Abstract
Food allergies are becoming more common globally. The strict elimination diet, as the main approach so far, has a negative impact on different areas of the lives of children suffering from food allergies, as well as on their caregivers. Oral immunotherapy (OIT), which consists of ingesting small, gradually increasing amounts of food allergens, is a promising approach. Although efficient, this approach is accompanied by frequent adverse reactions (AR), some of which are as severe as anaphylaxis. It seems that, generally, slow dose escalation, as well as low maintenance doses with longer durations, make OIT safer, especially in children with severe food allergies. Furthermore, less allergenic forms of allergens, such as baked milk or egg, also contribute to OIT safety. Adjuvant therapy in combination with OIT has the potential to improve OIT efficiency and safety. Treatment with monoclonal antibodies such as omalizumab and dupilumab in combination with OIT is promising. While both could improve efficiency, omalizumab seems to have a better effect on safety. Interferon γ shows promising results. In contrast, the effect of probiotics and vitamin D supplementation in combination with OIT is still controversial, and new trials about their synergistic effect are needed.
1. Introduction
With a prevalence of around 1.6–5.6% in children across Europe, food allergies represent a significant burden for the pediatric population. In Europe, the prevalence of food allergies confirmed by double-blind, placebo-controlled food challenges ranges from 0.4% to 4.6% among children aged four to ten years. The most commonly reported symptoms of allergic reactions are cutaneous manifestations (up to 70%) and gastrointestinal symptoms (up to 40%). Among the most prevalent food allergens in almost all European centers are cow’s milk, hen’s eggs, and peanuts [1]. The quality of life of these patients is affected by the impairment of social activities, the fear of accidentally eating the suspect food and the risk of life-threatening anaphylaxis, leading to emotional stress and anxiety. Therefore, an active approach to ameliorate these difficulties is sought in contrast to the elimination diet and the application of medicines in emergencies.
Oral immunotherapy (OIT) for food allergies has proven to be a promising intervention that offers several benefits to patients: reducing the risk of allergic reactions due to contaminated foods, reducing the severity of reactions when foods are accidentally consumed, improving quality of life by minimizing dietary and social restrictions and alleviating anxiety, and allowing some patients to consume small amounts of allergenic food safely [2]. Although the first report of OIT dates back to 1908, when Schofield successfully desensitized a 13-year-old boy with a history of anaphylactic reactions to eggs through the oral intake of gradually increasing amounts of egg, OIT was neglected for most of the 20th century [3].
Food oral immunotherapy (F-OIT) consists of the oral ingestion of small, gradually increasing amounts of the culprit food to reach the maintenance dose, which is taken over a prolonged period [4]. Despite different OIT protocols, most protocols start with an initial escalation day to determine the tolerance threshold, followed by a build-up period over several months to years until the final target maintenance dose is achieved [4]. For some patients, F-OIT may protect them from accidental exposure, while others may achieve food intake [5].
As the treatment consists of ingesting allergenic foods, it is clear that it carries some risk. During OIT, the majority of children experience mild to moderate reactions, while severe reactions occur in about 5–10% of cases [6].
The most common adverse reactions (ARs) during OIT are mild to moderate and include gastrointestinal symptoms, skin reactions, and respiratory symptoms. However, there is a risk of severe reactions, including anaphylaxis [5]. The frequency of ARs tends to be higher during the initial escalation phase and decreases once the maintenance dose is reached [2]. Since there are no standardized F-OIT protocols, the goal of this review is to identify the differences between published F-OIT protocols in (a) milk OIT, (b) egg OIT and (c) peanut OIT in children, focusing on safety. The additional goal is to evaluate the adjuvant methods in improving safety in F-OIT protocols.
2. Materials and Methods
Relevant articles were selected by searching the electronic database PUBMED; the search was carried out independently by four authors, all of whom were experienced in performing OIT. Three authors searched for OIT protocols for milk, egg and peanut, while one author was charged with OIT protocols accompanied by adjuvant therapy. The keywords used in the literature search were oral immunotherapy OR OIT AND milk, OR egg OR peanut AND adjuvants in OIT, AND safety OR adverse events OR adverse reaction. The inclusion criteria were: original research articles published in English, free full-text access, designed as randomized clinical trials (RCTs) or clinical trials (CTs), no time limit for publication, the study populations were limited to the age group of children (<18 years), and safety concerns should be clearly reported. After reading the abstracts, articles that meet the inclusion criteria were considered. In total, we included thirty randomized controlled trials—ten for each food—as well as eighteen additional studies focusing on adjuvants used in OIT. Different studies that reported AR during OIT were included, although safety was usually not the primary outcome of these studies. To compare different OIT protocols, the following data for each OIT protocol were extracted in predefined tables: authors, years of publications, trial designs, population size and age, including/excluding criteria for participants, the form of allergen, duration, maintenance dose, efficacy, and safety. Data analysis was performed manually by four authors, all of whom are medical doctors with expertise in pediatric allergy and oral immunotherapy. Each author independently reviewed and extracted data from the selected studies, and the results were cross-checked and harmonized through discussion to ensure consistency and accuracy. No automated tools or software were used in the analysis.
3. Food Allergen Immunotherapy
3.1. Milk Oral Immunotherapy
Cow’s milk allergy (CMA) is one of the most common food allergies in infancy and early childhood. The prevalence of self-reporting CMA is estimated to be 1.2–17%, but it is much lower if only cases confirmed by oral food challenges (OFC) are considered, with a range of up to 3% [7]. The cow’s milk (CM) elimination diet carries a high burden because CM is the staple food in children’s diets and can cause nutritional deficit and impaired growth [8], as well as increase the risk of feeding difficulties [9]. Alongside nuts and peanuts, CM is among the most common causes of food-induced anaphylaxis [7]. Fortunately, CMA is commonly outgrown by the age of five years. For children with persistent CMA, OIT is a potential therapeutic approach that reduces reactivity or induces tolerance to CM protein [2]. Almost all of the published articles about milk OIT included children older than four years. Selected articles are listed in Table 1.
Although the first studies on milk OIT date back to the beginning of the century, the first RCT was published by Skripak et al. in 2008 [10]. In this placebo-controlled RCT, the milk threshold reaction significantly increased in all children in the OIT group, with no change in the placebo group. ARs were common, but nearly 90% were transient and did not require treatment. However, it should be noted that this study did not include children with severe allergies, which may have contributed to the low rate of severe AR. In a follow-up study, desensitized children were advised to regularly consume dairy products at home. ARs were common, but the rate of reactions decreased over time, regardless of increasing milk doses. Several reactions were multisystemic but they were often linked to exercise or viral illness [11].
The first RCT with OIT among children who suffered severe CMA was published by Longo et al. in 2008 [12]. Although they had severe allergies, 36% of children in the OIT group were able to consume dairy products without restrictions after one year of treatment, as opposed to none in the control group. Almost all children had ARs during OIT, mainly cutaneous or abdominal. Epinephrine was applied in 13.3% of administered doses. A more recent RCT reported similar results in 30 children with severe milk allergies [13]. The treatment efficacy for desensitization was 50%, compared to 0% in the control group, with a threshold increase of 3090%. Unfortunately, the incidence of ARs was also high. More than 80% of the children in the OIT group had ARs, while 43% needed adrenaline.
As OIT in children with severe CMA was accompanied by frequent ARs, with high rates of severe reactions, Yanagida et al. designed a study with a very low maintenance dose of milk (3 mL) [14]. After one year of treatment, unresponsiveness in the OIT group was significantly higher than in the control group. More than 50% of children tolerated 3 mL of milk, and 33% tolerated 25 mL of milk, significantly higher than the maintenance dose. ARs were more frequent at the beginning of the build-up phase, with antihistamines as the most common treatment. Therefore, low-dose-induction OIT may be a relatively safe and effective treatment method, reducing symptoms after accidental exposure to small amounts of milk. Slow low-dose OIT for different allergens (milk, egg, wheat) in children with severe food allergies also showed significant efficacy and good safety (more than 60% of children on milk OIT did not have any ARs) [15].
To further increase the safety of OIT while maintaining its effectiveness, efforts have been made to use less allergenic forms of milk. It is known that the majority of children with CMA can tolerate heated or baked milk [16]. The heating process could change the allergenicity of milk proteins through conformational changes in specific epitopes of whey protein, especially in combination with wheat flour matrix [17]. Incorporating baked milk (BM) into the diets of BM-tolerant children could induce tolerance to more allergenic (less heat-denatured) forms of milk. Children who tolerated BM but reacted to unheated milk (UM) were advised to introduce BM products into their diet, gradually introducing more allergenic forms of milk (muffins, pizza, rice pudding and UM). After 36 months, 48% of children who followed the BM diet became tolerant to UM. Severe ARs were not reported in children who could tolerate at least pizza, suggesting that OIT in BM-tolerant children could be a safe option [18].
Two recent RCTs have shown promising results regarding the safety of OIT with heat-processed milk [19,20]. OIT with heated milk induced tolerance development in a safer manner than OIT with UM in children with a risk of CMA anaphylaxis, but with somewhat lower efficiency. AR rates were significantly lower in BM than in the UM group [19]. An RCT comparing BM OIT and a placebo in children with severe CMA showed that 79% of children in the BM OIT group achieved tolerance of 4 g of BM protein after one year of treatment, compared to 0% in the placebo group [20].

Table 1.
Review of safety in milk OIT.
Table 1.
