlier diagnostic work-up for EoE. As for its persistence, symptoms remitted in 12.7% of patients after a median period of 4 years, allowing for speculation that FIRE syndrome may not be a permanent condition [16]. Additional data are necessary to make a definite conclusion in this regard.
Another important aspect to consider is the broad atopic profile of FIRE syndrome patients. EoE is strongly linked to other atopic conditions [20]. In a large retrospective study on 450 thousand patients diagnosed with EoE, asthma was found in almost 60% of them, 17.8% had atopic dermatitis, and 60% presented with allergic rhinitis [21]. Regarding FIRE syndrome, allergic comorbidities marked a higher prevalence in EoE patients with FIRE syndrome compared to their counterparts without it. Biedermann et al. found a 69.2% prevalence of allergic diseases in general and a 51.6% prevalence of allergic rhinoconjunctivitis in FIRE syndrome patients [16].
3. Clinical Presentation of FIRE Syndrome
FIRE syndrome manifests with atypical complaints among EoE patients. It is characterized by intense, retrosternal discomfort or pain that occurs rapidly and reproducibly after ingestion of specific foods or beverages [16,19]. Unlike solid food dysphagia in EoE, these manifestations are instead described as a tightening or pressure sensation in the mid-sternum, a choking sensation that could be accompanied by anxiety, chest pain, or heartburn [16,17]. The series of patients surveyed by Biedermann et al. reported FIRE characteristics such as narrowing, pressure, burning, choking, locking, cramps, or swelling; 33% of patients also reported anxiety caused by these sensations [16].
The symptoms have a remarkably sudden onset, typically developing within the first 5 min following exposure to the trigger, with interindividual variations ranging from 1 to 60 min. This abrupt development of symptoms is correlated with an impressive intensity, most often rated 7 or higher on a 1–10 visual analog scale (VAS) by patients. Due to the severity of symptoms, many patients actively avoid exposure to identified triggers [16,17,18,19].
Another striking feature is the limited duration of symptoms. A FIRE syndrome episode may last between a few minutes and approximately 6 h. Data show that 72.4% of patients reported by Biedermann et al. had symptoms lasting less than 30 min, almost all becoming symptom-free in under 2 h (96.5%) [16,17,18,19]. Interestingly, even if the symptoms subside in a relatively short time, there are, however, patients that report experiencing lasting dysphagia in the following days after an episode [16].
In EoE patients, symptoms suggestive of FIRE syndrome or pollen-food allergy syndrome (PFAS) may coexist or occasionally overlap, both with one another but also with the typical symptoms of EoE. Given this potential clinical crossover, a careful clinical distinction among these entities is essential to formulate the precise diagnostic [16,19,22,23].
The symptoms in EoE are triggered by solid, fibrous food ingestion, the size of the food being of importance as well [24]. This serves as the rationale for the adaptive eating behaviors observed in these patients [25]. Unlike them, FIRE syndrome patients present with symptoms triggered by even the smallest serving of the triggering food, and compensatory eating habits do not alleviate esophageal discomfort. An intriguing aspect of FIRE syndrome is that symptoms can be triggered by beverages such as milk, beer, or wine, a characteristic that is not observed in EoE [16,17,18,19]. Consequently, the absence of symptom relief through modifications of serving size and food texture, along with ubiquitous trigger avoidance, can be distinctive features to help identify FIRE syndrome in EoE patients [16,17,18,19].
PFAS is a frequent comorbidity in EoE patients and was reported in approximately one-third of EoE patients, commonly triggered by fresh (raw) fruits or vegetables, including apples (21.1%), carrots (15.5%), and peaches (15.5%) [26]. Like in FIRE syndrome, PFAS symptoms appear immediately after ingestion of food triggers. However, the two conditions vary considerably in the location and quality of symptoms.
PFAS typically causes itching, tingling, or mild swelling usually limited to the oropharynx, while FIRE syndrome is characterized by intense retrosternal discomfort, such as pressure or pain, often described by patients as “esophageal constriction” [16,27]. The two conditions may share the same triggers; however, FIRE syndrome may also be triggered by liquids (such as vinegar, milk, or wine), a feature that is not observed in PFAS, where the main elicitors are raw plant-based foods [16,19].
Both FIRE syndrome and PFAS can coexist in the same patient, as reported in 75% of cases in Holbreich et al.’s series of adult patients [17]. Patients seem to be able to clearly differentiate between the two entities, based on symptom quality and location [17]. Interestingly, a small pediatric study conducted by Koken et al. reported a much higher prevalence of PFAS compared with FIRE syndrome among children with EoE (15.3% vs. 1.2%) [19]. The coexistence of both conditions in EoE individuals and their similar characteristics with immediate onset and common triggers mark the potential for clinical confusion. To address this diagnostic challenge, a summary of the main clinical features of EoE, FIRE syndrome, and PFAS is provided in Table 2, which may assist in distinguishing these conditions in clinical practice.

Table 2.
The main characteristics of EoE, FIRE syndrome, and PFAS.
4. The Main Elicitors of FIRE Syndrome Symptoms
Food proteins are known inductors of EoE [28]. The clinical, endoscopic, and histopathological remission following an elimination diet therapy illustrates the importance of food triggers in EoE pathophysiology. The rate of remission in patients undergoing an elemental diet is approximately 90% [29]. Milk has been shown to induce EoE in ⅔ of EoE patients, followed by wheat and eggs in about ¼ of patients, but also other foods belonging to the “Big 8” allergen list have been shown to induce EoE in less common situations [29,30,31].
The main triggers in FIRE syndrome partially overlap with foods known to induce EoE, namely milk and dairy derivatives, wheat, soybeans, and peanuts [20,30]. Data from the available reports reveal that in almost half of patients, the symptoms are induced by fruits or vegetables. Unlike EoE, in which various foods contribute to inflammation without necessarily causing the characteristic dysphagia, making trigger identification more challenging, in FIRE syndrome there is a clear correlation between the ingestion of a specific trigger and the immediate onset of clinical symptoms [16,17,18,19]. Interestingly, patients have reported other unusual triggers of FIRE syndrome, including mustard, chestnut, meat, chocolate, and rice [16]. This aspect may complicate the identification of a pattern among these patients. Consequently, analyzing causal connections becomes more difficult.
Furthermore, regarding EoE and FIRE syndrome triggers, there are several differences in both the type of food and their texture. It is known that solid and fibrous foods induce dysphagia in EoE patients [32]. The texture seems to be irrelevant in FIRE syndrome patients, as these individuals experience symptoms upon ingestion of both small and large quantities of food, either solid or liquid, fibrous or soft. Additionally, it appears that FIRE syndrome symptoms are triggered by both foods and beverages. Among the latter, wine, beer, milk, or vinegar seem to be the most frequently reported triggers [16,17,18,19].
5. Diagnostic Criteria
The limited data available and the lack of clarity involving the pathophysiological mechanisms involved in FIRE syndrome make it difficult to formulate diagnostic criteria. Drawing from extensive clinical experience of European and United States experts, coupled with thorough examination of patient-reported outcomes, Biedermann et al. [16] were able to identify the most important clinical characteristics, which can now inform a preliminary diagnostic algorithm. While objective biomarkers for FIRE syndrome are still lacking, the pattern of clinical presentation, particularly in the context of EoE, provides the basis for a stepwise clinical diagnostic framework formulation (see Figure 2) [16,17,18,19].
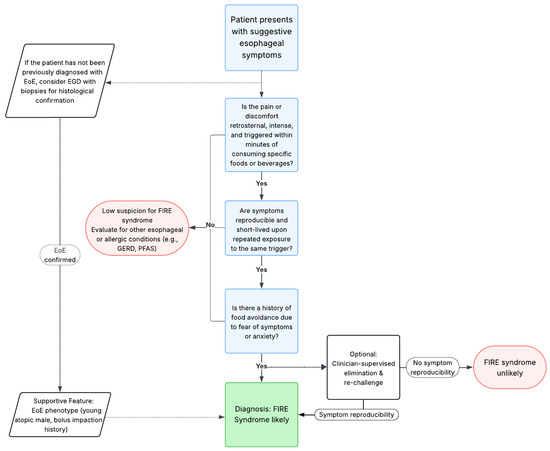
Figure 2.
Proposed diagnostic algorithm for FIRE syndrome. We summarized the main clinical features of FIRE syndrome from the available literature and formulated a potential diagnostic algorithm [16,17,18,19].
Firstly, the primary complaint is defined by intense retrosternal discomfort or pain, typically appearing within a timeframe of 5 min, lasting for about 30 min, strictly linked to the ingestion of a specific food or beverage. The symptoms manifest as angina-like or burning discomfort [16].
Secondly, this intense sensation is reproducible after re-exposure to the same trigger and appears with high intensity, often leading to avoidance behavior and food-related anxiety. Approximately 30–40% of patients report coexisting symptoms, such as throat discomfort, burning sensation, or panic-like symptoms [16].
Thirdly, these patients appear to have a particular EoE phenotype, having a younger age at diagnosis and a high rate of comorbid atopic disease. Male patients with a history of food bolus impaction necessitating endoscopic intervention for removal tend to display a higher prevalence of FIRE syndrome [16].
Additionally, an optional diagnostic step could include a temporary elimination diet followed by a clinician-supervised re-challenge, which may be taken into consideration in order to confirm the reproducibility of symptoms and avoid misdiagnosis. This approach must follow a shared decision with the informed patient and should be conducted with caution, particularly in patients with a history of severe symptoms.
