Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Black Currant (Ribes nigrum) Pomace
Abstract
1. Introduction
2. Materials and Methods
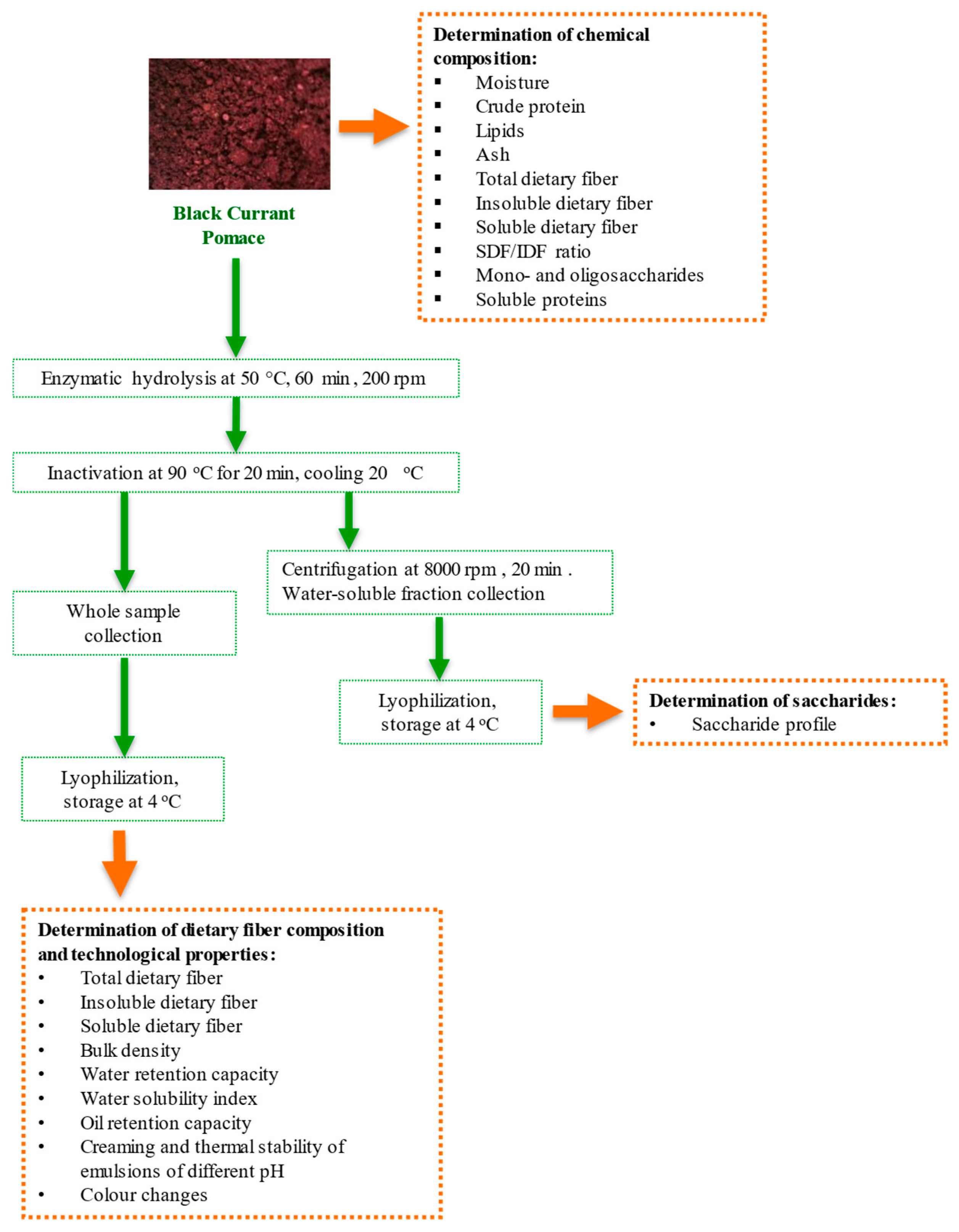
2.1. Black Currants Pomace Characteristics
2.2. Enzymatic Treatment of Black Currants Pomace
2.3. Chemical Composition Determination
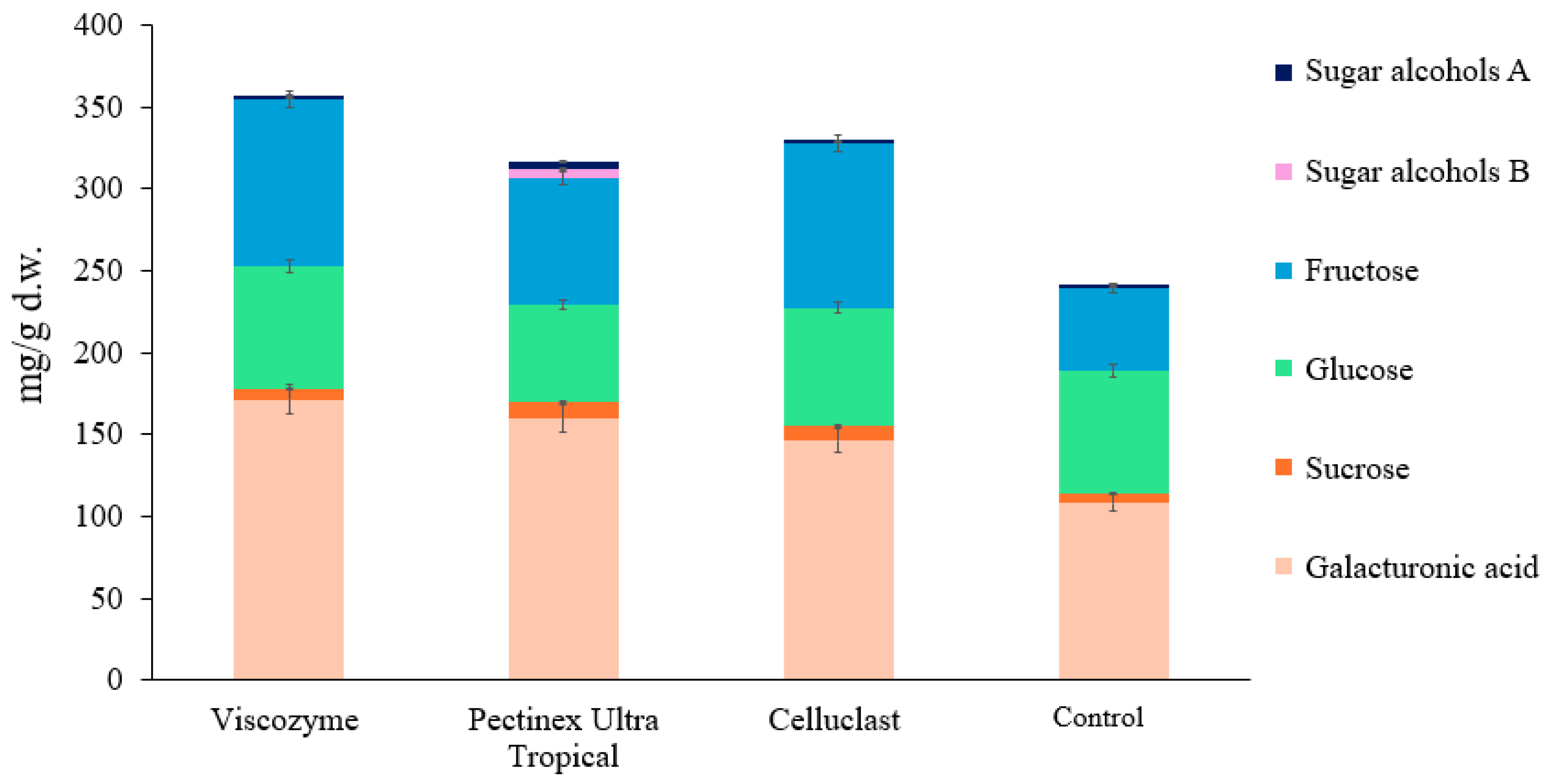
2.4. Analysis of Saccharides
2.5. The Evaluation of Technological Properties
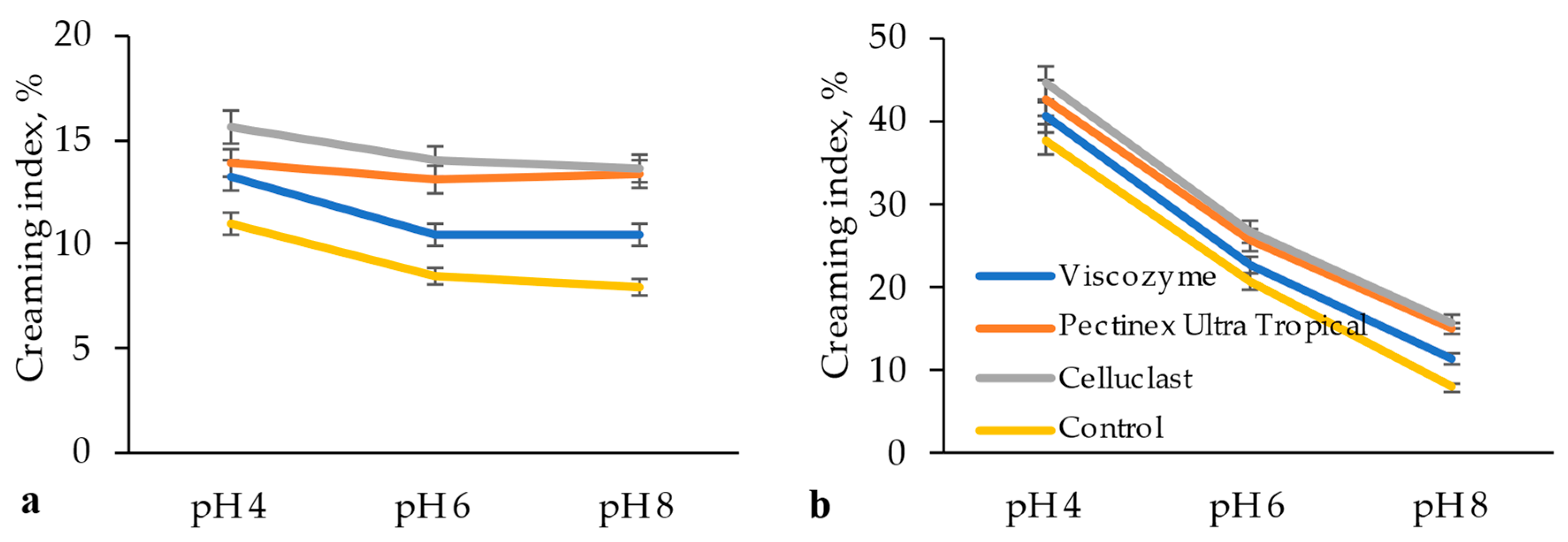
2.6. Evaluation of Emulsion Stability
2.7. Color Analysis
2.8. Determination of Water-Soluble Protein Content
2.9. Statistical Analyses
3. Results and Discussion
3.1. Chemical Composition of BCP
3.2. DF Composition of Enzymatically Treated BCP
3.3. Technological Properties of Modified BCP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCP | black currant pomace |
| TDF | total dietary fiber |
| SDS | soluble dietary fiber |
| IDF | insoluble dietary fiber |
| ORC | oil retention capacity |
| WSI | water solubility index |
| WRC | eater retention capacity |
References
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, Antioxidant and Hypoglycemic Activities of Degraded Polysaccharides from Blackcurrant (Ribes nigrum L.) Fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of Berry Pomace to Design Functional Foods. Food Rev. Int. 2023, 39, 3204–3224. [Google Scholar] [CrossRef]
- Al Faruq, A.; Farahnaky, A.; Torley, P.J.; Buckow, R.; Eri, R.; Majzoobi, M. Sustainable Approaches to Boost Soluble Dietary Fibre in Foods: A Path to Healthier Foods. Food Hydrocoll. 2025, 162, 110880. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the “Fiber Gap”. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and Characterisation of Dietary Fibre from Blackcurrant Pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Ayuso, P.; Peñalver, R.; Quizhpe, J.; Rosell, M.d.l.Á.; Nieto, G. Broccoli, Artichoke, Carob and Apple By-Products as a Source of Soluble Fiber: How It Can Be Affected by Enzymatic Treatment with Pectinex® Ultra SP-L, Viscozyme® L and Celluclast® 1.5 L. Foods 2024, 14, 10. [Google Scholar] [CrossRef]