Review of safety in milk OIT.
| Reference | Design | Sample | Participants Characteristics | Form of Allergen | Duration | Maintenance Dose | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| Skripak et al. (2008) [10] | Double-blind, placebo-controlled RCT | N = 19 (6–17 y) A = 13 P = 7 | DBPCFC < 2.5 g MP. Excluding: history of severe anaphylaxis; severe persistent asthma | non-fat milk powder | 3–4 months | 500 mg MP | Desensitization: DBPCFC dose threshold of MP: A: median-5140 mg (p = 0.002) P: <40 mg (p = 0.0003) | AE: total reaction per doses: A vs. P = 45.4% vs. 112. Most common local and mild. |
| Narisety et al. (2009) [11] | Open label fellow-up | N = 15 (6–16 y) | DBPCFC > 2.5 g MP | Dairy product | 3–17 months | 1–16 g MP (median 7 g) | Desensitization: 6/13 >16 g MP 7/13 1–16 g MP | AE per total doses: 17% SR: 5.8% |
| Yanagida et al. (2015) [14] | CCT | N = 37 (>5 g) A = 12 C = 25 | OFC < 3 mL milk. history of anaphylaxis. | Fresh milk | 1 y | 3 mL of fresh milk | Desensitization to 3 mL of milk: A vs. C = 66.7% vs. 13.8% (p = 0.001) US to 3 mL of milk: A vs. C = 58.3% vs. 13.8% (p = 0.031) US to 25 mL of milk: A vs. C = 33% vs. 0% (p = 0.007) | AE per dose: hospital vs. home = 57.1% vs. 19.5% Severe AE: hospital vs. home = 0% vs. 0.03% |
| Inuo et al. (2018) [21] | Double-blind RCT | N = 22 (1–9 y) pHF = 11 eHF = 11 | History of SR to CM. Positive OFC to <20 mL rCMF; Excluding: severe persistent asthma | pHF | 8 w | <20 mL pHF | Threshold increase: pHF (p < 0.048) eHF (p = 0.23) | Mild AE: pHF = 18% eHF = 0% Severe AE: 0% |
| Nowak-Węgrzyn et al. (2018) [18] | CT | N = 160 (4–10 y) A = 126 RBM = 41 TBM = 85 C = 34 | Positive DBPCFC to different milk form Excluding: history of severe anaphylaxis; severe asthma/EoE | muffin -> pizza -> rice pudding-> unheated milk | 10 months | Unrestricted daily ingestion of dairy products | SU: OFC to 8 g unheated milk: RBM = 0%; TBM = 41% C: 0% | AE: 35% during escalation-OFC SR: no to BM |
| Sugiura et al. (2020) [15] | CCT | N = 50 (4–7 y) A = 41 C = 9 | Severe allergy to CM; OFC< 0.264 g MP. Excluding: history of anaphylaxis to <1 mL CM; tolerant to Baked mil | Whole milk | 1 y | 10× > initial dose | Desensitization: OFC to 0.264 g MP: A vs. C = 34.7% vs. 0% (p = 0.042) | % of AE in total doses: 0.47% (subjective 0.28%, objective 0.19%) |
| Nagakura et al. (2021) [19] | RCT | N = 33 (5–11 y) HM = 17 UM = 16 | DBPCFC < 3 mL of HM >5 y; history of anaphylaxis to CM; Excluding: uncontrolled AD; bronchial asthma | HM or UM | <12 months | 3 mL of HM or UM | Desensitization: OFC to 3 mL CM: HM vs. UM = 94% vs. 75% (p = 0.17) SU: OFC to 3 mL CM HM vs. UM = 35% vs. 50% (p = 0.34) OFC to 25 mL CM HM vs. UM = 31% vs. 18% (p = 0.43) | % AE: HM vs. UM = 8.1% vs. 9.6% (p = 0.01) |
| Maeda et al. (2021) [13] | RCT | N = 28 (3–12 y) A = 14 C = 14 | OFC < 10 mL CM. Excluding: history of severe anaphylaxis to CM; uncontrolled asthma or AD; | Whole milk | 1 y | 100 mL whole milk | Desensitisation to 100 mL CM: A vs. C = 50% vs. 0% (p < 0.01) SU: 8.5% in A group (2 y) | % participants with AE: A = 12/14 (severe 2/14) C = 3/14 (severe 0) |
| Dantzer et al. (2022) [20] | Double-blind, placebo-controlled RCT | N = 30 (3–18 y) A = 15 P = 15 | DBPCFC to 3–444 mg of baked MP Excluding: history of severe anaphylaxis; uncontrolled asthma or AD; EoE; | Milk baked in muffin | 8 w–1 y | 2 g MP baked in muffin | Desensitisation: DBPCFC to 4044 mg baked MP: A vs. P = 73% vs. 0% (p < 0.0001) DBPCFC to 2044 mg baked MP: A vs. P = 80% vs. 20% (p < 0.003) Change in threshold= A vs. P = 3.9 g vs. 0 g (p < 0.0001) | AE: % participants: A vs. P = 100% vs. 73% AE: % doses: A vs. P = 42% vs. 2% 95%-mild AE |
| Dantzer et al. (2025) [22] | Open label follow-up | N = 13 (3–18 y) | Follow up participants desensitized to 2044 BM | Milk baked in muffin | 1 y | 2 g MP baked in muffin | Per protocol analysis: DBPCFC to 2044 mg of BMP: 75% DBPCFC to 2 g UMP: 73% DBPCFC to 8 g UMP: 45% | AE: % participants: 61.5% % doses: 18.6% 98%- mild AE |
A—active; AD—atopic dermatitis; AE—adverse events; BM—baked milk; BMP—baked milk protein; C—control; CCT—controlled clinical trial; CM—cow milk; CT—clinical trial; DBPCFC—double-blind placebo-controlled food challenge; eHF—extensively hydrolyzed CM-based formula; EoE—eosinophil esophagitis; RCT—randomized controlled trial; HM—heated milk; MP—milk protein; OFC—oral food challenge; P—placebo; pHF—partially CM-based formula; rCMF—regular CM-based formula; RBM—reactive to baked milk; TBM—tolerant to baked milk; SR—severe reactions; SU—sustain unresponsiveness; UM—unheated milk; w—weeks; y—years.
ARs were common, but, in 98% of cases, they were mild, and less than 1% had severe reactions. Gastrointestinal side effects, which dominated, decreased during the maintenance period and mainly did not require treatment. After two years of BM OIT, 43% of children could tolerate 2 g or more of UM protein, suggesting that there may be increased efficacy with a longer treatment duration. Dose-related symptoms decreased in the second year compared to the first year, supporting the idea that safety likely increases over time with regular ingestion [22]. Alongside heat processing, the allergenicity of CM can be decreased or lost after enzymatic hydrolysis breaks down CM proteins. The effect of allergenicity depends on the final peptide fragment size. Extensively hydrolyzed formulas (eHF) with peptides < 3000 Da are intended for feeding infants with CMA. Partially hydrolyzed formulas (pHF) contain more peptides (3000–5000 Da). Although not intended for infants with CMA, pHF could potentially induce immunological changes minimalizing AR due to lower allergenicity. OIT involving pHF ingestion in a safe manner improved tolerance in children suffering from severe CMA, compared to children consuming eHF [21].
3.2. Egg Oral Immunotherapy
Alongside milk, egg allergy is one of the commonest forms of food allergy among children, spontaneously resolving in 50–60% of cases by school age [2]. Strict allergen avoidance seriously affects the nutritional status and quality of life of patients and their families [23]. In children with persistent egg allergy, OIT is the treatment of choice [2].
In 2007, Buchan et al. published a pilot study of hen’s egg (HE) OIT [24]. Seven subjects completed the protocol. They reported the desensitization of all patients after two years of OIT. Three subjects tolerated known or possible accidental egg ingestions while receiving OIT, and two subjects demonstrated oral tolerance during the second OFC. This study suggested that OIT can be safely used for patients with egg allergies without a history of anaphylaxis to eggs. After this promising study, over a dozen studies were published about HE OIT in children. We chose ten of them to show a spectrum of published protocols and their safety (Table 2).
In 2012, Burks et al. published the first double-blind RCT in 55 participants aged 5 to 11 (OIT 40: Placebo 15) [25]. The OIT protocol included three phases: an initial dose escalation, a build-up phase, and a maintenance phase. During the maintenance phase, participants consumed up to 2 g of egg-white powder daily, roughly equivalent to one-third of an egg. After 10 months, all participants completed an OFC involving a cumulative dose of 5 g of egg-white powder. At 22 months, children who had received OIT underwent a second OFC with a 10-g dose of egg-white powder. The results showed a desensitization rate of 75% of children. Children who successfully passed the challenge at 22 months discontinued OIT and avoided all egg consumption for four to six weeks. In the OIT group, 28% (11 of 40 children) passed the OFC at 24 months and were considered to have tolerance or sustained unresponsiveness (SU). ARs were reported in 78% of children in the OIT group and 20% in the placebo group (p < 0.001). ARs manifested as oral or pharyngeal, and no severe ARs were reported. In 2015, Ciminiti et al. published a double-blind placebo-controlled study that enrolled 31 participants (OIT 17: Placebo 14) [26]. Of the 17 children (1 dropout), 16 achieved desensitization and started the six-month egg-containing diet. After three months of egg avoidance, tolerance was maintained in only one third of them. ARs were encountered, but only one child needed adrenaline treatment.

Table 2.
Review of safety in egg OIT.
Table 2.