Figure 2 represents a proposed diagnostic algorithm synthesizing these features into a structured potential clinical decision tool.
6. Proposed Pathogenic Mechanisms
The pathophysiological mechanisms underlying FIRE syndrome are still unknown and remain largely speculative due to limited available data. The immediate onset of symptoms, their reproducibility upon trigger exposure, and the common association with other allergic diseases point to the involvement of a rapid immune-mediated response [17]. Furthermore, the confinement of symptoms to the esophagus strongly suggests the association of localized immunological mechanisms [16].
The esophagus is not merely a passive conduit for food but also serves as an immunologically active organ [33]. In a healthy esophagus, immune cells are present across all tissue layers and contribute to both tissue homeostasis and defense mechanisms. T lymphocytes make up the most abundant immune cell population [34]. CD8+ cytotoxic T cells are scattered throughout the epithelium and muscularis layers, whereas CD4+ helper T cells are primarily situated in the lamina propria [34]. Unlike T lymphocytes, the B lymphocytes are found in a small number in the esophageal mucosa, mainly situated in the vascular papillae and lamina propria, and are absent in the muscularis mucosae and muscularis externa [34]. Mastocytes, with a previously underestimated presence in the esophageal mucosa, have been shown to comprise the most abundant immune cell population in the muscular layers, suggesting a potential role in motility regulation and sensory perception [34,35]. Alongside mast cells, macrophages represent another prevalent immune cell type within the muscle layers of the esophagus, but their exact function is still unclear. Their presence suggests a potential role in the regulation of esophageal motility, similar to their function in other intestinal segments [34,36]. These findings highlight the esophageal role in initiating and modulating immune responses, with significant implications for both physiological and pathological processes [37].
The pathophysiology of EoE involves epithelial barrier defects with dysregulated epithelial–immune cell interaction and Th2-driven eosinophilic mucosal infiltration [37]. These complex mechanisms lead to chronic, progressive inflammation and tissue remodeling with fibrosis, leading to esophageal dysfunction [37]. Unlike chronic Th2-driven inflammation, which dictates EoE pathophysiology, immediate immune responses may be involved in FIRE syndrome, with some potential key pathways including IgE-mediated hypersensitivity response, mast cell activation response, or neuroimmune interactions [16,17,18,19].
Although B lymphocytes are not abundant in healthy esophageal tissue, EoE patients exhibit a significantly higher number of B cells, particularly localized in the vascular papillae and peripapillary region, compared with healthy controls [38,39]. In murine models of EoE induced by intranasal exposure to Aspergillus fumigatus, the esophageal B lymphocyte population exhibited an approximate twofold increase [40]. B cells have been shown to be actively recruited in the esophageal mucosa of EoE patients independently of patients’ atopic status [39]. Their implication in FIRE syndrome may depend on local IgE class switching occurring in response to local allergen exposure. Upon activation, B lymphocytes (CD20+) undergo class switch recombination (CSR), expressing other antibody isotypes such as IgG, IgA, and IgE [41]. The process involves the recombination of heavy chain gene segments and the generation of circular transcripts, leading to the synthesis of mature IgE mRNA and protein expression [41]. The CSR to IgE, a key player in atopic diseases, is driven by IL-4 and IL-13 signaling, in conjunction with CD40-CD40L interactions and the upregulation of activation-induced cytidine deaminase (AID) [39,42,43]. This phenomenon, which has been thought to be restricted to lymphoid organs, has been described in other mucosal sites such as the gastrointestinal, nasal, or bronchial mucosa [44,45,46]. In their study, Vicario et al. demonstrated the presence of germline transcripts (GLTs), AID, and IgE heavy chain (Cε) mRNA in the esophageal mucosa of EoE patients, which are essential for CSR [39]. The authors state that a limitation of this study would be that biopsies were taken with a substantial delay after allergen exposure, so the presence of circular transcripts or switch circles, which is needed to confirm the ongoing process of CSR, could not be determined. Consequently, the possibility that CSR occurred in another location and primed B cells migrated to the esophagus cannot be excluded [39]. On that basis, together with significantly upregulated IL-4 and IL-13 expression in EoE patients and the presence of IgE-bound mast cells, these findings suggest the local esophageal priming for IgE synthesis and a potential local IgE-mediated response in EoE that could consequently explain FIRE syndrome manifestations [39].
A hypothesis concerning the pathophysiology of this syndrome suggests the involvement of a local, immediate hypersensitivity reaction. While skin prick tests (SPTs) are used to identify IgE-mediated sensitization to foods, their utility in EoE and particularly in FIRE syndrome is limited [9,17]. In fact, SPTs with suspected foods may be negative, likely due to the esophagus being the primary site of the sensitization [9,17].
A study by Warners et al. introduced the use of esophageal prick testing (EPT) involving direct injection of suspected allergens into the esophageal mucosa [47]. They demonstrated that 63% of known trigger foods elicited a positive result in EPT, characterized by erythema, edema, and muscle contraction with esophageal obstruction. These responses were absent in healthy controls, supporting the idea of a localized IgE-mediated mechanism in a subset of EoE patients [47]. In contrast, only 18% of suspected triggers were identified through SPTs [47]. Furthermore, transcriptomic analysis of the esophageal mucosa following EPT exposure revealed an upregulation of genes associated with acute allergic inflammation, such as TNFSF18 (GITRL) [48]. This gene is also known to play a role in other atopic diseases, such as asthma and atopic dermatitis, but its role in the early phase of the food-induced immune response is still to be determined [48,49,50]
Although these findings support the presence of an immediate, localized esophageal immune response, their clinical application in FIRE syndrome remains limited. It is still unclear whether positive EPT or SPTs results in EoE patients corelate directly with FIRE syndrome symptoms. No studies have yet evaluated the diagnostic accuracy of EPT or SPT in relation to this syndrome. While EPT may prove useful in identifying esophageal-specific sensitization and distinguishing immune-mediated reactions from mucosal irritation, its sensitivity, specificity, and diagnostic value in FIRE syndrome remain to be formally established though prospective studies [19]. Moreover, EPT is invasive, not yet standardized, and may not fully replicate natural exposure during eating [47,48]. Nevertheless, the epithelial barrier defect due to the inflamed esophageal mucosa may compensate for this shortcoming.
Dysfunction of the epithelial barrier has been described in EoE [46,51]. It is characterized by a decreased mRNA expression of key epithelial barrier proteins such as filaggrin, involucrin, desmoglein-1, and the antiprotease encoded by the SPINK7 gene [52]. Decreased esophageal impedance further indicates an epithelial barrier impairment in EoE [53]. The decreased epithelial barrier function involves dilated intercellular spaces and likely facilitates allergen penetration in the esophageal mucosa [54]. Esophageal intramucosal antigen deposition has been demonstrated in a study conducted by Marietta et al., showing detectable intraepithelial gluten deposition in active EoE patients [55]. Furthermore, the presence of aeroallergens such as Dermatophagoides farinae has been described at the epithelial level in adults with EoE [56]. Their inherent proteolytic quality may further contribute to esophageal epithelial dysfunction, as demonstrated in other allergic conditions such as asthma [57]. Additionally, the altered microbiome seen in EoE patients could contribute to epithelial barrier dysfunction by decreasing beneficial metabolite synthesis (e.g., short-chain fatty acids) and increasing the production of lipopolysaccharides and pathogen-associated molecular patterns (PAMPs), which can damage the epithelium and increase its permeability [58,59]. Subsequent to the impairment of the esophageal epithelial barrier, an increased number of type 2 innate lymphoid cells (ILC2s) is observed. The activation of these cells, mediated by thymic stromal-derived lymphopoietin (TSLP) and IL-33, triggers an early inflammatory response [60,61]. Combined with local antigen presence, these findings create a favorable environment for a local immediate hypersensitivity response to food allergens in FIRE syndrome.
Expanding upon Th2-mediated inflammation, sustained Th2 immune response to allergens involves an increase in proinflammatory cytokines such as IL-4, IL-13, and IL-5, with a subsequent increase in eosinophil count [37]. Eosinophil-derived IL-9 has been shown to display an increased expression in active EoE, stimulating esophageal mast cell accumulation and contributing to an altered epithelial barrier [62]. Notably, the number of mast cells significantly increases during active EoE compared to healthy individuals [39,63]. Furthermore, the percentage of IgE-positive cells is increased in atopic compared to non-atopic EoE patients [63]. The infiltrated mast cells may be activated through various pathways and degranulate, releasing a variety of proinflammatory mediators [37,64]. Importantly, mast cells are the main immune cell population in the muscularis externa and therefore can affect esophageal motility upon activation, potentially leading to a transient esophageal motor dysfunction [34,65]. This effect is partially due to their capacity to release a diverse range of mediators upon activation, including prostaglandins, leukotrienes, and thromboxanes, that contribute to increased vascular permeability and smooth muscle contraction [37,66]. This mechanism may be particularly relevant in FIRE syndrome, where symptoms such as pain, tightening, or pressure-like sensations occur immediately after food exposure, consistent with mast-cell-driven hypersensitivity and smooth muscle contraction.