- Nguyen, T.T.A.; Nguyen, H.H.; Ton, N.M.N.; Tran, T.T.T.; Le, V.V.M. Enzymatic Treatment of Pineapple Pomace and Its Application into Fiber-Rich Biscuit Making. J. Agric. Food Res. 2024, 15, 100936. [Google Scholar] [CrossRef]
- Spadoni Andreani, E.; Karboune, S. Comparison of Enzymatic and Microwave-Assisted Alkaline Extraction Approaches for the Generation of Oligosaccharides from American Cranberry (Vaccinium macrocarpon) Pomace. J. Food Sci. 2020, 85, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Baskaya-Sezer, D. The Characteristics of Microwave-Treated Insoluble and Soluble Dietary Fibers from Grape and Their Effects on Bread Quality. Food Sci. Nutr. 2023, 11, 7877–7886. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic Review on Modification Methods of Dietary Fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Jagelaviciute, J.; Basinskiene, L.; Cizeikiene, D.; Syrpas, M. Technological Properties and Composition of Enzymatically Modified Cranberry Pomace. Foods 2022, 11, 2321. [Google Scholar] [CrossRef]
- Iqbal, S.; Tirpanalan-Staben, Ö.; Franke, K. Modification of Dietary Fibers to Valorize the By-Products of Cereal, Fruit and Vegetable Industry—A Review on Treatment Methods. Plants 2022, 11, 3466. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry Pomace Valorization into Food Ingredients by Enzyme-Assisted Extraction: Process Optimization and Product Characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- Yoshida, B.Y.; Prudencio, S.H. Physical, Chemical, and Technofunctional Properties of Okara Modified by a Carbohydrase Mixture. LWT 2020, 134, 110141. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Total Dietary Fiber Assay Procedure. Megazyme 2017; pp. 1–19. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 1 May 2022).