Review of safety in egg OIT.
| Reference | Design | Sample | Participants Characteristic | Form of Allergen | Duration | Maintenance Dose | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| Burks et al. (2012) [25] | Double-blind, placebo RCT | n = 55 (5–11 y) OIT: 40 Placebo: 15 | Clinical history of egg-allergy | EWP | 24 months | 2 g EWP | At 10 and 22 months: Desensitization to 5 and 10 g of EPW: OIT: 55% and 75%; Placebo: 0% and 0% (p < 0.00, p < 0.0011) At 24 months: SU to 10 g EPW + 1 cooked egg: OIT: 11%, placebo: 0% (p < 0.03) | AE: % participants OIT: 78%; Placebo: 20% (p < 0.01) Severe AE: no |
| Caminiti et al. (2015) [26] | Double-blind placebo-controlled study | N = 31 (4–11 y. median 6) OIT: 17 Placebo: 14 | Age ≥ 4 Clinical history; HE specific IgE & SPT Pos DBPCFC to 3.7 g EW protein | OIT: EWP Placebo: corn flour | 10 months (4 months OIT, 6 months egg containing diet) Control group avoided HE for 9 months after trial | 4 g EWP | Desensitization 4 months: ITT: OIT 16/17; Cont 0/14 SU 13 months ITT: OIT 5/17; Cont 1/14 | AE: % participants OIT: 29 P: 0% Adrenaline OIT 1/17 P 0/14 |
| Jones et al. (2016) [27] | Extension of previous study at 4 y | n = 55 (5–11 y) OIT: 40 Placebo: 15 | Clinical history of egg-allergy | EWP | 2 y | 2 g EWP | SU to 10 g EPW + whole cooked egg by 3 and 4 y: OIT: 45% and 50% | AE during OIT dosing: 54% OIT participants, mostly mild symptoms |
| Pérez-Rangel et al. (2017) [28] | Open-label, RCT ROIT1 group: rush egg OIT for 5 months Control group: egg avoidance for 5 months before rush egg OIT | ROIT1: n = 19, 10.9 ± 2.7 years Control: n = 14, 9.7 ± 2.3 years | Positive DBPCFC with 2808 mg of EW protein | Dehydrated EWP | 5 months | 1 undercooked egg | Desensitized to 1 undercooked egg ROIT1: 89% Control: 0% (p < 0.001) | Patients with any AE in build-up: 69% |
| Itoh-Nagato et al. (2018) [29] | Randomized, parallel-group, delayed-start study. 1st stage: early start group on rush OIT. 2nd stage: all participants on OIT | n = 45 (5–15 y) early start: n = 23 (rush OIT for 3 months) late-group: n = 22 (egg elimination for 3 months before OIT) | positive DBPCFC to ≤500 mg dried raw EPW | Raw EWP | 1 y | 60 g of cooked egg ~1 medium size egg or 1 g EWP | Desensitization to 1000 mg EPW after 3 months: Early star: 87% Late start: 22% (p < 0.001). | AE during first stage: Early start: 80% Late-star: 0% AE requiring adrenaline; 11.6% |
| Martín-Muñoz et al. (2019) [30] | Multicenter open-label RCT Group A (AG): Daily maintenance dose Group B (BG): Maintenance dose every two days | AG: n = 38. 81.4 ± 12.5 months BG: n = 38. 81.7 ± 13.4 months | Positive DBPCFC with 3.3 g EPW protein | Pasteurized EW | 1 year | 3.3 g of | Desensitized to 3.3 g at 6 months (T18) and 12 (T24) months after OIT T18: AG, 93.1% and BG, 83.3% T24: AG, 100% and BG, 92% | Patients with any AE: 86.8% More frequent in the BG |
| Kim et al. (2020) [31] | Open label randomized trial | n = 50 (3–36 y) BE-R: n = 27 OIT-R: n = 23. OIT-assigned (OIT-A) comparison: 39 | Negative DBPCFC to BE Positive DBPCFC to unbaked egg (1444 mg of EWP) | BE vs. EWP | 2 y | 2000 mg egg white protein | SU to 7444 mg white egg protein: BE-R = 11.1% OIT-R: 43.15% OIT-A 17.9% | AE: % participants: BE-R: 2.8% OIT-R: 3.9% OIT-A: 12.6% Severe AE: only in OIT groups |
| Kim et al. (2020) [32] | Extension of previous published study at 5 y | n = 55 (5–11 y) OIT: 40 Placebo: 15 | Clinical history of egg-allergy, Completed previous OIT study | All forms of egg | 1 y | Unlimited consumption all forms of egg | Ingestion all form of egg: SU-OIT: 100% Desensitized OIT: 43% Non-desensitized OIT: 17% Placebo: 36% | AE: no OIT participants reported symptoms to any baked egg consumption |
| Palosuo et al. (2021) [33] | Open-label randomized trial | n = 50 (6–17 y) OIT: 32 Control: 18 | Positive DBPCFC to heated egg white | EWP | 8 months | 1 g egg-white protein | Desensitization to 1 g of egg white protein: OIT: 44% Control: 4.8% | AE: 82% participants during build-up phase No severe reactions |
| Ogura et al. (2020) [34] | Multicenter, open-label, randomized, uncontrolled trial | Low-dose OIT group: n = 25, 5.2–8.9 year High-dose OIT group: n = 26, 4.7–9.2 year | Positive OFC with 194–1550 mg of egg protein | Scrambled egg | 1 y | Low dose: 1550 mg High dose: 6200 mg | Short term SU to 6200 mg Low-dose OIT: 20% High-dose OIT: 26.9% (p = 0.743) | Any AE per dose Low-dose OIT: 8.74% High-dose OIT: 10.9% (p < 0.05) |
AE—adverse events; BE-R—baked egg randomized; DBPCFC—double-blind placebo-controlled food challenge; EWP—egg-white powder; OIT—oral immunotherapy; OIT-A—OIT assigned; OIT-R—OIT randomized; RCT—randomized controlled trial; SU—sustained unresponsiveness; y—years.
The study by Jones et al. published in 2016 showed that 55% of children with OIT were desensitized to 5 g of egg white powder after 10 months of therapy, and 75% of OIT subjects were desensitized to 10 g of egg white powder after 22 months of therapy. The number of OIT subjects who achieved desensitization was 77.5% in years three and four. SU in year two occurred in 27.5% of OIT subjects and increased to 45.0% in year three and 50.0% in year four. In 54% of OIT participants, ARs during OIT dosing were reported, and all were categorized as mild [27]. In a multicenter RCT published in 2019, Martin-Munoz et al. randomized children into three groups: egg-free diet (CG), daily OIT maintenance (AG), and OIT every two days (BG). Daily OIT maintenance improved adherence, effectiveness, and safety. ARs affected 86.8% of children, and they were more frequent in the BG group [30]. Reducing the maintenance dose may effectively lower the AR compared to the target OIT dose, while at the same time maintaining efficiency [34].
The safety of egg OIT is influenced by protocol factors, including the food products used, the dosing schedule, and the maintenance dose (see the table). In a study examining the efficacy of baked versus unbaked eggs, OIT with unbaked eggs was significantly more effective in achieving SU in children who were allergic to unbaked eggs [31]. A protocol involving daily intake may offer better efficacy and safety compared to intake every two days [30]. The majority of ARs in egg OIT are categorized as mild or moderate [25,27,30,31,33]. ARs were most strongly associated with OIT dosing [25,33]. Severe reactions were rare but still significant for the affected patient [26,28,29,32].
3.3. Peanut Oral Immunotherapy
Peanut allergy is a significant concern, particularly among children, due to its potential to cause severe and life-threatening reactions. It is estimated that the prevalence of allergic reactions to peanuts lies at around 2% [35]. These facts have led to allergen-specific immunotherapy for peanuts becoming the most researched approach for peanut allergy treatment and food allergen-specific immunotherapy in general [36].
Different OIT protocols vary in terms of the initial dose, the rate of dose escalation, and the maintenance dose [4]. Some protocols start with very low doses (e.g., 0.1 mg of peanut protein) and increase gradually [37,38], while others begin with higher initial doses or an initial dose that is determined after performing OFC [39]. The rate of escalation can also vary, with some protocols increasing the dose weekly, every two weeks, or monthly. Maintenance doses are categorized into either low-dose or high-dose groups, with only one protocol utilizing an intermediate maintenance dose [40]. The comparison data between the peanut OIT studies are shown in Table 3.
The first double-blind RCT on peanut allergy was conducted by Varshney in 2011, involving a small group of children with peanut allergies. All children treated with OIT achieved desensitization to a cumulative dose of 5 g of peanut protein, experiencing only mild clinical symptoms during the build-up phase. The patients with severe anaphylaxis were excluded from this trial [41].
In a STOP II study, RCT was performed in children 7–16 years with immediate reactions after peanut ingestion; this involved a positive double-blind placebo-controlled food challenge (DBPCFC) with a maintenance dose of 800 mg of peanut protein. The ARs in most participants were mild [39]. These first RCTs were conducted with peanut flour [37,39,41]. In addition to conventional food forms, commercially standardized food products were used for this purpose [42,43,44,45].

Table 3.
Review of safety in peanut OIT.
Table 3.