Given the high intensity and rapid onset of FIRE symptoms, it is plausible that local mast cell degranulation could contribute to this phenomenon, either by mediator release or neuroimmune interactions amplifying sensory hypersensitivity. Zhang et al.’s study demonstrated that increased esophageal mast cell density was strongly correlated with pain perception, whereas no significant association was observed between mast cell number and dysphagia [67]. The pain perception is linked to high mast cell numbers but not to eosinophil count. This was further supported by the findings of a clinical trial where anti-IL-5 therapy reduced eosinophilic infiltration in EoE but had no effect on esophageal pain, suggesting mast cells rather than eosinophils are the main drivers of pain perception and symptom severity [68]. While mast cell activation involvement in FIRE is a plausible contributor, it remains speculative and is not yet confirmed by dedicated studies.
Local mast cell degranulation, followed by tryptase release, can increase the sensitivity of ion channels found on esophageal nociceptive C-fibers, such as transient receptor potential vanilloid 1 (TRPV1) and acid-sensing ion channels (ASICs) [69,70]. This results in increased ATP release, leading to an amplified response in sensory neurons, potentially contributing to esophageal pain signaling [71,72]. The activation of these channels further adds to esophageal enhanced pain perception through the release of neuropeptides, including substance P and calcitonin gene-related peptide (CGRP) [73]. Data show that a prolonged increase in esophageal C-fiber excitability with intensified vagal nociceptor stimulation is correlated with local mast cell activation [70]. Interestingly, TRPV1 has been found to be upregulated in active EoE and inactive EoE with associated persistent pain [67]. Krarup et al. showed that esophageal hypersensitivity could also be attributed to other ion channels like ASIC1 and ASIC3, which are upregulated in patients with reflux esophagitis and in rodent models of esophagitis [74,75]. Taken together, these data underscore the importance of the connection between esophageal mast cell infiltration and pain perception in patients with EoE. The contribution of chemical irritation in the pathogenesis of FIRE syndrome could account for the immediate onset and high intensity of pain. However, this hypothesis remains theoretical in nature and further research is needed to confirm it.
To summarize, the pathophysiological mechanisms underlying FIRE syndrome have not been investigated to date. However, several hypotheses could be formulated based on the key pathways implicated in EoE etiology. The increase in esophageal B cell population accompanied by evidence suggesting a potential local CSR mechanism and an elevated IgE-bearing cell number within the esophageal mucosa supports the idea that an IgE-mediated mechanism could be involved in the generation of the localized immediate hypersensitivity reaction observed in FIRE syndrome [39,41]. This immediate response has been demonstrated before in EoE patients, where esophageal prick tests with suspected triggers evoked signs suggestive of immediate reactivity [47]. Furthermore, the mucosal barrier dysfunction known to occur in EoE may create a favorable environment for allergen penetration, thereby promoting their interaction with the immune cells [46,51,52,53,54,55,56]. The resulting immediate response appears to lead to mast cell degranulation with the release of important proinflammatory mediators such as prostaglandins, leukotrienes, and thromboxanes. Given the increased presence of mastocytes in the muscular layers of the esophagus, mast cell degranulation could potentially explain the motility dysfunction and pain seen in FIRE syndrome [37,66]. Additionally, the stimulation of acid-sensing ion channels such as TRPV1 and ASICs further promotes pain generation, suggesting a potential role of the neuroimmune interaction in the pathology [69,70,71,72,73,74,75]. These hypotheses about the mechanisms will necessitate further investigations and thoroughly conducted studies to elucidate their implication in patient management. The key postulated ideas are depicted in Figure 3.
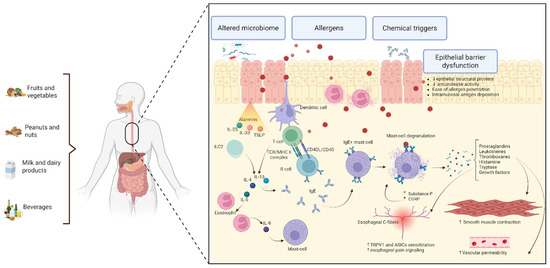
Figure 3.
Proposed pathophysiological mechanisms for FIRE syndrome, extrapolated from EoE and related allergic conditions. The impaired mucosal barrier due to epithelial dysfunction and abnormal protease activity leads to the facilitation of food allergen translocation and their presentation to the dendritic cells. These antigen-presenting cells continue to process and present food allergens to Th2 CD4+ cells, generating an inflammatory response, with potential local IgE formation and mast cell degranulation. Alarmins such as IL-33 and TSLP may contribute to the process initiation. IL-4 and IL-13 may promote potential local CSR. Mast cell degranulation leads to mediator release, which are thought to play a role in sensitizing esophageal C-fibers containing TRPV1 and ASICs with pain generation; they could also be involved in smooth muscle contraction with hyperreactivity and dysmotility, and in increasing vascular permeability. This conceptual model highlights the proposed mechanism pathways based on current understanding of FIRE syndrome, EoE, and other allergic disorders. Created with https://BioRender.com. ASICs—acid-sensing ion channels; CD40L—CD40 ligand; IgE—immunoglobulin E; IL—interleukin; ILC2s—type 2 innate lymphoid cells; MHC II—major histocompatibility complex class II; TCR—T cell receptor; TRPV1—transient receptor potential vanilloid 1; TSLP—thymic stromal lymphopoietin.
Given the novelty of FIRE syndrome, most of the proposed mechanisms discussed in this section are extrapolated from EoE, or other related allergic disorders and should be considered exploratory. While some pathways are supported by experimental or histologic findings in EoE, others remain theoretical. Table 3 outlines the key proposed mechanisms, their biological basis, and the types of evidence available.

Table 3.
Proposed pathophysiological mechanisms in FIRE syndrome.
7. Potential Management Strategies and Challenges
The established management of eosinophilic esophagitis implies several key treatment options. There is no gold standard of therapy, with available treatment choices including dietary therapy (empirical elimination of six, four, two, or one foods, or elemental diet), swallowed topical corticosteroids (CSs), proton pump inhibitors (PPIs), endoscopic dilation, and the use of biologics [76]. Given that FIRE syndrome is described in EoE patients, and the fact that there are no established treatment protocols for this condition, therapeutic approaches remain challenging to determine and are primarily extrapolated from treatments used in EoE or in IgE-mediated food allergy.
Data derived from published case series indicate that trigger food avoidance represents the main strategy adopted by patients [16]. One pediatric case report documented a favorable evolution under swallowed topical corticosteroid treatment, while no improvement in both FIRE syndrome and EoE was noted during PPI treatment [18].
To improve conceptual clarity, proposed interventions can be categorized based on their underlying pathophysiological rationale, such as immediate hypersensitivity, mast cell activation, neuroimmune interactions, or epithelial barrier dysfunction. The main therapeutic approaches proposed in the following sections are summarized in Table 4.
7.1. Dietary Interventions
The avoidance of triggering foods and beverages appears to be the primary strategy adopted by patients. Given the intensity of symptoms, most patients are able to identify the culprit and consequently avoid it [16]. In cases where it might be difficult to identify triggers, a food diary may help patients track their symptoms and pinpoint specific foods or beverages responsible. In selected cases, oral food challenges could be useful in confirming immediate food hypersensitivity. However, extensive exclusion diets, particularly when multiple foods are implicated, could lead to nutritional deficiencies, food-related anxiety, and an overall decreased quality of life [77]. We have also observed this pattern in some patients whose symptoms were triggered by fresh fruits or vegetables, leading in some cases to the complete exclusion of all raw fruits or vegetables from the diet. This illustrates the challenges patients with FIRE syndrome may face in maintaining a balanced diet and underscores the need for multidisciplinary management.
In this context, the involvement of a registered dietitian is essential to ensure adequate nutritional intake while keeping a restrictive diet. Patient education holds an important place in FIRE syndrome management as informing patients on safe food practices and label reading may empower them to make suitable dietary choices. These strategies may be essential in the management of pediatric patients, where dietary restrictions may affect growth and development. In selected cases involving anxiety or avoidant eating behavior, psychological counseling may be useful. Overall, an individualized care plan is needed to balance symptom control with quality of life and nutritional intake.
7.2. Pharmacologic Approaches Targeting Immediate Hypersensitivity
Given the partial symptomatic overlap with PFAS, the association with other atopic diseases, and the proposed involvement of an IgE-mediated mechanism or mast cell activation, several pharmacologic agents used in other allergic conditions could be considered for FIRE syndrome. Although data are lacking in this subset of patients, their potential application seems to be biologically plausible.
7.2.1. Histamine Receptor Antagonists
Drawing from the observation that FIRE syndrome implies an immediate hypersensitivity reaction, possibly mediated by mast cell histamine release, it might be postulated that rapidly acting pharmacological agents targeting histaminic pathways could offer symptomatic relief. Evidence showing elevated H1 (H1R), H2 (H2R), and H4 (H4R) receptor expression in active EoE biopsies further supports the potential use of H1R antagonists in FIRE syndrome [78]. If acid plays a role in the pathogenesis, anti-H2R medications such as famotidine could represent a potential treatment, although evidence supporting the use of H2R antagonists in eosinophilic gastrointestinal disorders is lacking [79]. These proposed therapies remain speculative and while many more pathways could be implicated in the generation of symptoms, antihistamine use in FIRE syndrome has not been formally studied and will require further investigation.