- Bytautaitė, M.; Kitrytė-Syrpa, V.; Jonutė, P.; Endriulaitytė, U.; Petrikaitė, V.; Syrpas, M. Short-chain fructooligosaccharides synthesis using a commercial enzyme complex from Aspergillus sp. and anti-cancer activity on HCT116 and HT-29 cell lines. Food Biosci. 2024, 61, 104995. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of Carrot (Daucus carota Linn. Var. Sativa hoffm.) Pomace Insoluble Dietary Fiber with Complex Enzyme Method, Ultrafine Comminution, and High Hydrostatic Pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef]
- Jagelavičiutė, J.; Čižeikienė, D.; Bašinskienė, L. Enzymatic Modification of Apple Pomace and Its Application in Conjunction with Probiotics for Jelly Candy Production. Appl. Sci. 2025, 15, 599. [Google Scholar] [CrossRef]
- Keršiene, M.; Jasutiene, I.; Eisinaite, V.; Venskutonis, P.R.; Leskauskaite, D. Designing Multiple Bioactives Loaded Emulsions for the Formulations for Diets of Elderly. Food Funct. 2020, 11, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Quek, S.; Perera, C.O. Physicochemical Properties of Bread Dough and Finished Bread with Added Pectin Fiber and Phenolic Antioxidants. J. Food Sci. 2011, 76, H97–H107. [Google Scholar] [CrossRef]
- He, F. Bradford Protein Assay. Bio. Protoc. 2011, e45. [Google Scholar] [CrossRef]
- Schneeman, B.O. Soluble vs Insoluble Fiber: Different Physiological Responses. Food Technol. 1987, 47, 81–82. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Wang, J.; Guo, Z.; Li, Z.; Qiu, J.; Wang, L. Soluble and Insoluble Dietary Fiber at Different Ratios: Hydration Characteristics, Rheological Properties, and Ameliorative Effects on Constipation. Food Chem. X 2024, 24, 101996. [Google Scholar] [CrossRef]
- Reißner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef]
- Sezer, D.B.; Ahmed, J.; Sumnu, G.; Sahin, S. Green Processing of Sour Cherry (Prunus cerasus L.) Pomace: Process Optimization for the Modification of Dietary Fibers and Property Measurements. J. Food Meas. Charact. 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Šimkutė, S.; Bašinskienė, L.; Syrpas, M.; Čižeikienė, D. Composition and Technological Properties of Modified Lingonberry (Vaccinium vitis-idaea L.) Pomace. Appl. Sci. 2025, 15, 3661. [Google Scholar] [CrossRef]