Review of safety in peanut OIT.
| Reference | Design | Sample | Participants Characteristic | Form of Allergen | Duration | Maintenance Dose | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| Du Toit et al. (2023) [46] | RCT | 146 (12–48 m) | Positive DBPCFC | peanut flour | 12 months | 300 mg | Desensitization | AE: mild to moderate 75.5%, systemic 2%, none severe Control: mild to moderate 58.3%, severe none |
| Bird et al. (2018) [42] | Double-blind, placebo-RCT | 55 (4–26 y) OIT:29 Control:26 | Positive DBPCFC | peanut flour | 24 weeks | 300 mg | Desensitization OIT cumulative doses of ≥443 mg: 79% 1043 mg: 62% Placebo cumulative doses of ≥443 mg: 19% 1043 mg: 0% | AE: Severe symptoms OIT: 0% Control: 38% |
| Fernandez-Rivas et al. (2022) [45] | Open-label follow-on study | 142 (4–17 y) OIT 1,5 y: n = 110 OIT 2 y; n = 32 | Positive DBPCFC | peanut flour | 1.5–2 years | 300 mg | Desensitization to 2000 mg of peanut protein: OIT: n = 50 (48.1%) Control: n = 21 (80.8%) | n AE/n participants during maintenance: PALISADE vs. ARC004= OIT 1.5 y: 20.7 vs. 12.8 OIT 2 y: 31.8 vs. 17.5 |
| IMPACT trial (2022) [38] | Double-blind, placebo-RCT | 146 (median age 39.3 mo) OIT: 96 Control: 50 | Positive DBPCFC reactive to 500 mg or less of peanut protein | peanut flour | 160 weeks | 2000 mg | Desensitization to 5000 mg OIT: 71% Control: 2% | AE (dosing reaction): Mild to moderate OIT: 98% Control: 80% |
| PALISADE study (2018) [43] | Double-blind, placebo-RCT | 496 (4–17 y) OIT: n = 372 Control: 124 | Positive DBPCFC | peanut flour | 6 months | 300 mg | Desensitization to 600 mg of peanut protein: OIT: N = 250 (67.2%) Control: N = 5 (4.0%) | AE: Moderate OIT: 25% Control: 59% Severe OIT: 0.8% Control: 4.3% |
| POISEID study (2019) [47] | Double-blind, placebo-RCT | 120 (7–55 y) OIT: 60 OIT 300 mg:35 Placebo: n = 25 | Positive DBPCFC | peanut flour | 156 weeks | 4000 mg | Desensitization to 4000 mg OIT: 37% OIT 300 mg:13% Placebo: 4% | AE: OIT: 83.3% OIT 300 mg: 82.3% Placebo: 44% |
| STOP II study (2014) [39] | Open-label randomized trial | 99 (7–15 y) OIT: n = 39 Control: n = 46 | Positive DBPCFC | peanut flour | 6 months | 800 mg | Desensitization OIT: 62% Control: 0% | AE (mild/severe): OIT: 79.5%/0.01% Control: 0% |
| Varshney P et al. (2011) [41] | Double-blind, placebo-RCT | 25 (1–16 y) OIT: n = 16 Control: n = 9 | Clinical history peanut reaction within 60 min of ingestion, peanut CAP-FEIA >15 kU/L or >7 kU/L if reaction within 6 months of enrollment, and a positive SPT | peanut flour | 12 months | 4000 mg | Desensitization to 5000 mg of peanut protein: OIT: N = 16 (100%) Control: N = 1 | AE: OIT: 6.25% Control: 100% |
| Vickery et al. (2021) [44] | Open label RCT | 24 (1–16 y) | Positive DBPCFC | peanut flour | 5 years | 4000 mg | Desensitization: 50% per protocol 31% ITT | AE: Treatment success: 0% Treatment failure: 14% (mild) |
| Vickery et al. (2017) [37] | Open label RCT | 37 (9–36 mo) Low dose: 20 High dose: 17 | Positive DBPCFC | peanut flour | 3 years | Low dose 300 mg High dose 3000 mg | Desensitization (low/high dose): 85%/76% | AE (mild to moderate): 99% |
AEs—adverse events; DBPCFC—double-blind placebo-controlled food challenge; ITT—intent to treat; OIT—oral immunotherapy; RCT—randomized controlled trial; y—years; mo—months.
The study conducted by Bird et al., two studies by Vickery et al., and a survey by Fernandez Rivas used commercial products in studies, all with a maintenance dose of 300 mg [42,43,44,45]. The ARC001 and PALISADE studies were conducted in patients positive for DBPCFC of 143 mg peanut protein and 100 mg peanut protein. ARs were high among the treatment group even though the rate of ARs between the treatment group and placebo group differed only regarding serious ARs, which were rare (4.3% vs. 0.8%) [42,43].
The open-label follow-on studies conducted by Vickery et al. (ARC004) and Fernandez Rivas et al. both used the same commercial product in a maintenance dose of 300 mg, but, in patients negative to DBPCFC, in a dose of 300 mg of peanut proteins [44,45]. The study by Vickery randomized the patients according to the dosing schedule, showing that the rate of ARs was lower in patients using maintenance doses every day compared to the different maintenance dosing regimens. The prolonged exposure to the maintenance dose led to a decrease in ARs, still with significant immunomodulation and desensitization [44].
The IMPACT trial was performed with peanut flour and had a higher level of peanut protein in the maintenance dose, with 71% of patients developing desensitization, of whom 21% were SU. Peanut OIT in children younger than four years old was linked to increased desensitization and SU [38].
The POISED study demonstrated that OIT leads to desensitization to 4 g of peanut protein in most peanut-allergic children. However, discontinuing treatment or reducing the daily dose to 300 mg decreases the likelihood of maintaining tolerance to peanuts at the previously achieved thresholds, highlighting the importance of ongoing daily allergen ingestion [47].
3.4. Adjuvants in Oral Immunotherapy
ARs during OIT are not rare, and, in some patients, a favorable outcome is not so clear. Anaphylaxis pronounced gastrointestinal symptoms, eosinophilic esophagitis, and other ARs are responsible for 10–30% of OIT withdrawals [48].
A preferred outcome of OIT would be long-term allergen tolerance or SU, defined as a prolonged state of immune unresponsiveness that persists after allergen withdrawal [48]. Unfortunately, only one-third of the patients suffering from milk, egg, or peanut allergies achieve SU after OIT. Additionally, half of those patients will lose tolerance during the first six months following OIT [49]. On the other hand, a common outcome of OIT in food allergies is desensitization. In these cases, the threshold for allergic reaction is increased when regular allergen ingestion is maintained [50]. Patients are protected against the incidental ingestion of the culprit allergen, but only to a limited quantity. ARs will follow if the threshold amount is breached. Another negative side of desensitization is the vast number of patients experiencing ARs. There are data showing that three in four desensitized individuals experienced ARs to previously tolerated doses of OIT allergens [51,52]. ARs resulted in the discontinuation of OIT in almost half of the patients. Additionally, desensitization can be lost over time [52]. One approach to ameliorate the mentioned limitations of OIT is the introduction of adjuvant therapy. By suppressing acute ARs and modulating the immune response, adjuvant therapy aims to reduce the frequency of ARs and promote tolerance in patients receiving OIT.
3.4.1. Monoclonal Antibodies
- (a)
- Anti-immunoglobulin E monoclonal antibodies
The rationale behind talizumab and omalizumab (OMB) usage as an adjuvant therapy of OIT is the role of IgE in allergic reactions. These humanized anti-IgE monoclonal antibodies (mabs) prevent IgE binding to receptors on basophils and mast cells. They also downregulate the expression of IgE receptors on mast cells, basophils, and dendritic cells [53]. Administered as a monotherapy, anti-IgE mabs showed a significant effect in increasing the threshold sensitivity to food allergens [54,55,56]. Additionally, most research data have shown the synergistic effect of mab as an adjuvant to OIT. OMB as an adjuvant therapy had a superior effect compared to mab monotherapy in achieving desensitization in CMA [57]. OMB makes OIT more effective in achieving desensitization, and it is also safer due to the lower frequency of ARs [58,59,60]. It also makes the up-dosing process faster with higher maintenance OIT doses [48,57,60,61]. The introduction of OMB to OIT in high-risk patients with cow’s milk allergy facilitated desensitization. In the same research, the discontinuation of OMB resulted in ARs [62]. In the studied egg OIT, OMB helped desensitization, but ending the treatment resulted in the recurrence of symptoms; once adjuvant therapy was reinstated, the symptoms disappeared [63]. In peanut allergies, OMB facilitated rapid oral desensitization and improved the quality of the desensitization process, but it had no effect on SU [58,59]. Participants with multiple food allergies subjected to combined OIT and OMB therapy developed long-term desensitization faster and more safely [61,64,65].
- (b)
- Dupilumab
Dupilumab is a human monoclonal antibody that binds to interleukin (IL) 4R alpha receptor [66,67]. IL4Rα is a shared receptor for IL-4 and IL-13, and dupilumab inhibits the signaling of both IL-4 and IL-13, key components of type 2 inflammation [68,69]. Dupilumab is currently approved for the treatment of atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis (EoE), and prurigo nodularis [70,71,72,73]. Dupilumab as an adjunct to OIT moderately improved desensitization to peanut protein in children and adolescents. It had no significant effect on the frequency of ARs [74].
3.4.2. Interferon γ
Interferon (IFN)-γ is a pro-inflammatory cytokine involved in IgE regulation and the development of allergic reactions. IFN-γ facilitates T helper 1 and suppresses the Th2 response [75]. Desensitization for house dust mites and milk in atopic dermatitis has been successfully achieved using IFN-γ [76,77]. In two small case-control studies, IFN-γ showed promising results as adjunctive therapy to OIT. Patients with food allergies treated with a combination of OIT and IFN-γ or IFN-γ alone developed desensitization and a three-month tolerance [78,79].