7.2.2. Mast-Cell-Targeted Therapies
Additionally, considering that mast cells may hold a pivotal role in the pathology of FIRE syndrome, the use of mast cell stabilizers (e.g., cromolyn sodium, ketotifen) could provide additional benefits in symptom control by limiting their activation with a consequent reduction in histamine, tryptase, prostaglandin, and leukotriene release. This could contribute to a reduction in symptom frequency and severity, as demonstrated in other mast-cell-driven diseases such as mastocytosis [80]. Cromolyn sodium is the most used mast cell stabilizer, preventing degranulation by specifically binding to ion channels and reducing Ca2+ cellular influx. Due to its high molecular weight, it has a low systemic absorption and could be preferred to topical corticosteroids in EoE-associated FIRE syndrome [81]. In spite of this, a 4-week trial of viscous oral cromolyn use in pediatric EoE patients showed no significant improvement, neither in symptoms nor in eosinophilic infiltrate [82]. In contrast, a later study on a murine model demonstrated that disodium cromoglycate effectively reduced Th2-driven inflammation, eosinophil, and mast cell count [83]. Ketotifen, a dual-action mast-cell stabilizer and H1-antihistamine that is used in many allergic diseases, has shown benefit in improving the symptoms of eosinophilic gastrointestinal disease in a patient unable to tolerate corticosteroid administration [84].
Montelukast, a leukotriene D4 receptor antagonist, showed symptomatic improvement when used in high doses in adult EoE patients, suggesting that blocking leukotriene effects could be a useful treatment option in some patients [85]. When assessed for maintaining corticoid-induced symptomatic remission, montelukast failed to show any benefit compared with placebo, implying that future studies are needed to evaluate its long-term use in FIRE syndrome [86].
7.3. Biologic Therapies
7.3.1. Anti-IL-4/IL-13 Therapy: Dupilumab
Targeting type 2 cytokine signaling pathways may be beneficial in FIRE syndrome, particularly where there is overlap with EoE and other allergic disorders where mast cells may play a central role [87]. Dupilumab, a fully human monoclonal antibody directed against the subunit alpha of the shared IL-4/IL-13 receptor, is approved for several allergic conditions, including moderate-to-severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, asthma, and eosinophilic esophagitis [88]. It showed significant benefit in alleviating symptoms and improving both histopathologic and endoscopic aspects of EoE [89]. By looking into its capacity to suppress inflammatory responses initiated by IL-13 and IL-4, including the production of proinflammatory cytokines, chemokines, and IgE, Dupilumab could prove to be a useful instrument in FIRE syndrome management [90]. In addition, by downregulating inflammatory genes, Dupilumab can reduce the probability of mast cell activation, reinforcing its potential use in this syndrome [91].
7.3.2. Anti-IgE Therapy: Omalizumab
Additionally, considering the probable role of IgE in inducing an immediate hypersensitivity reaction in FIRE syndrome, a potential approach could involve the use of anti-IgE therapy. Omalizumab, a humanized monoclonal antibody that binds to the Cε3 domain of circulating IgE, inhibits its ability to bind to FcεRI, thus reducing serum IgE levels and downregulating FcεRI on mast cells and basophils [92,93]. It is approved for the treatment of multiple atopic conditions including asthma in patients 6 years and older, urticaria from the age of 12, and chronic rhinosinusitis with nasal polyposis in adult patients [88]. Notably, in food allergy, Omalizumab showed significant benefit, used in monotherapy as adjuvant to oral immunotherapy (OIT) [94,95]. It increased reaction thresholds for various foods such as milk, eggs, wheat, and peanuts, reducing food-induced reactions while also improving the quality of life [94,95]. The promising results of the OUtMATCH study, which investigated the safety and effectiveness of Omalizumab as monotherapy in patients with multiple food allergies, led to the FDA approval, dated February 2024, for the use of Omalizumab for IgE-mediated food allergy in adults and children 1 year or older [96,97]. In contrast with its success in food allergy, Omalizumab did not demonstrate therapeutic benefit in eosinophilic esophagitis, a disease with a complex pathophysiology [98]. Its application in FIRE syndrome remains theoretical. However, if FIRE syndrome can be considered a distinct phenotype in EoE, with its potential IgE-driven mechanism, future studies evaluating the use of Omalizumab could yield significant benefit for patients.
7.4. Allergen Immunotherapy
Considering the potential role of IgE-mediated mechanisms in FIRE syndrome and its frequent coexistence with PFAS and atopic disease, allergen immunotherapy (AIT) may represent an interesting therapeutic direction. AIT may have a role in reducing symptoms by raising the reaction threshold while also reducing esophageal inflammation associated with EoE, in cases where PFAS and FIRE syndrome overlap. While EoE appears as a complication of OIT in approximately 5% of cases, epicutaneous immunotherapy (EPIT) has the potential to reduce eosinophilic esophageal inflammation [28]. A study involving pediatric patients with EoE induced by milk examined the impact of EPIT on reducing eosinophil infiltration [99]. The study revealed a 47% remission rate, with sustained response at 2-year follow-up, showing that EPIT could be an effective treatment in some patients with EoE [99,100]. Additionally, another study on peanut-sensitized mice demonstrated that EPIT prevented esophageal eosinophilic infiltration and consequently influenced esophageal inflammation [101]. As plant-derived foods represent the vast majority of triggers in FIRE syndrome, pollen immunotherapy could represent a hypothetical therapeutic strategy to improve tolerance to these foods [102], although clinical evidence for pollen AIT benefit in oral allergy syndrome is scarce [103].
7.5. Neuroimmune Modulators
In the absence of specific therapies for FIRE syndrome, a future direction could involve interfering with neuroimmune pathways by blocking TRPV1 and ASICs, which could reduce sensory neuron activation, diminishing the main symptoms, including pain, discomfort, or burning sensation. Studies using TRPV1 antagonists such as AMG-9810 showed a significant reduction in acid-induced responses in both healthy and EoE animal models, suggesting a potential role for preventing esophageal hypersensitivity and pain [71,104]. However, capsazepine, a synthetic capsaicin analog that acts as a TRPV1 receptor antagonist, showed promise in managing visceral pain, but its clinical application is limited by its potential risk of mucosal damage [105]. Blocking the upregulated ASIC1 and ASIC3 in gastroesophageal reflux disease showed promise in managing pain in a mice model of reflux esophagitis [75]. These results contrast with the limited clinical impact of amiloride, a diuretic known to inhibit ASICs, on acid-induced esophageal hypersensitivity in non-erosive reflux disease patients, despite its potential analgesic effect on skin nociception [106,107]. Although neuroimmune pathways have been implicated in esophageal pain perception, their therapeutic modulation in FIRE syndrome remains largely theoretical and unsupported by clinical evidence to date.
7.6. Mucosal Protective and Barrier-Enhancing Agents
Considering the potential role of barrier dysfunction in FIRE syndrome, an interesting therapeutic approach could target this level to prevent allergen penetration and exposure of immunologic cells to antigens and pathogens. Strategies could involve the use of cytoprotective agents such as sucralfate, which is a known mucosal protective agent used in reflux esophagitis. It acts by forming a defensive barrier, aiding in healing and defense of the mucosal layer [108]. Ongoing research into sucralfate slurry use in eosinophilic esophagitis aims to demonstrate its benefit in improving various aspects of esophageal mucosa in this condition, but no results have been posted so far [109]. Moreover, the addition of other biopolymers, including sodium hyaluronate and sodium alginate, has shown beneficial aspects in improving symptoms and mucosal health in patients with esophageal reflux disease [110]. Therefore, epithelial barrier repair and mucus barrier improvement could be an interesting strategy for relieving symptoms, but validation in future studies is needed.
A thorough understanding of FIRE syndrome mechanisms is crucial for the development of targeted therapeutic approaches. Given the lack of available data concerning the natural evolution of FIRE syndrome and the influence of EoE activity over its manifestations, the long-term course of this syndrome and its evolution from childhood to adulthood are difficult to predict. Many patients may require indefinite trigger avoidance, which can impact nutritional status. It is important to note that the intervention strategies described previously are based on limited evidence, remain hypothetical, and necessitate validation through future studies.

Table 4.
Summary of therapies for EoE and their potential use in FIRE syndrome [15,17,28,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].
Table 4.
Summary of therapies for EoE and their potential use in FIRE syndrome [15,17,28,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].
| Therapy | Use in EoE | Use in FIRE Syndrome |
|---|---|---|
| Dietary elimination | Effective (6-, 4-, 2-, or 1-food elimination diet, or elemental diet) | Main strategy |
| Swallowed topical corticosteroids | Effective | One case showed improvement; needs further study |
| Proton pump inhibitors | Used but not always effective | No improvement reported; needs further study |
| Antihistamines (H1R, H2R, H4R antagonists) | Potential use (H1R expression elevated in EoE) | Potential symptom relief; needs further study |
| Mast cell stabilizers (Cromolyn, Ketotifen) | Cromolyn showed no benefit in pediatric EoE; ketotifen helped EoGID patients | Could reduce histamine, prostaglandin, and leukotriene release; needs further study |
| Leukotriene antagonist (Montelukast) | High doses showed symptomatic improvement but no long-term benefit | Could be useful; long-term effectiveness unclear; needs further study |
| Anti-IL4R and IL13R therapy (Dupilumab) | Effective, symptomatic, histopathologic, and endoscopic benefit | Could be beneficial by reducing T2 inflammation; needs further study |
| Anti-IgE therapy (Omalizumab) | No proven benefit | Could help considering the IgE-mediated mechanism; needs further study |
| Allergen immunotherapy (AIT) | EPIT showed potential benefit in reducing eosinophilic inflammation | Hypothetical role (pollen AIT for plant-based triggers); needs further study |
| TRPV1 and ASIC blockers | AMG-9810 reduced acid-induced responses in animal models | Potential for pain relief; limited clinical evidence; needs further study |
| Mucosal protective agents (Sucralfate, sodium hyaluronate, sodium alginate) | Under investigation | Could improve mucosal barrier and symptom relief; needs further study |
H1R—H1 receptor, H2R—H2 receptor, H4R—H4 receptor, IL4R—Interleukin 4 receptor, IL13R—Interleukin 13 receptor, IgE—Immunoglobulin E, AIT—allergen immunotherapy, TRPV1—transient receptor potential vanilloid 1, ASICs—acid-sensing ion channels, EoGIDs—eosinophilic gastrointestinal disorders, EPIT—epicutaneous immunotherapy.