- Gu, M.; Fang, H.; Gao, Y.; Su, T.; Niu, Y.; Yu, L. Characterization of Enzymatic Modified Soluble Dietary Fiber from Tomato Peels with High Release of Lycopene. Food Hydrocoll. 2020, 99, 105321. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Hamza, H.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Sindic, M.; Jiménez-Araujo, A. Enzymatic Conversion of Date Fruit Fiber Concentrates into a New Product Enriched in Antioxidant Soluble Fiber. LWT 2017, 75, 727–734. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Cha, M.; Shin, S.R.; Kim, K.S. Enzymatic Production of a Soluble-Fibre Hydrolyzate from Carrot Pomace and Its Sugar Composition. Food Chem. 2005, 92, 151–157. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and Functional Properties of Water-Soluble Dietary Fiber from Apple Pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Xiao, Q.; Yang, L.; Guo, J.; Zhang, X.; Huang, Y.; Fu, Q. Preparation, Structural Characterization, and Hypoglycemic Activity of Dietary Fiber from Sea Buckthorn Pomace. Foods 2024, 13, 3665. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Zhang, X.; Li, T.; Ren, D.; Lu, J. Physicochemical, Functional, and Microstructural Properties of Modified Insoluble Dietary Fiber Extracted from Rose Pomace. J. Food Sci. Technol. 2020, 57, 1421–1429. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional Properties, Bioactive Compounds, and In Vitro Gastrointestinal Digestion Study of Dried Fruit Pomace Powders as Functional Food Ingredients. LWT 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Azman, E.M.; House, A.; Charalampopoulos, D.; Chatzifragkou, A. Effect of Dehydration on Phenolic Compounds and Antioxidant Activity of Blackcurrant (Ribes nigrum L.) Pomace. Int. J. Food Sci. Technol. 2021, 56, 600–607. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 308869. [Google Scholar] [CrossRef] [PubMed]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Sahni, P.; Shere, D. Comparative Evaluation of Physico-Chemical and Functional Properties of Apple, Carrot and Beetroot Pomace Powders. Intl. J. Food. Ferment. Technol. 