3.4.3. Probiotics
Similarly to IFN-γ, probiotics, beneficial bacteria, inhibit allergic diseases by suppressing the Th2 response with possible additional effects on dendritic and Th17 cells [80,81]. The suppression of IL-4, IL-5, and IL-13 production, accompanied by T-regulatory responses, increased IL-10 and IFN-γ production, making probiotics promising candidates for adjuvants in OIT [82]. One of the largest studies was conducted in 2017, where the effects of combined OIT and Lactobacillus rhamnosus CGMCC 1.3724 were compared to a placebo [83]. Even though desensitization was achieved in the majority of children who were allergic to peanuts, there was no control group subjected to OIT only for comparison. In the follow-up studies, SU and therapy benefits persisted at the 12-month and four-year checkpoints [84]. In a more recent multicenter RCT, children were assigned to receive peanut OIT and placebo, peanut OIT and probiotic Lactobacillus rhamnosus ATCC 53103, and placebo and placebo for 18 months [85]. In terms of SU, eight weeks after the end of treatment, the group receiving probiotics and OIT had no advantage over children treated with OIT only. During the 12-month post-treatment period, almost identical percentages were eating peanuts with few anaphylactic reactions.
3.4.4. Vitamin D
The immunomodulatory effect of vitamin D has gained a lot of attention in allergology recently. Vitamin D is responsible for the downregulation of proinflammatory signaling pathways and affects the Th1/Th2 balance. Vitamin D deficiency can be responsible for the overproduction of IgE and IL-4 in allergen responses [86,87]. Additionally, vitamin D possibly plays a role in the stability and integrity of the mucosal epithelial barrier, and its insufficiency leads to inappropriate exposure to antigens and food allergies [88]. Cross-sectional studies in infants found that CMA was related to lower vitamin D levels, and infants with deficiency had a few-fold increased risk of egg, peanut, and multiple food allergies [89,90]. While recent observations confirmed the positive effects of vitamin D adjuvant therapy in allergic rhinitis, such data for food allergy OIT are missing [91].
4. Discussion
The reviewed studies show marked variability in OIT protocols for milk, egg, and peanut allergies, reflecting differences in clinical goals, patient characteristics, and safety considerations. This section critically evaluates these variations, their rationale, and clinical implications. Food allergies have been characterized by an increasing prevalence over the past few decades, particularly within the pediatric population. This condition significantly impacts quality of life and may lead to nutritional deficits and growth impairments [8]. The most common food allergens in early childhood—milk and eggs—are staple foods that substantially contribute to the daily intake of energy, protein, calcium, vitamin A, vitamin D, and various B vitamins [8]. Conversely, peanuts rank among the leading causes of food-induced anaphylaxis resulting from unintentional ingestion [92]. Consequently, there is a growing emphasis on preventive strategies and proactive measures in the research surrounding food allergies.
The management of food allergies is complex and encompasses allergen avoidance, individualized dietary guidance, and a comprehensive treatment plan that includes emergency medications such as oral antihistamines, adrenaline auto-injectors, and inhaled salbutamol. It is essential for patients to possess a written treatment plan containing emergency contacts and to receive training on the correct use of adrenaline auto-injectors. While the avoidance of food allergens and the management of clinical symptoms remain the primary approaches in food allergy treatment, oral immunotherapy (OIT) has emerged as an alternative strategy that aims to address the underlying pathophysiology of the condition. Numerous studies have been published on OIT, exhibiting significant variability in study protocols—including differences in administration regimens such as the initial dosing, dose increments, timing between doses, and target maintenance doses. Furthermore, these studies present varying outcomes concerning efficacy, safety profiles, and quality of life assessments among treated individuals.
In this review, we concentrated on safety considerations and potential modifications to existing protocols to enhance safety during OIT. Regardless of the chosen protocol, specific preconditions must be met to maximize safety. Initiation of the OIT protocol should involve a shared decision-making process between healthcare providers and caregivers. Patients and caregivers must be informed of OIT-related goals, risks, and benefits, as adherence to the treatment plan is crucial [2]. It is advisable for OIT to be conducted under the supervision of a clinical team that is experienced in food immunotherapy and adept at managing allergic reactions of varying severities, including anaphylaxis [2]. The dose increases during the build-up phase, when patients are most vulnerable to adverse reactions, should be conducted in a hospital or day treatment center equipped with appropriate resources and personnel to address any type of allergic reaction [2].
Before commencing OIT, it is critical to determine the suitability of the patient. Although milk and eggs are prevalent allergens in children, these allergies are often outgrown early in life, and it may be prudent to await the natural development of tolerance. Thus, the European Academy of Allergy and Clinical Immunology (EAACI) guidelines recommend postponing OIT for milk and egg allergies until the child reaches four years of age. Nonetheless, these guidelines also advocate for the careful consumption of various forms of allergens that children can tolerate (e.g., baked milk or egg) [2]. In contrast, peanut allergies are more likely to persist into adulthood. Interestingly, OIT demonstrates high efficacy and safety in young children, likely attributable to the adaptive plasticity of the immune system [37,38]. The POSEIDON and IMPACT studies have indicated that younger intervention ages correlate with increased tolerability, suggesting that the age of OIT initiation may be a key factor in enhancing safety [38,46].
A further potential modification to the food oral immunotherapy (F-OIT) protocol involves reducing the maintenance dose of the offending food. Evidence suggests that low-dose OIT can suppress pro-allergic cytokines and basophil activation in children [93]. Reduced maintenance doses have been effectively utilized in single-food studies, such as those involving peanuts, cow’s milk, and eggs, demonstrating successful desensitization and sustained unresponsiveness, albeit with limited evidence. In children with severe cow’s milk allergy (CMA), transitioning to a lower maintenance dose has resulted in enhanced safety for OIT, and similar outcomes have been observed with low-dose OIT in pediatric patients with peanut allergies [14,15,44,45]. Administering lower maintenance doses could potentially prevent OIT-induced eosinophilic esophagitis (EoE) and EoE-like conditions associated with OIT, although the supporting evidence is limited. The application of low-dose OIT is currently being explored for multiple food allergens [94]. Maintaining a low dose over an extended duration has further improved patient safety and minimized the occurrence of allergic reactions [34,44]. Cross-comparisons of daily dosing with various dosing regimens reveal that daily dosing exhibits a superior safety profile [30,34,44]. Moreover, OIT utilizing less allergenic food forms, such as baked milk or eggs, may also enhance safety, albeit potentially at the expense of efficacy [20,31].
For patients with severe food allergies, the safety of OIT can be further enhanced through the utilization of adjuvants, with omalizumab emerging as the most promising candidate among these adjunct therapies. The role of other forms of biologic therapy in improving safety during OIT remains a subject of debate. Despite the observation that children with food allergies may exhibit gut dysbiosis and lower serum levels of vitamin D3 compared to their healthy peers, the use of probiotics and vitamin D3 as adjuncts in OIT has not yielded satisfactory results to date [89,95].
Collaboration among experts is essential to establishing universal access to validated protocols and standardized products. There remains a pressing need for high-quality evidence regarding optimal maintenance dose frequency and duration, appropriate initiation ages that correlate with individual patient profiles, and the effective use of biologics tailored to patient characteristics, alongside long-term follow-up and cost-effectiveness analysis. Our proposed measures to enhance the safety of OIT are outlined in Scheme 1.
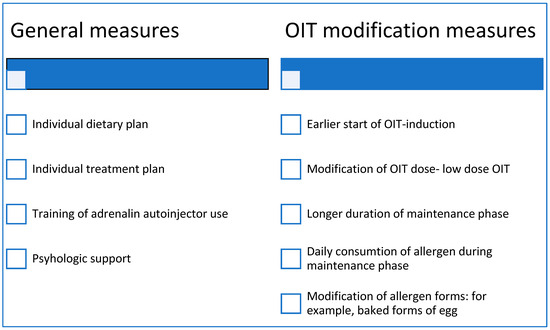
Scheme 1.
Measures to improve OIT safety.
In this review, we aimed to provide a concise overview of the safety of various OIT protocols documented thus far; however, certain limitations must be acknowledged. Notably, there is a dearth of placebo-controlled randomized controlled trials (RCTs), which complicates the estimation of placebo effects in terms of safety, particularly when assessing subjective symptoms reported during OIT. Furthermore, most existing studies primarily focus on the short-term effects of OIT. Additional follow-up investigations beyond the completion of OIT are warranted to evaluate long-term safety, particularly in light of the influence of co-factors such as exercise and infections. The absence of a standardized OIT protocol, coupled with significant disparities among protocols, renders comparisons of efficacy and safety challenging. Additionally, the lack of a uniform methodology for reporting allergic reactions across studies further complicates comparisons.
Despite the current limitations, OIT remains a promising approach for managing pediatric food allergies. By optimizing protocols—tailoring dose, duration, and adjunct therapy to individual patients—we can move toward safer, more effective treatments. Future research must aim to refine these elements while standardizing outcome reporting and ensuring long-term follow-up examinations.
5. Conclusions
Oral food immunotherapy is a very promising alternative for patients with food allergies, but it is not without risks. Safety can be improved under strict supervision, with a gradual increase in doses and good education on epinephrine use. When addressing different protocols, those using less allergenic forms of allergen with lower maintenance doses are generally safer. In cases where severe ARs are expected, the use of omalizumab improves safety. The effect of probiotics and vitamin D supplementation in combination with OIT is still controversial, and new trials regarding their synergistic effect are needed.