8. Conclusions and Future Directions
FIRE syndrome represents a novel and distinct esophageal hypersensitivity reaction, proposed as a potential clinical phenotype within the eosinophilic esophagitis spectrum. It is characterized by a rapid-onset, reproducible esophageal response, often manifesting as retrosternal discomfort or pain following specific trigger exposure. This distinct clinical presentation differentiates it from classical EoE or oral allergy syndrome. Given the limited number of cases reported so far, there is a lack of diagnostic criteria and optimal therapeutic approaches, which can contribute to misdiagnosis and suboptimal management of these patients, highlighting the need for greater awareness and research.
This review reflects the current state of knowledge, which is limited by the small number and nature of the available studies included. The reports on FIRE syndrome, represented by only observational or descriptive studies and case reports, are subject to significant limitations, including selection bias, reporting bias, and lack of standardized diagnostic criteria or specific biomarkers. However, the evidence is valuable for hypothesis generation and clinical awareness. The conclusions drawn in this paper represent early-stage observations that require further validation. Well-designed prospective studies, such as controlled trials and mechanistic investigations, are needed to validate the proposed diagnostic algorithm and therapeutic strategies.
Exploring the role of epithelial barrier disruption, local IgE-mediated pathways, mast cell–nerve interaction, and esophageal pain signaling might provide insights into the pathophysiology of FIRE syndrome while also marking the opportunity to advance knowledge regarding the immune processes in the esophagus.
FIRE syndrome remains an underdiagnosed clinical syndrome while our knowledge is still limited. We wish to underscore the importance of increased awareness of this syndrome and to encourage other clinicians or researchers to publish their cases. Establishing diagnostic criteria and targeted therapies through further research will enhance patient outcomes and quality of life.
Author Contributions
S.A. and R.S.B. conceptualized and designed the study; S.A. and M.C.C. conducted the literature search and collected and analyzed the data; S.A. and M.C.C. drafted the manuscript; M.R.V. reviewed the references; M.C.C. and M.R.V. prepared the figures and tables; E.C.B. and R.S.B. critically reviewed and edited the manuscript; R.S.B. supervised the research activities and contributed to the refinement of the intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflicts of Interest
The authors declare no conflicts of interest with respect to the research, authorship and/or publication of this study.
Abbreviations
The following abbreviations are used in this manuscript:
| AID | Activation-induced cytidine deaminase |
| AIT | Allergen immunotherapy |
| AR | Allergic rhinitis |
| ASICs | Acid-sensing ion channels |
| ATP | Adenosine triphosphate |
| CD40L | CD40 ligand |
| CGRP | Calcitonin gene-related peptide |
| CSs | Corticosteroids |
| CSR | Class switch recombination |
| EGIDs | Eosinophilic gastrointestinal diseases |
| EoE | Eosinophilic esophagitis |
| EPIT | Epicutaneous immunotherapy |
| EPT | Esophageal prick tests |
| FIRE | Food-Induced Immediate Response of the Esophagus |
| GERD | Gastroesophageal reflux disease |
| GITRL | Glucocorticoid-induced tumor-necrosis-factor-receptor-related protein ligand |
| GLTs | Germline transcripts |
| HPF | High-power field |
| HR | Histamine receptor |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| ILC2s | Type 2 innate lymphoid cells |
| MeSH | Medical Subject Heading |
| MHC II | Major Histocompatibility Complex Class II |
| mRNA | Messenger Ribonucleic Acid |
| OIT | Oral immunotherapy |
| PAMPs | Pathogen-associated molecular patterns |
| PFAS | Pollen-food allergy syndrome |
| PPIs | Proton pump inhibitors |
| sIgE | Specific immunoglobulin E |
| SPINK 7 | Serine Peptidase Inhibitor Kazal Type 7 |
| SPT | Skin prick test |
| TCR | T cell receptor |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TSLP | Thymic stromal-derived lymphopoietin |
| VAS | Visual analog scale |
References
- Hill, D.A.; Grundmeier, R.W.; Ramos, M.; Spergel, J.M. Eosinophilic Esophagitis Is a Late Manifestation of the Allergic March. J. Allergy Clin. Immunol. Pract. 2018, 6, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.A.; Winter, H.S.; Grand, R.J.; Allred, E.N. Are Endoscopic Changes Predictive of Histologic Esophagitis in Children? J. Pediatr. 1983, 103, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.E.A.; Smyrk, T.C.; Demeester, T.R.; Jones, J.B. Esophageal Eosinophilia with Dysphagia: A Distinct Clinicopathologic Syndrome. Dig. Dis. Sci. 1993, 38, 109–116. [Google Scholar] [CrossRef]
- Straumann, A.; Schoepfer, A. Update on Basic and Clinical Aspects of Eosinophilic Oesophagitis. Gut 2014, 63, 1355–1363. [Google Scholar] [CrossRef]
- Navarro, P.; Arias, Á.; Arias-González, L.; Laserna-Mendieta, E.J.; Ruiz-Ponce, M.; Lucendo, A.J. Systematic Review with Meta-analysis: The Growing Incidence and Prevalence of Eosinophilic Oesophagitis in Children and Adults in Population-based Studies. Aliment. Pharmacol. Ther. 2019, 49, 1116–1125. [Google Scholar] [CrossRef]
- Thomsen, S.F. Epidemiology and Natural History of Atopic Diseases. Eur. Clin. Respir. J. 2015, 2, 24642. [Google Scholar] [CrossRef]
- Muir, A.; Falk, G.W. Eosinophilic Esophagitis: A Review. JAMA 2021, 326, 1310. [Google Scholar] [CrossRef]
- Barni, S.; Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Liotti, L.; Saretta, F.; Castagnoli, R.; Caminiti, L.; et al. Pediatric Eosinophilic Esophagitis: A Review for the Clinician. Ital. J. Pediatr. 2021, 47, 230. [Google Scholar] [CrossRef]
- Ruano-Zaragoza, M.; Hill, S.; Nurmatov, U.; Reese, I.; Vieira, M.C.; Dupont, C.; Venter, C.; Walsh, J.; Yonamine, G.; Beauregard, A.; et al. Systematic Review of Feeding Difficulties in Children with Eosinophilic Esophagitis: An EAACI Task Force Report. Pediatr. Allergy Immunol. 2025, 36, e70087. [Google Scholar] [CrossRef]
- Chen, J.W.; Kao, J.Y. Eosinophilic Esophagitis: Update on Management and Controversies. BMJ 2017, 359, j4482. [Google Scholar] [CrossRef] [PubMed]
- Votto, M.; Castagnoli, R.; De Filippo, M.; Brambilla, I.; Cuppari, C.; Marseglia, G.L.; Licari, A. Behavioral Issues and Quality of Life in Children with Eosinophilic Esophagitis. Minerva Pediatr. 2020, 72, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portmann, S.; Simon, H.; Straumann, A. Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation in a Time-Dependent Manner. Gastroenterology 2013, 145, 1230–1236.e2. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.R.; Kreienbuehl, A.S.; Greuter, T.; Nennstiel, S.; Safroneeva, E.; Saner, C.; Schindler, V.; Schlag, C.; Schoepfer, A.M.; Schreiner, P.; et al. Diagnostic Delay in Patients with Eosinophilic Esophagitis Has Not Changed Since the First Description 30 Years Ago: Diagnostic Delay in Eosinophilic Esophagitis. Am. J. Gastroenterol. 2022, 117, 1772–1779. [Google Scholar] [CrossRef]
- Warners, M.J.; Van Rhijn, B.D.; Verheij, J.; Smout, A.J.P.M.; Bredenoord, A.J. Disease Activity in Eosinophilic Esophagitis Is Associated with Impaired Esophageal Barrier Integrity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G230–G238. [Google Scholar] [CrossRef]
- Biedermann, L.; Holbreich, M.; Atkins, D.; Chehade, M.; Dellon, E.S.; Furuta, G.T.; Hirano, I.; Gonsalves, N.; Greuter, T.; Gupta, S.; et al. Food-induced Immediate Response of the Esophagus—A Newly Identified Syndrome in Patients with Eosinophilic Esophagitis. Allergy 2021, 76, 339–347. [Google Scholar] [CrossRef]