2018, 7, 317–323. [Google Scholar]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of Drying Methods on the Physical Properties and Microstructures of Mango (Philippine ‘carabao’ Var.) Powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry Dietary Fibre: Chemical Properties, Functional Evaluation and Prebiotic In Vitro Effect. LWT 2020, 134, 110140. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.B.; Hwang, K.E.; Song, D.H.; Ham, Y.K.; Kim, H.W.; Sung, J.M.; Kim, C.J. Effect of Apple Pomace Fiber and Pork Fat Levels on Quality Characteristics of Uncured, Reduced-Fat Chicken Sausages. Poult. Sci. 2016, 95, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Ma, Y.S. The Effect of Extrusion Processing on the Physiochemical Properties of Extruded Orange Pomace. Food Chem. 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Huc-Mathis, D.; Almeida, G.; Michon, C. Pickering Emulsions Based on Food Byproducts: A Comprehensive Study of Soluble and Insoluble Contents. J. Colloid Interface Sci. 2021, 581, 226–237. [Google Scholar] [CrossRef]
- Xu, W.; Sun, H.; Li, H.; Li, Z.; Zheng, S.; Luo, D.; Ning, Y.; Wang, Y.; Shah, B.R. Preparation and Characterization of Tea Oil Powder with High Water Solubility Using Pickering Emulsion Template and Vacuum Freeze-Drying. LWT 2022, 160, 113330. [Google Scholar] [CrossRef]
| Parameter | Content |
|---|---|
| Moisture, g/100 g | 7.97 ± 0.10 |
| Crude protein, g/100 g d.w. | 9.05 ± 0.33 |
| Soluble proteins, g/100 g d.w. | 5.09 ± 0.01 |
| Lipids, g/100 g d.w. | 13.85 ± 0.27 |
| Ash, g/100 g d.w. | 3.82 ± 0.02 |
| TDF, g/100 g d.w. | 49.24 ± 0.82 |
| IDF, g/100 g d.w. | 40.95 ± 0.78 |
| SDF, g/100 g d.w. | 8.29 ± 0.17 |
| SDF/IDF ratio | 1:5 |
| Mono-/oligosaccharides, g/100 g d.w. * | 24.04 ± 0.64 |
| Control | Viscozyme® L | Pectinex® Ultra Tropical | Celluclast® 1.5 L | |
|---|---|---|---|---|
| TDF, g/100 g d.w. | 49.24 ± 0.82 c | 37.22 ± 0. 74 a | 36.46 ± 0.33 a | 40.06 ± 0.90 b |
| Decrease of TDF, % | - | 24.42 ± 1.45 b | 25.95 ± 0.64 b | 18.64 ± 1.76 a |
| SDF, g/100 g d.w. | 8.29 ± 0.17 b | 3.33 ± 0.09 a | 3.29 ± 0.02 a | 8.88 ± 0.42 b |
| Decrease of SDF, % | - | 59.88 ± 1.11 a | 60.30 ± 0.22 a | - |