Author Contributions
Conceptualization, I.M.K.; structure, I.M.K. and A.M.G.; literature search and selection, I.M.K., M.J., A.M.G., H.M.Š. and A.M.; writing—original draft preparation, I.M.K., M.J., A.M.G., H.M.Š. and A.M.; writing—review and editing, I.M.K., M.J. and A.M.G.; supervision, I.M.K. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AR | Adverse reaction |
| BM | Baked milk |
| CM | Cow’s milk |
| CMA | Cow’s milk allergy |
| CT | Controlled trial |
| DBPCFC | Double-blind placebo-controlled food challenge |
| F-OIT | Food oral immunotherapy |
| HE | Hen’s egg |
| IFN | Interferon |
| IL | Interleukin |
| mab | Monoclonal antibody |
| OFC | Oral food challenge |
| OIT | Oral immunotherapy |
| OMB | Omalizumab |
| RCT | Randomized controlled trial |
| SU | Sustained unresponsiveness |
| UM | Unheated milk |
| EoE | Eosinophilic esophagitis |
References
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Riggioni, C.; Agache, I.; Akdis, C.A.; Akdis, M.; Alvarez-Perea, A.; Alvaro-Lozano, M.; Ballmer-Weber, B.; Barni, S.; Beyer, K.; et al. EAACI Guidelines on the Management of IgE-mediated Food Allergy. Allergy 2025, 80, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Schofield, A. A case of egg poisoning. Lancet 1908, 171, 716. [Google Scholar] [CrossRef]
- Graham, F. Variations in Protocol Development during Oral Immunotherapy. J. Food Allergy 2022, 4, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on Allergen Immunotherapy: IgE-mediated Food Allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef]
- Anagnostou, A. Weighing the Benefits and Risks of Oral Immunotherapy in Clinical Practice. Allergy Asthma Proc. 2021, 42, 118–123. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef]
- Parlak, Z.; İlgün Gürel, D.; Soyer, Ö.; Şekerel, B.E.; Şahiner, Ü.M. Nutritional Risks in Children with Food Allergy. Turk. J. Med. Sci. 2023, 53, 845–858. [Google Scholar] [CrossRef]
- Hill, S.A.; Nurmatov, U.; DunnGalvin, A.; Reese, I.; Vieira, M.C.; Rommel, N.; Dupont, C.; Venter, C.; Cianferoni, A.; Walsh, J.; et al. Feeding Difficulties in Children with Food Allergies: An EAACI Task Force Report. Pediatr. Allergy Immunol. 2024, 35, e14119. [Google Scholar] [CrossRef]
- Skripak, J.M.; Nash, S.D.; Rowley, H.; Brereton, N.H.; Oh, S.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. A Randomized, Double-Blind, Placebo-Controlled Study of Milk Oral Immunotherapy for Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2008, 122, 1154–1160. [Google Scholar] [CrossRef]
- Narisety, S.D.; Skripak, J.M.; Steele, P.; Hamilton, R.G.; Matsui, E.C.; Burks, A.W.; Wood, R.A. Open-Label Maintenance after Milk Oral Immunotherapy for IgE-Mediated Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2009, 124, 610–612. [Google Scholar] [CrossRef]
- Longo, G.; Barbi, E.; Berti, I.; Meneghetti, R.; Pittalis, A.; Ronfani, L.; Ventura, A. Specific Oral Tolerance Induction in Children with Very Severe Cow’s Milk-Induced Reactions. J. Allergy Clin. Immunol. 2008, 121, 343–347. [Google Scholar] [CrossRef]
- Maeda, M.; Imai, T.; Ishikawa, R.; Nakamura, T.; Kamiya, T.; Kimura, A.; Fujita, S.; Akashi, K.; Tada, H.; Morita, H.; et al. Effect of Oral Immunotherapy in Children with Milk Allergy: The ORIMA Study. Allergol. Int. 2021, 70, 223–228. [Google Scholar] [CrossRef]
- Yanagida, N.; Sato, S.; Asaumi, T.; Okada, Y.; Ogura, K.; Ebisawa, M. A Single-Center, Case-Control Study of Low-Dose-Induction Oral Immunotherapy with Cow’s Milk. Int. Arch. Allergy Immunol. 2015, 168, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Kitamura, K.; Makino, A.; Matsui, T.; Furuta, T.; Takasato, Y.; Kando, N.; Ito, K. Slow Low-Dose Oral Immunotherapy: Threshold and Immunological Change. Allergol. Int. 2020, 69, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to Extensively Heated Milk in Children with Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347.e2. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Fiocchi, A. Rare, Medium, or Well Done? The Effect of Heating and Food Matrix on Food Protein Allergenicity. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.T.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495.e5. [Google Scholar] [CrossRef]
- Nagakura, K.; Sato, S.; Miura, Y.; Nishino, M.; Takahashi, K.; Asaumi, T.; Ogura, K.; Ebisawa, M.; Yanagida, N. A Randomized Trial of Oral Immunotherapy for Pediatric Cow’s Milk-Induced Anaphylaxis: Heated vs Unheated Milk. Pediatr. Allergy Immunol. 2021, 32, 161–169. [Google Scholar] [CrossRef]
- Dantzer, J.; Dunlop, J.; Psoter, K.J.; Keet, C.; Wood, R. Efficacy and Safety of Baked Milk Oral Immunotherapy in Children with Severe Milk Allergy: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. J. Allergy Clin. Immunol. 2022, 149, 1383–1391.e17. [Google Scholar] [CrossRef]
- Inuo, C.; Tanaka, K.; Suzuki, S.; Nakajima, Y.; Yamawaki, K.; Tsuge, I.; Urisu, A.; Kondo, Y. Oral Immunotherapy Using Partially Hydrolyzed Formula for Cow’s Milk Protein Allergy: A Randomized, Controlled Trial. Int. Arch. Allergy Immunol. 2018, 177, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, J.A.; Lewis, S.A.; Psoter, K.J.; Sutherland, A.; Frazier, A.; Richardson, E.; Maiche, S.; Seumois, G.; Peters, B.; Wood, R.A. Clinical and Immunological Outcomes after Randomized Trial of Baked Milk Oral Immunotherapy for Milk Allergy. JCI Insight 2025, 10, e184301. [Google Scholar] [CrossRef]
- Meyer, R.; Wright, K.; Vieira, M.C.; Chong, K.W.; Chatchatee, P.; Vlieg-Boerstra, B.J.; Groetch, M.; Dominguez-Ortega, G.; Heath, S.; Lang, A.; et al. International Survey on Growth Indices and Impacting Factors in Children with Food Allergies. J. Hum. Nutr. Diet. 2019, 32, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.D.; Green, T.D.; Jones, S.M.; Scurlock, A.M.; Christie, L.; Althage, K.A.; Steele, P.H.; Pons, L.; Helm, R.M.; Lee, L.A.; et al. Egg Oral Immunotherapy in Nonanaphylactic Children with Egg Allergy. J. Allergy Clin. Immunol. 2007, 119, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Burks, A.W.; Jones, S.M.; Wood, R.A.; Fleischer, D.M.; Sicherer, S.H.; Lindblad, R.W.; Stablein, D.; Henning, A.K.; Vickery, B.P.; Liu, A.H.; et al. Oral Immunotherapy for Treatment of Egg Allergy in Children. N. Engl. J. Med. 2012, 367, 233–243. [Google Scholar] [CrossRef]
- Caminiti, L.; Pajno, G.B.; Crisafulli, G.; Chiera, F.; Collura, M.; Panasci, G.; Ruggeri, P.; Guglielmo, F.; Passalacqua, G. Oral Immunotherapy for Egg Allergy: A Double-Blind Placebo-Controlled Study, with Postdesensitization Follow-Up. J. Allergy Clin. Immunol. Pract. 2015, 3, 532–539. [Google Scholar] [CrossRef]
- Jones, S.M.; Burks, A.W.; Keet, C.; Vickery, B.P.; Scurlock, A.M.; Wood, R.A.; Liu, A.H.; Sicherer, S.H.; Henning, A.K.; Lindblad, R.W.; et al. Long-Term Treatment with Egg Oral Immunotherapy Enhances Sustained Unresponsiveness That Persists after Cessation of Therapy. J. Allergy Clin. Immunol. 2016, 137, 1117–1127.e10. [Google Scholar] [CrossRef]
- Pérez-Rangel, I.; Rodríguez del Río, P.; Escudero, C.; Sánchez-García, S.; Sánchez-Hernández, J.J.; Ibáñez, M.D. Efficacy and Safety of High-Dose Rush Oral Immunotherapy in Persistent Egg Allergic Children. Ann. Allergy Asthma Immunol. 2017, 118, 356–364.e3. [Google Scholar] [CrossRef]
- Itoh-Nagato, N.; Inoue, Y.; Nagao, M.; Fujisawa, T.; Shimojo, N.; Iwata, T.; Adachi, Y.; Arakawa, K.; Arima, T.; Fukushima, K.; et al. Desensitization to a Whole Egg by Rush Oral Immunotherapy Improves the Quality of Life of Guardians: A Multicenter, Randomized, Parallel-Group, Delayed-Start Design Study. Allergol. Int. 2018, 67, 209–216. [Google Scholar] [CrossRef]
- Martín-Muñoz, M.F.; Alonso Lebrero, E.; Zapatero, L.; Fuentes Aparicio, V.; Piquer Gibert, M.; Plaza Martín, A.M.; Muñoz, C.; Belver, M.T.; Martorell-Calatayud, C.; Martorell-Aragonés, A.; et al. Egg OIT in Clinical Practice (SEICAP II): Maintenance Patterns and Desensitization State after Normalizing the Diet. Pediatr. Allergy Immunol. 2019, 30, 214–224. [Google Scholar] [CrossRef]