- Holbreich, M.; Straumann, A. Features of Food-Induced Immediate Response in the Esophagus (FIRE) in a Series of Adult Patients with Eosinophilic Esophagitis. Allergy 2021, 76, 2893–2895. [Google Scholar] [CrossRef]
- Votto, M.; Naso, M.; De Filippo, M.; Marseglia, A.; Raffaele, A.; Marseglia, G.L.; Licari, A. Food-induced Immediate Response of the Esophagus: A First Report in the Pediatric Age. Allergy 2022, 77, 711–712. [Google Scholar] [CrossRef]
- Koken, G.; Ertoy Karagol, H.I.; Polat Terece, S.; Cavdar, Z.; Cetin, K.; Egritas Gurkan, O.; Sari, S.; Dalgic, B.; Bakirtas, A. Food-Induced Immediate Response of the Esophagus in Pediatric Eosinophilic Esophagitis. Allergy 2023, 78, 3235–3240. [Google Scholar] [CrossRef]
- Williamson, P.; Aceves, S. Allergies and Eosinophilic Esophagitis—Current Updates for the Pediatric Gastroenterologist. Curr. Gastroenterol. Rep. 2019, 21, 56. [Google Scholar] [CrossRef]
- Capucilli, P.; Cianferoni, A.; Grundmeier, R.W.; Spergel, J.M. Comparison of Comorbid Diagnoses in Children with and Without Eosinophilic Esophagitis in a Large Population. Ann. Allergy Asthma. Immunol. 2018, 121, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Savarino, E.; Sciume, G.; Chio, T.D.; Bronzini, F.; Tolone, S.; Frazzoni, M.; Pugno, C.; Ghisa, M.; Bertani, L.; et al. Eosinophilic Esophagitis: Clinical, Endoscopic, Histologic and Therapeutic Differences and Similarities between Children and Adults. Ther. Adv. Gastroenterol. 2021, 14, 1756284820980860. [Google Scholar] [CrossRef] [PubMed]
- Poncet, P.; Sénéchal, H.; Charpin, D. Update on Pollen-Food Allergy Syndrome. Expert Rev. Clin. Immunol. 2020, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.; Epstein, J. Eosinophilic Oesophagitis: Recent Advances and Practical Management. Frontline Gastroenterol. 2021, 12, 644–649. [Google Scholar] [CrossRef]
- Yapar, D.; Karagol, H.I.E.; Terece, S.P.; Duztas, D.T.; Gurkan, O.E.; Sari, S.; Dalgic, B.; Bakirtas, A. A Novel Pediatric Eosinophilic Esophagitis Symptoms and Adaptive Behavior Scale for Different Ages: GaziESAS v2.0. Pediatr. Allergy Immunol. 2023, 34, e13974. [Google Scholar] [CrossRef]
- Letner, D.; Farris, A.; Khalili, H.; Garber, J. Pollen-Food Allergy Syndrome Is a Common Allergic Comorbidity in Adults with Eosinophilic Esophagitis. Dis. Esophagus 2018, 31, dox122. [Google Scholar] [CrossRef]
- Al-Shaikhly, T.; Cox, A.; Nowak-Wegrzyn, A.; Cianferoni, A.; Katelaris, C.; Ebo, D.G.; Konstantinou, G.N.; Brucker, H.; Yang, H.-J.; Protudjer, J.L.P.; et al. An International Delphi Consensus on the Management of Pollen-Food Allergy Syndrome: A Work Group Report of the AAAAI Adverse Reactions to Foods Committee. J. Allergy Clin. Immunol. Pract. 2024, 12, 3242–3249.e1. [Google Scholar] [CrossRef]
- Burk, C.M.; Shreffler, W.G. Triggers for Eosinophilic Esophagitis (EoE): The Intersection of Food Allergy and EoE. J. Allergy Clin. Immunol. 2024, 153, 1500–1509. [Google Scholar] [CrossRef]
- Spergel, J.M.; Brown-Whitehorn, T.F.; Cianferoni, A.; Shuker, M.; Wang, M.-L.; Verma, R.; Liacouras, C.A. Identification of Causative Foods in Children with Eosinophilic Esophagitis Treated with an Elimination Diet. J. Allergy Clin. Immunol. 2012, 130, 461–467.e5. [Google Scholar] [CrossRef]
- Spergel, J.; Aceves, S.S. Allergic Components of Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2018, 142, 1–8. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; González-Cervera, J.; Yagüe-Compadre, J.L.; Guagnozzi, D.; Angueira, T.; Jiménez-Contreras, S.; González-Castillo, S.; Rodríguez-Domíngez, B.; De Rezende, L.C.; et al. Empiric 6-Food Elimination Diet Induced and Maintained Prolonged Remission in Patients with Adult Eosinophilic Esophagitis: A Prospective Study on the Food Cause of the Disease. J. Allergy Clin. Immunol. 2013, 131, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.V.; Umeweni, C.N.; Alston, M.; Dolinsky, L.; McCormack, S.M.; Taylor, L.A.; Bendavid, A.; Benitez, A.; Mitchel, E.; Karakasheva, T.; et al. Esophageal Remodeling Correlates with Eating Behaviors in Pediatric Eosinophilic Esophagitis. Am. J. Gastroenterol. 2024, 119, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, T.; Hruz, P.; Niess, J.H. Immune System and Microbiome in the Esophagus: Implications for Understanding Inflammatory Diseases. FEBS J. 2022, 289, 4758–4772. [Google Scholar] [CrossRef] [PubMed]
- Fortea, M.; Hacour, L.; Sancho, F.; Boada, C.; Sevillano-Aguilera, C.; González-Castro, A.M.; Salvo-Romero, E.; Lobo, B.; Guagnozzi, D.; Ceulemans, L.J.; et al. Characterization of Immune Cell Populations and Acid-Sensing Receptors in the Human Esophagus. Gastroenterol. Insights 2024, 15, 819–834. [Google Scholar] [CrossRef]
- Mastracci, L.; Bruzzone, M.; Pacella, E.; Tinelli, C.; Zentilin, P.; Savarino, E.; De Silvestri, A.; Fiocca, R.; Grillo, F. The Contribution of Intraepithelial Inflammatory Cells to the Histological Diagnosis of Microscopic Esophagitis. Esophagus 2016, 13, 80–87. [Google Scholar] [CrossRef]
- Cipriani, G.; Gibbons, S.J.; Kashyap, P.C.; Farrugia, G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 120–130.e1. [Google Scholar] [CrossRef]
- Underwood, B.; Troutman, T.D.; Schwartz, J.T. Breaking down the Complex Pathophysiology of Eosinophilic Esophagitis. Ann. Allergy Asthma. Immunol. 2023, 130, 28–39. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Navarro, M.; Comas, C.; Pascual, J.M.; Burgos, E.; Santamaría, L.; Larrauri, J. Immunophenotypic Characterization and Quantification of the Epithelial Inflammatory Infiltrate in Eosinophilic Esophagitis Through Stereology: An Analysis of the Cellular Mechanisms of the Disease and the Immunologic Capacity of the Esophagus. Am. J. Surg. Pathol. 2007, 31, 598–606. [Google Scholar] [CrossRef]
- Vicario, M.; Blanchard, C.; Stringer, K.F.; Collins, M.H.; Mingler, M.K.; Ahrens, A.; Putnam, P.E.; Abonia, J.P.; Santos, J.; Rothenberg, M.E. Local B Cells and IgE Production in the Oesophageal Mucosa in Eosinophilic Oesophagitis. Gut 2010, 59, 12–20. [Google Scholar] [CrossRef]
- Mishra, A.; Schlotman, J.; Wang, M.; Rothenberg, M.E. Critical Role for Adaptive T Cell Immunity in Experimental Eosinophilic Esophagitis in Mice. J. Leukoc. Biol. 2007, 81, 916–924. [Google Scholar] [CrossRef]
- Stavnezer, J.; Schrader, C.E. IgH Chain Class Switch Recombination: Mechanism and Regulation. J. Immunol. 2014, 193, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Mingler, M.K.; Vicario, M.; Abonia, J.P.; Wu, Y.Y.; Lu, T.X.; Collins, M.H.; Putnam, P.E.; Wells, S.I.; Rothenberg, M.E. IL-13 Involvement in Eosinophilic Esophagitis: Transcriptome Analysis and Reversibility with Glucocorticoids. J. Allergy Clin. Immunol. 2007, 120, 1292–1300. [Google Scholar] [CrossRef]
- Stavnezer, J.; Guikema, J.E.J.; Schrader, C.E. Mechanism and Regulation of Class Switch Recombination. Annu. Rev. Immunol. 2008, 26, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’Connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class Switch Recombination to IgE in the Bronchial Mucosa of Atopic and Nonatopic Patients with Asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Fagarasan, S.; Muramatsu, M.; Suzuki, K.; Nagaoka, H.; Hiai, H.; Honjo, T. Critical Roles of Activation-Induced Cytidine Deaminase in the Homeostasis of Gut Flora. Science 2002, 298, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Takhar, P.; Smurthwaite, L.; Coker, H.A.; Fear, D.J.; Banfield, G.K.; Carr, V.A.; Durham, S.R.; Gould, H.J. Allergen Drives Class Switching to IgE in the Nasal Mucosa in Allergic Rhinitis. J. Immunol. 2005, 174, 5024–5032. [Google Scholar] [CrossRef]
- Warners, M.J.; Terreehorst, I.; Van Den Wijngaard, R.M.; Akkerdaas, J.; Van Esch, B.C.A.M.; Van Ree, R.; Versteeg, S.A.; Smout, A.J.P.M.; Bredenoord, A.J. Abnormal Responses to Local Esophageal Food Allergen Injections in Adult Patients with Eosinophilic Esophagitis. Gastroenterology 2018, 154, 57–60.e2. [Google Scholar] [CrossRef]