| IDF, g/100 g d.w. | 40.95 ± 0.78 c | 33.89 ± 0.65 b | 33.17 ± 0.31 b | 31.18 ± 0.48 a |
| Decrease of IDF, % | - | 17.24 ± 1.58 a | 18.99 ± 0.76 a | 23.86 ± 1.18 b |
| SDF/IDF ratio | 0.202 | 0.099 | 0.099 | 0.255 |
| Control | Viscozyme® L | Pectinex® Ultra Tropical | Celluclast® 1.5 L | |
|---|---|---|---|---|
 |  |  |  | |
| L* | 25.05 ± 0.39 b | 24.38 ± 0.35 b | 21.36 ± 0.64 a | 24.79 ± 0.21 b |
| a* | 19.26 ± 0.22 b | 20.19 ± 0.28 c | 15.94 ± 0.32 a | 21.08 ± 0.08 d |
| b* | 4.04 ± 0.05 b | 4.61 ± 0.11 d | 3.08 ± 0.04 a | 4.30 ± 0.05 c |
| ∆E | - | 1.28 ± 0.09 a | 5.06 ± 0.27 b | 1.87 ± 0.13 a |
| Chroma | 19.68 ± 0.22 b | 20.71 ± 0.30 c | 16.24 ± 0.32 a | 21.51 ± 0.08 d |
| ORC, g/g d.w. | 3.5 ± 0.1 d | 2.2 ± 0.1 a | 2.5 ± 0.2 ab | 3.2 ± 0.1 cd |
| WRC, g/g d.w. | 5.0 ± 0.2 a | 6.8 ± 0.2 c | 6.2 ± 0.1 b | 5.6 ± 0.3 a |
| WSI, % | 32.3 ± 1.2 a | 37.6 ± 1.7 b | 48.1 ± 0.1 c | 37.7± 2.1 b |
| Bulk density, g/mL | 0.17 ± 0.01 a | 0.16 ± 0.01 a | 0.18 ± 0.00 a | 0.17 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairė, A.; Jagelavičiūtė, J.; Bašinskienė, L.; Syrpas, M.; Čižeikienė, D. Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Black Currant (Ribes nigrum) Pomace. Appl. Sci. 2025, 15, 6207. https://doi.org/10.3390/app15116207
Kairė A, Jagelavičiūtė J, Bašinskienė L, Syrpas M, Čižeikienė D. Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Black Currant (Ribes nigrum) Pomace. Applied Sciences. 2025; 15(11):6207. https://doi.org/10.3390/app15116207
Chicago/Turabian StyleKairė, Aurelija, Jolita Jagelavičiūtė, Loreta Bašinskienė, Michail Syrpas, and Dalia Čižeikienė. 2025. "Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Black Currant (Ribes nigrum) Pomace" Applied Sciences 15, no. 11: 6207. https://doi.org/10.3390/app15116207
APA StyleKairė, A., Jagelavičiūtė, J., Bašinskienė, L., Syrpas, M., & Čižeikienė, D. (2025). Influence of Enzymatic Hydrolysis on Composition and Technological Properties of Black Currant (Ribes nigrum) Pomace. Applied Sciences, 15(11), 6207. https://doi.org/10.3390/app15116207