- Kim, E.H.; Perry, T.T.; Wood, R.A.; Leung, D.Y.M.; Berin, M.C.; Burks, A.W.; Cho, C.B.; Jones, S.M.; Scurlock, A.; Sicherer, S.H.; et al. Induction of Sustained Unresponsiveness after Egg Oral Immunotherapy Compared to Baked Egg Therapy in Children with Egg Allergy. J. Allergy Clin. Immunol. 2020, 146, 851–862.e10. [Google Scholar] [CrossRef]
- Kim, E.H.; Jones, S.M.; Burks, A.W.; Wood, R.A.; Sicherer, S.H.; Leung, D.Y.M.; Henning, A.K.; Lindblad, R.W.; Dawson, P.; Keet, C.; et al. A 5-Year Summary of Real-Life Dietary Egg Consumption after Completion of a 4-Year Egg Powder Oral Immunotherapy (EOIT) Protocol. J. Allergy Clin. Immunol. 2020, 145, 1292–1295.e1. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K.; Karisola, P.; Savinko, T.; Fyhrquist, N.; Alenius, H.; Mäkelä, M.J. A Randomized, Open-Label Trial of Hen’s Egg Oral Immunotherapy: Efficacy and Humoral Immune Responses in 50 Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 1892–1901.e1. [Google Scholar] [CrossRef]
- Ogura, K.; Yanagida, N.; Sato, S.; Imai, T.; Ito, K.; Kando, N.; Ikeda, M.; Shibata, R.; Murakami, Y.; Fujisawa, T.; et al. Evaluation of Oral Immunotherapy Efficacy and Safety by Maintenance Dose Dependency: A Multicenter Randomized Study. World Allergy Organ. J. 2020, 13, 100463. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Gupta, R.S.; Knibb, R.C.; Haselkorn, T.; Tilles, S.; Mack, D.P.; Pouessel, G. The Global Burden of Illness of Peanut Allergy: A Comprehensive Literature Review. Allergy 2021, 76, 1367–1384. [Google Scholar] [CrossRef]
- Reinwald, S.; Rolland, J.M.; O’Hehir, R.E.; van Zelm, M.C. Peanut Oral Immunotherapy: Current Trends in Clinical Trials. Immunother. Adv. 2022, 2, ltac004. [Google Scholar] [CrossRef]
- Vickery, B.P.; Berglund, J.P.; Burk, C.M.; Fine, J.P.; Kim, E.H.; Kim, J.I.; Keet, C.A.; Kulis, M.; Orgel, K.G.; Guo, R.; et al. Early Oral Immunotherapy in Peanut-Allergic Preschool Children Is Safe and Highly Effective. J. Allergy Clin. Immunol. 2017, 139, 173–181.e8. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and Safety of Oral Immunotherapy in Children Aged 1–3 Years with Peanut Allergy (the Immune Tolerance Network IMPACT Trial): A Randomised Placebo-Controlled Study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef]
- Anagnostou, K.; Islam, S.; King, Y.; Foley, L.; Pasea, L.; Bond, S.; Palmer, C.; Deighton, J.; Ewan, P.; Clark, A. Assessing the Efficacy of Oral Immunotherapy for the Desensitisation of Peanut Allergy in Children (STOP II): A Phase 2 Randomised Controlled Trial. Lancet 2014, 383, 1297–1304. [Google Scholar] [CrossRef]
- Laubach, S.; Kim, E.H.; Greenhawt, M.; Bailey, S.; Anagnostou, A. A Review of Shared Decision-Making, Published Protocols, and Post-Desensitization Strategies in Oral Immunotherapy (OIT). Curr. Allergy Asthma Rep. 2024, 24, 173–197. [Google Scholar] [CrossRef]
- Varshney, P.; Jones, S.M.; Scurlock, A.M.; Perry, T.T.; Kemper, A.; Steele, P.; Hiegel, A.; Kamilaris, J.; Carlisle, S.; Yue, X.; et al. A Randomized Controlled Study of Peanut Oral Immunotherapy: Clinical Desensitization and Modulation of the Allergic Response. J. Allergy Clin. Immunol. 2011, 127, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Spergel, J.M.; Jones, S.M.; Rachid, R.; Assa’ad, A.H.; Wang, J.; Leonard, S.A.; Laubach, S.S.; Kim, E.H.; Vickery, B.P.; et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 476–485.e3. [Google Scholar] [CrossRef] [PubMed]
- PALISADE Group of Clinical Investigators. AR101 Oral Immunotherapy for Peanut Allergy. N. Engl. J. Med. 2018, 379, 1991–2001. [Google Scholar] [CrossRef]
- Vickery, B.P.; Vereda, A.; Nilsson, C.; du Toit, G.; Shreffler, W.G.; Burks, A.W.; Jones, S.M.; Fernández-Rivas, M.; Blümchen, K.; Hourihane, J.O.; et al. Continuous and Daily Oral Immunotherapy for Peanut Allergy: Results from a 2-Year Open-Label Follow-On Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 1879–1889.e13. [Google Scholar] [CrossRef]
- Fernandez-Rivas, M.; Vereda, A.; Vickery, B.P.; Sharma, V.; Nilsson, C.; Muraro, A.; Hourihane, J.O.; DunnGalvin, A.; du Toit, G.; Blumchen, K.; et al. Open-label Follow-on Study Evaluating the Efficacy, Safety, and Quality of Life with Extended Daily Oral Immunotherapy in Children with Peanut Allergy. Allergy 2022, 77, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Brown, K.R.; Vereda, A.; Irani, A.-M.; Tilles, S.; Ratnayake, A.; Jones, S.M.; Vickery, B.P. Oral Immunotherapy for Peanut Allergy in Children 1 to Less Than 4 Years of Age. NEJM Evid. 2023, 2, EVIDoa2300145. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Purington, N.; Andorf, S.; Long, A.; O’Laughlin, K.L.; Lyu, S.C.; Manohar, M.; Boyd, S.D.; Tibshirani, R.; Maecker, H.; et al. Sustained Outcomes in Oral Immunotherapy for Peanut Allergy (POISED Study): A Large, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Study. Lancet 2019, 394, 1437–1449. [Google Scholar] [CrossRef]
- Wood, R.A. Food Allergen Immunotherapy: Current Status and Prospects for the Future. J. Allergy Clin. Immunol. 2016, 137, 973–982. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Hsiao, K.-C. An Update on Oral Immunotherapy for the Treatment of Food Allergy. Paediatr. Child. Health 2016, 26, 304–309. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M. Adjuvant Therapies in Food Immunotherapy. Immunol. Allergy Clin. N. Am. 2018, 38, 89–101. [Google Scholar] [CrossRef]
- Barbi, E.; Longo, G.; Berti, I.; Matarazzo, L.; Rubert, L.; Saccari, A.; Lenisa, I.; Ronfani, L.; Radillo, O.; Ventura, A. Adverse Effects during Specific Oral Tolerance Induction: In Home Phase. Allergol. Immunopathol. 2012, 40, 41–50. [Google Scholar] [CrossRef]
- Keet, C.A.; Seopaul, S.; Knorr, S.; Narisety, S.; Skripak, J.; Wood, R.A. Long-Term Follow-up of Oral Immunotherapy for Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2013, 132, 737–739.e6. [Google Scholar] [CrossRef] [PubMed]
- Prussin, C.; Griffith, D.T.; Boesel, K.M.; Lin, H.; Foster, B.; Casale, T.B. Omalizumab Treatment Downregulates Dendritic Cell FcεRI Expression. J. Allergy Clin. Immunol. 2003, 112, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Sampson, H.A.; Yunginger, J.W.; Burks, A.W.; Schneider, L.C.; Wortel, C.H.; Davis, F.M.; Hyun, J.D.; Shanahan, W.R. Effect of Anti-IgE Therapy in Patients with Peanut Allergy. N. Engl. J. Med. 2003, 348, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Leung, D.Y.M.; Burks, A.W.; Lack, G.; Bahna, S.L.; Jones, S.M.; Wong, D.A. A Phase II, Randomized, Double-blind, Parallel-group, Placebo-controlled Oral Food Challenge Trial of Xolair (Omalizumab) in Peanut Allergy. J. Allergy Clin. Immunol. 2011, 127, 1309–1310.e1. [Google Scholar] [CrossRef]
- Savage, J.H.; Courneya, J.-P.; Sterba, P.M.; MacGlashan, D.W.; Saini, S.S.; Wood, R.A. Kinetics of Mast Cell, Basophil, and Oral Food Challenge Responses in Omalizumab-Treated Adults with Peanut Allergy. J. Allergy Clin. Immunol. 2012, 130, 1123–1129.e2. [Google Scholar] [CrossRef]
- Nadeau, K.C.; Schneider, L.C.; Hoyte, L.; Borras, I.; Umetsu, D.T. Rapid Oral Desensitization in Combination with Omalizumab Therapy in Patients with Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2011, 127, 1622–1624. [Google Scholar] [CrossRef]
- Yee, C.S.K.; Albuhairi, S.; Noh, E.; El-Khoury, K.; Rezaei, S.; Abdel-Gadir, A.; Umetsu, D.T.; Burke-Roberts, E.; LeBovidge, J.; Schneider, L.; et al. Long-Term Outcome of Peanut Oral Immunotherapy Facilitated Initially by Omalizumab. J. Allergy Clin. Immunol. Pract. 2019, 7, 451–461.e7. [Google Scholar] [CrossRef]
- MacGinnitie, A.J.; Rachid, R.; Gragg, H.; Little, S.V.; Lakin, P.; Cianferoni, A.; Heimall, J.; Makhija, M.; Robison, R.; Chinthrajah, R.S.; et al. Omalizumab Facilitates Rapid Oral Desensitization for Peanut Allergy. J. Allergy Clin. Immunol. 2017, 139, 873–881.e8. [Google Scholar] [CrossRef]
- Schneider, L.C.; Rachid, R.; LeBovidge, J.; Blood, E.; Mittal, M.; Umetsu, D.T. A Pilot Study of Omalizumab to Facilitate Rapid Oral Desensitization in High-Risk Peanut-Allergic Patients. J. Allergy Clin. Immunol. 2013, 132, 1368–1374. [Google Scholar] [CrossRef]