- Kleuskens, M.T.A.; Haasnoot, M.L.; Garssen, J.; Bredenoord, A.J.; Van Esch, B.C.A.M.; Redegeld, F.A. Transcriptomic Profiling of the Acute Mucosal Response to Local Food Injections in Adults with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2024, 153, 780–792. [Google Scholar] [CrossRef]
- Byrne, A.M.; Goleva, E.; Chouiali, F.; Kaplan, M.H.; Hamid, Q.A.; Leung, D.Y.M. Induction of GITRL Expression in Human Keratinocytes by T H2 Cytokines and TNF-α: Implications for Atopic Dermatitis. Clin. Exp. Allergy 2012, 42, 550–559. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Niu, C.; Zou, W.; Yang, L.; Wang, T.; Tian, D.; Luo, Z.; Dai, J.; Li, Q.; et al. Blockade of GITRL/GITR Signaling Pathway Attenuates House Dust Mite-Induced Allergic Asthma in Mice through Inhibition of MAPKs and NF-κB Signaling. Mol. Immunol. 2021, 137, 238–246. [Google Scholar] [CrossRef]
- Simon, D.; Radonjic-Hösli, S.; Straumann, A.; Yousefi, S.; Simon, H.-U. Active Eosinophilic Esophagitis Is Characterized by Epithelial Barrier Defects and Eosinophil Extracellular Trap Formation. Allergy 2015, 70, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Kleuskens, M.T.A.; Bek, M.K.; Al Halabi, Y.; Blokhuis, B.R.J.; Diks, M.A.P.; Haasnoot, M.L.; Garssen, J.; Bredenoord, A.J.; van Esch, B.C.A.M.; Redegeld, F.A. Mast Cells Disrupt the Function of the Esophageal Epithelial Barrier. Mucosal Immunol. 2023, 16, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.A.; Vaezi, M.F.; Correa, H.; Higginbotham, T.; Slaughter, J.C.; Acra, S. Mucosal Impedance Measurements Differentiate Pediatric Patients with Active Versus Inactive Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Katzka, D.A.; Tadi, R.; Smyrk, T.C.; Katarya, E.; Sharma, A.; Geno, D.M.; Camilleri, M.; Iyer, P.G.; Alexander, J.A.; Buttar, N.S. Effects of Topical Steroids on Tight Junction Proteins and Spongiosis in Esophageal Epithelia of Patients with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1824–1829.e1. [Google Scholar] [CrossRef]
- Marietta, E.V.; Geno, D.M.; Smyrk, T.C.; Becker, A.; Alexander, J.A.; Camilleri, M.; Murray, J.A.; Katzka, D.A. Presence of Intraepithelial Food Antigen in Patients with Active Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2017, 45, 427–433. [Google Scholar] [CrossRef]
- Ravi, A.; Marietta, E.V.; Geno, D.M.; Alexander, J.A.; Murray, J.A.; Katzka, D.A. Penetration of the Esophageal Epithelium by Dust Mite Antigen in Patients with Eosinophilic Esophagitis. Gastroenterology 2019, 157, 255–256. [Google Scholar] [CrossRef]
- Reithofer, M.; Jahn-Schmid, B. Allergens with Protease Activity from House Dust Mites. Int. J. Mol. Sci. 2017, 18, 1368. [Google Scholar] [CrossRef]
- Angerami Almeida, K.; De Queiroz Andrade, E.; Burns, G.; Hoedt, E.C.; Mattes, J.; Keely, S.; Collison, A. The Microbiota in Eosinophilic Esophagitis: A Systematic Review. J. Gastroenterol. Hepatol. 2022, 37, 1673–1684. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, D.; Zhuang, Q.; Hou, X.; Jia, X.; Chen, J.; Lin, H.; Zhang, M.; Tan, N.; Xiao, Y. Esophageal Microbial Dysbiosis Impairs Mucosal Barrier Integrity via Toll-like Receptor 2 Pathway in Patients with Gastroesophageal Reflux Symptoms. J. Transl. Med. 2024, 22, 1145. [Google Scholar] [CrossRef]
- Aceves, S.S. Local Antigen Deposition in Eosinophilic Esophagitis: Implications for Immune Activation. Gastroenterology 2019, 157, 17–20. [Google Scholar] [CrossRef]
- Doherty, T.A.; Baum, R.; Newbury, R.O.; Yang, T.; Dohil, R.; Aquino, M.; Doshi, A.; Walford, H.H.; Kurten, R.C.; Broide, D.H.; et al. Group 2 Innate Lymphocytes (ILC2) Are Enriched in Active Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2015, 136, 792–794.e3. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Khamishon, R.; Rawson, R.; Duong, L.; Dohil, L.; Myers, S.J.; Bell, B.; Dohil, R.; Newbury, R.O.; Barrett, K.E.; et al. Interleukin 9 Alters Epithelial Barrier and E-cadherin in Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Bokhary, R.; Marcon, M.A.; Cutz, E. Activated Mucosal Mast Cells Differentiate Eosinophilic (Allergic) Esophagitis from Gastroesophageal Reflux Disease. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bolton, S.M.; Kagalwalla, A.F.; Arva, N.C.; Wang, M.-Y.; Amsden, K.; Melin-Aldana, H.; Dellon, E.S.; Bryce, P.J.; Wershil, B.K.; Wechsler, J.B. Mast Cell Infiltration Is Associated with Persistent Symptoms and Endoscopic Abnormalities Despite Resolution of Eosinophilia in Pediatric Eosinophilic Esophagitis. Am. J. Gastroenterol. 2020, 115, 224–233. [Google Scholar] [CrossRef]
- Nelson, M.; Zhang, X.; Pan, Z.; Spechler, S.J.; Souza, R.F. Mast Cell Effects on Esophageal Smooth Muscle and Their Potential Role in Eosinophilic Esophagitis and Achalasia. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G319–G327. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Zhang, S.; Shoda, T.; Aceves, S.S.; Arva, N.C.; Chehade, M.; Collins, M.H.; Dellon, E.S.; Falk, G.W.; Gonsalves, N.; Gupta, S.K.; et al. Mast Cell-pain Connection in Eosinophilic Esophagitis. Allergy 2022, 77, 1895–1899. [Google Scholar] [CrossRef]
- Otani, I.M.; Anilkumar, A.A.; Newbury, R.O.; Bhagat, M.; Beppu, L.Y.; Dohil, R.; Broide, D.H.; Aceves, S.S. Anti–IL-5 Therapy Reduces Mast Cell and IL-9 Cell Numbers in Pediatric Patients with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2013, 131, 1576–1582.e2. [Google Scholar] [CrossRef]
- Dusenkova, S.; Ru, F.; Surdenikova, L.; Nassenstein, C.; Hatok, J.; Dusenka, R.; Banovcin, P.; Kliment, J.; Tatar, M.; Kollarik, M. The Expression Profile of Acid-Sensing Ion Channel (ASIC) Subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 in the Esophageal Vagal Afferent Nerve Subtypes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G922–G930. [Google Scholar] [CrossRef]
- Yu, S.; Kollarik, M.; Ouyang, A.; Myers, A.C.; Undem, B.J. Mast Cell-Mediated Long-Lasting Increases in Excitability of Vagal C Fibers in Guinea Pig Esophagus. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G850–G856. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Z.; Yu, X.; Pasricha, P.J.; Undem, B.J.; Yu, S. Increased Acid Responsiveness in Vagal Sensory Neurons in a Guinea Pig Model of Eosinophilic Esophagitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G149–G157. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Oshima, T.; Shan, J.; Sei, H.; Tomita, T.; Ohda, Y.; Fukui, H.; Watari, J.; Miwa, H. PAR-2 Activation Enhances Weak Acid-Induced ATP Release through TRPV1 and ASIC Sensitization in Human Esophageal Epithelial Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G695–G702. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chang, C.; Undem, B.J.; Yu, S. Capsaicin-Sensitive Vagal Afferent Nerve-Mediated Interoceptive Signals in the Esophagus. Molecules 2021, 26, 3929. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.L.; Villadsen, G.E.; Mejlgaard, E.; Olesen, S.S.; Drewes, A.M.; Funch-Jensen, P. Acid Hypersensitivity in Patients with Eosinophilic Oesophagitis. Scand. J. Gastroenterol. 2010, 45, 273–281. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Lee, A.; Li, Z.; Gao, J.; Wu, X.; Zhao, J.; Wang, H.; Chen, D.; Zou, D.; et al. Upregulation of Acid Sensing Ion Channels Is Associated with Esophageal Hypersensitivity in GERD. FASEB J. 2022, 36, e22083. [Google Scholar] [CrossRef]
- Ferreira, C.T.; Vieira, M.C.; Furuta, G.T.; Barros, F.C.L.F.D.; Chehade, M. Eosinophilic Esophagitis—Where Are We Today? J. Pediatr. 2019, 95, 275–281. [Google Scholar] [CrossRef]
- Kotchetkoff, E.C.D.A.; De Oliveira, L.C.L.; Sarni, R.O.S. Elimination Diet in Food Allergy: Friend or Foe? J. Pediatr. 2024, 100, S65–S73. [Google Scholar] [CrossRef]
- Merves, J.; Chandramouleeswaran, P.M.; Benitez, A.J.; Muir, A.B.; Lee, A.J.; Lim, D.M.; Dods, K.; Mehta, I.; Ruchelli, E.D.; Nakagawa, H.; et al. Altered Esophageal Histamine Receptor Expression in Eosinophilic Esophagitis (EoE): Implications on Disease Pathogenesis. PLoS ONE 2015, 10, e0114831. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Amil-Dias, J.; Auth, M.K.; Chehade, M.; Collins, M.H.; Gupta, S.K.; Gutiérrez-Junquera, C.; Orel, R.; Vieira, M.C.; Zevit, N.; et al. Joint ESPGHAN/NASPGHAN Guidelines on Childhood Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 122–152. [Google Scholar] [CrossRef]
- Tefferi, A.; Pardanani, A. Systemic Mastocytosis: Current Concepts and Treatment Advances. Curr. Hematol. Rep. 2004, 3, 197–202. [Google Scholar]
- Sinniah, A.; Yazid, S.; Flower, R.J. The Anti-Allergic Cromones: Past, Present, and Future. Front. Pharmacol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Zhang, J.; Whitworth, J.; Cavender, C. A Randomized, Double-Blinded, Placebo-Controlled Study of the Use of Viscous Oral Cromolyn Sodium for the Treatment of Eosinophilic Esophagitis. Ann. Allergy. Asthma. Immunol. 2018, 120, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.D.C.E.; De Oliveira, E.E.; Ambrósio, M.G.E.; Ayupe, M.C.; De Souza, V.P.; Menegati, L.M.; Reis, D.R.D.L.; Machado, M.A.; Macedo, G.C.; Ferreira, A.P. Disodium Cromoglycate Treatment Reduces TH2 Immune Response and Immunohistopathological Features in a Murine Model of Eosinophilic Esophagitis. Int. Immunopharmacol. 2020, 83, 106422. [Google Scholar] [CrossRef]
- Suzuki, J.; Kawasaki, Y.; Nozawa, R.; Isome, M.; Suzuki, S.; Takahashi, A.; Suzuki, H. Oral Disodium Cromoglycate and Ketotifen for a Patient with Eosinophilic Gastroenteritis, Food Allergy and Protein-Losing Enteropathy. Asian Pac. J. Allergy Immunol. 2003, 21, 193–197. [Google Scholar] [PubMed]
- Attwood, S.E.A. Eosinophilic Oesophagitis: A Novel Treatment Using Montelukast. Gut 2003, 52, 181–185. [Google Scholar] [CrossRef]
- Alexander, J.A.; Ravi, K.; Enders, F.T.; Geno, D.M.; Kryzer, L.A.; Mara, K.C.; Smyrk, T.C.; Katzka, D.A. Montelukast Does Not Maintain Symptom Remission After Topical Steroid Therapy for Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 214–221.e2. [Google Scholar] [CrossRef]
- McLeod, J.J.A.; Baker, B.; Ryan, J.J. Mast Cell Production and Response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef]
- Sindher, S.B.; Barshow, S.; Tirumalasetty, J.; Arasi, S.; Atkins, D.; Bauer, M.; Bégin, P.; Collins, M.H.; Deschildre, A.; Doyle, A.D.; et al. The Role of Biologics in Pediatric Food Allergy and Eosinophilic Gastrointestinal Disorders. J. Allergy Clin. Immunol. 2023, 151, 595–606. [Google Scholar] [CrossRef]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef]
- Burchett, J.R.; Dailey, J.M.; Kee, S.A.; Pryor, D.T.; Kotha, A.; Kankaria, R.A.; Straus, D.B.; Ryan, J.J. Targeting Mast Cells in Allergic Disease: Current Therapies and Drug Repurposing. Cells 2022, 11, 3031. [Google Scholar] [CrossRef] [PubMed]
- Wedi, B.; Traidl, S. Anti-IgE for the Treatment of Chronic Urticaria. ImmunoTargets Ther. 2021, 10, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Rocha, C.; Pereira, A.; Song, Y.; Alonso-Coello, P.; Solà, I.; Beltran, J.; Posso, M.; Akdis, C.A.; Akdis, M.; et al. Efficacy and Safety of Treatment with Omalizumab for Chronic Spontaneous Urticaria: A Systematic Review for the EAACI Biologicals Guidelines. Allergy 2021, 76, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Riggioni, C.; Oton, T.; Carmona, L.; Du Toit, G.; Skypala, I.; Santos, A.F. Immunotherapy and Biologics in the Management of IgE-mediated Food Allergy: Systematic Review and Meta-analyses of Efficacy and Safety. Allergy 2024, 79, 2097–2127. [Google Scholar] [CrossRef]
- Zuberbier, T.; Wood, R.A.; Bindslev-Jensen, C.; Fiocchi, A.; Chinthrajah, R.S.; Worm, M.; Deschildre, A.; Fernandez-Rivas, M.; Santos, A.F.; Jaumont, X.; et al. Omalizumab in IgE-Mediated Food Allergy: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1134–1146. [Google Scholar] [CrossRef]
- Wood, R.A.; Togias, A.; Sicherer, S.H.; Shreffler, W.G.; Kim, E.H.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; Bird, J.A.; Spergel, J.M.; et al. Omalizumab for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 889–899. [Google Scholar] [CrossRef]
- Casale, T.B.; Fiocchi, A.; Greenhawt, M. A Practical Guide for Implementing Omalizumab Therapy for Food Allergy. J. Allergy Clin. Immunol. 2024, 153, 1510–1517. [Google Scholar] [CrossRef]
- Clayton, F.; Fang, J.C.; Gleich, G.J.; Lucendo, A.J.; Olalla, J.M.; Vinson, L.A.; Lowichik, A.; Chen, X.; Emerson, L.; Cox, K.; et al. Eosinophilic Esophagitis in Adults Is Associated with IgG4 and Not Mediated by IgE. Gastroenterology 2014, 147, 602–609. [Google Scholar] [CrossRef]
- Spergel, J.M.; Elci, O.U.; Muir, A.B.; Liacouras, C.A.; Wilkins, B.J.; Burke, D.; Lewis, M.O.; Brown-Whitehorn, T.; Cianferoni, A. Efficacy of Epicutaneous Immunotherapy in Children with Milk-Induced Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2020, 18, 328–336.e7. [Google Scholar] [CrossRef]
- Spergel, J.M.; Muir, A.B.; Liacouras, C.A.; Burke, D.; Lewis, M.O.; Brown-Whitehorn, T.; Cianferoni, A. Sustained Milk Consumption after 2 Years Post–Milk Epicutaneous Immunotherapy for Eosinophilic Esophagitis. Allergy 2021, 76, 1573–1576. [Google Scholar] [CrossRef]
- Dioszeghy, V.; Mondoulet, L.; Dhelft, V.; Ligouis, M.; Puteaux, E.; Dupont, C.; Benhamou, P.-H. The Regulatory T Cells Induction by Epicutaneous Immunotherapy Is Sustained and Mediates Long-term Protection from Eosinophilic Disorders in Peanut-sensitized Mice. Clin. Exp. Allergy 2014, 44, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Furci, F.; Ricciardi, L. Plant Food Allergy Improvement after Grass Pollen Sublingual Immunotherapy: A Case Series. Pathogens 2021, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. Allergen Immunotherapy in Children User’s Guide. Pediatr. Allergy Immunol. 2020, 31, 1–101. [Google Scholar] [CrossRef] [PubMed]
- Peles, S.; Medda, B.K.; Zhang, Z.; Banerjee, B.; Lehmann, A.; Shaker, R.; Sengupta, J.N. Differential Effects of Transient Receptor Vanilloid One (TRPV1) Antagonists in Acid-Induced Excitation of Esophageal Vagal Afferent Fibers of Rats. Neuroscience 2009, 161, 515–525. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Devi, K.; Kumar, A.; Khan, R.; Singh, R.P.; Rajarammohan, S.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Intrarectal Capsazepine Administration Modulates Colonic Mucosal Health in Mice. Int. J. Mol. Sci. 2022, 23, 9577. [Google Scholar] [CrossRef]
- Ugawa, S.; Ueda, T.; Ishida, Y.; Nishigaki, M.; Shibata, Y.; Shimada, S. Amiloride-Blockable Acid-Sensing Ion Channels Are Leading Acid Sensors Expressed in Human Nociceptors. J. Clin. Investig. 2002, 110, 1185–1190. [Google Scholar] [CrossRef]
- Bulsiewicz, W.J.; Shaheen, N.J.; Hansen, M.B.; Pruitt, A.; Orlando, R.C. Effect of Amiloride on Experimental Acid-Induced Heartburn in Non-Erosive Reflux Disease. Dig. Dis. Sci. 2013, 58, 1955–1959. [Google Scholar] [CrossRef]
- Savarino, E. Drugs for Improving Esophageal Mucosa Defense: Where Are We Now and Where Are We Going? Ann. Gastroenterol. 2017, 30, 585. [Google Scholar] [CrossRef]
- Goyal, A.; Cheng, Y. Recent Discoveries and Emerging Therapeutics in Eosinophilic Esophagitis. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 21. [Google Scholar] [CrossRef]
- Dorofeyev, A.E.; Dyadyk, O.O.; Zvyagintseva, T.D.; Chychula, Y.V.; Rudenko, N.N.; Stukalo, A.A. Mucus Barrier Correction as a Target in Complex Treatment of Gastroesophageal Reflux Disease. Gastroenterol. Hepatol. Open Access 2021, 12, 22–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).