- Bégin, P.; Dominguez, T.; Wilson, S.P.; Bacal, L.; Mehrotra, A.; Kausch, B.; Trela, A.; Tavassoli, M.; Hoyte, E.; O’Riordan, G.; et al. Phase 1 Results of Safety and Tolerability in a Rush Oral Immunotherapy Protocol to Multiple Foods Using Omalizumab. Allergy Asthma Clin. Immunol. 2014, 10, 7. [Google Scholar] [CrossRef]
- Takahashi, M.; Soejima, K.; Taniuchi, S.; Hatano, Y.; Yamanouchi, S.; Ishikawa, H.; Irahara, M.; Sasaki, Y.; Kido, H.; Kaneko, K. Oral Immunotherapy Combined with Omalizumab for High–Risk Cow’s Milk Allergy: A Randomized Controlled Trial. Sci. Rep. 2017, 7, 17453. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, I.; Mazon, A.; Nieto, M.; Uixera, S.; Pina, R.; Nieto, A. Possible Recurrence of Symptoms after Discontinuation of Omalizumab in Anti-IgE-assisted Desensitization to Egg. Pediatr. Allergy Immunol. 2014, 25, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Andorf, S.; Manohar, M.; Dominguez, T.; Block, W.; Tupa, D.; Kshirsagar, R.A.; Sampath, V.; Chinthrajah, R.S.; Nadeau, K.C. Observational Long-Term Follow-up Study of Rapid Food Oral Immunotherapy with Omalizumab. Allergy Asthma Clin. Immunol. 2017, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Andorf, S.; Purington, N.; Kumar, D.; Long, A.; O’Laughlin, K.L.; Sicherer, S.; Sampson, H.; Cianferoni, A.; Whitehorn, T.B.; Petroni, D.; et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-Facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. eClinicalMedicine 2019, 7, 27–38. [Google Scholar] [CrossRef]
- Macdonald, L.E.; Karow, M.; Stevens, S.; Auerbach, W.; Poueymirou, W.T.; Yasenchak, J.; Frendewey, D.; Valenzuela, D.M.; Giallourakis, C.C.; Alt, F.W.; et al. Precise and in Situ Genetic Humanization of 6 Mb of Mouse Immunoglobulin Genes. Proc. Natl. Acad. Sci. USA 2014, 111, 5147–5152. [Google Scholar] [CrossRef]
- Murphy, A.J.; Macdonald, L.E.; Stevens, S.; Karow, M.; Dore, A.T.; Pobursky, K.; Huang, T.T.; Poueymirou, W.T.; Esau, L.; Meola, M.; et al. Mice with Megabase Humanization of Their Immunoglobulin Genes Generate Antibodies as Efficiently as Normal Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 5153–5158. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 Pathway in Atopic Diseases. Expert. Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual Blockade of IL-4 and IL-13 with Dupilumab, an IL-4Rα Antibody, Is Required to Broadly Inhibit Type 2 Inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Busse, W.W.; Maspero, J.F.; Lu, Y.; Corren, J.; Hanania, N.A.; Chipps, B.E.; Katelaris, C.H.; FitzGerald, J.M.; Quirce, S.; Ford, L.B.; et al. Efficacy of Dupilumab on Clinical Outcomes in Patients with Asthma and Perennial Allergic Rhinitis. Ann. Allergy Asthma Immunol. 2020, 125, 565–576.e1. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, R.S.; Sindher, S.B.; Nadeau, K.C.; Leflein, J.G.; Spergel, J.M.; Petroni, D.H.; Jones, S.M.; Casale, T.B.; Wang, J.; Carr, W.W.; et al. Dupilumab as an Adjunct to Oral Immunotherapy in Pediatric Patients with Peanut Allergy. Allergy 2025, 80, 827–842. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The Role of Interferon-Gamma on Immune and Allergic Responses. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. 1), 137–144. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.; Young Lee, K. Pilot study of ifn-γ-induced specific hyposensitization for house dust mites in atopic dermatitis: Ifn-γ-induced immune deviation as a new therapeutic concept for atopic dermatitis. Cytokine 2000, 12, 472–476. [Google Scholar] [CrossRef]
- Noh, G.; Lee, S.S. Effects of IFN-γ on Milk-Specific Oral Tolerance Induction for Milk Allergy in Atopic Dermatitis: Food-Specific Oral Tolerance Induction Using IFN-γ as Adjuvant. In Clinical Immunology and Allergy in Medicine; Marone, G., Ed.; JGC Editions: Naples, Italy, 2003; pp. 475–496. [Google Scholar]
- Noh, G.; Jang, E.H. Dual Specific Oral Tolerance Induction Using Interferon Gamma for IgE-Mediated Anaphylactic Food Allergy and the Dissociation of Local Skin Allergy and Systemic Oral Allergy: Tolerance or Desensitization? J. Investig. Allergol. Clin. Immunol. 2014, 24, 87–97. [Google Scholar]
- Noh, G.; Lee, S.-S. A Pilot Study of Interferon-γ–Induced Specific Oral Tolerance Induction (ISOTI) for Immunoglobulin E–Mediated Anaphylactic Food Allergy. J. Interferon Cytokine Res. 2009, 29, 667–676. [Google Scholar] [CrossRef]
- Jang, S.-O.; Kim, H.-J.; Kim, Y.-J.; Kang, M.-J.; Kwon, J.-W.; Seo, J.-H.; Kim, H.Y.; Kim, B.-J.; Yu, J.; Hong, S.-J. Asthma Prevention by Lactobacillus rhamnosus in a Mouse Model Is Associated with CD4 + CD25 + Foxp3 + T Cells. Allergy Asthma Immunol. Res. 2012, 4, 150. [Google Scholar] [CrossRef]
- Jan, R.-L.; Yeh, K.-C.; Hsieh, M.-H.; Lin, Y.-L.; Kao, H.-F.; Li, P.-H.; Chang, Y.-S.; Wang, J.-Y. Lactobacillus gasseri Suppresses Th17 pro-Inflammatory Response and Attenuates Allergen-Induced Airway Inflammation in a Mouse Model of Allergic Asthma. Br. J. Nutr. 2012, 108, 130–139. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of Regulatory Dendritic Cells and CD4 + Foxp3 + T Cells by Probiotics Administration Suppresses Immune Disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Ponsonby, A.-L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a Probiotic with Peanut Oral Immunotherapy: A Randomized Trial. J. Allergy Clin. Immunol. 2015, 135, 737–744.e8. [Google Scholar] [CrossRef] [PubMed]
- Dunn Galvin, A.; McMahon, S.; Ponsonby, A.-L.; Hsiao, K.-C.; Tang, M.L.K. The Longitudinal Impact of Probiotic and Peanut Oral Immunotherapy on Health-related Quality of Life. Allergy 2018, 73, 560–568. [Google Scholar] [CrossRef]
- Hsiao, K.-C.; Ponsonby, A.-L.; Axelrad, C.; Pitkin, S.; Tang, M.L.K.; Burks, W.; Donath, S.; Orsini, F.; Tey, D.; Robinson, M.; et al. Long-Term Clinical and Immunological Effects of Probiotic and Peanut Oral Immunotherapy after Treatment Cessation: 4-Year Follow-up of a Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Child. Adolesc. Health 2017, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the Development of Allergic Disease: How Important Is It? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Roider, E.; Ruzicka, T.; Schauber, J. Vitamin D, the Cutaneous Barrier, Antimicrobial Peptides and Allergies: Is There a Link? Allergy Asthma Immunol. Res. 2013, 5, 119. [Google Scholar] [CrossRef]
- Silva, C.M.; da Silva, S.A.; Antunes, M.M. de C.; Silva, G.A.P. da; Sarinho, E.S.C.; Brandt, K.G. Do Infants with Cow’s Milk Protein Allergy Have Inadequate Levels of Vitamin D? J. Pediatr. 2017, 93, 632–638. [Google Scholar] [CrossRef]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.-L.; Gurrin, L.C.; Wake, M.; Vuillermin, P.; Martin, P.; Matheson, M.; Lowe, A.; Robinson, M.; et al. Vitamin D Insufficiency Is Associated with Challenge-Proven Food Allergy in Infants. J. Allergy Clin. Immunol. 2013, 131, 1109–1116.e6. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Ren, Y.; Yang, H.; Sun, X.; Huang, H. Clinical Efficacy of Vitamin D3 Adjuvant Therapy in Allergic Rhinitis: A Randomized Controlled Trial. Iran. J. Immunol. 2020, 17, 283–291. [Google Scholar] [CrossRef]
- Cianferoni, A.; Muraro, A. Food-Induced Anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 165–195. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Yue, X.; Guo, R.; Zhang, H.; Orgel, K.; Ye, P.; Li, Q.; Liu, Y.; Kim, E.; Burks, A.W.; et al. High- and Low-dose Oral Immunotherapy Similarly Suppress Pro-allergic Cytokines and Basophil Activation in Young Children. Clin. Exp. Allergy 2019, 49, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, P. Low Dose Multi-Nut Oral Immunotherapy in Pre-Schoolers with a Multi-Nut Allergy (LMNOP). Clinical Trial NCT05049512. 2021; manuscript in preparation. [Google Scholar]
- Akagawa, S.; Kaneko, K. Gut Microbiota and Allergic Diseases in Children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).